Background: Thymine starvation causes irreversible toxicity and chromosomal damage, by unknown mechanisms.
Results: Replication in thymine-starved cells leads to preferential fragmentation and degradation of new DNA and culminates in RecA-dependent destruction of replication origins.
Conclusion: DNA replicated in thymine-starved cells is unstable, precipitates irreparable damage of the chromosome.
Significance: The mechanisms behind this potent way to kill any organism are becoming clearer.
Keywords: Chromosomes, DNA Repair, DNA Replication, Genomic Instability, Microarray, Chromosomal Fragmentation, RecA, RecBCD, Replication Origin Destruction, Thymineless Death
Abstract
Thymineless death strikes cells unable to synthesize DNA precursor dTTP, with the nature of chromosomal damage still unclear. Thymine starvation stalls replication forks, whereas accumulating evidence indicates the replication origin is also affected. Using a novel DNA labeling technique, here we show that replication slowly continues in thymine-starved cells, but the newly synthesized DNA becomes fragmented and degraded. This degradation apparently releases enough thymine to sustain initiation of new replication bubbles from the chromosomal origin, which destabilizes the origin in a RecA-dependent manner. Marker frequency analysis with gene arrays 1) reveals destruction of the origin-centered chromosomal segment in RecA+ cells; 2) confirms origin accumulation in the recA mutants; and 3) identifies the sites around the origin where destruction initiates in the recBCD mutants. We propose that thymineless cells convert persistent single-strand gaps behind replication forks into double-strand breaks, using the released thymine for new initiations, whereas subsequent disintegration of small replication bubbles causes replication origin destruction.
Introduction
Thymine starvation (T-starvation),3 the inability to synthesize DNA precursor dTTP due to the combination of thymidylate synthase (thyA) deficiency and unavailability of exogenous thymine or thymidine, causes death in bacterial (Fig. 1A) and eukaryotic cells alike (1–3). The resulting so-called “thymineless death” (TLD) is used in popular antimicrobial and anticancer treatments that seek to inhibit thymidylate synthase itself (for example, by fluorodeoxyuridine), or the metabolic circuit that recharges its cofactor, methylene tetrahydrofolate (for example, by dehydrofolate reductase inhibitor trimethoprim) (4, 5). At the same time, the underlying mechanisms of cell death remain an enigma for over a half a century. Until recently, the most popular explanations of TLD assumed massive wrong nucleotide incorporation with subsequent excision, causing either double-strand breaks or uncontrolled homologous recombination (1, 6–9). Although confirming that certain homologous recombination functions do play a key role in TLD, and double-strand breaks are indeed induced by thymine starvation, we and others have recently shown that wrong nucleotide incorporation does not contribute to TLD in bacteria (10, 11), and that the real trigger of the lethal events is the replication fork stalling, which precipitates poisoning via recombinational repair of persistent single-strand gaps (10, 12).
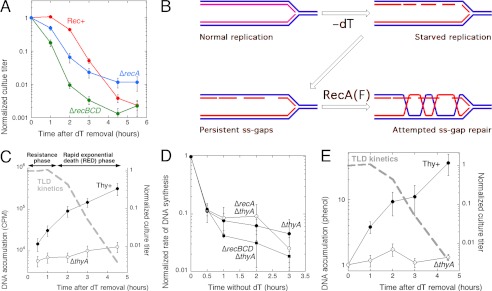
FIGURE 1.
Significant DNA replication during thymine starvation and gradual disappearance of replication points. A, the standard TLD curve (red) of a ΔthyA (Rec+) mutant E. coli, grown in the presence of thymidine (dT) and at time = 0 transferred into the same growth medium, but without thymidine. For the first 90 min the viable titer of such culture does not change (the resistance phase); once the cells exhaust their resistance, the rapid exponential death (RED) phase ensues, whose mechanisms are still unknown. For comparison, TLD curves of the ΔrecA (blue) and ΔrecBCD (green) variants of the same ΔthyA mutant are shown (adapted from Ref. 10). B, hypothetical events at T-starved replication forks: the toxic ss-gap repair. Blue lines, old DNA; red lines, DNA synthesized during T-starvation. As less and less dTTP is available, the initiated Okazaki fragments are left unfinished, leading to persistent single-strand gaps in the nascent DNA strands. These gaps trigger RecA(RecF)-catalyzed recombinational repair in an attempt to close them, which leads to formation of long-lived RecA filaments and further slows gap closure. C, the accumulation of newly synthesized DNA during T-starvation. DNA accumulation was measured by chronic 32P labeling after removal of dT from the growth medium in half of the culture. The TLD kinetics (10) is overlaid in a dashed gray line, with resistance and rapid exponential death phases indicated. The strains are KKW58 ΔthyA and KKW 59 Thy+. D, the rate of DNA synthesis during T-starvation, as measured by 10 min 32P incorporation at the indicated times after removal of dT at the indicated strains, and normalized to the level of incorporation of the same culture grown with dT at the time = 0. E, chromosomal DNA accumulation during T-starvation, as quantified by Hoefer DyNA quant 200 fluorometer in samples of total DNA isolated by phenol extraction. The TLD kinetics (10) is overlaid in dashed gray line.
