Background: Male sex determination is dependent on the SRY and SOX9 transcription factors.
Results: SRY activates Er71 transcription. Then, ER71 and SOX9 may sustain each other's expression.
Conclusion: The transcription factor ER71 may be part of the SRY-SOX9 axis.
Significance: Understanding how male gonadal development is induced explains how the Y chromosome leads to a male phenotype.
Keywords: Development, Ets Family Transcription Factor, Gene Regulation, Gene Transcription, Testis, ER71, SOX9, SRY
Abstract
ER71, also known as ETV2, is an ETS transcription factor that is expressed during embryogenesis and in adult testes. We show that Er71 transcription can be up-regulated by SRY, the key determinant of male differentiation. Accordingly, SRY bound to and activated the Er71 promoter, and mutation of a putative SRY binding site abolished this promoter activation. In turn, ER71 was able to bind to the promoter of Sox9, the primary target of SRY and a critical transcription factor for maintenance of the Sertoli cell phenotype. Mutation of the ER71 binding site in the Sox9 promoter suppressed ER71-dependent up-regulation of Sox9 transcription, and a dominant-negative ER71 molecule severely reduced Sox9 transcription in a Sertoli cell line. Conversely, SOX9 bound the Er71 promoter in vivo and Sox9 down-regulation reduced Er71 transcript levels. Together, these data suggest a mechanism by which SRY induces Sox9 and Er71 transcription early in testis differentiation, whereas ER71 and SOX9 participate in an autoregulatory loop to sustain each other's expression after Sry expression has subsided in mice. Thereby, ER71 and SOX9 may affect late testis development as well as the function of the adult male gonad.
Introduction
In most mammals, including humans and mice, development of testes is dependent on the Y-chromosomal Sry (sex-determining region Y)3 gene. Initially, gonads emerge as bipotential structures that develop by default into ovaries, but the correct timing of Sry expression triggers the path to testis differentiation (1, 2). Accordingly, transgenic expression of Sry in XX mice leads to male development (3), indicating that no other gene on the Y chromosome is needed for testis determination.
Sry encodes a protein whose high mobility group (HMG) domain binds to the minor groove of DNA and induces strong DNA bending. Aside from the HMG domain, SRY is surprisingly unconserved during evolution. For instance, whereas mouse SRY consists of 395 amino acids with a glutamine-rich repeat, the 204 amino acid human SRY protein lacks this repeat but possesses 57 amino acids preceding the HMG domain that are absent from mouse SRY (4). Therefore, it is commonly concluded that the conserved HMG domain is crucial for SRY action in sex determination and, consistently, most mutations in human SRY that cause male to female sex reversal are located within the HMG domain (5).
One downstream effector of SRY in testis development is SOX9 (SRY-type HMG box protein 9). Mutation of one SOX9 allele leads to campomelic dysplasia (6, 7), a skeletal dysmorphology syndrome that is commonly characterized by bowing and angulation of long bones, hypoplastic scapulae, and a missing pair of ribs. Most patients die soon after birth, probably due to the hypoplasia of the tracheobronchial cartilage and small thoracic cage causing respiratory distress. Analysis of SOX9 expression and knock-out models revealed that SOX9 is essential for the proper differentiation of chondrocytes, which explains how SOX9 haploinsufficiency leads to defects in skeletal elements derived from cartilage (8–10). However, another feature of campomelic dysplasia is that about 75% of XY patients develop as phenotypic females or intersexes (11), indicating that SOX9 is crucial for male development.
Consistently, SOX9 overexpression causes XX mice to develop as males (12–14), indicating that SOX9 in itself is sufficient for the male sex determination process in mice. Similarly, XX sex reversal has been observed in a human upon duplication of the SOX9 gene (15). Moreover, conditional knock-out models revealed that the absence of Sox9 in the developing gonads of XY mice leads to their differentiation into ovaries (16, 17), clearly demonstrating that SOX9 is not only sufficient but also necessary for male gonad development. Like SRY, SOX9 binds and bends DNA by virtue of its HMG domain. In addition, SOX9 contains two transactivation domains allowing it to stimulate gene transcription (4). The discovery that the anti-Müllerian hormone gene, which is required for male duct development, is a target of SOX9 was the first revelation of how SOX9 induces the development of the male sexual phenotype (18, 19), and subsequently more SOX9 target genes relevant for sex determination have been uncovered (1, 2).
An obvious hypothesis would be that SRY directly activates the transcription of the Sox9 gene and thereby exerts its sex determining power. In fact, a recent study identified an enhancer located ∼10 kb upstream of the Sox9 transcription start site that stimulates Sox9 transcription upon binding of steroidogenic factor 1 (SF1) and SRY (20). However, this does not exclude the presence of other regulatory elements in the Sox9 promoter, or the involvement of other factors in Sox9 gene expression. Further, this does not explain how Sox9 transcription remains elevated in the developing testes after the transient, short burst of Sry expression at the beginning of male gonadal differentiation. Notably, Sox9 transcription is also elevated in the adult testes, where SRY is not expressed (4).
We speculated that the transcription factor ER71 (ETS related 71; also called ETV2 for ETS variant 2) might be a candidate regulator of Sox9 transcription, as Er71 is testis-specifically expressed in the adult mouse and also present in mouse embryos at the onset of gonadal differentiation (21, 22). ER71 belongs to the ETS class of transcription factors characterized by a conserved, ∼85 amino acids long DNA binding domain (23–25). It is a constitutively nuclear protein and is capable of activating gene transcription (26). In addition, ER71 is an essential protein whose absence leads to hematopoietic, vascular and endocardial defects (27–29). Here, we present evidence that the Er71 gene is a target of SRY. Further, the Er71 and Sox9 gene products up-regulate each other's transcription. This implicates ER71 in the formation of testes in the embryo as well as in testicular function in adults.
EXPERIMENTAL PROCEDURES
Luciferase Assay
Cells were grown in 6-cm dishes in DMEM supplemented with 10% fetal bovine serum (30) and transiently transfected with a total of 9 μg of DNA by the calcium phosphate coprecipitation method (31, 32). 36 h after transfection, cells were washed once with phosphate-buffered saline, and lysed in 25 mm Tris-HCl (pH 7.8), 2 mm EDTA, 10% glycerol, 1% Triton X-100, and 2 mm DTT (33). Luciferase activity of the cleared supernatant was then determined in a luminometer (34, 35). Shown are averages (with standard error) of triplicate experiments.
Electrophoretic Mobility Shift Assay
GST (glutathione S-transferase) and GST-SRY1–130 protein were produced in Escherichia coli and purified on glutathione-agarose according to standard procedures (36, 37). ER71 was produced by in vitro transcription and translation using Promega's TNT kit (26). Proteins were incubated with 32P-labeled, double-stranded oligonucleotides and separated on 5% polyacrylamide gels as previously described (38, 39). Gels were dried and respective autoradiograms obtained by exposure to film (40).
32P-labeled probes were obtained by annealing complementary oligonucleotides, whose ends were filled in with Klenow enzyme in the presence of [α-32P]dATP (41, 42). Probes were purified on NucTrap columns (Stratagene) before utilization in binding reactions. The following oligonucleotides were employed: SRY site (sense): 5′-GCCGATTGTGACGTAGGCT. SRY site (antisense): 5′-CATCAGCCTACGTCACAATCGGC. −5/−8 (sense): 5′-AGCTATTTATTAGAGACCCTGAGCTGGAAGTCGG. −5/−8 (antisense): 5′-AGCTCCGACTTCCAGCTCAGGGTCTCTAATAAAT. −111/−114&−118/−121 (sense): 5′-AGTCCCCTTCCAAAATCCGGTCCAATCAGC. −111/−114&−118/−121 (antisense): 5′-AGTCGCTGATTGGACCGGATTTTGGAAGGG. −135/−138 (sense): 5′-AGCTCACCCAACCCGGAGCCACAATCCTCCCC. −135/−138 (antisense): 5′-AGCTGGGGAGGATTGTGGCTCCGGGTTGGGTG. −204/−207 (sense): 5′-AGTCCCCTCACCCCACCATCCACCCTCTGG. −204/−207 (antisense): 5′-AGCTCCAGAGGGTGGATGGTGGGGTGAGG. −292/−295&−308/−311 (sense): 5′-GCTCGGAGACCGTTCCAAAACTGTGACATTCCGAG. −292/−295&−308/−311 (antisense): 5′-AGCTCTCGGAATGTCACAGTTTTGGAACGGTCTCCG.
