Background: Thiazolidinediones may have insulin-sensitizing effects independent of the nuclear receptor PPARγ.
Results: A novel PPARγ-sparing thiazolidinedione ameliorated insulin resistance and inflammation in obese mice.
Conclusion: The insulin-sensitizing effects of thiazolidinediones are separable from the ability to bind PPARγ.
Significance: Identification of other molecular targets of thiazolidinediones may generate new therapeutics for treatment of insulin resistance and diabetes.
Keywords: Diabetes, Glucose Metabolism, Insulin Resistance, Lipogenesis, Liver Metabolism
Abstract
Currently approved thiazolidinediones (TZDs) are effective insulin-sensitizing drugs that may have efficacy for treatment of a variety of metabolic and inflammatory diseases, but their use is limited by side effects that are mediated through ectopic activation of the peroxisome proliferator-activated receptor γ (PPARγ). Emerging evidence suggests that the potent anti-diabetic efficacy of TZDs can be separated from the ability to serve as ligands for PPARγ. A novel TZD analog (MSDC-0602) with very low affinity for binding and activation of PPARγ was evaluated for its effects on insulin resistance in obese mice. MSDC-0602 treatment markedly improved several measures of multiorgan insulin sensitivity, adipose tissue inflammation, and hepatic metabolic derangements, including suppressing hepatic lipogenesis and gluconeogenesis. These beneficial effects were mediated at least in part via direct actions on hepatocytes and were preserved in hepatocytes from liver-specific PPARγ−/− mice, indicating that PPARγ was not required to suppress these pathways. In conclusion, the beneficial pharmacology exhibited by MSDC-0602 on insulin sensitivity suggests that PPARγ-sparing TZDs are effective for treatment of type 2 diabetes with reduced risk of PPARγ-mediated side effects.
Introduction
Clinically approved thiazolidinediones (TZDs4; pioglitazone and rosiglitazone) are effective insulin-sensitizing agents. However, their use is associated with side effects, including weight gain, edema, and increased risk of fractures (1, 2). Compound-specific effects, including liver toxicity with troglitazone (3) and increased risk of cardiac mortality with rosiglitazone (4, 5), have also arisen. These side effects of TZDs have limited their potential applications for a variety of inflammatory and obesity-related metabolic diseases. The future widespread use of TZDs as insulin sensitizers and for treatment of other metabolic and inflammatory diseases clearly requires a breakthrough that would allow better therapeutic profiles.
Although discovered without respect to mechanism, many TZDs are now known to be ligands for a nuclear receptor transcription factor, the peroxisome proliferator activated receptor γ (PPARγ) (6). Activation of PPARγ in adipose tissue is thought to mediate several of the beneficial effects of TZDs (7, 8). Unfortunately, there are clear data that many of the side effects of TZDs are also mediated through direct and ectopic activation of PPARγ. Various tissue-specific knockouts of PPARγ are protected from increased adiposity (adipocyte-specific (9, 10)), plasma fluid volume expansion (collecting duct-specific (11, 12)), and hepatic steatosis (hepatocyte-specific (13, 14)) in response to the strong PPARγ agonist, rosiglitazone.
Although TZDs are widely regarded as purely PPARγ agonists, evidence also exists for PPARγ-independent, anti-diabetic pharmacology (15, 16). Pioglitazone, although 10-fold less potent at activating PPARγ compared with rosiglitazone (6), is equally efficacious as an insulin-sensitizing agent and has superior effects on lipids, hepatic steatosis, and cardiovascular outcomes (17–19). Studies of isolated perfused liver, which expresses little PPARγ, have shown effects of TZDs on hepatic glucose production in a matter of minutes (20, 21). Supratherapeutic doses of rosiglitazone and pioglitazone also activate AMP-activated protein kinase (AMPK) within minutes in myocytes and adipocytes (22), which is again consistent with a non-genomic effect. Interestingly, TZDs are also known to bind specifically to mitochondrial membrane proteins and may modulate mitochondrial metabolism through this direct binding (23). We have hypothesized that TZDs with potent anti-diabetic pharmacology that do not activate PPARγ at physiologic concentrations (PPARγ-sparing TZDs (PsTZDs)) can be identified (24) and used to treat insulin resistance, diabetes, and other metabolic and inflammatory conditions with reduced risk of PPARγ-driven side effects.
Herein, we demonstrate that a PsTZD (MSDC-0602), which is in Phase 2 clinical trials, markedly improved measures of systemic insulin sensitivity and metabolism and reduced adipose tissue inflammation. We also provide evidence that at least some of the beneficial pharmacology of the TZDs on hepatocytes is direct and independent of PPARγ. These studies suggest that PsTZDs could prove efficacious for treatment of obesity-related derangements in insulin sensitivity and metabolism and provide the framework for further evaluation of PPAR-sparing mechanisms to treat aspects of metabolic disease.
EXPERIMENTAL PROCEDURES
Materials
MSDC-0602 was synthesized initially at Kalexsyn (Kalamazoo, MI) as part of a series of TZDs designed to have reduced ability to bind to PPARγ. Production scale synthesis of all TZDs for mouse studies was conducted by USV Ltd. (Mumbai, India), and TZDs were incorporated into the experimental diets by Research Diets Inc.
Animal Studies
Male mice were used in all studies. Liver-specific (LS)-PPARγ−/− mice were generated by intercrossing mice harboring floxed PPARγ alleles (9, 25) with mice expressing Cre recombinase in a liver-specific manner by using the albumin promoter (26) and extensively back-crossed into the C57BL/6 background. LS-PPARγ−/− mice were compared with littermate PPARγflox/flox mice not expressing Cre. Ob/ob and ob/+ control mice were purchased from The Jackson Laboratory. To cause diet-induced obesity (DIO), C57BL/6 wild-type or LS-PPARγ−/− mice were fed a diet providing 60% of its calories as fatty acids (Research Diets Inc. (catalog #D12492i)) starting at 6 weeks of age. DIO mice were compared with age-matched C57BL/6J male mice maintained on a matched low fat diet (10% fat; #D12450Bi).
For in vivo drug treatment, 6-week-old ob/ob mice were placed on a diet (Purina #5015) containing rosiglitazone, pioglitazone, or MSDC-0602 (300 ppm) for 4 weeks before sacrifice. This dose was chosen based on pilot studies showing that 300 ppm in diet resulted in a concentration of 2–5 μm MSDC-0602 in the blood of obese mice. DIO mice and that had been maintained on the high fat (60% fat) diet for 8 weeks were randomized to receive 60% fat diet containing MSDC-0602 (300 ppm) for the last 2 or 4 weeks of the trial or to receive 60% fat diet not containing MSDC-0602 for the duration. The dosage selected for these studies provides ∼30 mg/kg/day of MSDC-0602.
Mice were sacrificed for tissue collection after a 4-h fast. To examine insulin-stimulated protein phosphorylation, a subset of mice was injected intraperitoneal with human insulin (10 milliunits/g of body weight) 5 min before sacrifice, and tissue was dissected and snap-frozen for protein isolation.
Glucose and insulin tolerance test studies were performed as described (27). Tail blood glucose was determined at 0, 30, 60, and 120 min after challenge using a One-Touch Ultra glucometer (LifeScan, Inc.). Total area under the curve was calculated using the trapezoidal rule. All animal experiments were approved by the Animal Studies Committee of Washington University School of Medicine.
Hyperinsulinemic-Euglycemic Clamp Studies
The right jugular vein and carotid artery of DIO mice that were untreated or treated for 3 weeks with MSDC-0602 were surgically catheterized and mice recovered for 1 week. After a 5–6-h fast, hyperinsulinemic clamp studies were performed on conscious mice using the protocol adopted from the Vanderbilt Mouse Metabolic Phenotyping Center (28) by the University of Michigan Animal Phenotyping Core consisting of a 90-min equilibration period followed by a 120-min experimental period (t = 0–120 min). Insulin was infused at 2.5 milliunits/kg/min. To estimate insulin-stimulated glucose uptake in individual tissues, a bolus injection of 2-[1-14C]deoxyglucose (PerkinElmer Life Sciences) (10 μCi) was given at t = 78 min while continuously maintaining the hyperinsulinemic-euglycemic steady state. At the end of the experiment, animals were anesthetized with an intravenous infusion of sodium pentobarbital, and tissues were collected and immediately frozen in liquid nitrogen for later analysis of tissue 14C radioactivity.