We proposed at that time that replication with diminishing supplies of one of the DNA precursors should produce persistent single-strand gaps in the nascent DNA strands behind the replication forks (10) (Fig. 1B). Similar daughter-strand gaps accumulate upon replication of DNA with unremoved UV lesions (13) and become substrates for the daughter-strand gap repair pathway, initiated by RecFOR and catalyzed by RecA in Escherichia coli (Ref. 14, reviewed in Ref. 15). We proposed a similar scenario for the resistance phase of T-starvation (defined in Fig. 1C), hypothesizing formation of persistent repair structures behind the slowly advancing replication forks (detectable as branched DNA), in which ss-gap closure is further delayed by RecA interference (10) (Fig. 1B). Then, by still unknown mechanisms, these persistent single-strand gaps are converted into irreparable chromosomal lesions during the rapid exponential death (RED) phase of T-starvation (defined in Fig. 1C).
In contrast to UV-irradiated cells, where daughter-strand gap repair helps the cells to recover replication and survive the DNA damage (reviewed in Ref. 15), the same daughter-strand gap repair somehow becomes poisonous during T-starvation. The idea that unfinished daughter-strand gap repair is toxic during T-starvation is supported by the following observations: 1) mutations in recF and recO suppress TLD (10, 16, 17); 2) inactivation of the UvrD helicase, which inhibits the RecFOR-promoted ss-gap repair, accelerates TLD (10, 12); 3) T-starvation sensitizes E. coli cells to subsequent UV irradiation (18, 19); and 4) formation of branched DNA, dependent on RecA and RecF, is a hallmark of T-starvation (20).
However, it is still unclear how this attempted ss-gap repair contributes to the prominent formation of double-strand breaks during T-starvation that yields both the observed chromosomal fragmentation and the strong dependence on RecBCD-catalyzed double-strand break repair during the ∼1.5-h long resistance phase preceding TLD (10, 16, 21) (Fig. 1A). The role of RecA in TLD is also unclear: according to the “toxic ss-gap repair” idea, the recA mutation should alleviate TLD, and although recA mutants indeed alleviate TLD later (10, 22–24), the early effect of recA inactivation is detrimental for the T-starved cells (10, 23) (Fig. 1A). Finally, the severity of killing during TLD clearly correlates with the number of pre-existing replication forks (25), yet a mechanistic explanation is lacking.
Part of the reason TLD is still an enigma is the absence of methods to label DNA during T-starvation, as addition of [3H]thymidine, the only known DNA-specific label, instantly relieves starvation. Recently, we described a novel method of DNA labeling during T-starvation using [32P]orthophosphoric acid (26). Here we employed this new DNA labeling technique, together with differential labeling for chromosomal fragmentation analysis with pulsed-field gels, as well as the marker frequency analysis with gene arrays, to show how the postulated single-strand gaps are converted into irreparable chromosomal lesions by toxic repair during T-starvation. We did it by examining the extent of initiation, replication, and chromosome damage in T-starved cells, in both recombinational repair proficient (Rec+) and deficient (recA or recBCD mutants) cells.
