Background: RNA polymerases (RNAPs) from Thermus aquaticus and Escherichia coli differ in many aspects of transcription initiation.
Results: Regions 1.1 and 1.2 of the σ subunit determine instability and cold sensitivity of promoter complexes of T. aquaticus RNAP.
Conclusion: Substitutions in σ regions 1.1 and 1.2 modulate RNAP-promoter interactions.
Significance: Evolutionary changes in the σ subunit determine functional differences between bacterial RNAPs during transcription initiation.
Keywords: Bacterial Transcription, Gene Regulation, Promoters, RNA Polymerase, Transcription Initiation Factors, Abortive Initiation, Open Promoter Complex, Promoter Complex Stability
Abstract
RNA polymerase (RNAP) from thermophilic Thermus aquaticus is characterized by higher temperature of promoter opening, lower promoter complex stability, and higher promoter escape efficiency than RNAP from mesophilic Escherichia coli. We demonstrate that these differences are in part explained by differences in the structures of the N-terminal regions 1.1 and 1.2 of the E. coli σ70 and T. aquaticus σA subunits. In particular, region 1.1 and, to a lesser extent, region 1.2 of the E. coli σ70 subunit determine higher promoter complex stability of E. coli RNAP. On the other hand, nonconserved amino acid substitutions in region 1.2, but not region 1.1, contribute to the differences in promoter opening between E. coli and T. aquaticus RNAPs, likely through affecting the σ subunit contacts with DNA nucleotides downstream of the −10 element. At the same time, substitutions in σ regions 1.1 and 1.2 do not affect promoter escape by E. coli and T. aquaticus RNAPs. Thus, evolutionary substitutions in various regions of the σ subunit modulate different steps of the open promoter complex formation pathway, with regions 1.1 and 1.2 affecting promoter complex stability and region 1.2 involved in DNA melting during initiation.
Introduction
The σ subunit of bacterial RNAP3 has been implicated in various steps of transcription initiation, from promoter recognition and melting to initiation of RNA synthesis and promoter escape (1–6). All primary σ subunits (σ70 in Escherichia coli; σA in other bacteria) that are involved in transcription of housekeeping genes contain four conserved regions, further divided into subregions (7, 8). Structural analyses of E. coli (Eco) σ70 (9) and Thermus aquaticus (Taq) σA (8) subunits, as well as Taq (3) and closely related Thermus thermophilus (4) RNAP holoenzymes revealed that σs contain four domains, σ1.1 (comprised of region 1.1), σ2 (regions 1.2 through 2.4), σ3 (regions 3.0–3.2), and σ4 (regions 4.1–4.2). Based on genetic, biochemical, and structural studies, the σ regions were assigned various functions in transcription initiation. In particular, regions 4.2, 2.3–2.4, and 3.0 were implicated in recognition of the −35, −10, and TG (extended −10) promoter elements, respectively (reviewed in Refs. 1 and 2). Region 1.2 in Eco σ70 and Taq σA was shown to recognize an additional promoter element (GGGA motif in Taq) located downstream of the −10 element (10, 11).
Recently, the three-dimensional structure of domain 2 of the Taq σA subunit in complex with a short DNA oligonucleotide containing the −10 element was determined (12). In this complex the nontemplate DNA strand forms tight contacts with σ region 2 (Fig. 1A), incompatible with double-stranded DNA conformation, explaining previously established roles of region 2 in promoter recognition, DNA melting, and stabilization of RNAP-DNA interactions (1, 13–17). Although the exact position of the nontemplate DNA strand downstream of the −10 element remains unknown, structural modeling, site-specific cross-linking, and biochemical analyses suggest that this DNA segment may directly contact σ region 1.2, depending on the DNA sequence context (10, 11, 18, 19). Amino acid substitutions at various positions of region 1.2 in Eco σ70 were shown to decrease RNAP activity, inhibit promoter DNA melting, and destabilize the open promoter complex (Fig. 1B) (19, 20). In addition to its direct role in DNA recognition, region 1.2 was shown to allosterically modulate the DNA binding activity of region 2 (21).
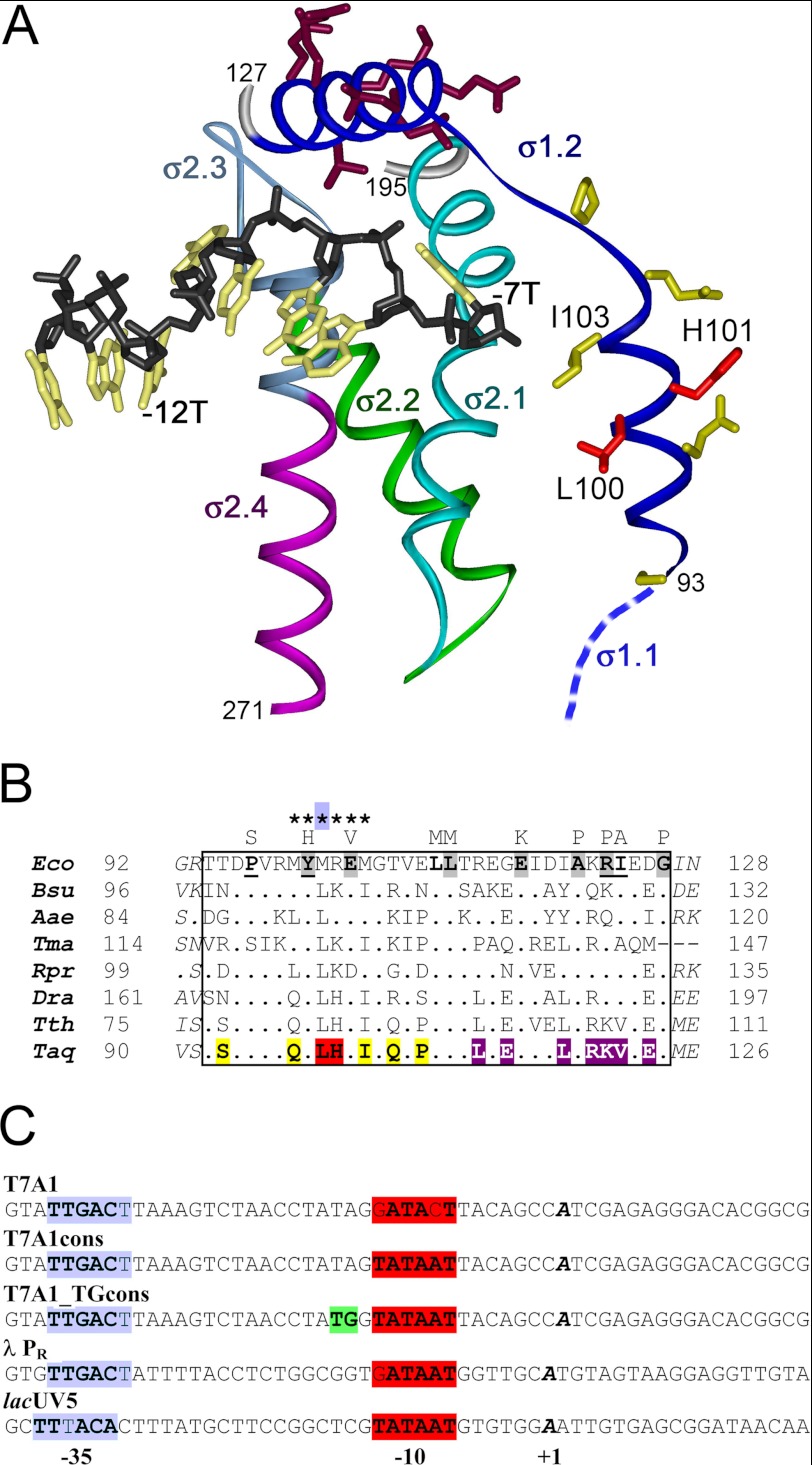
FIGURE 1.
Recognition of the nontemplate promoter strand by the σ subunit and sequence comparisons of σ region 1.2 from various bacteria. A, the structure of Taq σA domain 2 (amino acids 93–271) in complex with the −10 DNA element (Protein Data Bank code 3UGO) (12). The nonconserved spacer between regions 1.2 and 2.1 is not shown on the structure. The DNA segment containing the −10 element (TGTACAAT) is shown in black (backbone) and light yellow (bases), and the first (−12T) and the last (−7T) thymines of the −10 element are indicated. Region 1.2 (dark blue) connects to region 1.1 (blue), the position of which remains unknown. Amino acids substituted in σA region 1.2 in comparison with the Eco σ70 subunit are shown in yellow (first α helix) and dark violet (second α helix). Residues Leu-100 and His-101 that probably contact DNA nucleotides downstream of the −10 element are shown in red. B, sequences of region 1.2 of primary σ subunits from various bacteria: Bsu, Bacillus subtilis; Aae, Aquifex aeolicus; Tma, T. maritima; Rpr, Rickettsia prowazekii; Dra, Deinococcus radiodurans; Tth, T. thermophilus. Region 1.2 is boxed; amino acid numbers are indicated at both sides of the alignment. Mutations of conserved amino acids in Eco σ70 that were shown to strongly affect promoter-dependent activity of RNAP (20) are shown above the Eco σ70 sequence. Those mutations that were demonstrated to impair promoter opening or promoter escape are shadowed and underlined, respectively. The positions of alanine substitutions that were proposed to decrease promoter complex stability by affecting σ70 contacts with DNA downstream of the −10 element are indicated with asterisks (19). Amino acid substitutions in Taq σA region 1.2 are colored in the same way as in A. C, sequences of promoters used in this study (only nontemplate strands are shown). The −35, TG, and −10 elements are shown in blue, green, and red, respectively; the +1 nucleotide is shown in bold italics.