RT-PCR Analysis of Transfected Cells
Cells were grown in 12-well plates and transfected utilizing 2 μg of DNA and 2 μl of Lipofectamine 2000 reagent (Invitrogen). Total RNA was isolated from cells employing Trizol (Invitrogen). Approximately 0.5 μg of RNA was utilized to generate and amplify cDNA with the Access RT-PCR kit (Promega) (43, 44). Briefly, after 45 min of reverse transcription at 48 °C, DNA was amplified for 36 cycles (Er71) or 30 cycles (Sox9) at 98 °C for 1 min, 60 °C for 1 min and 68 °C for 2 min. Amplification for Gapdh with respective primers was done as described before (22). DNA was finally separated on 1.5% agarose gels and visualized by ethidium bromide staining (45). Er71 primers yielding a 348 bp product and Sox9 primers yielding a 256 bp product were: Er71: 5′-CAAGAGGACACACCGATCACACC/5′-CACAGAACAGTCCCAGCTGGTAGTG. Sox9: 5′-AGGAAGCTGGCAGACCAGTA/5′-CCTTGAAGATAGCATTAGGAGAGATGTG.
Mouse Embryos
Embryos were collected from timed matings, with noon of the day on which the mating plug was observed designated 0.5 days post coitum (dpc). CD1 mice were used for in situ hybridization and We/We mice (46) for quantitative RT-PCR analysis. When necessary, embryo sexing was carried out by analysis of tail tissue for the presence of Zfy as described (47). All animal work was conducted according to protocols approved by the University of Queensland Animal Ethics Committee.
In Situ Hybridization, Microarray Analysis, and RT-PCR on Embryos
Whole-mount in situ hybridization was performed as described (48). Section in situ hybridization was performed on 10 μm sections of paraffin-embedded embryos as described previously (49). The Er71 probe used spans nucleotides 641–937 of the mouse Er71 transcript (L10427), encoding most of the 3′-UTR (untranslated region). Expression of mouse Sox9 was detected using the probe Sox9.5a (8).
A genome-wide Affymetrix microarray analysis of gene expression in mouse fetal gonads, with a focus on time points 11.5 and 12.0 dpc, was conducted and described previously (50). Data are shown for Sry (1450578_at), Sox9 (1433889_at), Cbln4 (1433607_at), and Er71 (1421773_at).
Relative cDNA levels were analyzed by the comparative cycle time method of quantitative RT-PCR with reactions including Taqman PCR master mix (ABI) and Taqman gene expression sets as described (51). Endogenous control, used to normalize gene expression levels, was Tbp (encoding TATA box binding protein). Taqman gene expression sets were as follows: Mm0044973_m1 (Tbp), Mm00448840_m1 (Sox9), and Mm00468389_m1 (Er71).
Chromatin Immunoprecipitation
Where indicated, cells were transfected utilizing Lipofectamine 2000 reagent (Invitrogen). 36 h after transfection, chromatin immunoprecipitation (ChIP) assays were performed essentially as described (52, 53). To ascertain that equal amounts of chromatin were present in each sample, we split cell extracts after formaldehyde treatment and sonication into identical portions. Sox9 promoter DNA was amplified utilizing nested PCR with the ChIP-Sox9-forward/ChIP-Sox9-reverse-new primer pair in the first PCR reaction (20 repeats) and ChIP-Sox9-forward-new/ChIP-Sox9-reverse primer pair in the second PCR reaction (22 repeats). The PCR program was: 98 °C for 2 min, 8 cycles of 98 °C for 30 s, 64 °C for 30 s (minus 1 °C per cycle), 72 °C for 25 s followed by the indicated number of repeats for the cycle 98 °C for 30 s, 56 °C for 30 s, 72 °C for 25 s (plus 1 s per cycle), and finally by a 4 min extension at 72 °C. The expected 286 bp DNA product was visualized by ethidium bromide staining after electrophoresis on a 1.5% agarose gel (54).
Similarly, a 309 bp Flk1 promoter fragment was amplified by utilizing ChIP-Flk-forward and ChIP-Flk-reverse primers. Moreover, Sox9 3′-UTR sequences were amplified with 2646-for/3044-rev or 3268-for/3599-rev primers in the first PCR reaction followed by 2664-for/3023-rev or 3293-for/3572-rev primers, respectively, in the second PCR reaction. To amplify a 362 bp Er71 sequence around the SRY binding-site, a first amplification with ChIP-Er71-for-1/ChIP-Er71-rev1 primers was followed by a second amplification with ChIP-Er71-for-2/ChIP-Er71-rev2 primers. As a control, a 306 bp Er71 fragment spanning intron 6 and parts of exons 6 and 7 was amplified with ChIP-Er71-end-for-1/ChIP-Er71-end-rev1 primers followed by a second amplification with ChIP-Er71-end-for-3/ChIP-Er71-end-rev2 primers. Lastly, a 329 bp fragment from the beginning of the Sry coding sequence was amplified with SRY-for-1/SRY-rev-1 primers in the first PCR reaction followed by SRY-for-2/SRY-rev-2 primers in the second PCR reaction. The primers used were: ChIP-Sox9-forward: 5′-TCGGTTCACACGGAGACCGTTCC. ChIP-Sox9-forward-new: 5′-CCGTTCCAAAACTGTGACATTCCGAG. ChIP-Sox9-reverse: 5′-TACTCCTCCTTCACGTTAGATACC. ChIP-Sox9-reverse-new: 5′-TCGGCTCTCCGACTTCCAGCTCAG. ChIP-Flk-forward: 5′-AGGGCTAATCAGGTAACTTCGGAC. ChIP-Flk-reverse: 5′-CTCATTAGGAGCCTGCAAGTGCAG. 2646-for: 5′-GCTTTGATTAATTCCCCAGGCTCTTG. 2664-for: 5′-GGCTCTTGGATTTCAAGAGTAGCTG. 3023-rev: 5′-GTGCTTGGGCACTTAGGGCTGCGTG. 3044-rev: 5′-CAGGAGGCAACCAGGGAAAATGTGC. 3268-for: 5′-TCTTTAAGGTAGATTGTTGGCGCCTTC. 3293-for: 5′-TCCTCAAAGGGTATGGTCATCTGTTG. 3572-rev: 5′-GCTCCTCACTGCATCTGAAACCTCTC. 3599-rev: 5′-GGCTGAAAACATTGCAAAGGACTCAG. ChIP-Er71-for-1: 5′-CTCGAAATTCTCCAATGGTTCGGGAC. ChIP-Er71-for-2: 5′-GCTATAGCCCAGAAAGCCCAGACAG. ChIP-Er71-rev-1: 5′-CTGGGCAGCTCTGGGCTTATCTGCAAC. ChIP-Er71-rev-2: 5′-CAACAGGAAGAGGGATTTCCGGCCAG. ChIP-Er71-end-for-1: 5′-CATTCAGCTGTGGCAATTCCTCCTG. ChIP-Er71-end-for-3: 5′-GGCAATAGCCGCGAGTTCCAGCTG. ChIP-Er71-end-rev-1: 5′-CATCCTGATAGGCGAGGACAGGCACAC. ChIP-Er71-end-rev-2: 5′-CGCCCACCACTCTTGAGCACGATGTC. SRY-for-1: 5′-ATGGAGGGCCATGTCAAGCG. SRY-for-2: 5′-CCCATGAATGCATTTATGGTGTGG. SRY-rev-1: 5′-CAGTGGGGATATCAACAGGCTG. SRY-rev-2: 5′-CAGTCTTGCCTGTATGTGATGGC.
RNA Interference
The sequence 5′-GGACAGACCUUAAUUCUUA in the 3′-UTR of mouse Sox9 mRNA was targeted with shRNA, which was cloned into pSIREN-RetroQ (Clontech). Respective retrovirus was then produced in 293T cells according to standard procedures (55). 15P-1 cells were then twice infected with control or Sox9 shRNA expressing retrovirus (56), selected for 5 days with 1 μg/μl puromycin and then grown for another 2 days before preparation of RNA utilizing Trizol (Invitrogen). RNA was then amplified with mSox9-for-604/627 (5′- GTCAGCCAGGTGCTGAAGGGCTAC) and mSox9-rev-993/969 (5′-CGCCTTGAAGATAGCATTAGGAGAG) primers in a MiniOpticon (Bio-Rad) real-time PCR machine utilizing the iScript One-Step RT-PCR kit with SYBR green (Bio-Rad) and the following PCR program: 50 °C for 15 min; 95 °C for 5 min; 35 cycles of 95 °C for 15 s, 58 °C for 15 s, 65 °C for 45 s. Likewise, Gapdh was amplified with previously described primers (22). Er71 mRNA was first amplified with the Access RT-PCR kit (Promega) and then with the iQ SYBR Green Supermix (Bio-Rad) using nested primer pairs published before (22). Sox9 and Er71 mRNA levels were normalized to Gapdh by the comparative cycle time method.