EXPERIMENTAL PROCEDURES
Bacterial Strains
All E. coli strains are K-12 and are derivatives of AB1157. The following strains were used: ΔthyA, KKW58; Thy+, KKW59; ΔrecA, KKW60; ΔthyA ΔrecA, KJK61; ΔthyA ΔrecBCD, KJK63; dut recBC(Ts), AK107; they are all published (10, 26, 27) and all, with the exception of AK107, carry deletion of the entire deoCABD operon. Alleles were moved between strains by P1 transduction (28, 29). Precise deletion-replacement alleles of the selected genes were generated by the Datsenko and Wanner method (30) and confirmed by PCR and phenotypic tests. The deo mutants were confirmed by their sensitivity to 5-fluorodeoxyuridine (31). The thyA mutants cannot grow on minimal medium without thymine or thymidine. The rec mutants were confirmed by their characteristic sensitivities to UV irradiation (32). In addition, the recBCD mutants were confirmed by their ability to plate the T4 2 mutant (33).
Growth Conditions
All strains were propagated on LB plates with appropriate antibiotics, supplemented with 10 μg/ml of thymidine in the case of all thyA mutants. LB broth per 1 liter contained: 10 g of tryptone, 5 g of yeast extract, 5 g of NaCl, pH 7.4, with 250 μl of 4 m NaOH; LB agar contained 15 g of agar per 1 liter of LB broth; M9CAA medium contained 1× M9 salts (Difco), 2 mm MgSO4, 0.1 mm CaCl2, 10 mg/liter of thiamine (B1), 0.2% glucose, and 0.2% casamino acids (Difco). MOPS-minimal phosphate medium was as described (34). Antibiotics were used in the following concentrations: kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; tetracycline, 10 μg/ml; and chloramphenicol, 12.5 μg/ml.
To measure TLD kinetics, cells were grown at 30 °C in M9CAA or MOPS-minimal phosphate medium with 10 μg/ml of thymidine until mid-logarithmic phase (A600 ∼ 0.2), the supplementing thymidine was removed by collecting cells on nitrocellulose membrane filters (type 0.22 μm GS, Millipore), followed by washes with 3 volumes of 1% NaCl and final resuspension in the original medium, but this time without thymidine. Viability of cultures was measured by spotting 10 μl of serial dilutions in 1% NaCl on LB plates (supplemented with 10 μg/ml of thymidine for thy mutants). Although still small (after 1–3 days incubation of the plates at room temperature), colonies at each spot were counted under the stereomicroscope.
Measurement of Replication Points in T-starved Cells via Determination of the Rate of DNA Synthesis with [3H]Thymidine (Modified from Refs. 26 and 35)
Cells were grown at 30 °C with shaking to the desired density in a M9CAA supplemented with 10 μg/ml of thymidine, then spun down and washed with equal volumes of M9CAA, then resuspended in M9CAA without thymidine. At different time points, 200-μl aliquots of the cultures were mixed with equal volumes of M9CAA medium supplemented with 1 μCi/ml of [3H]thymidine (equivalent to the final concentration of 4 ng/ml of dT) and incubated at 37 °C for 1 min. 5 ml of ice-cold 5% TCA was then added to stop the reaction, and the TCA-cracked cells were filtered through a 25-mm Fisher G6 glass fiber filter, using manifold, to separate labeled DNA from unincorporated label. 5 ml of 5% TCA, followed by 5 ml of ethanol, was used to wash the filter, and 100 μl of 0.1 m KOH was deposited on the filter to quench the intrinsic fluorescence during counting. The filters were dried, soaked in scintillation mixture overnight, and the radioactivity was determined in the LS 6500 Beckman scintillation counter. Counts were first normalized to A600 taken at each time point, then to the initial time point.
Isolation of the Total DNA by Phenol Extraction
Cells were processed according to the standard protocol for TLD kinetics. At the indicated time points, 2-ml aliquots of T-starved cultures were taken, cells were collected by centrifugation, and pellets were resuspended in 50 μl of 30% sucrose in TE buffer. To this suspensions, 350 μl of 2% SDS in TE buffer were added, the content of the tubes was mixed by inversion, and the tubes were incubated at 70 °C for 5 min or until suspensions were completely cleared. These lysates were extracted once with 400 μl of phenol, followed by a 5-min centrifugation at 13,000 × g and organic phase removal, then once with 400 μl of 1:1 mixture of phenol/chloroform, then once with 400 μl of chloroform. After chloroform removal and one more 1-min centrifugation, the aqueous phase was transferred into a fresh microcentrifuge tube, and DNA was precipitated by adding 20 μl of 4 m KCl and 1 ml of ethanol. DNA was dissolved for at least 1 h in 480 μl of TE with periodic vortexing, then reprecipitated as above. After the second ethanol removal, DNA was dissolved in 100 μl of TE and quantified by Hoefer DyNA Quant 200 fluorometer. The amount of DNA at each time point was then normalized to the amount of DNA extracted from the culture at the point of resuspension in the medium without thymidine.