Plasma insulin was measured using the Linco rat/mouse insulin ELISA kits. For determination of plasma radioactivity of [3-3H]glucose and 2-[1-14C]deoxyglucose, plasma samples were deproteinized and counted using a liquid scintillation counter. For analysis of tissue 2-[1-14C]deoxyglucose 6-phosphate, tissues were homogenized in 0.5% perchloric acid, and the supernatants were neutralized with KOH. Aliquots of the neutralized supernatant with and without deproteinization were counted for determination of the content of 2-[1-14C]deoxyglucose P.
Cell Culture Studies
Primary cultures of mouse hepatocytes were prepared as described (29). For isolation of hepatocytes from DIO mice, mice that had been fed a high fat diet for 12 weeks were used. Rates of fatty acid synthesis were assessed 2–3 h after cells were plated and were performed using [14C]acetate (30). TG synthesis rates were quantified by using [3H]glycerol in the presence of 300 μm oleate in the culture media as previously described (31, 32). To measure the rate of glucose output, after a pretreatment period of 18 h with MSDC-0602 or vehicle, the hepatocytes were washed extensively with PBS and then incubated for 3 h in 1 mm Krebs-Ringer-Hepes buffer containing 10 mm lactate and 0.5 μm glucagon with or without insulin (0.5 μm) and MSDC-0602. Glucose contents in the media were determined enzymatically (Sigma).
Hepatocytes from WT or LS-PPARγ−/− mice were isolated, plated, and then treated with vehicle (DMSO), rosiglitazone (1 μm), or MSDC-0602 (1 μm). Protein and RNA was collected 18 h after treatment. Rates of glucose production were determined in hepatocytes after 24 h of drug treatment.
3T3-L1 mouse preadipocytes were purchased from Zen-Bio. Preadipocytes were grown to confluence in complete medium containing 10% calf serum. Two days after reaching confluence, adipocyte differentiation was initiated by adding complete medium containing 10% fetal bovine serum and a hormone differentiation mixture (115 μg/ml methylisobutylxanthine, 10 μg/ml insulin, and 390 ng/ml dexamethasone). In addition to the differentiation mixture, cells were treated with vehicle (DMSO), rosiglitazone (0.1 μm), pioglitazone (0.1 μm), or MSDC-0602 (0.1 μm). RNA was collected after 48 h of drug treatment.
Plasma and Tissue Metabolite Quantification
Plasma glucose concentrations were determined using a colorimetric assay (Sigma) using plasma collected at time of sacrifice. Plasma insulin and adiponectin concentrations were determined by commercially available ELISAs from Crystal Chemical Co. and Linco Research Inc., respectively. Hepatic triglyceride content was determined using a colorimetric assay as previously described (29).
Western Blotting Analyses
Total cell proteins were collected using radioimmune precipitation assay buffer containing protease and phosphatase inhibitors. Western blotting studies were performed using antibodies directed against AKT (Cell Signaling), phospho-Ser-473 or phospho-Thr-308 AKT (Cell Signaling), fatty acid synthase (Abcam Inc.), glucose-6-phosphatase α (Santa Cruz Biotechnology), phosphoenolpyruvate carboxykinase (Cayman Chemical), and actin (Sigma). Densitometries of the bands were conducted using ImageJ software (NIH).
Quantitative RT-PCR
Total RNA was isolated using the RNAzol method (Tel-Test). Real-time PCR was performed using the ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, CA) and the SYBR Green kit. The sequence of the oligonucleotides used in quantitative real-time-PCR analyses can be provided upon request.
PPAR Binding Assays
TZD binding to the ligand binding domain of PPARγ was assessed in a LanthaScreenTM TR-FRET competitive binding assay performed according the protocol of the manufacturer (Invitrogen). IC50 values for the PPARγ LanthaScreen were determined using Gen5 software (BioTek Instruments, Inc.). R2 values for individual IC50 values ranged from 0.983 to 0.999 based on three separate assays for each compound.
Gal4-PPAR Activation Studies
HepG2 hepatoma cells were co-transfected by calcium phosphate coprecipitation with (i) expression vectors for Gal4-PPARγ (ligand binding domain only; a gift of J. Reddy (33)) or Gal4-PPARα (a gift of D. Kelly), (ii) heterologous firefly luciferase reporter construct driven by five copies of a Gal4 response element, and (iii) SV40-driven renilla luciferase reporter construct. Transfected cells were treated with PPARγ agonists (rosiglitazone and pioglitazone; Santa Cruz Biotechnology), PPARα agonist (GW7647; Cayman Chemical), or MSDC-0602 for 24 h. Cell lysate firefly and renilla luciferase activity was assessed 48 h later by using the Dual-Glo kit (Promega). Firefly luciferase activity was corrected for renilla luciferase activity, and DMSO (vehicle) values were normalized to 1.0.
Mitochondrial Binding
The 125I-labeled probe (MSDC-1101; see chemical structure Fig. 1E) was synthesized by coupling a carboxylic acid analog of pioglitazone to a p-azido-benzyl group containing ethylamine. The purified compound was iodinated carrier-free with Iodogen (Pierce). [125I]MSDC-1101 was purified on a C18 column and stored in the dark. Cross-linking reactions were incubated at room temperature for 15 min in a total volume of 40 μl (50 mm Tris, pH 8.0) containing 20 μl of mitochondrial membranes (1 μg/μl), 10 μl of 4% DMSO with or without competing TZDs (n = 3/concentration), and 10 μl (0.1 μCi) of carrier free 125I-labeled MSDC-1101. The reaction was stopped by UV exposure (180,000 μJ), cross-linked proteins were run on SDS/PAGE under reducing conditions, and the gels were dried and exposed to imaging film. Densitometries of the specifically labeled bands were conducted using ImageJ software (NIH).
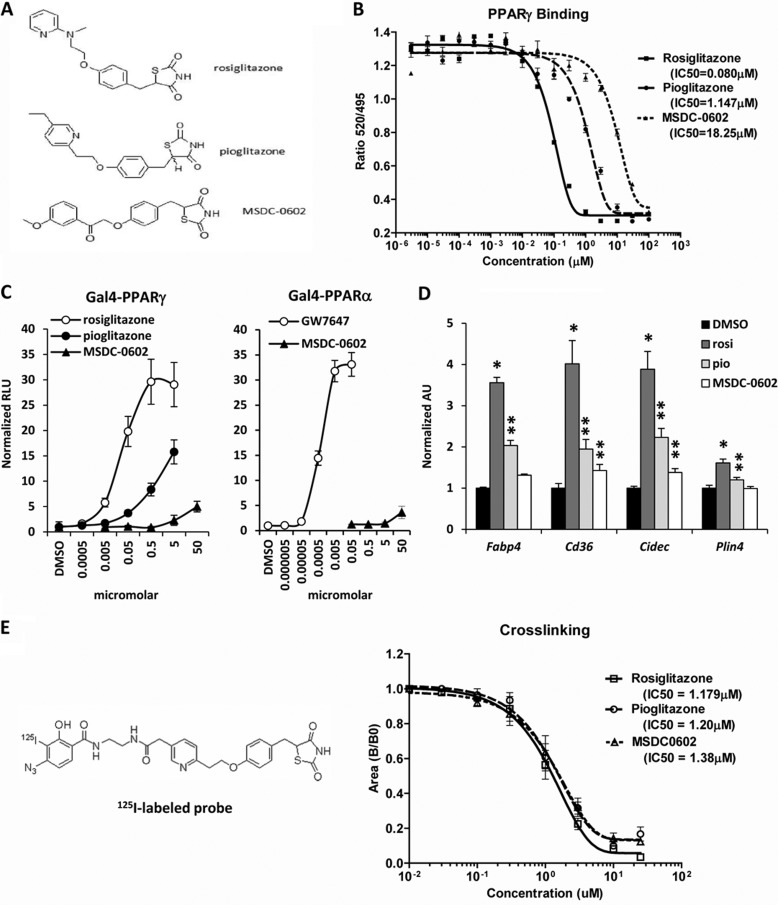
FIGURE 1.