The DNA binding activity of primary σ subunits is inhibited in free σs, in part because of the presence of a weakly conserved region 1.1 at their N terminus. Removal of this region in Eco σ70 increases specific interactions of σ70 with promoters (22, 23). As revealed by site-specific cross-linking, in Thermotoga maritima σA region 1.1 is physically close to the promoter recognition regions 2 and 4 (24). Although the structures of regions 1.1 in Eco and Taq σ subunits remain unknown, the structure of region 1.1 from T. maritima was solved by NMR showing that it folds into three α helixes and possesses a negative electrostatic potential on its surface (supplemental Fig. S1) (24). The presence of a high number of negatively charged amino acids is also a characteristic feature of region 1.1 in other primary σ subunits, including Taq and T. thermophilus σA subunits that have highly divergent region 1.1 sequences (supplemental Fig. S1). In addition to the proposed functions in preventing interactions of free σ with DNA, region 1.1 likely plays an important role in holoenzyme assembly and open complex formation (25–28). Several studied amino acid substitutions and deletions in this region in Eco σ70 (supplemental Fig. S1) decreased RNAP activity, impaired open complex formation, and/or decreased the stability of promoter complexes (25, 27–29).
Although a wealth of biochemical and genetic data on promoter recognition and transcription initiation were accumulated for Eco RNAP, most structural information on the mechanisms of bacterial transcription was obtained for RNAPs from thermophilic Taq and T. thermophilus that are distantly related to Eco. Although all multisubunit RNAPs share a highly conserved architecture, Thermus RNAPs significantly differ from the Eco RNAP in structural details (30–33) and transcription properties (18, 34–39). In particular, Taq RNAP has a higher temperature optimum of activity than the Eco RNAP and is inactive at low and moderate temperatures. These differences were shown to be associated with the catalytic properties of Taq core RNAP and with the properties of the Taq σA subunit that is unable to induce promoter DNA melting at temperatures below 45 °C (36–38, 40). The cold sensitivity of promoter opening by the Taq σA subunit in comparison with the Eco σ70 subunit was explained by substitutions of nonconserved amino acids in region 2 and by differences in the structures of the N-terminal parts of these σ subunits (36–38). However, individual roles of σA regions 1.1 and 1.2 in transcription initiation by Taq RNAP were not investigated.
A characteristic feature of most studied promoter complexes of Eco RNAP is their very high stability in vitro, with half-lives ranging from tens of minutes to hours. In contrast, promoter complexes of Taq RNAP were shown to be intrinsically unstable and to dissociate within seconds (10, 36). The structural features of Taq RNAP that can explain the low stability of promoter complexes formed by this RNAP remain unidentified.
In this work we extended analysis of functional differences between Eco and Taq RNAPs in transcription initiation, with particular emphasis on the roles of regions 1.1 and 1.2 of the σ70 and σA subunits in promoter complex formation. We demonstrated that both regions 1.1 and 1.2 of the σA subunit determine the low stability of promoter complexes of Taq RNAP, whereas substitutions in region 1.2 of σA contribute to the cold sensitivity of promoter opening by Taq RNAP, suggesting possible functions of these regions at different steps of the open complex formation pathway.
EXPERIMENTAL PROCEDURES
RNAPs and Promoters
Wild-type Eco and Taq core RNAPs were purified from E. coli BL21(DE3) cells overproducing all four core RNAP subunits from plasmids pVS10 and pET28ABCZ, respectively, as described previously (35, 36, 41). Genes coding for mosaic Eco and Taq σ subunits were generated by PCR mutagenesis of wild-type rpoD genes and cloned between NdeI and EcoRI sites into the pET28 plasmid. Wild-type Eco σ70 and Taq σA subunits and mosaic σ subunits, all containing His6 tags at their N termini, were overexpressed in E. coli BL21(DE3) and purified as described in Refs. 34 and 36.
Promoter DNA fragments for in vitro transcription were obtained as follows. The T7A1, T7A1cons, and T7A1_TGcons promoters (positions from −85 to +53 nucleotides relative to the starting point of transcription) were obtained by PCR from synthetic oligonucleotide templates. The lacUV5 promoter (positions −59 to +58) was obtained as described in Ref. 21. The λPR promoter fragment (positions −81 to +54) was obtained by PCR from plasmid pIA226. The sequences of all promoters are shown on Fig. 1C.
In Vitro Transcription
Transcription assays were performed in transcription buffer containing 40 mm Tris-HCl, pH 7.9, 40 mm KCl, and 10 mm MgCl2. Holoenzyme RNAPs were prepared by incubating core RNAP (final concentration, 100 nm) and either the wild type or mosaic σ subunits (500 nm) in the transcription buffer for 5 min at 25 °C. DNA template was added (10–30 nm), and the samples were incubated for 3–5 min at desired temperatures (45 °C for holoenzymes containing Eco core RNAP and 55 °C for holoenzymes containing Taq core RNAP in most experiments). For analysis of promoter complex stabilities on T7A1 and λPR promoters, heparin was added to 10 μg/ml. Following incubation of the samples for different time intervals at the same temperatures, transcription reactions were initiated by the addition of dinucleotide primer CpA (25 μm) and UTP (10 μm, with the addition of [α-32P]UTP). The reactions were stopped after 1 min by the addition of an equal volume of buffer containing 8 m urea and 20 mm EDTA; the 3-nucleotide RNA products were analyzed by 23% denaturing PAGE followed by phosphorimaging. For analysis of temperature dependence of transcription on the lacUV5 promoter, reactions were performed in the presence of trinucleotide primer ApApU and UTP (with the addition of [α-32P]UTP); the samples were transferred to desired temperatures 5 min prior to addition of nucleotide substrates. For analysis of promoter escape, the reactions were performed in transcription buffer containing 100 mm KCl; all four nucleotide substrates were added (100 μm of ATP, CTP, and GTP and 10 μm of UTP with the addition of [α-32P]UTP), either in the absence or in the presence of the CpA primer (25 μm). The transcription reactions were stopped after 5 min, and RNA products were separated by 20% denaturing PAGE.
KMnO4 Footprinting
For the KMnO4 footprinting experiments, the lacUV5 promoter was labeled at the 3′-end of the template DNA strand with the Klenow fragment of DNA polymerase I and [α-32P]dATP as described in Refs. 36 and 42. Holoenzyme RNAPs (100 nm core and 500 nm σ) were incubated with the labeled DNA fragment (10 nm) in the transcription buffer for 10 min at either 25 or 45 °C, followed by the addition of KMnO4 to 2 mm. The reaction was stopped after 20 s by the addition of equal volume of solution containing 1 m β-mercaptoethanol and 1 m sodium acetate. DNA was ethanol-precipitated, treated with piperidine as described (6), and analyzed on 10% denaturing polyacrylamide gel.
Nontemplate Oligonucleotide Binding and Cross-linking
Apparent dissociation constants (Kd) for the oligonucleotide binding to RNAP holoenzymes were determined by nitrocellulose filtration method (43). 5′-End-labeled nontemplate promoter oligonucleotide containing the −10 element (see Fig. 5C), taken at fixed 0.03 nm concentration, was mixed with RNAP holoenzyme, taken at varying concentrations (250 nm σ plus 0.1–100 nm core RNAP), in binding buffer containing 40 mm Tris-HCl, pH 7.9, 10 mm MgCl2, and 100 mm NaCl; incubated for 10 min at 25 °C; and filtered through 0.45-μm nitrocellulose filters (HAWP, Millipore), followed by phosphorimaging. The binding curves were fit to hyperbolic equation B = Bmax*[RNAP]/([RNAP] + Kd), where B is a fraction of bound DNA, Bmax is the maximum binding, and Kd is the apparent dissociation constant, using GraFit software (Erithacus Software). RNAP-DNA cross-linking experiments were performed in buffer containing 40 mm Hepes, pH 8.0, 5 mm MgCl2, and 100 mm NaCl as described (44). Core RNAP, σ subunits, and nontemplate oligonucleotide were taken at 50, 300, and 10 nm, respectively. After incubation for 10 min at 25 °C, the samples were irradiated for 10 min with a 254-nm UV lamp (4 watts; Spectroline). The DNA-protein complexes were separated by 5% SDS-PAGE.