Degradation of the Newly Synthesized DNA
Experiments were performed as described above in the protocol for rate measurements except that 20 μCi of [32P]orthophosphate were added to the whole culture right after ΔthyA mutant cells were resuspended in MOPS medium without thymidine, and the cells were incubated at 28 °C for 1.5 h to label DNA. [32P]Orthophosphate was removed from the medium by centrifugation, the cells were resuspended in MOPS medium only, and incubation was continued at 28 °C. At the indicated time points, 200-μl aliquots of cultures were taken to be processed into agarose plugs for subsequent alkaline gel electrophoresis.
Marker Frequency Analysis by Dot-blot Hybridization to Determine ori/ter Ratio
Overnight cultures grown in MOPS with thymidine at 30 °C were diluted 100-fold in the same medium and grown until OD ∼ 0.1. Then thymidine was removed by filtering as described above, and cells were resuspended in the MOPS medium. At the indicated time points, total DNA was extracted by either the phenol/chloroform method (above) or by the Genome Wizard kit (Promega). The same culture volume (10 ml) was used for DNA extraction at each time point. Samples were treated with RNase for 10 min at 37 °C (0.4 μl of 10 mg/ml) after lysing and before the phenol step, if DNA was isolated by phenol/chloroform extraction. Ori and Ter probes were made from gel-purified 2-kb PCR fragments as described (36). Probe was made using the random priming NEBlot kit (New England Biolabs). The amount of DNA isolated from each sample was quantified using DyNA Quant, and 200 ng from each samples was denatured in 0.1 m NaOH for 15 min at 37 °C and spotted on a positively charged Nylon membrane (Amersham Biosciences Hybond N+) using a vacuum manifold. After UV cross-linking, the membrane was divided in 2 halves with one-half being hybridized to the ori-probe and other half to the ter-probe. Hybridization conditions were as described (37).
Measuring the Rate of DNA Synthesis by [32P]Orthophosphate Incorporation and Alkaline Gel Electrophoresis
Experiments were performed as previously described (26), except for the following modifications: cells were grown at 28 °C in MOPS with 10 μg/ml of thymidine until A600 ∼ 0.1, then the supplementing thymidine was removed by collecting cells on nitrocellulose membrane filters (type 0.22 μm GS, Millipore), followed by washes with 3 volumes of 1% NaCl and final resuspension in MOPS, but this time without thymidine. At each indicated time point, 1.8-ml aliquots of cultures were taken for A600 measurement, and then 20 μCi of [32P]orthophosphate was added to the aliquot, and the aliquot was shaken for 10 min at 28 °C for [32P]orthophosphate incorporation before cells were collected for making agarose plugs. The same volume of cells was used in each plug regardless of the A600 increase, because the A600 increase is mostly due to cell filamentation instead of the actual increase of chromosomal content. Because DNase I treatment of the plug confirmed that everything at the top of the gel is in fact DNA species, the signals from both the wells and the lanes were counted. As positive controls, ΔthyA cultures supplemented with thymidine throughout were used.
Accumulation of the Newly Synthesized DNA
Experiments were performed as described above in the protocol for rate measurements, except that 20 μCi of [32P]orthophosphate were added to the whole culture right after ΔthyA mutant cells were resuspended in MOPS medium without thymidine, and at each time point the same volume of labeled cultures was taken to be processed into agarose plugs for subsequent alkaline gel electrophoresis.
Marker Frequency Analysis by Gene Arrays
Marker frequency analysis was carried out with the Affymetrix GeneChip E. coli Genome 2.0 Arrays. Sequence information for this array was provided from the ASAP data base (E. coli Genome Project; University of Wisconsin-Madison) and the NCBI public data base. Signals from the 4070 coding sequences (CDS) corresponding to K-12 (MG1655 strain) was used. Each sequence in the arrays is represented by the probe set containing 11 probe pairs of 25-mer oligonucleotides.