RESULTS
SRY Activates Transcription of Er71
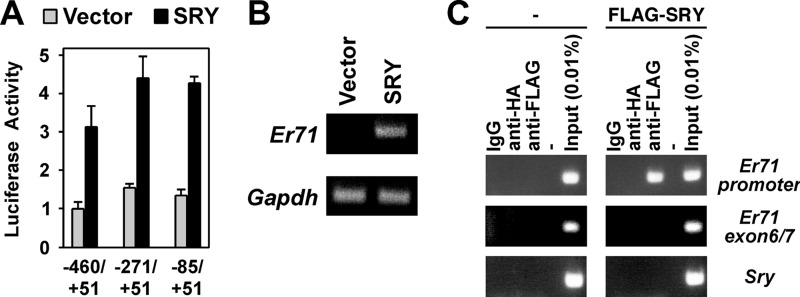
Previously, we cloned the mouse Er71 gene promoter (22), allowing us to set out to analyze whether SRY activates Er71 transcription and may thus contribute to the embryonic expression of ER71. To this end, successively smaller Er71 promoter fragments were cloned in front of luciferase cDNA and their activation by mouse SRY investigated. As shown in Fig. 1A, SRY activated all three Er71 promoter fragments utilized, and the degree of activation was basically unchanged from the longest (−460 to +51) to the shortest (−85 to +51) promoter fragment. Moreover, we expressed SRY in the mouse Leydig tumor cell 1 (MLTC-1) line that expresses no significant amounts of Sry or Er71 (22) and found that endogenous Er71 transcription was activated by ectopic SRY expression (Fig. 1B). In addition, we performed ChIP assays in MLTC-1 cells transfected with a FLAG-tagged SRY expression construct. We thereby found that mouse SRY bound to the Er71 promoter within the region −334 to +28 (Fig. 1C), whereas SRY did not interact with a downstream region of the Er71 gene or the Sry coding region. These data strongly indicate that the Er71 gene is a potential target of SRY.
FIGURE 1.
Activation of the Er71 promoter by SRY. A, indicated Er71 promoter fragments were cloned into the pGL2-Basic luciferase reporter plasmid and cotransfected with 1.5 μg of empty vector or mouse SRY expression vector into MLTC-1 cells. Resultant luciferase activities were measured. B, RT-PCR revealing Er71 and Gapdh mRNA levels in MLTC-1 cells transfected with empty vector or 0.4 μg of mouse SRY expression vector. C, untransfected MLTC-1 cells or transfected with FLAG-SRY2–130 were subjected to ChIP assays. Immunoprecipitations were performed with control mouse IgG or mouse monoclonal anti-HA or anti-FLAG antibodies. DNA from the Er71 promoter (−334 to +28), an Er71 region ranging from exon 6 to exon 7, or the coding region of Sry was amplified by PCR. IgG and anti-HA immunoprecipitations served as negative controls.
SRY Binds to the Er71 Promoter
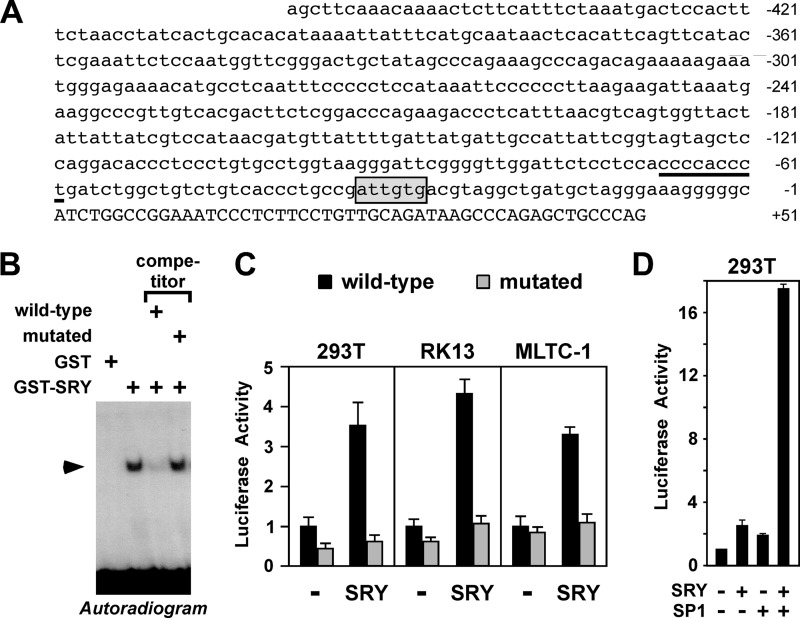
The smallest Er71 promoter fragment identified above to be responsive for activation by SRY spans the Er71 promoter sequences from −85 to +51. If SRY directly up-regulates Er71 transcription, SRY should bind within this region of the Er71 promoter. Mouse SRY binds preferentially to sites of the sequence 5′-NACAAT-3′ (or reverse: 5′-ATTGTN-3′) (57) and inspection of the Er71 promoter revealed one such site from −34 to −29 (Fig. 2A). Thus, we tested in electrophoretic mobility shift assays whether SRY binds to this site. Indeed, bacterially expressed, mouse SRY formed a complex with a radiolabeled oligonucleotide spanning the −34/−29 site (Fig. 2B). This binding was specific, as this complex formation was suppressed by an excess of unlabeled oligonucleotide, but not by an unlabeled oligonucleotide in which the −34/−29 site was mutated. Thus, SRY can bind to the Er71 promoter and may thereby regulate its activity.
FIGURE 2.
Binding of SRY to the Er71 promoter. A, sequence of the mouse Er71 promoter from −460 to +51. A potential SRY binding site (−34 to −29) is boxed and a previously identified SP1 binding site (−68 to −60) underlined. B, electrophoretic mobility shift assays with a probe spanning the putative SRY site (−29/−34) in the Er71 promoter. GST or GST-SRY1–130 protein were incubated with 32P-labeled probe and resultant DNA:protein complexes resolved by native polyacrylamide gel electrophoresis. Where indicated, a 20-fold excess of unlabeled wild-type probe or mutated at the SRY binding site (5′-ATTGTG-3′ to 5′-ATTACG-3′ at −34/-29) was utilized for competition. C, activation of wild-type or binding site-mutated −460/+51 Er71 luciferase reporter by SRY in 293T, RK13 or MLTC-1 cells. Either 1 μg of wild-type or mutated (5′-ATTGTG-3′ to 5′-ATTACG-3′ at −34/−29) reporter plasmid was cotransfected with 1.5 μg of SRY plasmid. D, synergistic activation of the −460/+51 Er71 luciferase reporter by SRY (375 ng expression vector) and SP1 (625 ng expression vector) in 293T cells.
To further support this hypothesis, we analyzed how mutating the −34/−29 site would affect the ability of SRY to activate the Er71 promoter. In three different cell lines, mutation of the putative SRY site in the Er71 promoter abolished its responsiveness to SRY (Fig. 2C), thus underscoring the importance of this SRY site for Er71 promoter activity.
Previously, the Er71 promoter was shown to be regulated by the ubiquitously expressed transcription factor SP1 (22). Thus, we also assessed whether SP1 and SRY might cooperate in inducing Er71 transcription. To this end, we cotransfected suboptimal amounts of SP1 and SRY expression plasmids and indeed observed a synergistic stimulation of the Er71 promoter by these two transcription factors (Fig. 2D).