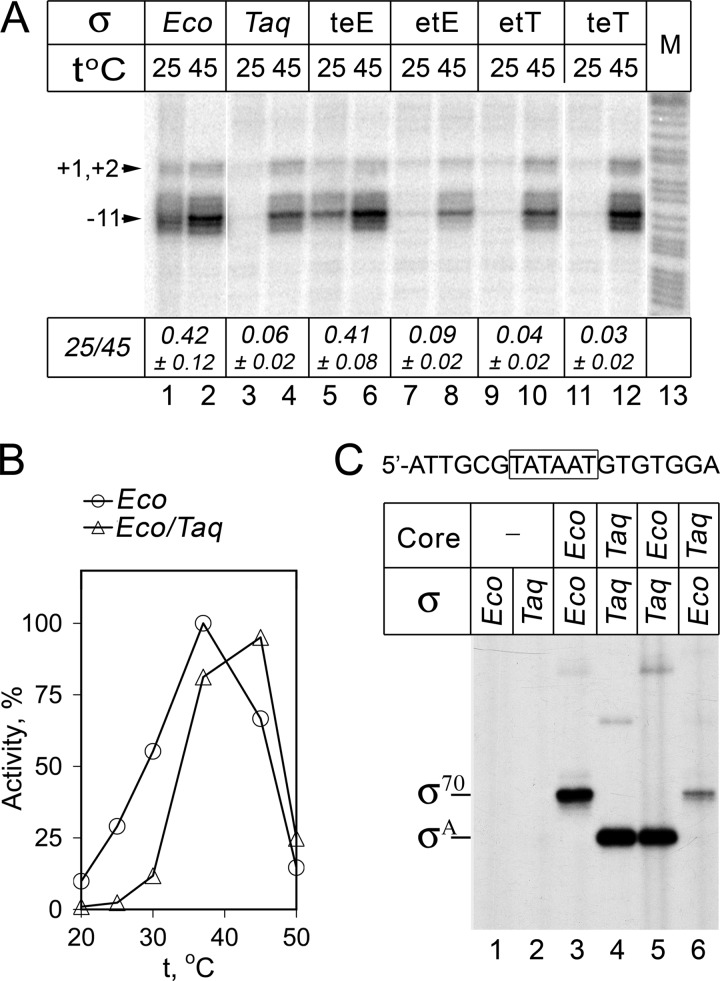
FIGURE 5.
Promoter recognition and opening by RNAP holoenzymes containing Eco core and various σ subunits. A, KMnO4 probing of the lacUV5 promoter complexes. Footprinting was performed at either 25 or 45 °C on a linear DNA fragment containing the lacUV5 promoter; positions of modified thymines in the template promoter strand relative to the starting point of transcription are shown. Lane 13 contains an A+G cleavage marker. The ratio of modification efficiencies at 25 and 45 °C (measured for position −11) for each RNAP is indicated below the figure (averages and standard deviations from two-three independent experiments). B, activities of wild-type Eco and hybrid Eco/Taq RNAPs measured in the reaction of abortive synthesis on the lacUV5 promoter DNA fragment at different temperatures (in percent of the maximal Eco RNAP activity). C, cross-linking of the nontemplate promoter oligonucleotide (shown on the top, the −10 element is boxed) with Eco, Taq, and hybrid Eco/Taq RNAP holoenzymes. The experiment was performed at 25 °C. The positions of cross-linked complexes containing the σ70 and σA subunits are indicated on the left.
RESULTS
The Instability of Taq RNAP Promoter Complexes Is Determined by the N Terminus of the σA Subunit
To determine which component of the Taq RNAP holoenzyme determines the instability of promoter complexes formed by this RNAP in comparison with the Eco RNAP holoenzyme, we compared stabilities of complexes formed on the T7A1 promoter by wild-type Eco and Taq RNAPs and by a hybrid RNAP containing Eco core and Taq σA (Eco/Taq). It should be noted that the properties of promoter complexes formed by a reciprocal hybrid holoenzyme containing Taq core and Eco σ70 could not be tested because such holoenzyme is inactive (35, 36). For each of the three RNAPs, we measured the kinetics of promoter complex dissociation in the presence of heparin (Fig. 2A). The measurements were performed at 45 °C in the case of Eco and hybrid Eco/Taq RNAPs and at 55 °C in the case of Taq RNAP. In agreement with published data, we found that promoter complexes of Eco RNAP were stable with half-life exceeding 10 min. In contrast, promoter complexes of Taq RNAP were unstable and almost completely dissociated within 20 s. The hybrid Eco/Taq holoenzyme also displayed much lower stability of promoter complexes, with a half-life of about 40 s (Fig. 2A). Importantly, the hybrid holoenzyme was highly active and fully melted promoter DNA around the starting point of transcription under the same conditions (at 45 °C), demonstrating that the low promoter complex stability does not result from its inability to form the open promoter complex (36, 38) (see also Fig. 5). In addition to the heparin challenge experiments, we analyzed activity of the T7A1 promoter complexes formed by the three RNAPs under various ionic strength conditions. Promoter complexes of Taq RNAP were much more salt-sensitive than promoter complexes of Eco RNAP, and promoter complexes of the hybrid RNAP displayed intermediate salt sensitivity.4 Thus, we conclude that the low stability of promoter complexes of Taq RNAP holoenzyme is in a large part determined by the properties of the Taq σA subunit (but also by the properties of the Taq core enzyme, because the Taq holoenzyme has lower promoter complex stability than the hybrid Eco/Taq holoenzyme).
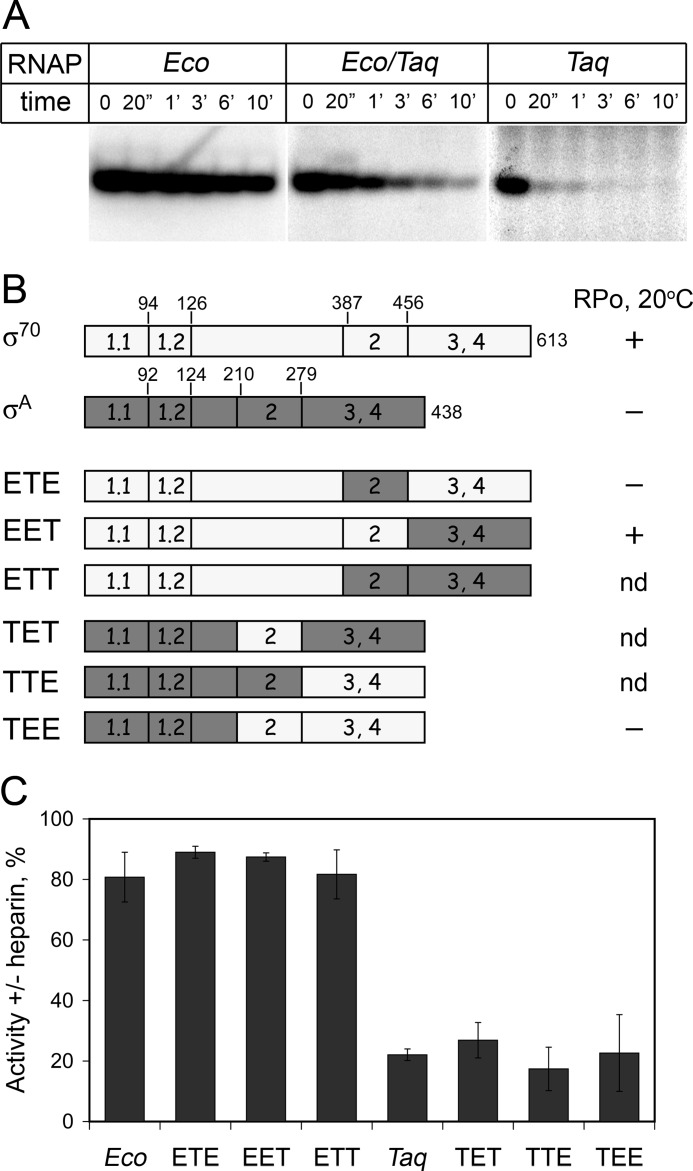
FIGURE 2.
Stabilities of promoter complexes formed by RNAPs containing Eco, Taq σ subunits, and their mosaic variants. A, stabilities of promoter complexes formed by Eco, Taq, and hybrid Eco/Taq RNAPs on the T7A1 promoter measured in the reaction of abortive synthesis in the presence of heparin (10 μg/ml). B, schematics of mosaic σ subunits with substitutions of regions 1, 2, and 3/4. The numbers above the schemes correspond to amino acid positions at the borders of conserved regions that were used for exchanging σ segments. The ability of various σ subunits to induce formation of the open promoter complex by Eco RNAP at 20 °C is indicated at right (the data from Ref. 38). nd, no data. C, relative stabilities of promoter complexes formed by holoenzymes containing Eco core and mosaic σ subunits. The plot shows RNAP activities measured after 3′ incubation of preformed promoter complexes with heparin relative to activities measured in the absence of heparin (averages and standard deviations from three independent experiments).