Total DNA was purified by the Genome Wizard kit (Promega) from 15–20 ml of cultures grown in MOPS at the time point indicated after removal of thymidine. DNA was labeled with the BioPrime DNA labeling Kit (Invitrogen) as described (36) except that the amount of DNA used to setup the reaction ranged from 100 to 400 ng, which did not affect the yield. Hybridization conditions, scanning and data quality control were done as described (36). Copy numbers of individual open reading frames were normalized to the corresponding signal intensities of the reference strain, which was AB1157 grown in the presence of chloramphenicol for 3 h to align the chromosome (ori/ter = 1:1). Values were then further normalized to the average value of 76 CDSs covering about 100 kbp of the terminus region of the E. coli chromosome around the dif site or 100 CDSs covering chromosomal position 294,520 to 410,521 bp, based on the MG1655 genome.
RESULTS
Significant New DNA Accumulation during T-starvation
Although it was always assumed that DNA replication is grossly inhibited in the absence of dTTP (2), the idea of ss-gap formation and repair behind T-starved replication forks (Fig. 1B) depends on the replication fork progress in T-starved cells. Because DNA replication in T-starved cells was never measured directly via incorporation (see above for the reason), we developed a novel protocol to measure DNA synthesis in T-starved cells by employing [32P]orthophosphate, lysing cells in agarose plugs, and separating DNA from other 32P-labeled species by alkaline gel electrophoresis (26). Using continuous 32P labeling, we found that during the first 30 min of thymine starvation the accumulation of 32P label in chromosomal DNA in the absence of thymidine is only three times lower than in cells growing in the presence of thymidine, although this difference becomes 25-fold after a 4.5-h incubation (Fig. 1C). The measurements of the rate of thymineless DNA synthesis (by 10-min 32P incorporation) further corroborated our findings: the rate at 30 min of T-starvation is still about 10% of the rate in the presence of dT, and slowly diminishes to 5% by 3 h of thymine starvation (Fig. 1D). This rate is not significantly influenced by either recA or recBCD mutations (Fig. 1D), suggesting that double-strand break repair, although saving cells upon thymidine readdition during the first 2 h of T-starvation, does not actually restore functional replication forks until thymidine is provided. To verify this significant DNA synthesis in T-starved cells, we employed an integral measurement of both DNA replication and degradation by monitoring the overall chromosomal DNA mass evolution (18, 38). We found that the total DNA content gradually increases during the first 2 h of T-starvation up to 1.7 times (if the prestarvation DNA is taken for 1), but then decreases to 1.5 times the level, indicating some DNA degradation (Fig. 1E). Overall, we conclude that there is sufficient new DNA synthesis during T-starvation to sustain formation of a substantial number of ss-gaps.
T-starvation Destabilizes Replication Forks
The idea of multiple ss-gap repair events behind the T-starved replication forks envisions a significant pool of DNA polymerases tied up at unfinished Okazaki fragments, and even occasional replication fork frameworks formed by annealing of overlapping ss-gaps in the sister duplexes, which should attract complete replisomes (Fig. 2A). If true, then T-starvation should induce a severalfold increase in the number of functional replication points that could go active when thymidine is re-supplied. Assuming that, upon thymidine re-addition, all replisomes return to the normal rate of DNA synthesis, we determined the overall number of functional replication points during T-starvation by measuring the rate of [3H]thymidine incorporation in aliquots withdrawn at various times from T-starved cultures and re-supplemented with thymidine. We found that, instead of the expected jump, the DNA label incorporation per OD shows a biphasic fall (Fig. 2B), leveling off at around 50% after 1 h and then again starting to fall after 2 h without thymine, at the time that marks the end of the resistance period under our conditions (Figs. 1C and 2B). This biphasic replication fork loss may reflect two types of recently reported double-strand breaks during T-starvation: the early repairable breaks, detectable in the recBCD mutant only, versus the irreparable breaks after 2 h without thymine, detectable in the Rec+ cells (10).
FIGURE 2.