Chemical structure of MSDC-0602. A, the chemical structures of rosiglitazone, pioglitazone, and MSDC-0602 are shown. B, the graph depicts the binding of the indicated TZDs to recombinant PPARγ protein in a Lantha-Screen TR FRET competitive binding assay. The results of one representative experiment (of three) are shown, and each point represents the average of three samples. C, the graphs represent the results of mammalian one-hybrid assays using Gal4-PPARγ or Gal4-PPARα expression constructs cotransfected into HepG2 cells with a UAS-TK-luciferase reporter. Values are expressed as raw luciferase units (RLU) and represent the average of three independent experiments performed in triplicate. D, the expression of the PPARγ target genes in 3T3-L1 adipocytes 48 h after the induction of differentiation and treatment with 0.1 μm concentrations of the indicated TZDs is shown. *, p < 0.05 versus DMSO control. **, p < 0.05 versus DMSO control and rosiglitazone-treated cells. rosi, rosiglitazone; pio, pioglitazone. E, the affinity of the indicated TZDs for mitochondrial binding is depicted in the graph, and the chemical structure of the cross-linker is shown. The results of one representative experiment (of three) are shown, and each point represents the average of three samples.
In Vitro Assays of Isolated Mitochondrial Respiration
Hepatic mitochondria from the caudate lobe of the liver were isolated from CO2-euthanized mice by sucrose gradient centrifugation (34). Mitochondrial respiration assays were performed at 37 °C using a water-jacketed Clark electrode (Hansatech Instruments). After measurement of basal (state 2) respiration, 1 mm ADP was added to isolated mitochondria in respiration buffer, and maximal (state 3) respiration was defined. Thereafter, state 4 (ADP-depleted) respiration was mimicked by adding 1 μg/ml oligomycin to inhibit ATP synthase. Uncoupled respiration was measured using 5 μm carbonylcyanide-p-trifluoromethoxyphenylhydrazone; Sigma).
Statistical Analyses
Statistical comparisons were made using analysis of variance or a t test. All data are presented as the means ± S.E., with a statistically significant difference defined as a p value <0.05.
RESULTS
MSDC-0602 Is a Ps-TZD
MSDC-0602 is a modified TZD with alterations in the carbon backbone (Fig. 1A) that limit the ability to bind PPARγ (Fig. 1B). The IC50 for PPARγ binding was 0.08 μm for rosiglitazone, 1.147 μm for pioglitazone, and 18.25 μm for MSDC-0602. Using a Gal4-PPARγ construct containing the ligand binding domain of mouse PPARγ, we confirmed that MSDC-0602 only minimally activated Gal4-PPARγ in HepG2 cells even at a concentration of 50 μm, whereas rosiglitazone and pioglitazone activated the Gal4-PPARγ construct at much lower concentrations (Fig. 1C). MSDC-0602 also did not activate Gal4-PPARα (Fig. 1C) or PPARδ5 at physiologic concentrations. The EC50 for activation of PPARα or PPARδ is >100 μm for MSDC-0602, rosiglitazone, and pioglitazone. Rosiglitazone and pioglitazone (0.1 μm) induced the expression of the known PPARγ target genes Fabp4, Cidec, Cd36, and Plin4 in differentiating 3T3-L1 adipocytes (Fig. 1D). In contrast, MSDC-0602 (0.1 μm) did not lead to a significant induction in the expression of these genes. Finally, TZDs are also known to bind competitively to mitochondrial membranes (23), and rosiglitazone, pioglitazone, and MSDC-0602 bound to mitochondrial membranes with an equivalent affinity (Fig. 1E). These data demonstrate that, although these TZDs differ greatly in their ability to bind and activate PPARγ, they each have similar affinity for mitochondrial binding, which we hypothesize mediates at least some of the anti-diabetic pharmacology of TZDs.
Comparison of the Anti-diabetic Effects of MSDC-0602 with Other TZDs
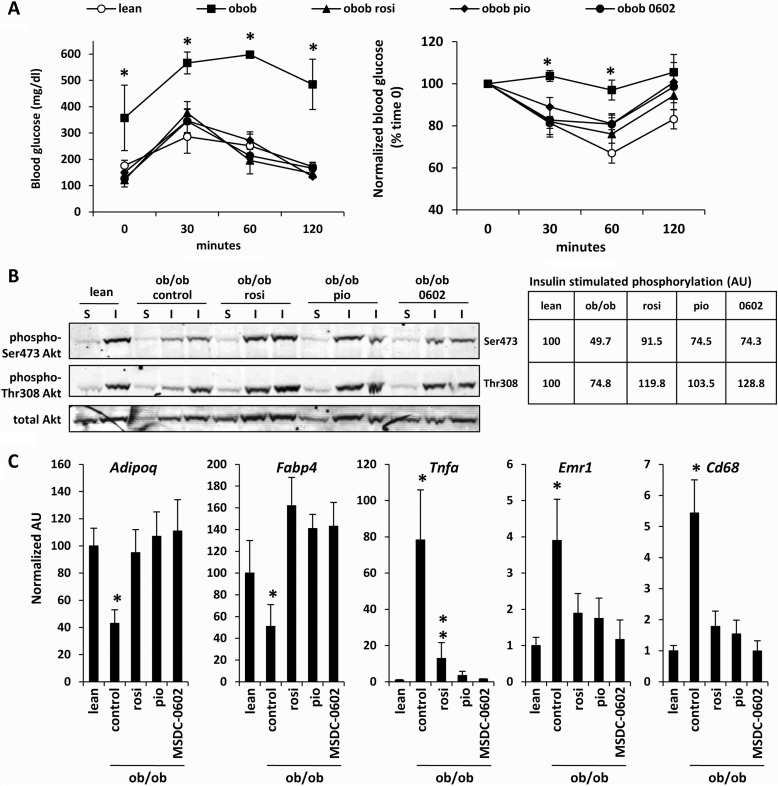
We compared the efficacy of MSDC-0602 with pioglitazone and rosiglitazone in ob/ob mice. Treatment with any of the three TZDs corrected plasma glucose, non-esterified fatty acids, triglycerides, cholesterol, and insulin concentrations, which were elevated in ob/ob mice compared with lean controls (Table 1). Treatment with MSDC-0602, pioglitazone, and rosiglitazone also significantly improved glucose and insulin tolerance in ob/ob mice (Fig. 2A). Consistent with this, insulin-mediated phosphorylation of Ser-473 and Thr-308 of Akt in gastrocnemius muscle was significantly improved by TZD treatment (Fig. 2B). Thiazolidinediones are known to increase insulin sensitivity by acting in adipose tissue to increase expression of beneficial adipokines and to reduce inflammation and macrophage infiltration. All three TZDs corrected the expression of Fabp4, adiponectin (Adipoq), and TNFα (Tnfa), which were dysregulated in ob/ob adipose tissue compared with lean controls (Fig. 2C). Plasma adiponectin was also increased by all three TZDs (Table 1). MSDC-0602, rosiglitazone, and pioglitazone also reduced the expression of macrophage cell surface markers (Cd68 and F4/80 (Emr1)) (Fig. 2C). Collectively, these data indicate that these TZDs are equally efficacious with similar pharmacology despite marked differences in the ability to bind and activate PPARγ.
TABLE 1.