Expression of Er71 in Developing Gonads
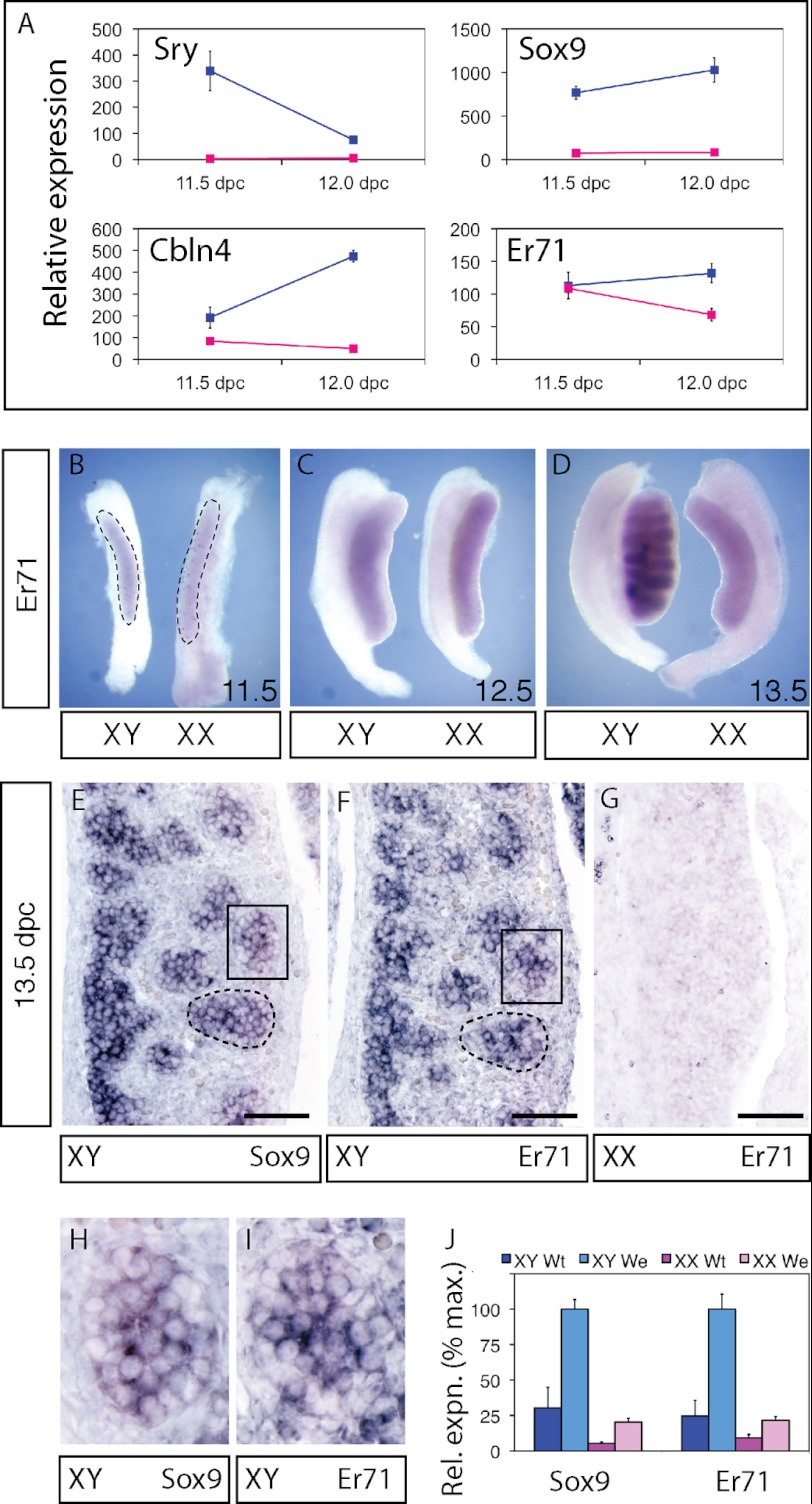
Sry transcription in the genital ridge of XY mice starts at 10.5 dpc, peaks at around 11.5 dpc and vanishes by 13 dpc (58–60). If Er71 is a true SRY target gene, its expression should closely follow that of Sry. We assessed the dynamics of Er71 expression in fetal mouse gonads, in comparison to expression patterns of Sry, Sox9 and Cbln4 (cerebellin 4 precursor), a known target of both SRY and SOX9 (61). As expected, Sry expression was high at 11.5 dpc and was diminishing by 12.0 dpc in XY embryos (Fig. 3A). In contrast, expression of known SRY targets, Sox9 and Cbln4, along with Er71 increased from 11.5 dpc to 12.0 dpc. The similar profiles of Er71, Sox9, and Cbln4 expression are consistent with all three genes being direct targets of SRY.
FIGURE 3.
Expression of Er71 in 11. 5–13.5 dpc mouse gonads. A, microarray expression data for Sry, Sox9, Cbln4, and Er71 in gonad samples at 11.5 dpc and 12.0 dpc. XY samples are shown in blue, XX samples in red. Whole-mount in situ hybridization for Er71 in developing testes and ovaries at (B) 11.5 dpc, (C) 12.5 dpc, and (D) 13.5 dpc. The extent of the gonad tissue within the gonad/mesonephros complex is outlined in B. Section in situ hybridization for Sox9 (E) and Er71 (F) in XY 13.5 dpc gonads and for Er71 in XX 13.5 dpc gonads (G). Testis cords are outlined (E, F); scale bar, 100 μm. Areas marked with rectangles are shown at higher magnification (H, I). J, quantitative RT-PCR analysis of Sox9 and Er71 expression in wild-type (wt) and We gonads at 13.5 dpc.
Next, we assessed the expression of Er71 mRNA by in situ hybridization in genital ridges of 11.5–13.5 dpc embryos. Er71 expression was present at low amounts in 11.5 dpc genital ridges of both XX and XY animals (Fig. 3B). By 12.5 dpc, the expression level of Er71 had increased in both XX and XY animals, but we noted that Er71 expression appeared to be more interior (i.e. within the developing gonads) in XY than in XX animals (Fig. 3C). By 13.5 dpc, there was a clear distinction between XY and XX animals, with Er71 expression being much higher in XY than XX gonads (Fig. 3D). This result was corroborated by RT-PCR of 13.5 dpc male and female gonads (see wild-type in Fig. 3J). Section in situ hybridization at 13.5 dpc confirmed that Er71 was male-specifically expressed and that, as was also the case for Sox9, expression was localized to testicular cords (Fig. 3, E–I). To confirm that Er71 is expressed in somatic (Sertoli) cells of the cords rather than in the germ cells, we analyzed expression in We/We mutant gonads, which are devoid of germ cells (46). Like Sox9, Er71 was more highly expressed in XY wild-type gonads than in XX wild-type gonads at 13.5 dpc (Fig. 3J). Further, Er71 expression was increased rather than diminished in XY We/We mutants compared with XY wild-type samples, consistent with Er71 expression being restricted to Sertoli cells. Together these data show that Er71 is up-regulated in the Sertoli lineage shortly after SRY is expressed in XY gonads, consistent with Er71 being an in vivo target gene of SRY.
Binding of ER71 to the Sox9 Promoter
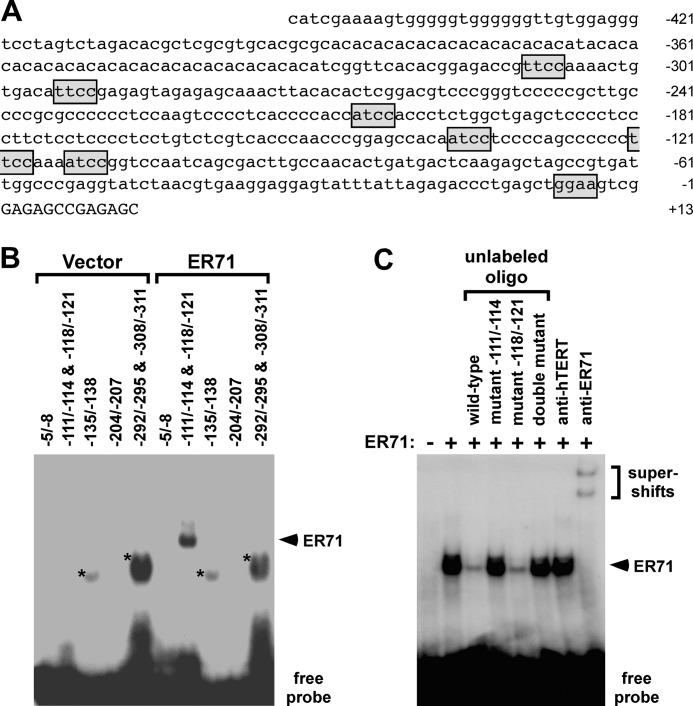
Sox9 expression is induced shortly after Sry in the genital ridges and remains high even after Sry transcription has ceased (62, 63). Since our studies identified Er71 to have a similar expression profile as Sox9, we wondered whether ER71 could be involved in the regulation of the Sox9 promoter. Indeed, inspection of the Sox9 promoter revealed seven putative ETS core binding sites that might be bound by ER71 (Fig. 4A). Therefore, we systematically investigated by gel electrophoretic mobility shift assays whether any of these sites could be bound by mouse ER71 in vitro. As shown in Fig. 4B, an oligonucleotide spanning both ETS sites at −111/−114 and −118/−121 was bound by ER71, but oligonucleotides spanning the other five putative ETS sites at −5/−8, −135/−138, −204/−207, −292/−295, or −308/−311 did not interact with ER71. Binding to the −111/−114&−118/−121 oligonucleotide was specific, since (i) simultaneous incubation with anti-ER71 antibodies but not control anti-hTERT antibodies resulted in supershifts (Fig. 4C) and (ii) competition with unlabeled −111/−114&−118/−121 oligonucleotide but not when both ETS sites were mutated resulted in suppression of mobility shift. Moreover, when mutating either the ETS site −111/−114 or the ETS site −118/−121 alone (Fig. 4C), we observed that only unlabeled oligonucleotide mutated at the −111/−114 site lost the ability to suppress mobility shift, indicating that ER71 binds to the −111/−114 site.