To determine which region of the σA subunit is responsible for instability of promoter complexes formed by the hybrid RNAP, we tested several mosaic σ subunits consisting of various parts of the σ70 and σA subunits. The exchanged segments of the σ subunits included the N-terminal part, including conserved region 1 and a nonconserved spacer between regions 1 and 2 (amino acids 1–386 and 1–209 in σ70 and σA, respectively); conserved region 2 (amino acids 387–455 and 210–278 in σ70 and σA, respectively); and the C-terminal part, including conserved regions 3 and 4 (amino acids 456–613 and 279–438 in σ70 and σA, respectively). In total, six mosaic σ subunits with all possible combinations of these segments (ETE, EET, ETT, TET, TTE, and TEE) were studied (Fig. 2B). All of the mosaic subunits were shown to form active holoenzymes with Eco core RNAP (38).5 It was found that RNAP holoenzymes that contained the σ subunits with the N-terminal part from σ70 (ETE, EET, and ETT) formed stable promoter complexes, whereas holoenzymes that contained the σ subunits with the N-terminal part from σA formed unstable complexes when challenged with heparin (Fig. 2C). Importantly, the promoter complex stabilities of RNAPs containing mosaic σs did not correlate with their abilities to form the open promoter complex at low temperatures (20 °C) (Fig. 2B). In particular, the holoenzyme containing the ETE σ subunit displayed cold sensitivity of promoter opening (38) but formed stable promoter complexes.
σ Regions 1.1 and 1.2 Together determine the Differences in Promoter Complex Stabilities between Eco and Taq RNAPs
To more precisely locate the region(s) in the N-terminal part of the σ subunit that can affect the promoter complex stability in Eco and Taq RNAPs, we designed a second set of mosaic σ subunits with substitutions of regions 1.1 and 1.2. The mosaic σ subunits were based either on the σ70 or the σA sequence and contained replacements of region 1.1 (σ teE, amino acid residues 1–93 in σ70 replaced with residues 1–91 from σA; σ etT, residues 1–91 in σA replaced with residues 1–93 from σ70), region 1.2 (σ etE, residues 94–125 in σ70 replaced with residues 92–123 from σA; σ teT, residues 92–123 in σA replaced with residues 94–125 from σ70), or both (σ ttE that contained regions 1.1 and 1.2 from σA) (Fig. 3A). Whereas regions 1.1 in σ70 and σA significantly differ in their sequences, region 1.2 is highly conserved and contains only 14 substitutions in σA in comparison with σ70 (Fig. 1B). Thus, to reveal possible effects of individual substitutions in region 1.2, we obtained a σ70 mutant that contained two σA-specific substitutions, M102L and R103H (σ MR-LH), that changed amino acids likely involved in contacts with the nontemplate DNA strand downstream of the −10 element (see Introduction and Fig. 1).
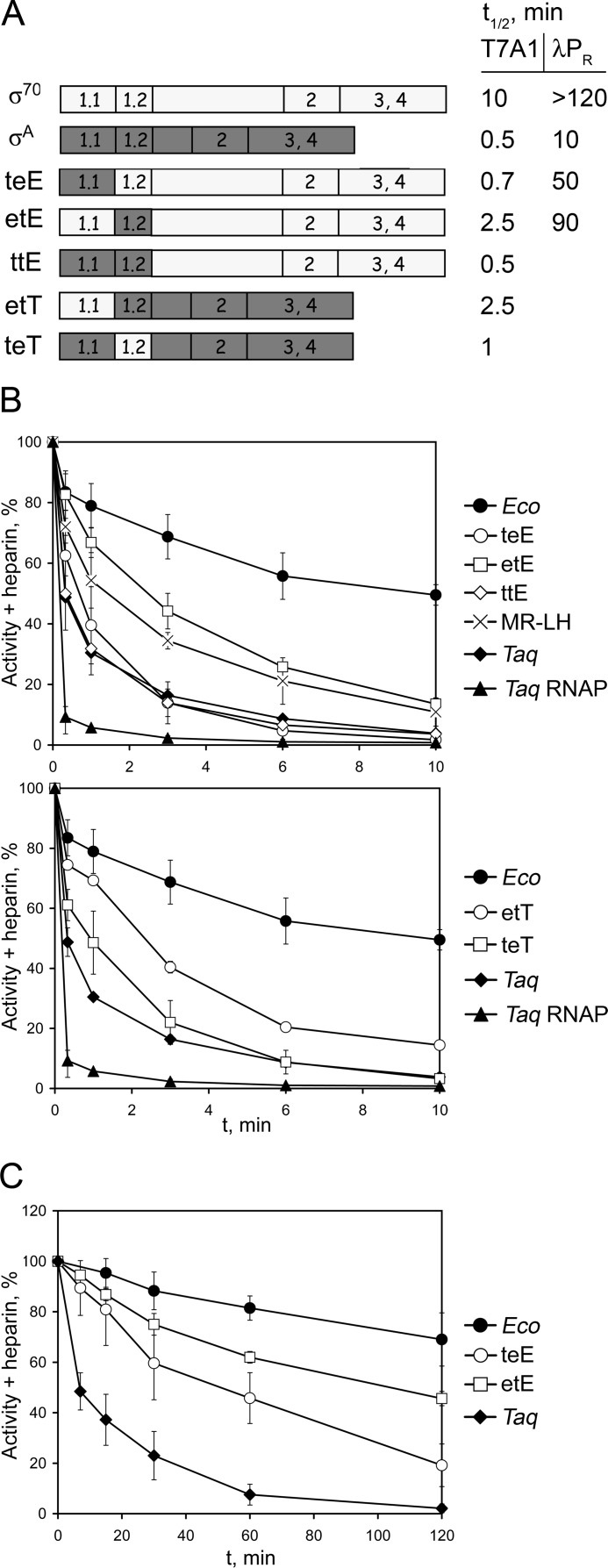
FIGURE 3.
Effects of substitutions of regions 1.1 and 1.2 in the Eco and Taq σ subunits on promoter complex stability. A, schematics of Eco σ70, Taq σA, and mosaic σ subunits with substitutions of regions 1.1 and 1.2. The half-life times of complexes formed by holoenzyme RNAPs containing Eco core RNAP and corresponding σ subunits on the T7A1 and λPR promoters, measured in the presence of heparin (10 μg/ml), are shown on the right. B, kinetics of dissociation of the T7A1 promoter complexes formed by Eco, Taq RNAPs, and RNAP holoenzymes containing Eco core RNAP and various σ subunits. The data for σ70- and σA-based subunits are shown on the upper and lower plots, respectively. RNAP activities were measured after incubation of promoter complexes in the presence of heparin for various time intervals. For each time point, the level of heparin-resistant activity relative to the activity measured in the absence of heparin is shown. C, kinetics of dissociation of the λPR promoter complexes formed by RNAP holoenzymes containing Eco core RNAP and various σ subunits.
Analysis of the T7A1 promoter complex stabilities of RNAPs containing Eco core RNAP and σ70-based mosaic σ subunits revealed that the substitution of region 1.1 in the teE σ subunit had the most significant effect on promoter complex stability, measured in the presence of heparin, and decreased it almost to the level of the wild-type σA subunit (Fig. 3B, upper panel). Substitution of region 1.2 in σ etE also decreased promoter complex stability but to a lesser extent. Importantly, the effect of the MR → LH substitution in region 1.2 was comparable with the effect of the substitution of the whole region 1.2. Finally, RNAP containing σ ttE with substitutions of both regions 1.1 and 1.2 displayed the same promoter complex stability as RNAP containing wild-type σA (Fig. 3B). Thus, substitutions in these two regions can fully explain the lower stability of promoter complexes formed by the σA-containing RNAP on the T7A1 promoter.
We then tested the effects of substitutions in the σA-based σ etT and σ teT subunits on the promoter complex stability. Substitution of region 1.2 in σ teT slightly increased the stability of promoter complexes in comparison with σA Taq. At the same time, substitution of region 1.1 in σ etT had a stronger stabilizing effect on promoter complexes (Fig. 3B, lower panel).
To test whether the observed effects are general for various promoters, we repeated the experiment with holoenzymes containing Eco core RNAP and various σ subunits on the λ PR promoter that forms more stable complexes with Eco RNAP (Fig. 3C). Promoter complexes formed by RNAP containing wild-type σ70 were highly resistant to heparin challenge and did not dissociate within 120 min. In contrast, the dissociation kinetics was much faster in the case of σA-containing RNAP (t½ = ∼10 min). The half-life times of promoter complexes formed by holoenzymes containing mosaic subunits were also decreased in comparison with wild-type Eco RNAP, although the effects were less dramatic than in the case of the T7A1 promoter. In particular, the promoter complex half-lives for RNAPs containing σ teE and σ etE were ∼50 and ∼90 min, respectively (Fig. 3C). Thus, it can be concluded that substitutions of regions 1.1 and 1.2 from the σA subunit destabilize complexes formed by Eco RNAP on various promoters.