Effect of thiazolidinedione treatment on plasma metabolite and hormone concentrations in ob/ob mice
Values represent the mean ± S.E. for plasma and tissue metabolites or plasma hormone concentrations. (n ≥ 7 for each group). Groups denoted by different letters are significantly different (p < 0.05).
| Parameter | Lean |
ob/ob |
|||
|---|---|---|---|---|---|
| Control diet | Control diet | Rosiglitazone | Pioglitazone | MSDC-0602 | |
| Glucose (mg/dl) | 176.0 ± 20.5A | 357.5 ± 124.3B | 121.3 ± 13.3C | 150.0 ± 26.1A | 127.0 ± 5.3C |
| NEFA (mmol/liter) | 1.67 ± 0.09A | 2.53 ± 0.28B | 1.33 ± 0.28A | 1.19 ± 0.10A | 1.30 ± 0.10A |
| TG (mg/dl) | 103.8 ± 8.4A | 249.2 ± 35.16B | 72.8 ± 9.8A | 80.4 ± 7.0A | 72.2 ± 4.6A |
| Cholesterol (mg/dl) | 143.9 ± 9.3A | 293.7 ± 41.0B | 190.4 ± 18.9C | 184.2 ± 11.5C | 163.3 ± 14.7AC |
| Insulin (ng/ml) | 2.21 ± 0.23A | 12.08 ± 1.06B | 2.72 ± 0.16A | 3.03 ± 0.18A | 2.99 ± 0.25A |
| Adiponectin (μg/ml) | 11.6 ± 1.68A | 10.2 ± 1.38A | 34.0 ± 1.84B | 33.2 ± 0.69B | 31.9 ± 0.84B |
FIGURE 2.
TZD treatment improves insulin sensitivity and remedies adipose tissue inflammation in ob/ob mice. A, glucose and insulin tolerance curves for lean, ob/ob (control diet), and ob/ob mice treated with the indicated TZDs for 4 weeks are shown. *, p < 0.05 versus all other groups. rosi, rosiglitazone; pio, pioglitazone. B, representative Western blots using gastrocnemius lysates from mice injected with saline (S) or insulin (I) before sacrifice and the antibodies listed at the left are shown. The table inset represents the normalized and corrected (to total Akt) band intensity for insulin-stimulated Akt phosphorylation for n = 4 per group. C, expression of the indicated genes in epididymal adipose tissue is depicted in the graphs. n = 6 per group. *, p < 0.05 versus lean and TZD-treated mice. **, p < 0.05 versus ob/ob control and lean control mice.
Administration of MSDC-0602 Improves Insulin Sensitivity of DIO Mice
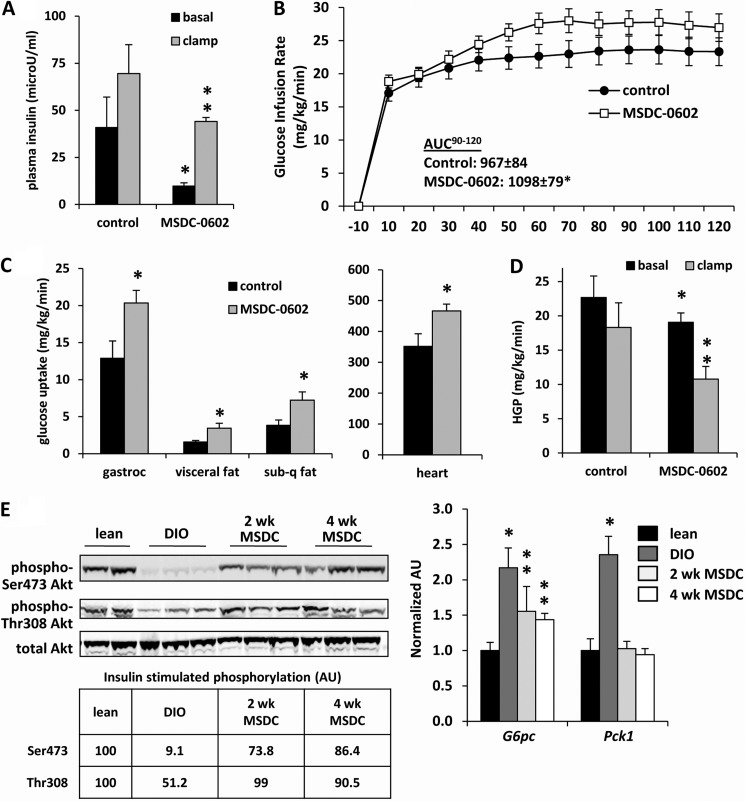
We also sought to evaluate the efficacy of MSDC-0602 endpoints germane to insulin sensitivity in DIO mice, which are more relevant to human obesity. At the time of sacrifice, plasma glucose and insulin levels were significantly lower, and adiponectin was increased in obese mice treated with MSDC-0602 for 2 or 4 weeks compared with DIO mice not receiving the drug (Table 2). Hyperinsulinemic-euglycemic clamp studies demonstrated that treatment of DIO mice with MSDC-0602 reduced basal plasma insulin concentration (Fig. 3A) and, under hyperinsulinemic conditions, MSDC-0602 increased the glucose rate of infusion (Fig. 3B). MSDC-0602 also increased glucose uptake into gastrocnemius, adipose tissue, and heart (Fig. 3C). MSDC-0602 treatment enhanced the suppression of hepatic glucose production by insulin (Fig. 3D). These data indicate that MSDC-0602 treatment improves insulin sensitivity in multiple tissues including striated muscle, adipose tissue, and liver. The phosphorylation status of Ser-473 and Thr-308 residues of Akt/PKB in liver (Fig. 3E) and skeletal muscle (data not shown) of DIO mice after a bolus insulin injection was again markedly improved by either 2 or 4 weeks of MSDC-0602 administration. Consistent with this, the expression of the gluconeogenic enzymes phosphoenolpyruvate carboxykinase (Pck1) and glucose-6-phosphatase (G6pc), which is suppressed by insulin action and elevated in DIO liver, was reduced by treatment with MSDC-0602 (Fig. 3E).
TABLE 2.
Effect of MSDC-0602 treatment on plasma metabolite and hormone concentrations and liver TG content in DIO mice
Values represent the mean ± S.E. for plasma and tissue metabolites or plasma hormone concentrations. n ≥ 8 for each group.
| Parameter | Lean | DIO control | DIO 2 wk MSDC | DIO 4 wk MSDC |
|---|---|---|---|---|
| Weight (g) | 31.1 ± 0.45 | 43.9 ± 1.65a | 45.1 ± 1.83a | 42.7 ± 1.91a |
| Glucose (mg/dl) | 149.6 ± 3.10 | 180.5 ± 5.32a | 145.2 ± 5.71 | 152.8 ± 6.17 |
| Insulin (ng/ml) | 0.79 ± 0.09 | 4.34 ± 0.93a | 1.56 ± 0.36b | 1.61 ± 0.24b |
| TG (mg/dl) | 99.2 ± 19.6 | 146.5 ± 26.4 | 103.9 ± 21.9 | 122.2 ± 20.9 |
| Adiponectin (mg/dl) | 7.3 ± 0.72 | 15.6 ± 1.54a | 22.5 ± 2.20b | 27.05 ± 1.60b |
| Liver TG (mg/g) | 13.3 ± 1.2 | 121.8 ± 10.8a | 95.4 ± 21.6a | 109.7 ± 15.1a |
a p < 0.05 versus lean controls.
b p < 0.05 versus DIO control and lean controls.
FIGURE 3.
MSDC-0602 treatment improves whole body and tissue insulin sensitivity in DIO mice. Hyperinsulinemic clamp studies performed after 3 weeks of treatment with MSDC-0602 in DIO mice demonstrate a significant improvement in plasma insulin concentration (A), glucose infusion rate (B), tissue glucose uptake (C), and hepatic glucose production (D). n = 11 per group. *, p < 0.05 versus DIO control diet mice. **, p < 0.05 versus clamped DIO control mice and basal values for MSDC-0602-treated mice. AUC, area under the curve. E, representative Western blots using liver lysates from mice injected with insulin (I) before sacrifice and the antibodies listed at the left are shown. The table inset represents the normalized and corrected (to total Akt) band intensity for insulin-stimulated Akt phosphorylation for n = 3 per group. Expression of the indicated genes in liver is depicted in the graphs. n = 8 per group. *, p < 0.05 versus lean and MSDC-0602-treated mice. **, p < 0.05 versus DIO control and lean control mice. AU, absorbance units.