FIGURE 4.
Interaction of ER71 with the Sox9 promoter. A, sequence of the mouse Sox9 promoter from −453 to +13. Sequences matching the ETS core sequence (5′-GGAA/T-3′, or 5′-T/ATCC-3′ in reverse) are boxed. B, electrophoretic mobility shift assays with indicated 32P-labeled probes. Either in vitro transcribed/translated ER71 or vector control was employed for the binding reactions. Asterisks mark unspecific complexes, whereas the arrowhead points to an ER71:DNA complex. C, −111/−114&−118/−121 radiolabeled probe was incubated with ER71 protein and, as indicated, with a 20-fold excess of unlabeled oligonucleotide, in which the ETS sites at −111/−114 and/or −118/−121 were mutated. In the last two lanes, either antibodies directed against hTERT or ER71 were included in the binding reaction.
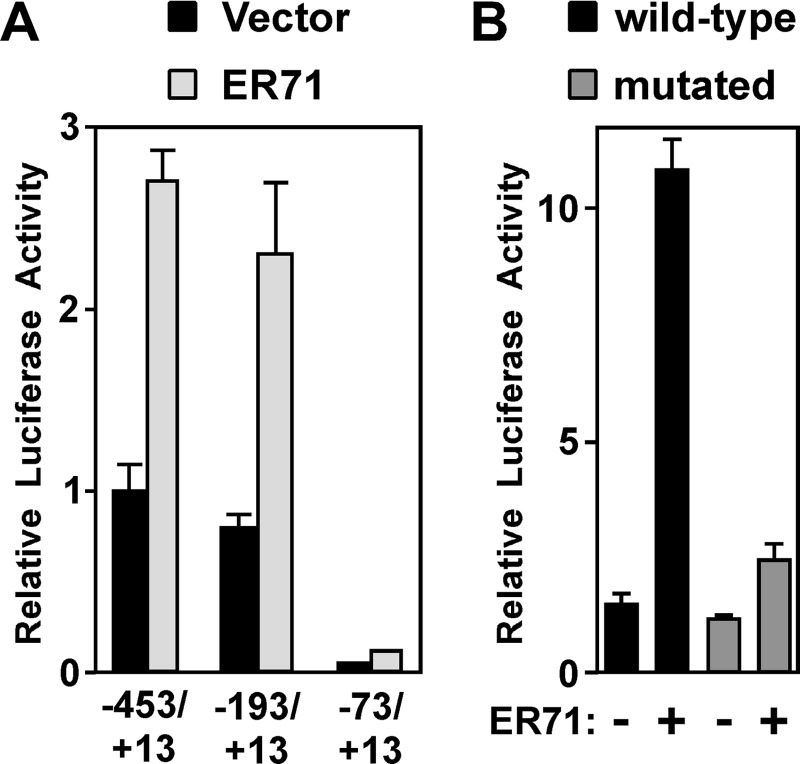
Activation of Sox9 Transcription by ER71
The fact that ER71 can bind to the Sox9 promoter prompted us to test whether ER71 might stimulate Sox9 transcription. To this end, we first assessed whether mouse ER71 activates a Sox9 promoter fragment spanning sequences from −453 to +13. Indeed, ER71 did so in two different cell lines tested (MLTC-1 and Mv1Lu; Fig. 5, A and B). Moreover, deletion of 260 bp of 5′-terminal base pairs (−193/+13 construct) did not compromise the ability of ER71 to stimulate the Sox9 promoter, whereas further deletion of 120 base pairs (−73/+13 construct) resulted in transcriptional inactivity (Fig. 5A). This is consistent with a previous report suggesting that Sox9 promoter activity in the gonads is dependent on sequences between −193 and −73 (64), and accordingly we have above identified ER71 to bind an ETS site (−111/−114) within this region (see Fig. 4). Next, we mutated the −111/−114 ETS site to determine whether this would compromise the ability of ER71 to activate the Sox9 promoter (Fig. 5B). Indeed, mutation of the −111/−114 ETS site greatly diminished the ability of ER71 to activate the −453/+13 Sox9 promoter, proving the functionality of the −111/−114 ETS site and corroborating that Sox9 is a potential target gene of ER71.
FIGURE 5.
Stimulation of the Sox9 promoter by ER71. A, indicated Sox9 promoter-luciferase constructs were cotransfected with 4 μg of ER71 expression construct or control vector into MLTC-1 cells. B, −453/+13 Sox9 luciferase reporter plasmid (wild-type or mutated at the ETS site −111/−114) was cotransfected with ER71 expression vector into Mv1Lu cells. Resultant luciferase activities are shown.
ER71 Is Involved in Endogenous Sox9 Regulation
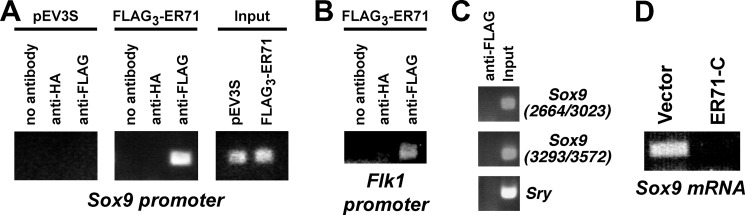
Next, we asked whether ER71 may also regulate the endogenous Sox9 gene promoter. First, we determined whether ER71 binds to the Sox9 promoter. To this end, we employed a mouse Sertoli cell line (15P-1) that robustly expresses Sox9. We then transfected 15P-1 cells with either the empty control vector (pEV3S) or a FLAG-tagged ER71 expression vector. Thereafter, cells were subjected to ChIP assays. When the control vector pEV3S was transfected, no Sox9 promoter DNA was immunoprecipitated with anti-FLAG antibodies (Fig. 6A, left panel). However, when equivalent amounts, as evidenced by comparable input levels (see Fig. 6A, right panel), of chromatin were immunoprecipitated from FLAG3-ER71-transfected cells, Sox9 promoter DNA was immunoprecipitated with anti-FLAG antibodies but not with anti-HA or no antibody (Fig. 6A, middle panel). These results indicate that ER71 is capable of binding to the Sox9 promoter.
FIGURE 6.
ER71 targets the endogenous Sox9 promoter. A, ChIP assays testing the binding of ER71 to the Sox9 promoter. As indicated, the vector control pEV3S or FLAG3-ER71 expression vector were transfected into 15P-1 cells and immunoprecipitations performed with no, anti-HA, or anti-FLAG antibodies. 0.04% of the input for each reaction was analyzed (right panel) as a control. B, corresponding ChIP assay to assess binding of ER71 to the Flk1 promoter. C, negative controls showing absence of ER71 binding to two regions (2664–3023 and 3293–3572) within the 3′-UTR of Sox9 and the coding region of Sry. D, equal amounts of RNA from 15P-1 cells transfected with vector control or ER71-C were subjected to RT-PCR to determine Sox9 mRNA levels.
As a positive control for our ChIP assay, we also assessed whether ER71 would bind to another previously reported target gene, Flk1 (27). Indeed, ER71 expectedly interacted with the Flk1 promoter (Fig. 6B). Furthermore, we performed negative controls by assessing whether ER71 would bind to two different regions in the Sox9 3′-UTR or within the coding region of Sry. No binding of ER71 to these genomic regions was observable (Fig. 6C), attesting to the specificity of our ChIP assay.
Thereafter, we assessed if ER71 can stimulate Sox9 transcription in 15P-1 Sertoli cells. Overexpression of ER71 did not result in changes of Sox9 mRNA (data not shown), possibly because Sox9 transcription may already be maximally induced in 15P-1 cells by endogenous ER71. Therefore, we took a dominant-negative approach expressing ER71-C, a molecule that consists of the last 129 amino acids of ER71 encompassing its DNA binding domain but lacking any transactivation domain. As previously shown (26), ER71-C can still bind to ER71 target sites and thereby prevent any productive transcriptional activation mediated by full-length endogenous ER71. Accordingly, we observed that ER71-C led to a down-regulation of Sox9 transcription in 15P-1 cells (Fig. 6D), corroborating that ER71 can target the Sox9 promoter.