Region 1.1 from σ70 Can Stabilize Promoter Complexes Formed by Taq RNAP
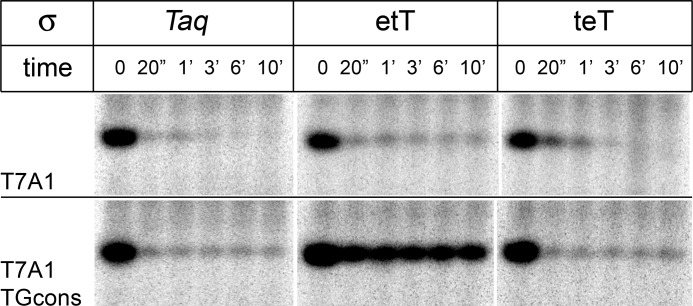
To determine whether substitutions of regions 1.1 and 1.2 could also affect the stability of promoter complexes formed by Taq RNAP, we analyzed the dissociation kinetics of promoter complexes formed by RNAPs containing Taq core and σ subunits etT and teT. In contrast to σ70, these two mosaic σ subunits were shown to form fully active holoenzymes with Taq core RNAP. In the case of the T7A1 promoter, promoter complexes formed by holoenzymes containing both mosaic σ subunits were unstable and rapidly dissociated after the addition of heparin (Fig. 4). We then repeated the experiment on promoter T7A1_TGcons, a variant of the T7A1 promoter containing three consensus promoter elements (−10, TG, and −35 elements; Fig. 1C), to increase the strength of RNAP-promoter interactions. On this promoter, holoenzymes containing σA and σ teT also formed unstable complexes. However, holoenzyme containing σ etT displayed increased promoter complex stability, and a significant fraction of complexes remained active even after 10 min of incubation with heparin (Fig. 4). Thus, substitution of region 1.1 in σA with region 1.1 from σ70 can stabilize promoter complexes formed by Taq RNAP.
FIGURE 4.
Heparin challenge of promoter complexes formed by Taq RNAP holoenzymes containing either wild-type σA or mosaic etT and teT σ subunits. RNAP activities were measured at different time intervals after the addition of heparin to preformed T7A1 (top panel) or T7A1TGcons (bottom panel) promoter complexes at 55 °C.
Cold Sensitivity of Transcription by Hybrid Eco/Taq RNAP
Previously, hybrid RNAP containing Eco core and Taq σA was shown to be unable to open promoters at moderate temperatures (20–25 °C), suggesting that the σA subunit is responsible for the cold sensitivity of promoter opening by Taq RNAP (see Introduction and Refs. 36 and 38). We compared DNA melting by the wild-type Eco and hybrid Eco/Taq RNAP holoenzymes on the model lacUV5 promoter by the KMnO4 footprinting and confirmed that, in contrast to the Eco holoenzyme, the hybrid holoenzyme was unable to open the promoter at 25 °C (Fig. 5A, lanes 1 and 3). At the same time, both holoenzymes fully opened the transcription bubble at 45 °C (Fig. 5A, lanes 2 and 4). Similarly, the hybrid holoenzyme was inactive in lacUV5-promoter-dependent transcription at temperatures below 30 °C but synthesized RNA as efficiently as wild-type Eco RNAP at higher temperatures (37–45 °C) (Fig. 5B).
To confirm that Taq σA is able to bind Eco core RNAP and recognize promoter DNA at low temperatures, we analyzed RNAP interactions with short oligonucleotides corresponding to the nontemplate promoter strand and containing the −10 element (Fig. 5C). Previously, recognition of such oligonucleotides by holoenzyme RNAP was shown to mimic the recognition of the −10 element in the open promoter complex (44–46). We found that both wild-type Eco and hybrid Eco/Taq holoenzymes bound the nontemplate oligonucleotide with high affinity, with apparent Kd values of 3.1 ± 1.6 and 3.2 ± 2.6 nm at 25 °C (see “Experimental Procedures” for details on Kd measurements). Furthermore, both RNAPs formed highly efficient cross-links between the corresponding σ subunit and the nontemplate oligonucleotide upon UV irradiation (Fig. 5C, lanes 3 and 5). Notably, the efficiencies of Taq σA-oligonucleotide cross-linking were similar in the case of the hybrid Eco/Taq RNAP and the Taq RNAP holoenzyme (compare lanes 4 and 5), suggesting that the Taq σA subunit similarly interacts with the Eco and Taq core RNAPs under our experimental conditions. In contrast, no efficient cross-linking was observed in the case of free σ70 and σA subunits (lanes 1 and 2) and in the complex of Taq core RNAP and the Eco σ70 subunit, which do not form an active holoenzyme (lane 6). These results suggest that the observed cold sensitivity of promoter opening by the hybrid Eco/Taq RNAP is not due to defects in the binding of the heterologous σA subunit to the Eco core RNAP and/or promoter DNA recognition but likely results from hampered promoter DNA melting at low temperatures.
σA-specific Substitutions in Region 1.2 Increase the Temperature of Promoter Opening by Eco RNAP
Previously, it was shown that the cold sensitivity of DNA melting by the Taq σA subunit is explained by structural features of region 2 and of the N-terminal part of σA, including regions 1.1 and 1.2 (38). In particular, substitutions of either region 2 or the N-terminal part in Eco σ70 with corresponding regions from σA (in the mosaic ETE and TEE σ subunits; Fig. 2B) resulted in cold sensitivity of promoter opening by Eco RNAP (38). We therefore tested whether substitutions of regions 1.1 and 1.2 in the mosaic σ70 and σA subunits obtained in this work can affect promoter opening by Eco RNAP. We found that holoenzymes containing the σ etT and σ teT subunits did not open the lacUV5 promoter at 25 °C (lanes 9–12), an expected result because these σs contained region 2 from σA Taq, which by itself imposes the cold sensitivity of promoter opening (38). In contrast, the mosaic σ teE subunit with substitution of region 1.1 was able to induce DNA melting at both 25 and 45 °C (lanes 5 and 6). However, the σ etE subunit with substitution of region 1.2 did not support DNA melting at 25 °C, although it opened the promoter at 45 °C (lanes 7 and 8). Thus, σA-specific substitutions in region 1.2, but not in region 1.1, increase the temperature of promoter opening by Eco RNAP. Importantly, RNAP containing the σ etE subunit formed more stable promoter complexes than the σ teE-containing holoenzyme, suggesting that substitutions of regions 1.1 and 1.2 independently affect different promoter complex properties.
Differences between Eco and Taq RNAPs in Abortive Synthesis and Promoter Escape Are Partially Determined by σ Regions 2–4
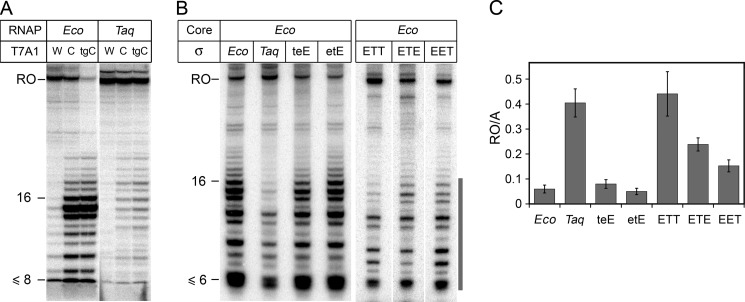
Changes in stabilities of promoter complexes of Eco RNAP were shown to significantly affect the efficiencies of abortive synthesis and promoter escape (47). Because Taq RNAP forms much less stable promoter complexes than the Eco RNAP, one could expect that it should also differ from Eco RNAP in the promoter escape efficiency. We found that this indeed was the case. To analyze promoter escape, we performed transcription on the T7A1 promoter and its two consensus variants, T7A1cons (containing −10 and −35 consensus elements) and T7A1_TGcons (containing −10, TG and −35 elements) (Fig. 1C) that are expected to form strong σ-mediated contacts with RNAP. In the case of Eco RNAP, large amounts of abortive RNAs of various lengths (up to 16 nucleotides) were synthesized during transcription initiation on the consensus promoters (Fig. 6A) (6, 41). Furthermore, the efficiency of the full-length RNA synthesis was significantly decreased in the case of the T7A1_TGcons promoter. In contrast, Taq RNAP synthesized much lower amounts of abortive RNAs, although the level of abortive synthesis was increased on the consensus promoters (Fig. 6A). Taq RNAP was also able to efficiently synthesize the full-length RNA on all three promoter variants.
FIGURE 6.
Abortive synthesis and promoter escape by various RNAP holoenzymes. A, transcription by Eco and Taq RNAPs on wild-type T7A1 (W), T7A1cons (C), and T7A1_TGcons (tgC) promoters. Transcription was performed at either 37 °C (Eco RNAP) or 55 °C (Taq RNAP) in the presence of all four NTPs. B, transcription by Eco holoenzymes containing various σ subunits. Transcription was performed at 45 °C on the T7A1_TGcons promoter in the presence of NTPs and dinucleotide primer CpA. C, promoter escape efficiencies by RNAP holoenzymes containing Eco core RNAP and various σ subunits. For each RNAP, the promoter escape efficiency was determined as the ratio of the full-length (RO) to abortive (A) RNAs; positions of abortive transcripts used for calculation are indicated by a vertical gray line on Fig. 6B (the data from two-three independent experiments).