MSDC-0602 Treatment Increases Mitochondrial Respiratory Rate and Leads to Reduced Expression of Genes Encoding Lipogenic Enzymes
Oxygen consumption rates by liver mitochondria supplied with succinate as a metabolic substrate were increased by 4 weeks of treatment with MSDC-0602 (Fig. 4A). This was observed under basal conditions as well as when respiration was maximally stimulated by ADP or uncoupled with a chemical uncoupler. These data are consistent with a mitochondrial site of action of MSDC-0602 and suggest that treatment with this novel TZD enhances the mitochondrial oxidative capacity in hepatocytes.
FIGURE 4.
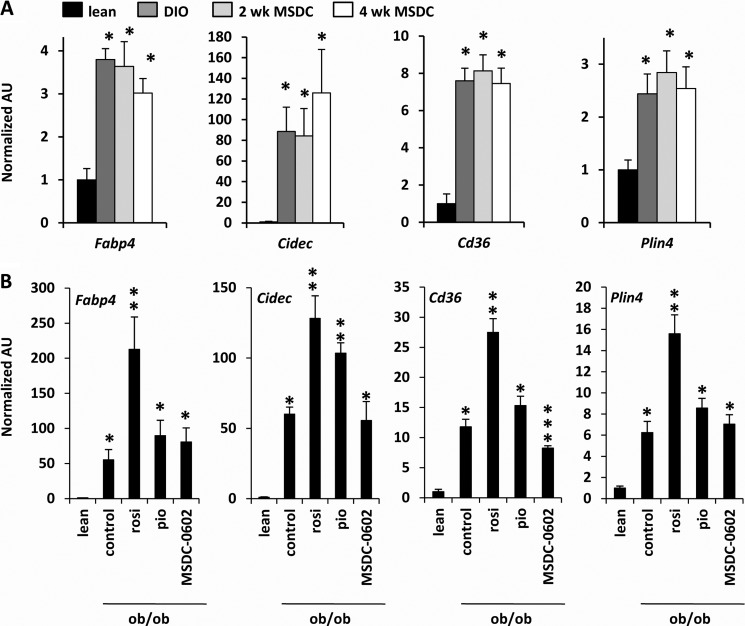
Mitochondrial oxygen consumption is increased and dysregulated lipogenesis in DIO mice is corrected by MSDC-0602 treatment. A, mitochondrial respiration was performed on isolated mitochondria and using succinate-rotenone as a substrate. *, p < 0.05 versus other groups mice. FCCP, carbonylcyanide-p-trifluoromethoxyphenylhydrazone. B, the graph depicts the hepatic expression of genes encoding glycolytic and lipogenic enzymes. n = 8 per group. *p < 0.05 versus lean mice. **p < 0.05 versus DIO and lean mice. Inset, representative Western blots using liver lysates and the antibodies listed at the left are shown. The table represents the normalized and corrected (to actin) band intensity for FAS (n = 6 per group). AU, absorbance units. C, the graph depicts rates of fatty acid synthesis in hepatocytes. *, p < 0.05 versus lean mice. **, p < 0.05 versus DIO and lean mice. D, the graph depicts the hepatic expression of genes encoding enzymes involved in TG esterification. *, p < 0.05 versus lean mice. **, p < 0.05 versus DIO and lean mice. E, the graph depicts rates of triglyceride synthesis in hepatocytes. *, p < 0.05 versus lean mice. **, p < 0.05 versus DIO and lean mice.
Obesity is known to increase hepatic de novo lipogenesis, and quantitative RT-PCR demonstrated that several genes involved in glycolysis (Gk and Lpk) and fatty acid synthesis (Acly, Fasn, Me1, and Thrsp) were robustly induced in liver of untreated DIO mice compared with lean controls (Fig. 4B). Treatment with MSDC-0602 for either 2 or 4 weeks led to a significant reduction in the expression of each of these genes in DIO mice, and this was confirmed at the protein level for FAS (Fig. 4B). The expression of many of these genes is known to be regulated by SREBP-1 (Srebf1) (35), the expression of which was up-regulated in liver of DIO mice compared with lean controls but down-regulated by administration of MSDC-0602 (Fig. 4B). As predicted by these changes in gene expression, rates of de novo fatty acid synthesis from [14C]acetate in isolated DIO hepatocytes were significantly suppressed by in vivo MSDC-0602 treatment (Fig. 4C). MSDC-0602 also significantly suppressed expression of enzymes involved in triglyceride synthesis (Lpin1 and Scd1) (Fig. 4D). Consistent with this, rates of oleate-stimulated TG synthesis in isolated hepatocytes were suppressed by in vivo MSDC-0602 (Fig. 4E). These data suggest that a molecular signature of MSDC-0602-mediated insulin sensitization is a down-regulation of lipogenic pathways regulated by SREBP-1.
Administration of rosiglitazone is known to activate the hepatic expression of PPARγ target genes in obese mice and can actually exacerbate hepatic steatosis (13, 14). MSDC-0602 did not affect the hepatic expression of various PPARγ genes that promote lipid storage including Fabp4, Cidec, Cd36, and Plin4 in DIO (Fig. 5A) or ob/ob (Fig. 5B) mice. In contrast, rosiglitazone increased the expression of Fabp4, Cidec, Cd36, and Plin4 in ob/ob liver, whereas pioglitazone also induced the expression of Cidec (Fig. 5B). Collectively, these data indicate that the PsTZD markedly improves measures of hepatic and systemic insulin sensitivity and does not lead to PPARγ activation in liver.
FIGURE 5.
MSDC-0602 does not induce hepatic expression of PPARγ target genes. A, the graphs depict the hepatic expression of PPARγ target genes in lean or DIO mice treated as indicated. n = 8 per group. *, p < 0.05 versus lean mice. B, the graphs depict the hepatic expression of PPARγ target genes in lean or ob/ob mice treated with TZDs. n = 6 per group. *, p < 0.05 versus lean mice. **, p < 0.05 versus lean, ob/ob control, and ob/ob MSDC-0602-treated mice. AU, absorbance units; rosi, rosiglitazone; pio, pioglitazone.
MSDC-0602 Suppresses Gluconeogenesis and Lipogenesis in Primary Hepatocytes
To determine whether MSDC-0602 might affect lipogenesis and gluconeogenesis directly, hepatocytes from drug-naïve DIO mice were isolated and treated for 18 h with MSDC-0602 or rosiglitazone. The expression of mRNA and protein of the lipogenic and gluconeogenic enzymes that are induced in DIO hepatocytes was diminished by MSDC-0602 or rosiglitazone treatment (Fig. 6A). Consistent with these signaling and protein expression results, MSDC-0602 treatment of isolated hepatocytes reduced rates of glucose production (Fig. 6B) and de novo lipogenesis (data not shown). These data are consistent with a direct hepatic effect of MSDC-0602 on metabolic pathways that are dysregulated in insulin-resistant hepatocytes.
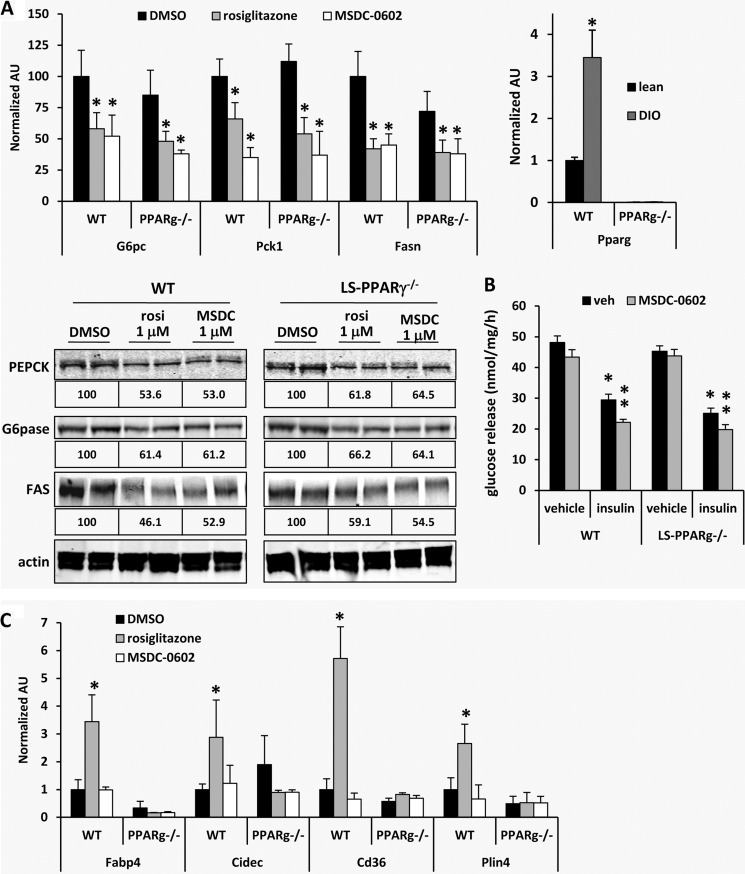
FIGURE 6.