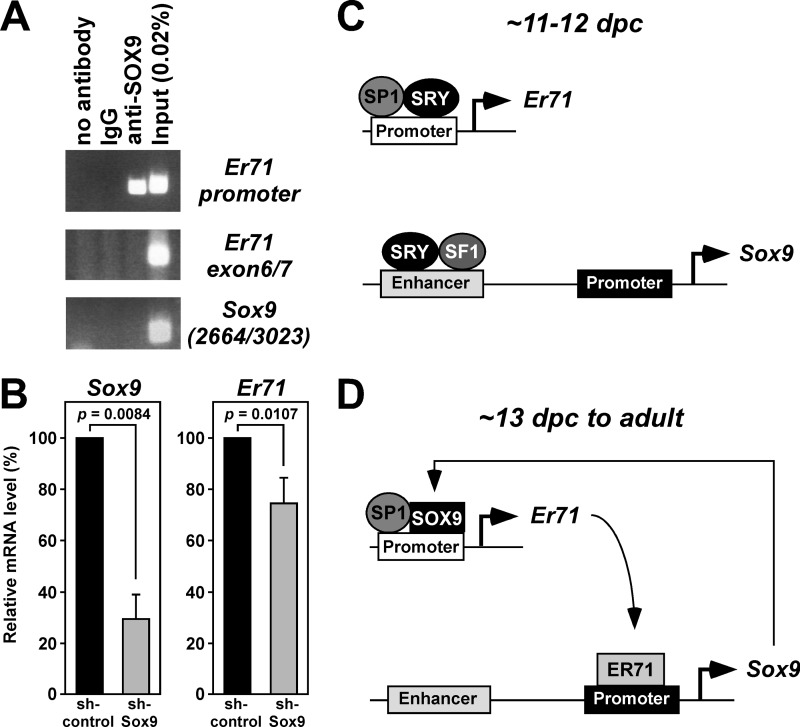
SOX9 as a Regulator of Er71 Transcription
SOX9 and SRY have similar DNA binding preferences (1), prompting us to investigate whether SOX9 may regulate Er71 expression similar to SRY. To test this, we first performed ChIP assays in 15P-1 Sertoli cells (Fig. 7A). Indeed, SOX9 occupied the Er71 promoter in vivo, but not a region ∼2.5 kbp downstream ranging from Er71 exon 6 to exon 7; neither did SOX9 bind to the Sox9 3′-UTR.
FIGURE 7.
SOX9-mediated regulation of Er71 transcription and models for the role of ER71 in male gonads. A, ChIP assay in 15P-1 cells. Immunoprecipitations were performed with anti-SOX9 antibodies (Abcam ab3697), control rabbit IgG or no antibody. DNA from the Er71 promoter (−334 to +28), an Er71 region ranging from exon 6 to exon 7, or the 3′-UTR of Sox9 (2664–3023) was PCR amplified. B, down-regulation of Sox9 with shRNA in 15P-1 cells. Shown are levels of Sox9 and Er71 mRNA. Statistical significance was determined with Student's t test. C, during gonadal differentiation, Er71 transcription is up-regulated by SRY in parallel to Sox9 gene expression. D, after SRY expression has subsided, ER71 and SOX9 mutually sustain their expression.
Finally, we tested whether SOX9 may not only bind to the Er71 promoter, but also regulate its activity. To this end, we down-regulated Sox9 with shRNA in 15P-1 cells and found as a consequence a 70% reduction of Sox9 mRNA (left panel, Fig. 7B). Simultaneously, a small, yet significant reduction of Er71 mRNA levels was observed (right panel, Fig. 7B). These data suggest that SOX9 is capable of activating Er71 transcription.
DISCUSSION
Our present data suggest that Er71 is a bona fide SRY target gene as evidenced by the following facts: (i) SRY can bind to the Er71 promoter in vitro and in vivo; (ii) SRY activates the Er71 promoter and mutation of the −29 to −34 SRY binding site compromises this activation; (iii) ectopic expression of SRY leads to the up-regulation of endogenous Er71 transcription; and (iv) Er71 mRNA levels are enhanced in male gonads shortly after Sry is up-regulated to trigger male differentiation of the bipotential gonads. Thus, our study has not only identified a novel direct SRY target gene but also for the first time implicates ER71 as a potential player in sex determination and gonadal development.
The fact that Er71 is also expressed in the absence of any SRY in the XX gonads, albeit at eventually lower levels than in XY gonads, indicates that SRY is not absolutely required for Er71 transcription. Possibly SP1, a ubiquitously expressed zinc finger transcription factor (65, 66) that we have previously shown to bind to and activate the Er71 promoter (22), may be responsible for the low levels of ER71 expression in XX gonads. Interestingly, SP1 is also required for efficient SRY expression during sexual differentiation (67, 68), emphasizing the important function of this transcription factor in the developing gonad. Therefore, SP1 and SRY may indeed collaborate to induce common target genes, and our data indicate that Er71 is such a target gene since its promoter was synergistically activated by SP1 and SRY.
Er71 mRNA levels are slightly up-regulated between 11.5 and 12.0 dpc in XY genital ridges and drastically increased at 13.5 dpc, whereas Sry transcription starts at 10.5 dpc, peaks at around 11.5 dpc and has subsided again by 12.5 dpc (58–60), indicating that Er71 transcription trails behind Sry transcription by about 1 day. This expression pattern of Er71 mRNA is consistent with Er71 being a target gene of SRY in vivo. Moreover, the Er71 expression pattern is reminiscent of that of Sox9: before overt sexual differentiation at 10.5 dpc, Sox9 expression is indiscriminate between XX and XY animals, but becomes elevated in XY and reduced in XX gonads after Sry induction (62, 63). This may suggest that Er71 and Sox9 are jointly up-regulated by SRY (Fig. 7C), with SRY cooperating with SP1 in upregulating the Er71 promoter and SRY and SF1 synergistically activating a gonad-specific Sox9 enhancer located ∼10 kb upstream of the transcription start site (20).
Sox9 expression persists in the absence of SRY past 13 dpc in the mouse embryo and is present in Sertoli cells of the adult testes (1, 4). Similarly, Er71 is also expressed in Sertoli cells (22), suggesting that Er71 and Sox9 might be coregulated. It has been hypothesized that the continued expression of Sox9 is due to an autoregulatory loop (2, 5). Alternatively, ER71 and SOX9 may act mutually in a positive feedback loop, with ER71 activating the Sox9 promoter, and SOX9 in turn activating the Er71 gene (Fig. 7D). In support of this model, our data show that ER71 can interact with the Sox9 promoter in vitro and in vivo and stimulate its activity, whereas the dominant-negative ER71-C molecule caused down-regulation of Sox9 transcription. Conversely, SOX9 occupied the Er71 promoter in vivo and Sox9 down-regulation resulted in a concomitant reduction of Er71 transcription. Possibly like SRY, SOX9 might cooperate with the ubiquitously expressed SP1 transcription factor in stimulating the Er71 promoter.
Clearly, such models are in need of testing, and the construction of transgenic mice overexpressing ER71 in the genital ridge or appropriate tissue-specific knock-outs will provide us with a more definite answer to ER71's role in male sex determination. Similarly, the role of ER71 in Sertoli cells of adult testes should be investigated utilizing mouse models. Notably, JMJD1A/TSGA/JHDM2A, a histone 3 lysine 9 demethylase (69), interacts with ER71 (70) and knocking-out this cofactor leads to defective spermatogenesis and infertility in male mice (71, 72), suggesting that ER71 and JMJD1A/TSGA/JHDM2A may cooperate in the physiological function of adult testes.
Apart from Sox9 transcriptional regulation, it is possible that ER71 affects male embryonic gonads and testicular function in adults by other mechanisms. Previously, we showed that ER71 activates transcription from the matrix metalloproteinase-1 promoter (26). It is likely that other matrix metalloproteinase genes are also induced by ER71, since many proteinase genes are transcriptionally controlled by ETS binding sites (24). Matrix metalloproteinases play an important role in early testicular development, including mesonephric cell migration (1), and also during spermatogenesis (73), indicating that ER71 may pleiotropically affect these processes through regulating the expression of one or more matrix metalloproteinases.
In conclusion, our study provides evidence that Er71 is a novel SRY target gene in the male developing gonad and that ER71 and SOX9 regulate each other's gene transcription. Thus, ER71 is strongly implicated in SRY-triggered male sex determination and to be a key regulator of adult testicular function in conjunction with SOX9.
Acknowledgment
We thank Chun-Wei Feng for technical assistance.
This work was funded in part by the National Health and Medical Research Council of Australia.
- SRY
- sex-determining region Y
- Cbln4
- cerebellin 4 precursor
- dpc
- days post coitum
- ER71
- ETS related 71
- ETV2
- ETS variant 2
- HMG
- high mobility group
- MLTC-1
- mouse Leydig tumor cell 1
- SF1
- steroidogenic factor 1
- SOX9
- SRY-type HMG box protein 9.