To determine whether the increased efficiency of promoter escape by Taq RNAP can be explained by the properties of the σA subunit, we analyzed transcription by the hybrid Eco/Taq RNAP. In the case of this RNAP, the amounts of abortive RNAs synthesized during initiation were significantly decreased, and the efficiency of promoter escape was increased in comparison with the σ70 RNAP holoenzyme (Fig. 6, B and C). Thus, the σA subunit promotes more efficient escape to elongation. At the same time, the hybrid RNAP was still less efficient in promoter escape than the Taq RNAP holoenzyme, demonstrating that the core enzyme also contributes to the higher promoter escape efficiency displayed by Taq RNAP (Fig. 6, compare A and B).
We then tested whether regions 1.1 and 1.2 of the σ70 and σA subunits contribute to the differences in promoter escape between Eco and Taq RNAPs. It was found that RNAP holoenzymes containing Eco core RNAP and σ teE or σ etE, bearing regions 1.1 or 1.2 from Taq, were characterized by the same promoter escape efficiencies as RNAP containing wild-type σ70 (Fig. 6, B and C). In contrast, RNAP containing the σ ETT subunit with regions 2–4 taken from the Taq σA subunit behaved similarly to the σA-containing RNAP holoenzyme. RNAPs containing σ subunits with individual substitutions of region 2 (σ ETE) or regions 3 and 4 (σ EET) displayed intermediate promoter escape efficiencies (Fig. 6C). Thus, substitutions of these regions in Eco σ70 with the corresponding regions from σA facilitate promoter escape by RNAP.
DISCUSSION
While sharing a conserved transcription mechanism, RNAPs from different bacteria may significantly differ in transcription properties, as a result of phylogenetic divergence or adaptation to various life conditions, as in the case of mesophilic and thermophilic bacteria. The differences between mesophilic Eco and thermophilic Taq RNAPs affect various steps of transcription, from promoter recognition and open complex formation to RNA elongation and termination (34, 36–40). In particular, at the transcription initiation step Taq RNAP displays cold sensitivity of promoter opening, lower promoter complex stability and higher promoter escape efficiency in comparison with Eco RNAP. In this work, we focused on analysis of the roles of the Eco σ70 and Taq σA subunits in defining specific differences between the Eco and Taq RNAPs during initiation. Below, we discuss possible impact of evolutionary variations in the structure of various regions of the σ subunit on different steps of the open complex formation by Eco and Taq RNAPs.
Formation of the open promoter complex by Eco RNAP was previously shown to proceed via at least three intermediates: RPc, the closed promoter complex with fully double-stranded DNA; intermediate complex I1, in which the downstream DNA duplex is partially bent and placed inside the DNA binding cleft of RNAP; and complex I2, containing the open transcription bubble at the starting point of transcription (reviewed in Ref 48). Further isomerization of the unstable I2 complex into the stable open promoter complex is accompanied by formation of tight contacts of RNAP with the downstream DNA duplex (49–51). Although forming highly stable complexes on most promoters, Eco RNAP was found to form unstable complexes on stringent-response promoters, such as the ribosomal rrnB P1 promoter (see Ref. 11 and references therein). The rrnB P1 promoter complexes were shown to have a shortened downstream DNA footprint (52) and to display high efficiency of promoter escape (53). It was therefore proposed that these complexes may be trapped at the I2 step of the open complex formation (49). The properties of complexes formed by Taq RNAP on most promoters are remarkably similar to the properties of the rrnB P1 promoter complexes. Furthermore, Taq RNAP also has a shortened downstream footprint on promoter DNA (54). We therefore speculate that the Taq RNAP promoter complexes may also correspond to the unstable I2 intermediate previously described for Eco RNAP. However, a detailed kinetic analysis is needed to establish the exact open complex formation pathway by Taq RNAP.
We demonstrated that the instability of promoter complexes of Taq RNAP in comparison with Eco RNAP is determined by both core RNAP and the σA subunit. The core-dependent variations in promoter complex stability may be probably explained by the differences in RNAP contacts with downstream DNA. In particular, Taq core RNAP lacks the SI3 domain that was previously hypothesized to form stabilizing contacts with the downstream DNA duplex in promoter complexes of Eco RNAP (49–51, 55). The σ-dependent variations in promoter-complex stability were shown to be mainly determined by σ region 1.1 and, to a lesser extent, region 1.2. Importantly, substitutions of regions 1.1 and 1.2 in the Eco σ70 and Taq σA subunits affected promoter complex stabilities on both the T7A1 and λPR promoters, which are characterized by different heparin sensitivities and may differ in the structures of intermediates formed by RNAP holoenzyme during promoter opening (49, 56).
Previously, region 1.1 was proposed to play an important role in the open promoter complex formation, illuminated by deleterious effects of several studied mutations in region 1.1 in the σ70 subunit on transcription by Eco RNAP (25, 27–29). Based on kinetic analysis and FRET measurements, it was hypothesized that region 1.1 binds within the downstream DNA binding cleft in the free Eco RNAP holoenzyme, likely remains bound inside the cleft in the I1 and I2 complexes, and is ejected from the cleft upon formation of the tight downstream RNAP-DNA contacts in the open promoter complex (49, 50, 57, 58). Thus, species-specific differences in the structures of σ region 1.1 in Eco and Taq RNAPs may affect its interactions with core RNAP and downstream DNA in intermediate and open promoter complexes, resulting in changes of their relative stabilities and shifting the equilibrium between them.
Nonconserved amino acids in region 1.2 also contribute to the lower promoter complex stability of Taq RNAP. Remarkably, substitution of just two amino acids in Eco σ70, M102L and R103H, located in the central part of the first of the two α helixes comprising region 1.2 had the same effect on promoter complex stability as substitution of the whole region 1.2. Thus, these two amino acids likely make the main contribution to the observed differences in promoter complex stabilities determined by σ region 1.2. Previously, alanine substitutions of residues Met-100 through Met-105 in Eco σ70 (corresponding to residues Gln-98–Ile-103 in σA; Fig. 1) were shown to destabilize promoter complexes of Eco RNAP, likely by disrupting RNAP contacts with the discriminator region located downstream of the −10 element (Fig. 1B and Ref. 19). In particular, residue Met-102 of Eco σ70 (corresponding to Leu-100 in Taq σA; Fig. 1A) was proposed to directly interact with DNA two nucleotides downstream of the −10 element and its alanine substitution decreased site-specific cross-linking of region 1.2 with DNA (19). Similarly, the instability of the rrnB P1 promoter complexes was proposed to be in part explained by the absence of favorable interactions of σ region 1.2 with the discriminator region at this promoter (11). Thus, nonconserved substitutions in region 1.2 in Taq σA may affect the open complex stability through weakening the σ-DNA contacts. Importantly, however, in contrast to the previously studied alanine substitutions in σ70, σA-specific substitutions do not disrupt interactions of region 1.2 with DNA, because σA region 1.2 was shown to specifically recognize the GGGA element downstream of the −10 element in promoter complexes of Taq RNAP (10, 18).
In addition to their effects on promoter complex stability, substitutions of nonconserved amino acids in region 1.2 of the σ70 subunit with corresponding residues from the σA subunit resulted in cold sensitivity of promoter opening by Eco RNAP, suggesting that region 1.2 is directly involved in DNA melting during initiation. In support of this, mutations in σ70 region 1.2 were previously shown to impair promoter opening by Eco RNAP holoenzyme (20). Substitutions of nonconserved amino acids located in the first α-helix of region 1.2, including the M102L and R103H (Fig. 1A), can likely directly affect interactions of the σ subunit with the nontemplate promoter strand and hinder DNA melting at low temperatures. Thus, both the cold sensitivity and the instability of promoter complexes may result from the loss of favorable contacts of region 1.2 with DNA downstream of the −10 element. In addition, substitutions of other nonconserved residues of region 1.2, not involved in direct contacts with DNA (in particular, in the second α helix of region 1.2), may indirectly affect DNA melting, either by changing σ-core interactions or by affecting the conformation of domain σ2. In support of this, substitutions of conserved residues in this part of region 1.2 in σ70 (shown in Fig. 1B above the σ70 sequence) also decreased RNAP activity and inhibited open complex formation by Eco RNAP holoenzyme (20).
Previous studies demonstrated that the main role in DNA melting during open complex formation is played by region 2 of the σ subunit, with conserved aromatic and positively charged amino acids from this region directly involved in interactions with the −10 promoter element (Fig. 1A) (12–17, 59). Substitutions of nonconserved amino acid residues in region 2 were demonstrated to modulate the temperature of promoter opening and to be in part responsible for the cold sensitivity of DNA melting by Taq RNAP in comparison with Eco RNAP (38). Our results suggest that nonconserved amino acid substitutions in regions 1.2 and 2 can together determine the natural variations in promoter DNA melting in various bacteria.
The instability of promoter complexes formed by Taq RNAP is paralleled by a higher promoter escape efficiency displayed by this RNAP in comparison with Eco RNAP. We propose that these differences in part depend on the properties of the Taq σA subunit that was shown to stimulate promoter escape in comparison with the σ70 subunit when combined with Eco core RNAP. However, the higher promoter escape efficiency displayed by the σA-containing RNAP does not depend on σ regions 1.1 or 1.2 but is apparently explained by structural features of the C-terminal part of the subunit, including regions 2, 3, and 4. In particular, nonconserved amino acid substitutions in region 2 may likely affect promoter escape through changes in interactions of the σ subunit with the −10 promoter element and/or with core RNAP (38), whereas substitutions in regions 3 and 4 may change σ contacts with the growing RNA transcript during initiation (5, 6).