MSDC-0602 suppresses gluconeogenesis and lipogenesis in primary hepatocytes independent of PPARγ. A, the graphs depict the expression of the indicated genes in mouse hepatocytes from WT or LS-PPARγ−/− DIO mice treated in vitro with vehicle (DMSO), rosiglitazone (1 μm), or MSDC-0602 (1 μm) for 18 h. *, p < 0.05 versus DMSO control hepatocytes. Representative Western blots using lysates from mouse hepatocytes from WT or LS-PPARγ−/− DIO mice treated in vitro with vehicle (DMSO), rosiglitazone (1 μm), or MSDC-0602 (1 μm) for 18 h and the antibodies listed at the left are shown. The values below each image represent the normalized and corrected (to actin) band intensity for the blot and group above (n = 4 per group). B, the graph depicts rates of glucose release by hepatocytes isolated from DIO mice treated for 24 h with vehicle (DMSO) or MSDC-0602 (5 μm). *, p < 0.05 versus DMSO control hepatocytes. C, the graphs depict the expression of PPARγ target genes in hepatocytes from WT or LS-PPARγ−/− DIO mice treated in vitro with vehicle (DMSO), rosiglitazone (1 μm), or MSDC-0602 (1 μm) for 18 h. *, p < 0.05 versus DMSO control hepatocytes. AU, absorbance units.
To determine the requirement for PPARγ in this response to TZDs, LS PPARγ−/− mice were generated and induced to become obese with high fat diet. The expression of PPARγ, which was increased in WT mice by DIO, was reduced by more than 99% in isolated LS-PPARγ−/− hepatocytes (Fig. 6A). Consistent with PPARγ-independent effects on insulin sensitivity, MSDC-0602 and rosiglitazone were equally effective at suppressing lipogenic and gluconeogenic enzyme expression (Fig. 6A) and hepatocyte glucose production (Fig. 6B) in hepatocytes isolated from DIO WT and LS-PPARγ−/− mice. On the other hand, hepatocytes from diet-induced obese LS-PPARγ−/− mice were refractory to the increase in PPARγ target gene expression after treatment with rosiglitazone, and again, MSDC-0602 did not induce PPARγ target gene expression (Fig. 6C). These data demonstrate that, although PPARγ is required for rosiglitazone to exacerbate hepatic “adiposis,” this nuclear receptor is dispensable for the beneficial effects of MSDC-0602 and other TZDs on hepatic lipogenesis and gluconeogenesis.
DISCUSSION
Although the past decade has proven that the TZDs are effective insulin-sensitizing agents, no new therapeutics with this mode of action have been approved since pioglitazone in 1999 due in part to side effects observed during clinical trials (24). Traditional drug discovery programs have been focused on the identification of direct, high affinity PPARγ agonists. However, of the two approved TZDs, pioglitazone is 10-fold less potent at activating PPARγ yet has equivalent or even superior therapeutic effects on hepatic steatosis, circulating lipid profile, and glycemia with fewer reported side effects (18, 36, 37). Mechanistic studies have demonstrated that many of the side effects of TZDs are mediated through ectopic activation of PPARγ. For example, mice lacking PPARγ in the collecting ducts of the kidney are protected from the plasma volume expansion and weight gain caused by rosiglitazone (11, 12). Activation of PPARγ is also the molecular trigger driving white adipose tissue expansion (9, 10). Finally, administration of high doses of rosiglitazone to obese mice is associated with an activation of PPARγ target gene expression profile in hepatocytes, which is abrogated by liver-specific deletion of PPARγ (this study and Refs. 13 and 14).
With these data in mind, we have designed TZD analogs with modifications to the carbon backbone to impair binding to PPARγ with the hypothesis that the anti-diabetic efficacy of TZDs can be separated from the PPARγ-activating functions to minimize the likelihood of side effects. The current studies reveal that the PsTZD, MSDC-0602, lowers blood glucose and insulin concentration, enhances hepatic and systemic measures of insulin sensitivity, and increases the circulating concentration of beneficial adipokines in obese rodents. These beneficial effects are likely translatable, as a Phase 2a clinical trial with a first generation PsTZD, MSDC-0160, demonstrated beneficial effects on glucose, insulin, circulating lipids, and blood pressure without causing weight gain and or blood volume expansion (38). These data indicate that a TZD with a PPARγ-sparing profile promotes systemic insulin sensitivity and has positive effects on adipocyte inflammation and hepatic lipid metabolism while potentially reducing other PPARγ-driven and dose-limiting side effects.
Recently, Choi et al. (39) have demonstrated that PPARγ activity can be modulated by TZDs that have reduced ability to interact with the ligand binding domain of PPARγ. These compounds instead bind PPARγ through other motifs to promote a conformational change that prevents phosphorylation by CDK5, a kinase that is activated by inflammatory signaling cascades in obesity and insulin resistance. Abrogating CDK5-mediated phosphorylation of PPARγ selectively enhanced its activity toward a subset of PPARγ target genes encoding important metabolic regulators, including adiponectin, that are believed to mediate anti-diabetic effects of TZDs. On the other hand, we have found that MSDC-0602 spares PPARγ binding altogether and thus likely does not affect CDK5-mediated phosphorylation of PPARγ. In support of this, the data obtained using LS-PPARγ knock-out mice indicate that PPARγ is dispensable altogether for the effects of MSDC-0602 on lipogenesis and gluconeogenesis.
If MSDC-0602 improves insulin sensitivity and hepatic metabolism independent of PPARγ phosphorylation or activation, what is the molecular mechanism of action of this compound? Our current hypothesis is that this pharmacology occurs after the direct interaction of the compound with a binding site in the mitochondrion. During the development of pioglitazone, an unbiased approach identified a mitochondrial binding site for [3H]pioglitazone (23). Initial work suggested that the mitochondrial target of pioglitazone was a protein termed mitoNEET (23), a protein of some interest as it contains a redox active iron-sulfur cluster and may be involved in regulating oxidative metabolism (40, 41). In unpublished studies, however, we have shown the binding of pioglitazone to mitochondria continues unabated in mitoNEET knock-out tissue. Experimentation to identify the protein(s) mediating the mitochondrial binding and to characterize its importance to the pharmacology of these novel agents is being conducted.
Regardless of the molecular target, the present data demonstrate that the effects MSDC-0602 on systemic insulin sensitivity and adipose tissue inflammation are comparable to equal doses of the two clinically effective TZDs. Hyperinsulinemic clamp studies detected strong effects of MSDC-0602 on insulin sensitivity in striated muscle, adipose tissue, and liver of DIO mice. The beneficial effects of MSDC-0602 treatment on skeletal muscle or liver could be indirect through the effects on systemic metabolism, as MSDC-0602 reduced circulating hyperinsulinemia and hyperglycemia, which are known to contribute to insulin resistance and activate hepatic lipogenesis (42). The increase in adiponectin concentration elicited by MSDC-0602 administration may also explain some of the observed suppression of hepatic lipogenesis and gluconeogenesis and increased skeletal muscle insulin sensitivity, as this adipokine is well established to play an important role in the insulin-sensitizing effects of TZDs (7, 43). Although generally described as a “PPARγ target gene,” several studies have shown that PPARγ does not mediate the induction of adiponectin expression during adipocyte differentiation and that other transcription factors control its expression (for review, see Ref. 44). Moreover, the regulation of adiponectin expression is not related to PPARγ affinity of the TZDs (45). There are a number of insulin sensitizing approaches unrelated to PPARγ biology that can normalize adiponectin expression in insulin-resistant rodents. Last, non-genomic control of adiponectin production by TZDs by regulation of its secretion has also been described (46). Collectively, these data suggest that regulation of adiponectin production by TZDs and the beneficial effects elicited by increased adiponectin secretion may not require activation of PPARγ.