REFERENCES
- 1. Wilhelm D., Palmer S., Koopman P. (2007) Sex determination and gonadal development in mammals. Physiol. Rev. 87, 1–28 [DOI] [PubMed] [Google Scholar]
- 2. Sekido R., Lovell-Badge R. (2009) Sex determination and SRY: down to a wink and a nudge? Trends Genet. 25, 19–29 [DOI] [PubMed] [Google Scholar]
- 3. Koopman P., Gubbay J., Vivian N., Goodfellow P., Lovell-Badge R. (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351, 117–121 [DOI] [PubMed] [Google Scholar]
- 4. Harley V. R., Clarkson M. J., Argentaro A. (2003) The molecular action and regulation of the testis-determining factors, SRY (sex-determining region on the Y chromosome) and SOX9 (SRY-related high-mobility group (HMG) box 9). Endocr. Rev. 24, 466–487 [DOI] [PubMed] [Google Scholar]
- 5. Polanco J. C., Koopman P. (2007) Sry and the hesitant beginnings of male development. Dev. Biol. 302, 13–24 [DOI] [PubMed] [Google Scholar]
- 6. Foster J. W., Dominguez-Steglich M. A., Guioli S., Kwok G., Weller P. A., Stevanovi M., Weissenbach J., Mansour S., Young I. D., Goodfellow P. N., Brook J. D., Schafer A. J. (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372, 525–530 [DOI] [PubMed] [Google Scholar]
- 7. Wagner T., Wirth J., Meyer J., Zabel B., Held M., Zimmer J., Pasantes J., Bricarelli F. D., Keutel J., Hustert E., Wolf U., Tommerup N., Schempp W., Scherer G. (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79, 1111–1120 [DOI] [PubMed] [Google Scholar]
- 8. Wright E., Hargrave M. R., Christiansen J., Cooper L., Kun J., Evans T., Gangadharan U., Greenfield A., Koopman P. (1995) The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat. Genet. 9, 15–20 [DOI] [PubMed] [Google Scholar]
- 9. Bi W., Deng J. M., Zhang Z., Behringer R. R., de Crombrugghe B. (1999) Sox9 is required for cartilage formation. Nat. Genet. 22, 85–89 [DOI] [PubMed] [Google Scholar]
- 10. Bi W., Huang W., Whitworth D. J., Deng J. M., Zhang Z., Behringer R. R., de Crombrugghe B. (2001) Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc. Natl. Acad. Sci. U.S.A. 98, 6698–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schafer A. J., Foster J. W., Kwok C., Weller P. A., Guioli S., Goodfellow P. N. (1996) Campomelic dysplasia with XY sex reversal: diverse phenotypes resulting from mutations in a single gene. Ann. N.Y. Acad. Sci. 785, 137–149 [DOI] [PubMed] [Google Scholar]
- 12. Bishop C. E., Whitworth D. J., Qin Y., Agoulnik A. I., Agoulnik I. U., Harrison W. R., Behringer R. R., Overbeek P. A. (2000) A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat. Genet. 26, 490–494 [DOI] [PubMed] [Google Scholar]
- 13. Vidal V. P., Chaboissier M. C., de Rooij D. G., Schedl A. (2001) Sox9 induces testis development in XX transgenic mice. Nat. Genet. 28, 216–217 [DOI] [PubMed] [Google Scholar]
- 14. Qin Y., Bishop C. E. (2005) Sox9 is sufficient for functional testis development producing fertile male mice in the absence of Sry. Hum. Mol. Genet. 14, 1221–1229 [DOI] [PubMed] [Google Scholar]
- 15. Huang B., Wang S., Ning Y., Lamb A. N., Bartley J. (1999) Autosomal XX sex reversal caused by duplication of SOX9. Am. J. Med. Genet. 87, 349–353 [DOI] [PubMed] [Google Scholar]
- 16. Chaboissier M. C., Kobayashi A., Vidal V. I., Lützkendorf S., van de Kant H. J., Wegner M., de Rooij D. G., Behringer R. R., Schedl A. (2004) Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131, 1891–1901 [DOI] [PubMed] [Google Scholar]
- 17. Barrionuevo F., Bagheri-Fam S., Klattig J., Kist R., Taketo M. M., Englert C., Scherer G. (2006) Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol. Reprod. 74, 195–201 [DOI] [PubMed] [Google Scholar]
- 18. De Santa Barbara P., Bonneaud N., Boizet B., Desclozeaux M., Moniot B., Sudbeck P., Scherer G., Poulat F., Berta P. (1998) Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol. Cell. Biol. 18, 6653–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arango N. A., Lovell-Badge R., Behringer R. R. (1999) Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 99, 409–419 [DOI] [PubMed] [Google Scholar]
- 20. Sekido R., Lovell-Badge R. (2008) Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453, 930–934 [DOI] [PubMed] [Google Scholar]
- 21. Brown T. A., McKnight S. L. (1992) Specificities of protein-protein and protein-DNA interaction of GABPα and two newly defined Ets-related proteins. Genes Dev. 6, 2502–2512 [DOI] [PubMed] [Google Scholar]
- 22. De Haro L., Janknecht R. (2005) Cloning of the murine ER71 gene (Etsrp71) and initial characterization of its promoter. Genomics 85, 493–502 [DOI] [PubMed] [Google Scholar]
- 23. Janknecht R., Nordheim A. (1993) Gene regulation by Ets proteins. Biochim. Biophys. Acta 1155, 346–356 [DOI] [PubMed] [Google Scholar]
- 24. Oikawa T., Yamada T. (2003) Molecular biology of the Ets family of transcription factors. Gene 303, 11–34 [DOI] [PubMed] [Google Scholar]
- 25. Hsu T., Trojanowska M., Watson D. K. (2004) Ets proteins in biological control and cancer. J. Cell. Biochem. 91, 896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Haro L., Janknecht R. (2002) Functional analysis of the transcription factor ER71 and its activation of the matrix metalloproteinase-1 promoter. Nucleic Acids Res. 30, 2972–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee D., Park C., Lee H., Lugus J. J., Kim S. H., Arentson E., Chung Y. S., Gomez G., Kyba M., Lin S., Janknecht R., Lim D. S., Choi K. (2008) ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell 2, 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sumanas S., Gomez G., Zhao Y., Park C., Choi K., Lin S. (2008) Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood 111, 4500–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferdous A., Caprioli A., Iacovino M., Martin C. M., Morris J., Richardson J. A., Latif S., Hammer R. E., Harvey R. P., Olson E. N., Kyba M., Garry D. J. (2009) Nkx2–5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc. Natl. Acad. Sci. U.S.A. 106, 814–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossow K. L., Janknecht R. (2001) The Ewing's sarcoma gene product functions as a transcriptional activator. Cancer Res. 61, 2690–2695 [PubMed] [Google Scholar]
- 31. Shin S., Janknecht R. (2007) Diversity within the JMJD2 histone demethylase family. Biochem. Biophys. Res. Commun. 353, 973–977 [DOI] [PubMed] [Google Scholar]
- 32. Mooney S. M., Grande J. P., Salisbury J. L., Janknecht R. (2010) Sumoylation of p68 and p72 RNA helicases affects protein stability and transactivation potential. Biochemistry 49, 1–10 [DOI] [PubMed] [Google Scholar]
- 33. Janknecht R. (2003) Regulation of the ER81 transcription factor and its coactivators by mitogen- and stress-activated protein kinase 1 (MSK1). Oncogene 22, 746–755 [DOI] [PubMed] [Google Scholar]
- 34. Shin S., Rossow K. L., Grande J. P., Janknecht R. (2007) Involvement of RNA helicases p68 and p72 in colon cancer. Cancer Res. 67, 7572–7578 [DOI] [PubMed] [Google Scholar]
- 35. Kim T. D., Shin S., Janknecht R. (2008) Repression of Smad3 activity by histone demethylase SMCX/JARID1C. Biochem. Biophys. Res. Commun. 366, 563–567 [DOI] [PubMed] [Google Scholar]
- 36. Mooney S. M., Goel A., D'Assoro A. B., Salisbury J. L., Janknecht R. (2010) Pleiotropic effects of p300-mediated acetylation on p68 and p72 RNA helicase. J. Biol. Chem. 285, 30443–30452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim J., Shin S., Subramaniam M., Bruinsma E., Kim T. D., Hawse J. R., Spelsberg T. C., Janknecht R. (2010) Histone demethylase JARID1B/KDM5B is a corepressor of TIEG1/KLF10. Biochem. Biophys. Res. Commun. 401, 412–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bosc D. G., Goueli B. S., Janknecht R. (2001) HER2/Neu-mediated activation of the ETS transcription factor ER81 and its target gene MMP-1. Oncogene 20, 6215–6224 [DOI] [PubMed] [Google Scholar]