Although analysis of hybrid RNAPs utilized in this work do have some caveats, several lines of evidence support the validity of this approach for comparison of functional properties of σ subunits from different bacteria. First, the levels of promoter-dependent activities at the temperature optima were similar for the wild-type Eco and hybrid RNAPs containing Taq σA and mosaic σ subunits. Second, Taq σA in complex with Eco core RNAP efficiently recognized the −10 promoter element in the nontemplate promoter strand, suggesting that the σ subunit adopts a proper conformation for DNA binding. Third, many properties of the hybrid RNAPs expectedly reproduced the properties of Taq holoenzyme RNAP. Finally, individual substitutions of σ regions 1.1 and 1.2 replaced relatively small parts of the σ subunit and affected a subset of promoter complex properties, thus implying specific functions for these regions in transcription initiation.
In conclusion, our results suggest that regions 1.1 and 1.2 of the Eco σ70 and Taq σA subunits modulate conformational transitions of RNAP during open complex formation and determine significant differences between the Eco and Taq RNAPs in transcription initiation. Although the functional importance of these differences for the expression of bacterial genes remains to be established, it can be proposed that the observed evolutionary variations in the properties of the σ subunit may have an important role in transcription regulation.
Acknowledgments
We thank I. Artsimovitch for plasmids, S. Malakho for help in obtaining mosaic rpoD genes, V. G. Nikiforov for helpful discussions, and D. Esyunina for Eco core RNAP purification.
This work was supported in part by funds from the Russian Academy of Sciences Presidium Program in Molecular and Cellular Biology (to A. K.), Russian Foundation for Basic Research Grant 10–04-00925, Federal Targeted Program “Scientific and scientific-pedagogical personnel of innovative Russia 2009–2013” State Contract 02.740.11.0771, and President of the Russian Federation Grants MD-618.2011.4 and MK-7156.2012.4.

This article contains supplemental references and Fig. S1.
N. Miropolskaya and A. Kulbachinskiy, unpublished data.
A. Kulbachinskiy, unpublished data.
- RNAP
- RNA polymerase
- Eco
- E. coli
- Taq
- T. aquaticus.
REFERENCES
- 1. Gross C. A., Chan C., Dombroski A., Gruber T., Sharp M., Tupy J., Young B. (1998) The functional and regulatory roles of σ factors in transcription. Cold Spring Harb. Symp. Quant. Biol. 63, 141–155 [DOI] [PubMed] [Google Scholar]
- 2. Murakami K. S., Darst S. A. (2003) Bacterial RNA polymerases. The wholo story. Curr. Opin. Struct. Biol. 13, 31–39 [DOI] [PubMed] [Google Scholar]
- 3. Murakami K. S., Masuda S., Darst S. A. (2002) Structural basis of transcription initiation. RNA polymerase holoenzyme at 4 A resolution. Science 296, 1280–1284 [DOI] [PubMed] [Google Scholar]
- 4. Vassylyev D. G., Sekine S., Laptenko O., Lee J., Vassylyeva M. N., Borukhov S., Yokoyama S. (2002) Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature 417, 712–719 [DOI] [PubMed] [Google Scholar]
- 5. Nickels B. E., Garrity S. J., Mekler V., Minakhin L., Severinov K., Ebright R. H., Hochschild A. (2005) The interaction between σ70 and the β-flap of Escherichia coli RNA polymerase inhibits extension of nascent RNA during early elongation. Proc. Natl. Acad. Sci. U.S.A. 102, 4488–4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kulbachinskiy A., Mustaev A. (2006) Region 3.2 of the σ subunit contributes to the binding of the 3′-initiating nucleotide in the RNA polymerase active center and facilitates promoter clearance during initiation. J. Biol. Chem. 281, 18273–18276 [DOI] [PubMed] [Google Scholar]
- 7. Lonetto M., Gribskov M., Gross C. A. (1992) The σ70 family. Sequence conservation and evolutionary relationships. J. Bacteriol. 174, 3843–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell E. A., Muzzin O., Chlenov M., Sun J. L., Olson C. A., Weinman O., Trester-Zedlitz M. L., Darst S. A. (2002) Structure of the bacterial RNA polymerase promoter specificity σ subunit. Mol. Cell 9, 527–539 [DOI] [PubMed] [Google Scholar]
- 9. Malhotra A., Severinova E., Darst S. A. (1996) Crystal structure of a σ70 subunit fragment from E. coli RNA polymerase. Cell 87, 127–136 [DOI] [PubMed] [Google Scholar]
- 10. Feklistov A., Barinova N., Sevostyanova A., Heyduk E., Bass I., Vvedenskaya I., Kuznedelov K., Merkiene E., Stavrovskaya E., Klimasauskas S., Nikiforov V., Heyduk T., Severinov K., Kulbachinskiy A. (2006) A basal promoter element recognized by free RNA polymerase σ subunit determines promoter recognition by RNA polymerase holoenzyme. Mol. Cell 23, 97–107 [DOI] [PubMed] [Google Scholar]
- 11. Haugen S. P., Berkmen M. B., Ross W., Gaal T., Ward C., Gourse R. L. (2006) rRNA promoter regulation by nonoptimal binding of σ region 1.2. An additional recognition element for RNA polymerase. Cell 125, 1069–1082 [DOI] [PubMed] [Google Scholar]
- 12. Feklistov A., Darst S. A. (2011) Structural basis for promoter-10 element recognition by the bacterial RNA polymerase σ subunit. Cell 147, 1257–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juang Y. L., Helmann J. D. (1994) A promoter melting region in the primary σ factor of Bacillus subtilis. Identification of functionally important aromatic amino acids. J. Mol. Biol. 235, 1470–1488 [DOI] [PubMed] [Google Scholar]
- 14. Panaghie G., Aiyar S. E., Bobb K. L., Hayward R. S., de Haseth P. L. (2000) Aromatic amino acids in region 2.3 of Escherichia coli σ70 participate collectively in the formation of an RNA polymerase-promoter open complex. J. Mol. Biol. 299, 1217–1230 [DOI] [PubMed] [Google Scholar]
- 15. Fenton M. S., Lee S. J., Gralla J. D. (2000) Escherichia coli promoter opening and −10 recognition. Mutational analysis of σ70. EMBO J. 19, 1130–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schroeder L. A., Choi A. J., DeHaseth P. L. (2007) The −11A of promoter DNA and two conserved amino acids in the melting region of σ70 both directly affect the rate limiting step in formation of the stable RNA polymerase-promoter complex, but they do not necessarily interact. Nucleic Acids Res. 35, 4141–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schroeder L. A., Gries T. J., Saecker R. M., Record M. T., Jr., Harris M. E., DeHaseth P. L. (2009) Evidence for a tyrosine-adenine stacking interaction and for a short-lived open intermediate subsequent to initial binding of Escherichia coli RNA polymerase to promoter DNA. J. Mol. Biol. 385, 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barinova N., Kuznedelov K., Severinov K., Kulbachinskiy A. (2008) Structural modules of RNA polymerase required for transcription from promoters containing downstream basal promoter element GGGA. J. Biol. Chem. 283, 22482–22489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haugen S. P., Ross W., Manrique M., Gourse R. L. (2008) Fine structure of the promoter-σ region 1.2 interaction. Proc. Natl. Acad. Sci. U.S.A. 105, 3292–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baldwin N. E., Dombroski A. J. (2001) Isolation and characterization of mutations in region 1.2 of Escherichia coli σ70. Mol. Microbiol. 42, 427–437 [DOI] [PubMed] [Google Scholar]
- 21. Zenkin N., Kulbachinskiy A., Yuzenkova Y., Mustaev A., Bass I., Severinov K., Brodolin K. (2007) Region 1.2 of the RNA polymerase σ subunit controls recognition of the −10 promoter element. EMBO J. 26, 955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dombroski A. J., Walter W. A., Record M. T., Jr., Siegele D. A., Gross C. A. (1992) Polypeptides containing highly conserved regions of transcription initiation factor σ70 exhibit specificity of binding to promoter DNA. Cell 70, 501–512 [DOI] [PubMed] [Google Scholar]
- 23. Dombroski A. J., Walter W. A., Gross C. A. (1993) Amino-terminal amino acids modulate σ-factor DNA-binding activity. Genes Dev. 7, 2446–2455 [DOI] [PubMed] [Google Scholar]
- 24. Schwartz E. C., Shekhtman A., Dutta K., Pratt M. R., Cowburn D., Darst S., Muir T. W. (2008) A full-length group 1 bacterial σ factor adopts a compact structure incompatible with DNA binding. Chem. Biol. 15, 1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson C., Dombroski A. J. (1997) Region 1 of σ70 is required for efficient isomerization and initiation of transcription by Escherichia coli RNA polymerase. J. Mol. Biol. 267, 60–74 [DOI] [PubMed] [Google Scholar]