Our data also suggest a direct hepatic effect of MSDC-0602, as treatment of isolated hepatocytes suppressed the rates of gluconeogenesis and lipogenesis. This is consistent with previous work demonstrating a direct effect of TZDs on hepatic glucose production in isolated perfused livers, effects that were evident within 20 min of TZD infusion (20, 21). Importantly, the effects observed in this study were preserved in hepatocytes from LS-PPARγ−/−, mice demonstrating that MSDC-0602 does not require PPARγ to elicit its beneficial pharmacology on hepatocytes. Interestingly, the strong PPARγ agonist rosiglitazone also suppressed hepatic gluconeogenic and lipogenic gene expression in PPARγ−/− hepatocytes, indicating that rosiglitazone, in addition to being a potent PPARγ agonist, also exhibits PPARγ-independent pharmacology. Whether the beneficial effects of TZDs can also be maintained in adipocytes completely lacking PPARγ cannot be determined due to the requirement for PPARγ in adipocyte differentiation. However, the present data show that MSDC-0602 is less adipogenic in differentiating 3T3-L1 adipocytes (Fig. 1D). These data demonstrate the potential utility of MSDC-0602 for treatment of insulin resistance and diabetes but more importantly provide the framework for the discovery and development of new insulin sensitizers that can operate independent of the activation of PPARγ.
Acknowledgment
Clamp studies were performed by the University of Michigan Animal Phenotyping Core directed by Dr. Nathan Qi.
This work was supported, in whole or in part, by National Institutes of Health Grants R41 DK084596 and R42 AA021228 and the core services of the Digestive Diseases Research Core Center (Grant P30 DK52574) and the Nutrition Obesity Research Center (Grant P30 DK56341) at Washington University School of Medicine. This work was also supported by a grant from the Barnes Jewish Hospital Foundation. We wish to disclose that Drs. McDonald, Colca, and Kletzien are employees and significant stockholders of Metabolic Solutions Development Co. The drugs provided in this study were provided by Metabolic Solutions Development Co.
Z. Chen, P. A. Vigueira, K. T. Chambers, A. M. Hall, M. S. Mitra, N. Qi, W. G. McDonald, J. R. Colca, R. F. Kletzien, and B. N. Finck, unpublished data.
- TZD
- thiazolidinedione
- PPAR
- peroxisome proliferator activated receptor γ
- PsTZD
- PPARγ-sparing TZD
- DIO
- diet-induced obesity
- LS
- liver-specific
- TG
- triglyceride.
REFERENCES
- 1. Malinowski J. M., Bolesta S. (2000) Rosiglitazone in the treatment of type 2 diabetes mellitus. A critical review. Clin. Ther. 22, 1151–1168; discussion 1149–1150 [DOI] [PubMed] [Google Scholar]
- 2. Kahn S. E., Zinman B., Lachin J. M., Haffner S. M., Herman W. H., Holman R. R., Kravitz B. G., Yu D., Heise M. A., Aftring R. P., Viberti G. (2008) Rosiglitazone-associated fractures in type 2 diabetes. An analysis from a diabetes outcome progression trial (ADOPT). Diabetes Care 31, 845–851 [DOI] [PubMed] [Google Scholar]
- 3. Funk C., Pantze M., Jehle L., Ponelle C., Scheuermann G., Lazendic M., Gasser R. (2001) Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and troglitazone sulfate. Toxicology 167, 83–98 [DOI] [PubMed] [Google Scholar]
- 4. Starner C. I., Schafer J. A., Heaton A. H., Gleason P. P. (2008) Rosiglitazone and pioglitazone utilization from January 2007 through May 2008 associated with five risk-warning events. J. Manag. Care Pharm. 14, 523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh S., Loke Y. K. (2008) The safety of rosiglitazone in the treatment of type 2 diabetes. Expert. Opin. Drug Saf. 7, 579–585 [DOI] [PubMed] [Google Scholar]
- 6. Lehmann J. M., Moore L. B., Smith-Oliver T. A., Wilkison W. O., Willson T. M., Kliewer S. A. (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor-γ (PPARγ). J. Biol. Chem. 270, 12953–12956 [DOI] [PubMed] [Google Scholar]
- 7. Nawrocki A. R., Rajala M. W., Tomas E., Pajvani U. B., Saha A. K., Trumbauer M. E., Pang Z., Chen A. S., Ruderman N. B., Chen H., Rossetti L., Scherer P. E. (2006) Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor-γ agonists. J. Biol. Chem. 281, 2654–2660 [DOI] [PubMed] [Google Scholar]
- 8. Chinetti G., Fruchart J. C., Staels B. (2000) Peroxisome proliferator-activated receptors (PPARs). Nuclear receptors at the cross-roads between lipid metabolism and inflammation. Inflamm. Res. 49, 497–505 [DOI] [PubMed] [Google Scholar]
- 9. He W., Barak Y., Hevener A., Olson P., Liao D., Le J., Nelson M., Ong E., Olefsky J. M., Evans R. M. (2003) Adipose-specific peroxisome proliferator-activated receptor-γ knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. U.S.A. 100, 15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones J. R., Barrick C., Kim K. A., Lindner J., Blondeau B., Fujimoto Y., Shiota M., Kesterson R. A., Kahn B. B., Magnuson M. A. (2005) Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. U.S.A. 102, 6207–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang H., Zhang A., Kohan D. E., Nelson R. D., Gonzalez F. J., Yang T. (2005) Collecting duct-specific deletion of peroxisome proliferator-activated receptor-γ blocks thiazolidinedione-induced fluid retention. Proc. Natl. Acad. Sci. U.S.A. 102, 9406–9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guan Y., Hao C., Cha D. R., Rao R., Lu W., Kohan D. E., Magnuson M. A., Redha R., Zhang Y., Breyer M. D. (2005) Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat. Med. 11, 861–866 [DOI] [PubMed] [Google Scholar]
- 13. Gavrilova O., Haluzik M., Matsusue K., Cutson J. J., Johnson L., Dietz K. R., Nicol C. J., Vinson C., Gonzalez F. J., Reitman M. L. (2003) Liver peroxisome proliferator-activated receptor-γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 278, 34268–34276 [DOI] [PubMed] [Google Scholar]
- 14. Matsusue K., Haluzik M., Lambert G., Yim S. H., Gavrilova O., Ward J. M., Brewer B., Jr., Reitman M. L., Gonzalez F. J. (2003) Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Invest. 111, 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cho M. C., Lee D. H., Kim E. J., Lee J. Y., Kang J. W., Song J. H., Chong Y., Kim Y., Hong J. T., Yoon D. Y. (2011) Novel PPARγ partial agonists with weak activity and no cytotoxicity. Identified by a simple PPARγ ligand screening system. Mol. Cell Biochem. 358, 75–83 [DOI] [PubMed] [Google Scholar]
- 16. Feinstein D. L., Spagnolo A., Akar C., Weinberg G., Murphy P., Gavrilyuk V., Dello Russo C. (2005) Receptor-independent actions of PPAR thiazolidinedione agonists. Is mitochondrial function the key? Biochem. Pharmacol 70, 177–188 [DOI] [PubMed] [Google Scholar]
- 17. Balkrishnan R., Arondekar B. V., Camacho F. T., Shenolikar R. A., Horblyuk R., Anderson R. T. (2007) Comparisons of rosiglitazone versus pioglitazone monotherapy introduction and associated health care utilization in medicaid-enrolled patients with type 2 diabetes mellitus. Clin. Ther. 29, 1306–1315 [PubMed] [Google Scholar]