The replication fork dynamics during T-starvation. A, hypothetical replication fork dynamics during T-starvation. The scheme of hypothetical gap formation and attempted recombinational repair. “AAA,” runs of several adenines in template DNA, at which T-starved replisomes are likely to stall first. Green ovals, replisomes; pink rectangles, RecA filaments; yellow rectangles, replication fork frameworks. B, disappearance of replication points during T-starvation. Cells of the indicated genotype were grown in the presence of thymidine and then switched to the same medium without thymidine at time = 0. At the indicated times, aliquots of these cells were taken, and the DNA synthesis rate was determined by adding [3H]thymidine (T-starvation was relieved). Counts/min (CPM) were normalized to the corresponding OD of the cultures. The TLD kinetics (10) is overlaid in dashed gray line. The strains are KKW58 ΔthyA and KKW 59 Thy+. C, rate of DNA synthesis per unit of culture volume. No normalization to culture OD was done in this case.
In growing cells, the rate of DNA synthesis is expressed per cell mass, by normalization to the optical density of the culture. However, cells undergoing TLD continue to elongate (23, 39), so OD of the culture increases, whereas completion of existing replication rounds is inhibited, a situation that may lead to overestimation of the replication fork loss during T-starvation by the standard protocol. Interestingly, when the rate of DNA synthesis was not normalized to OD, in this case reflecting the number of functional replication forks per cell, there was only a modest decrease during the resistance phase of T-starvation, whereas the 50% mark was reached only by 3 h of T-starvation (Fig. 2C). In other words, there is still a significant number of functional replication points per cell after hours of T-starvation, and the reason cells cannot recover when thymine is added is not due to irreparable loss of the DNA synthesis potential.
We measured the contribution of recombinational repair to the replication fork maintenance during T-starvation and found the same rate of replication fork inactivation in T-starved recA and recBCD mutant cells (Fig. 2C). Thus, the apparent “stability” of functional replication forks during thymine starvation may be mostly due to new initiations, rather than recombinational repair. This balance is disturbed during the RED phase, when the number of active replication forks is reduced to 20% of the original level. In other words (assuming a constant rate of inactivation of replication forks during T-starvation), the transition between the resistance phase and the RED phase is marked by a decrease in the ability of cells to initiate new replication rounds (the replication origins are inactivated). Interestingly, the rates of DNA synthesis in the recA mutant over the course of T-starvation were significantly elevated over the WT ones (Fig. 2C), suggesting that RecA+ activity contributes to fork and origin inactivation.
Altogether, these results show that, even if T-starvation initially induces new replication points, it destabilizes all the replication forks. Therefore, we looked for a possible mechanism of replication fork destabilization associated with persistent single-strand gaps behind the forks.
New DNA Synthesized during T-starvation Is Unstable
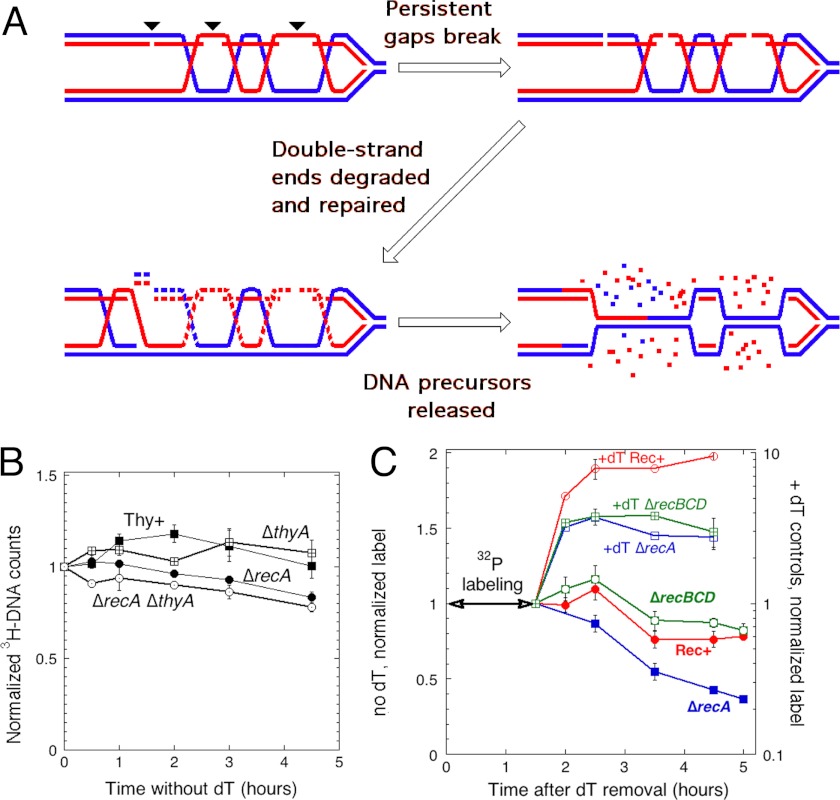
One such mechanism is the suspected instability of single-strand interruptions in new DNA, leading to ss-gap conversion into double-strand breaks, recently proposed to explain chromosomal degradation as a result of ligase-deficient replication (40). Such double-strand breaks at gaps behind replication forks should not only eliminate the replication points at unfinished gaps (green ovals in Fig. 2A), but could also affect the pre-existing replication forks. Remarkably, because recombinational repair not only reforms the parental duplex behind the replication fork, but also forms a duplex from newly synthesized strands, which contain persistent single-strand gaps, the duplex DNA, degraded as a result of the postulated breaks at ss-gaps should be mostly made of the newly synthesized strands (Fig. 3A, red strands). We tested this prediction, that the DNA strands synthesized during T-starvation should be preferentially degraded.