- 26. Gruber T. M., Markov D., Sharp M. M., Young B. A., Lu C. Z., Zhong H. J., Artsimovitch I., Geszvain K. M., Arthur T. M., Burgess R. R., Landick R., Severinov K., Gross C. A. (2001) Binding of the initiation factor σ70 to core RNA polymerase is a multistep process. Mol. Cell 8, 21–31 [DOI] [PubMed] [Google Scholar]
- 27. Bowers C. W., Dombroski A. J. (1999) A mutation in region 1.1 of σ70 affects promoter DNA binding by Escherichia coli RNA polymerase holoenzyme. EMBO J. 18, 709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bowers C. W., McCracken A., Dombroski A. J. (2000) Effects of amino acid substitutions at conserved and acidic residues within region 1.1 of Escherichia coli σ70. J. Bacteriol. 182, 221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vuthoori S., Bowers C. W., McCracken A., Dombroski A. J., Hinton D. M. (2001) Domain 1.1 of the σ70 subunit of Escherichia coli RNA polymerase modulates the formation of stable polymerase/promoter complexes. J. Mol. Biol. 309, 561–572 [DOI] [PubMed] [Google Scholar]
- 30. Iyer L. M., Koonin E. V., Aravind L. (2004) Evolution of bacterial RNA polymerase. Implications for large-scale bacterial phylogeny, domain accretion, and horizontal gene transfer. Gene 335, 73–88 [DOI] [PubMed] [Google Scholar]
- 31. Lane W. J., Darst S. A. (2010) Molecular evolution of multisubunit RNA polymerases. Sequence analysis. J. Mol. Biol. 395, 671–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lane W. J., Darst S. A. (2010) Molecular evolution of multisubunit RNA polymerases. Structural analysis. J. Mol. Biol. 395, 686–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Opalka N., Brown J., Lane W. J., Twist K. A., Landick R., Asturias F. J., Darst S. A. (2010) Complete structural model of Escherichia coli RNA polymerase from a hybrid approach. PLoS Biol. 8, e1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Minakhin L., Nechaev S., Campbell E. A., Severinov K. (2001) Recombinant Thermus aquaticus RNA polymerase, a new tool for structure-based analysis of transcription. J. Bacteriol. 183, 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuznedelov K., Minakhin L., Severinov K. (2003) Preparation and characterization of recombinant Thermus aquaticus RNA polymerase. Methods Enzymol. 370, 94–108 [DOI] [PubMed] [Google Scholar]
- 36. Kulbachinskiy A., Bass I., Bogdanova E., Goldfarb A., Nikiforov V. (2004) Cold sensitivity of thermophilic and mesophilic RNA polymerases. J. Bacteriol. 186, 7818–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kulbachinskiy A. V., Nikiforov V. G., Brodolin K. L. (2005) Differences in contacts of RNA polymerases from Escherichia coli and Thermus aquaticus with lacUV5 promoter are determined by core-enzyme of RNA polymerase. Biochemistry 70, 1227–1230 [DOI] [PubMed] [Google Scholar]
- 38. Barinova N., Zhilina E., Bass I., Nikiforov V., Kulbachinskiy A. (2008) Lineage-specific amino acid substitutions in region 2 of the RNA polymerase σ subunit affect the temperature of promoter opening. J. Bacteriol. 190, 3088–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schroeder L. A., deHaseth P. L. (2005) Mechanistic differences in promoter DNA melting by Thermus aquaticus and Escherichia coli RNA polymerases. J. Biol. Chem. 280, 17422–17429 [DOI] [PubMed] [Google Scholar]
- 40. Miropolskaya N., Artsimovitch I., Klimasauskas S., Nikiforov V., Kulbachinskiy A. (2009) Allosteric control of catalysis by the F loop of RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 106, 18942–18947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pupov D., Miropolskaya N., Sevostyanova A., Bass I., Artsimovitch I., Kulbachinskiy A. (2010) Multiple roles of the RNA polymerase β′ SW2 region in transcription initiation, promoter escape, and RNA elongation. Nucleic Acids Res. 38, 5784–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brodolin K., Mustaev A., Severinov K., Nikiforov V. (2000) Identification of RNA polymerase β′ subunit segment contacting the melted region of the lacUV5 promoter. J. Biol. Chem. 275, 3661–3666 [DOI] [PubMed] [Google Scholar]
- 43. Kulbachinskiy A., Feklistov A., Krasheninnikov I., Goldfarb A., Nikiforov V. (2004) Aptamers to Escherichia coli core RNA polymerase that sense its interaction with rifampicin, σ-subunit and GreB. Eur. J. Biochem. 271, 4921–4931 [DOI] [PubMed] [Google Scholar]
- 44. Kulbachinskiy A., Mustaev A., Goldfarb A., Nikiforov V. (1999) Interaction with free β′ subunit unmasks DNA-binding domain of RNA polymerase σ subunit. FEBS Lett. 454, 71–74 [DOI] [PubMed] [Google Scholar]
- 45. Savinkova L. K., Baranova L. V., Knorre V. L., Salganik R. I. (1988) [Binding of RNA-polymerase from Escherichia coli with oligodeoxyribonucleotides homologous to transcribed and non-transcribed DNA stands in the “−10”-promoter region of bacterial genes]. Mol. Biol. (Mosk.) 22, 807–812 [PubMed] [Google Scholar]
- 46. Marr M. T., Roberts J. W. (1997) Promoter recognition as measured by binding of polymerase to nontemplate strand oligonucleotide. Science 276, 1258–1260 [DOI] [PubMed] [Google Scholar]
- 47. Hsu L. M. (2002) Promoter clearance and escape in prokaryotes. Biochim. Biophys. Acta 1577, 191–207 [DOI] [PubMed] [Google Scholar]
- 48. Saecker R. M., Record M. T., Jr., Dehaseth P. L. (2011) Mechanism of bacterial transcription initiation. RNA polymerase-promoter binding, isomerization to initiation-competent open complexes, and initiation of RNA synthesis. J. Mol. Biol. 412, 754–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gries T. J., Kontur W. S., Capp M. W., Saecker R. M., Record M. T., Jr. (2010) One-step DNA melting in the RNA polymerase cleft opens the initiation bubble to form an unstable open complex. Proc. Natl. Acad. Sci. U.S.A. 107, 10418–10423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kontur W. S., Capp M. W., Gries T. J., Saecker R. M., Record M. T., Jr. (2010) Probing DNA binding, DNA opening, and assembly of a downstream clamp/jaw in Escherichia coli RNA polymerase-lambdaP(R) promoter complexes using salt and the physiological anion glutamate. Biochemistry 49, 4361–4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kontur W. S., Saecker R. M., Capp M. W., Record M. T., Jr. (2008) Late steps in the formation of E. coli RNA polymerase-lambda P R promoter open complexes. Characterization of conformational changes by rapid [perturbant] upshift experiments. J. Mol. Biol. 376, 1034–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rutherford S. T., Villers C. L., Lee J. H., Ross W., Gourse R. L. (2009) Allosteric control of Escherichia coli rRNA promoter complexes by DksA. Genes Dev. 23, 236–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gourse R. L. (1988) Visualization and quantitative analysis of complex formation between E. coli RNA polymerase and an rRNA promoter in vitro. Nucleic Acids Res. 16, 9789–9809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yuzenkova Y., Tadigotla V. R., Severinov K., Zenkin N. (2011) A new basal promoter element recognized by RNA polymerase core enzyme. EMBO J. 30, 3766–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Artsimovitch I., Svetlov V., Murakami K. S., Landick R. (2003) Co-overexpression of Escherichia coli RNA polymerase subunits allows isolation and analysis of mutant enzymes lacking lineage-specific sequence insertions. J. Biol. Chem. 278, 12344–12355 [DOI] [PubMed] [Google Scholar]
- 56. Sclavi B., Zaychikov E., Rogozina A., Walther F., Buckle M., Heumann H. (2005) Real-time characterization of intermediates in the pathway to open complex formation by Escherichia coli RNA polymerase at the T7A1 promoter. Proc. Natl. Acad. Sci. U.S.A. 102, 4706–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davis C. A., Bingman C. A., Landick R., Record M. T., Jr., Saecker R. M. (2007) Real-time footprinting of DNA in the first kinetically significant intermediate in open complex formation by Escherichia coli RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 104, 7833–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mekler V., Kortkhonjia E., Mukhopadhyay J., Knight J., Revyakin A., Kapanidis A. N., Niu W., Ebright Y. W., Levy R., Ebright R. H. (2002) Structural organization of bacterial RNA polymerase holoenzyme and the RNA polymerase-promoter open complex. Cell 108, 599–614 [DOI] [PubMed] [Google Scholar]
- 59. Tomsic M., Tsujikawa L., Panaghie G., Wang Y., Azok J., deHaseth P. L. (2001) Different roles for basic and aromatic amino acids in conserved region 2 of Escherichia coli σ70 in the nucleation and maintenance of the single-stranded DNA bubble in open RNA polymerase-promoter complexes. J. Biol. Chem. 276, 31891–31896 [DOI] [PubMed] [Google Scholar]