- 18. Beysen C., Murphy E. J., Nagaraja H., Decaris M., Riiff T., Fong A., Hellerstein M. K., Boyle P. J. (2008) A pilot study of the effects of pioglitazone and rosiglitazone on de novo lipogenesis in type 2 diabetes. J. Lipid Res. 49, 2657–2663 [DOI] [PubMed] [Google Scholar]
- 19. de Vries C. S., Russell-Jones D. L. (2009) Rosiglitazone or pioglitazone in type 2 diabetes? BMJ 339, b3076. [DOI] [PubMed] [Google Scholar]
- 20. Adams M. D., Raman P., Judd R. L. (1998) Comparative effects of englitazone and glyburide on gluconeogenesis and glycolysis in the isolated perfused rat liver. Biochem. Pharmacol. 55, 1915–1920 [DOI] [PubMed] [Google Scholar]
- 21. Nishimura Y., Inoue Y., Takeuchi H., Oka Y. (1997) Acute effects of pioglitazone on glucose metabolism in perfused rat liver. Acta Diabetol. 34, 206–210 [DOI] [PubMed] [Google Scholar]
- 22. Fryer L. G., Parbu-Patel A., Carling D. (2002) The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 277, 25226–25232 [DOI] [PubMed] [Google Scholar]
- 23. Colca J. R., McDonald W. G., Waldon D. J., Leone J. W., Lull J. M., Bannow C. A., Lund E. T., Mathews W. R. (2004) Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am. J. Physiol. Endocrinol. Metab. 286, E252–E260 [DOI] [PubMed] [Google Scholar]
- 24. Colca J. R., Kletzien R. F. (2006) What has prevented the expansion of insulin sensitisers? Expert. Opin. Investig. Drugs 15, 205–210 [DOI] [PubMed] [Google Scholar]
- 25. Hevener A. L., He W., Barak Y., Le J., Bandyopadhyay G., Olson P., Wilkes J., Evans R. M., Olefsky J. (2003) Muscle-specific Pparg deletion causes insulin resistance. Nat. Med. 9, 1491–1497 [DOI] [PubMed] [Google Scholar]
- 26. Postic C., Magnuson M. A. (2000) DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26, 149–150 [DOI] [PubMed] [Google Scholar]
- 27. Finck B. N., Bernal-Mizrachi C., Han D. H., Coleman T., Sambandam N., LaRiviere L. L., Holloszy J. O., Semenkovich C. F., Kelly D. P. (2005) A potential link between muscle peroxisome proliferator-activated receptor-α signaling and obesity-related diabetes. Cell Metab. 1, 133–144 [DOI] [PubMed] [Google Scholar]
- 28. Ayala J. E., Bracy D. P., Malabanan C., James F. D., Ansari T., Fueger P. T., McGuinness O. P., Wasserman D. H. (2011) Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J. Vis. Exp. 57, e3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Z., Gropler M. C., Norris J., Lawrence J. C., Jr., Harris T. E., Finck B. N. (2008) Alterations in hepatic metabolism in fld mice reveal a role for lipin 1 in regulating VLDL-triacylglyceride secretion. Arterioscler. Thromb. Vasc. Biol. 28, 1738–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin X., Schonfeld G., Yue P., Chen Z. (2002) Hepatic fatty acid synthesis is suppressed in mice with fatty livers due to targeted apolipoprotein B38.9 mutation. Arterioscler. Thromb. Vasc. Biol. 22, 476–482 [DOI] [PubMed] [Google Scholar]
- 31. Chen Z., Fitzgerald R. L., Averna M. R., Schonfeld G. (2000) A targeted apolipoprotein B-38.9-producing mutation causes fatty livers in mice due to the reduced ability of apolipoprotein B-38.9 to transport triglycerides. J. Biol. Chem. 275, 32807–32815 [DOI] [PubMed] [Google Scholar]
- 32. Finck B. N., Gropler M. C., Chen Z., Leone T. C., Croce M. A., Harris T. E., Lawrence J. C., Jr., Kelly D. P. (2006) Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab. 4, 199–210 [DOI] [PubMed] [Google Scholar]
- 33. Zhu Y., Qi C., Jain S., Rao M. S., Reddy J. K. (1997) Isolation and characterization of PBP, a protein that interacts with peroxisome proliferator-activated receptor. J. Biol. Chem. 272, 25500–25506 [DOI] [PubMed] [Google Scholar]
- 34. Boehm E. A., Jones B. E., Radda G. K., Veech R. L., Clarke K. (2001) Increased uncoupling proteins and decreased efficiency in palmitate-perfused hyperthyroid rat heart. Am. J. Physiol. Heart Circ. Physiol. 280, H977–H983 [DOI] [PubMed] [Google Scholar]
- 35. Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., Goldstein J. L. (2003) Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. U.S.A. 100, 12027–12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juurlink D. N., Gomes T., Lipscombe L. L., Austin P. C., Hux J. E., Mamdani M. M. (2009) Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone. Population based cohort study. BMJ 339, b2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Irons B. K., Greene R. S., Mazzolini T. A., Edwards K. L., Sleeper R. B. (2006) Implications of rosiglitazone and pioglitazone on cardiovascular risk in patients with type 2 diabetes mellitus. Pharmacotherapy 26, 168–181 [DOI] [PubMed] [Google Scholar]
- 38. Colca J. R., Kletzien R. F., VanderLugt J. T. (2009) A PPAR-sparing insulin sensitizer is effective in type 2 diabetic patients without causing weight gain. 20th World Diabetes Congress, October 18–22, 2009, Montreal, Abstr. D-0773, International Diabetes Foundation, Brussels, Belgium [Google Scholar]
- 39. Choi J. H., Banks A. S., Estall J. L., Kajimura S., Boström P., Laznik D., Ruas J. L., Chalmers M. J., Kamenecka T. M., Blüher M., Griffin P. R., Spiegelman B. M. (2010) Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature 466, 451–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wiley S. E., Paddock M. L., Abresch E. C., Gross L., van der Geer P., Nechushtai R., Murphy A. N., Jennings P. A., Dixon J. E. (2007) The outer mitochondrial membrane protein mitoNEET contains a novel redox-active 2Fe-2S cluster. J. Biol. Chem. 282, 23745–23749 [DOI] [PubMed] [Google Scholar]
- 41. Wiley S. E., Murphy A. N., Ross S. A., van der Geer P., Dixon J. E. (2007) MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc. Natl. Acad. Sci. U.S.A. 104, 5318–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laplante M., Sabatini D. M. (2009) An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 19, R1046–R1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kubota N., Terauchi Y., Kubota T., Kumagai H., Itoh S., Satoh H., Yano W., Ogata H., Tokuyama K., Takamoto I., Mineyama T., Ishikawa M., Moroi M., Sugi K., Yamauchi T., Ueki K., Tobe K., Noda T., Nagai R., Kadowaki T. (2006) Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and-independent pathways. J. Biol. Chem. 281, 8748–8755 [DOI] [PubMed] [Google Scholar]
- 44. Farmer S. R. (2005) Regulation of PPARγ activity during adipogenesis. Int. J. Obes. (Lond) 29, Suppl. 1, S13–S16 [DOI] [PubMed] [Google Scholar]
- 45. Maeda N., Takahashi M., Funahashi T., Kihara S., Nishizawa H., Kishida K., Nagaretani H., Matsuda M., Komuro R., Ouchi N., Kuriyama H., Hotta K., Nakamura T., Shimomura I., Matsuzawa Y. (2001) PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50, 2094–2099 [DOI] [PubMed] [Google Scholar]
- 46. Pereira R. I., Leitner J. W., Erickson C., Draznin B. (2008) Pioglitazone acutely stimulates adiponectin secretion from mouse and human adipocytes via activation of the phosphatidylinositol 3′-kinase. Life Sci. 83, 638–643 [DOI] [PubMed] [Google Scholar]