- 39. Wu J., Janknecht R. (2002) Regulation of the ETS transcription factor ER81 by the 90-kDa ribosomal S6 kinase 1 and protein kinase A. J. Biol. Chem. 277, 42669–42679 [DOI] [PubMed] [Google Scholar]
- 40. Dowdy S. C., Mariani A., Janknecht R. (2003) HER2/Neu- and TAK1-mediated up-regulation of the transforming growth factor β inhibitor Smad7 via the ETS protein ER81. J. Biol. Chem. 278, 44377–44384 [DOI] [PubMed] [Google Scholar]
- 41. Papoutsopoulou S., Janknecht R. (2000) Phosphorylation of ETS transcription factor ER81 in a complex with its coactivators CREB-binding protein and p300. Mol. Cell. Biol. 20, 7300–7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shin S., Kim T. D., Jin F., van Deursen J. M., Dehm S. M., Tindall D. J., Grande J. P., Munz J. M., Vasmatzis G., Janknecht R. (2009) Induction of prostatic intraepithelial neoplasia and modulation of androgen receptor by ETS variant 1/ETS-related protein 81. Cancer Res. 69, 8102–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goel A., Janknecht R. (2004) Concerted activation of ETS protein ER81 by p160 coactivators, the acetyltransferase p300 and the receptor tyrosine kinase HER2/Neu. J. Biol. Chem. 279, 14909–14916 [DOI] [PubMed] [Google Scholar]
- 44. Shin S., Janknecht R. (2007) Activation of androgen receptor by histone demethylases JMJD2A and JMJD2D. Biochem. Biophys. Res. Commun. 359, 742–746 [DOI] [PubMed] [Google Scholar]
- 45. Goel A., Janknecht R. (2003) Acetylation-mediated transcriptional activation of the ETS protein ER81 by p300, P/CAF, and HER2/Neu. Mol. Cell. Biol. 23, 6243–6254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buehr M., McLaren A. (1993) Isolation and culture of primordial germ cells. Methods Enzymol. 225, 58–77 [DOI] [PubMed] [Google Scholar]
- 47. Koopman P., Gubbay J., Collignon J., Lovell-Badge R. (1989) Zfy gene expression patterns are not compatible with a primary role in mouse sex determination. Nature 342, 940–942 [DOI] [PubMed] [Google Scholar]
- 48. Hargrave M., Bowles J., Koopman P. (2006) In situ hybridization of whole-mount embryos. Methods Mol. Biol. 326, 103–113 [DOI] [PubMed] [Google Scholar]
- 49. Wilkinson D. G., Nieto M. A. (1993) Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 225, 361–373 [DOI] [PubMed] [Google Scholar]
- 50. Holt J. E., Jackson A., Roman S. D., Aitken R. J., Koopman P., McLaughlin E. A. (2006) CXCR4/SDF1 interaction inhibits the primordial to primary follicle transition in the neonatal mouse ovary. Dev. Biol. 293, 449–460 [DOI] [PubMed] [Google Scholar]
- 51. Bowles J., Feng C. W., Knight D., Smith C. A., Roeszler K. N., Bagheri-Fam S., Harley V. R., Sinclair A. H., Koopman P. (2009) Male-specific expression of Aldh1a1 in mouse and chicken fetal testes: implications for retinoid balance in gonad development. Dev. Dyn. 238, 2073–2080 [DOI] [PubMed] [Google Scholar]
- 52. Goueli B. S., Janknecht R. (2003) Regulation of telomerase reverse transcriptase gene activity by upstream stimulatory factor. Oncogene 22, 8042–8047 [DOI] [PubMed] [Google Scholar]
- 53. Shin S., Janknecht R. (2007) Concerted activation of the Mdm2 promoter by p72 RNA helicase and the coactivators p300 and P/CAF. J. Cell. Biochem. 101, 1252–1265 [DOI] [PubMed] [Google Scholar]
- 54. Goueli B. S., Janknecht R. (2004) Upregulation of the catalytic telomerase subunit by the transcription factor ER81 and oncogenic HER2/Neu, Ras, or Raf. Mol. Cell. Biol. 24, 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim T. D., Shin S., Berry W. L., Oh S., Janknecht R. (2012) The JMJD2A demethylase regulates apoptosis and proliferation in colon cancer cells. J. Cell. Biochem. 113, 1368–1376 [DOI] [PubMed] [Google Scholar]
- 56. Shin S., Bosc D. G., Ingle J. N., Spelsberg T. C., Janknecht R. (2008) Rcl is a novel ETV1/ER81 target gene upregulated in breast tumors. J. Cell. Biochem. 105, 866–874 [DOI] [PubMed] [Google Scholar]
- 57. Dubin R. A., Ostrer H. (1994) Sry is a transcriptional activator. Mol. Endocrinol. 8, 1182–1192 [DOI] [PubMed] [Google Scholar]
- 58. Koopman P., Münsterberg A., Capel B., Vivian N., Lovell-Badge R. (1990) Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 348, 450–452 [DOI] [PubMed] [Google Scholar]
- 59. Hacker A., Capel B., Goodfellow P., Lovell-Badge R. (1995) Expression of Sry, the mouse sex determining gene. Development 121, 1603–1614 [DOI] [PubMed] [Google Scholar]
- 60. Jeske Y. W., Mishina Y., Cohen D. R., Behringer R. R., Koopman P. (1996) Analysis of the role of Amh and Fra1 in the Sry regulatory pathway. Mol. Reprod. Dev. 44, 153–158 [DOI] [PubMed] [Google Scholar]
- 61. Bradford S. T., Wilhelm D., Bandiera R., Vidal V., Schedl A., Koopman P. (2009) A cell-autonomous role for WT1 in regulating Sry in vivo. Hum. Mol. Genet. 18, 3429–3438 [DOI] [PubMed] [Google Scholar]
- 62. Kent J., Wheatley S. C., Andrews J. E., Sinclair A. H., Koopman P. (1996) A male-specific role for SOX9 in vertebrate sex determination. Development 122, 2813–2822 [DOI] [PubMed] [Google Scholar]
- 63. Morais da Silva S., Hacker A., Harley V., Goodfellow P., Swain A., Lovell-Badge R. (1996) Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat. Genet. 14, 62–68 [DOI] [PubMed] [Google Scholar]
- 64. Kanai Y., Koopman P. (1999) Structural and functional characterization of the mouse Sox9 promoter: implications for campomelic dysplasia. Hum. Mol. Genet. 8, 691–696 [DOI] [PubMed] [Google Scholar]
- 65. Bouwman P., Philipsen S. (2002) Regulation of the activity of Sp1-related transcription factors. Mol. Cell. Endocrinol. 195, 27–38 [DOI] [PubMed] [Google Scholar]
- 66. Safe S., Abdelrahim M. (2005) Sp transcription factor family and its role in cancer. Eur. J. Cancer 41, 2438–2448 [DOI] [PubMed] [Google Scholar]
- 67. Desclozeaux M., Poulat F., de Santa Barbara P., Soullier S., Jay P., Berta P., Boizet-Bonhoure B. (1998) Characterization of two Sp1 binding sites of the human sex determining SRY promoter. Biochim. Biophys. Acta 1397, 247–252 [DOI] [PubMed] [Google Scholar]
- 68. Assumpção J. G., Ferraz L. F., Benedetti C. E., Maciel-Guerra A. T., Guerra G., Jr., Marques-de-Faria A. P., Baptista M. T., de Mello M. P. (2005) A naturally occurring deletion in the SRY promoter region affecting the Sp1 binding site is associated with sex reversal. J. Endocrinol. Invest. 28, 651–656 [DOI] [PubMed] [Google Scholar]
- 69. Yamane K., Toumazou C., Tsukada Y., Erdjument-Bromage H., Tempst P., Wong J., Zhang Y. (2006) JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell 125, 483–495 [DOI] [PubMed] [Google Scholar]
- 70. Knebel J., De Haro L., Janknecht R. (2006) Repression of transcription by TSGA/Jmjd1a, a novel interaction partner of the ETS protein ER71. J. Cell. Biochem. 99, 319–329 [DOI] [PubMed] [Google Scholar]
- 71. Okada Y., Scott G., Ray M. K., Mishina Y., Zhang Y. (2007) Histone demethylase JHDM2A is critical for Tnp1 and Prm1 transcription and spermatogenesis. Nature 450, 119–123 [DOI] [PubMed] [Google Scholar]
- 72. Liu Z., Zhou S., Liao L., Chen X., Meistrich M., Xu J. (2010) Jmjd1a demethylase-regulated histone modification is essential for cAMP-response element modulator-regulated gene expression and spermatogenesis. J. Biol. Chem. 285, 2758–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Monsees T. K., Schill W. B., Miska W. (1997) Protease-protease inhibitor interactions in Sertoli cell-germ cell crosstalk. Adv. Exp. Med. Biol. 424, 111–123 [DOI] [PubMed] [Google Scholar]