FIGURE 3.
DNA synthesized during T-starvation is preferentially degraded. A, double-strand break formation at persistent single-strand gaps should lead to preferential instability of DNA synthesized during T-starvation. Blue and red lines denote old and newly synthesized DNA strands, respectively. Recombinational repair of persistent single-strand gaps pairs them with newly synthesized sister strands that targets these strands for gap-mediated breaks and subsequent degradation. Small arrowheads mark the position of future double-strand breaks. Note that this new DNA instability leads to the reformation of parental duplex behind the replication forks. B, stability of parental DNA during TLD. Chromosomal DNA was labeled with [3H]thymidine during growth in the presence of cold thymidine, then both the cold thymidine and the label were removed and the stability of the label in the chromosomal DNA was monitored. The strains are: Thy+ KKW 59; ΔthyA, KKW 58; ΔrecA, KKW 60; and ΔrecA ΔthyA, KJK 61. C, stability of newly synthesized DNA during TLD. After growth in the medium supplemented with thymidine, the cells were transferred to the medium without thymidine, but supplemented with [32P]orthophosphate (at time = 0). After 1.5 h at 30 °C, 32P label was removed, and its stability in the chromosomal DNA was monitored. Half of the cultures was processed the same way, but in the presence of thymidine (+dT controls). Note that values for T-starved cells are in linear scale (on the left), whereas values for the +dT controls are in logarithmic scale (on the right). The strains are: Rec+, KKW 58; ΔrecA, KJK 61; and ΔrecBCD, KJK 63.
We detected no loss of the pre-labeled DNA (strands synthesized before T-starvation) over the course of TLD in Rec+ cells (Fig. 3B). As a positive control for our ability to detect chromosomal DNA degradation, the recA mutant cells that are known to lose chromosomal DNA material even during normal growth (41), lost 22% of their prelabeled DNA, but did it independently of T-starvation (Fig. 3B). To measure the stability of the DNA synthesized during T-starvation, we labeled DNA with [32P]orthophosphate for 1.5 h after thymidine removal (during the resistance phase), then removed the 32P label and followed the stability of the 32P-labeled chromosomal DNA (Fig. 3C). It should be noted that the extent of the radiation damage is negligible with the amount of 32P radioactivity we use; for example, we could not detect any difference in viability of the wild type and recA uvrA mutant cells (hypersensitive to any DNA damage) even after long growth (42). We found that in the “+dT control” cells grown in the presence of thymidine throughout, the DNA label continues accumulating for another 30 min after [32P]orthophosphate removal, likely due to label redistribution from RNA, but then plateaus (Fig. 3C, top three curves). In contrast, T-starved Rec+ cells start losing newly synthesized DNA soon after the start of the RED phase, the total loss amounting to 25% over 3.5 h (Fig. 3C). The recBCD mutant T-starved cells lose a similar fraction of their 32P-labeled DNA radioactivity toward the end of the monitoring period (Fig. 3C). In the recA mutant cells, the ss-gaps should still be converted into double-strand breaks, but in the absence of preceding strand exchange, both old and new strands are expected to be degraded. Indeed, the T-starved recA mutant shows significant loss of the newly synthesized DNA strands (65% over 3.5 h) (Fig. 3C), suggesting that the loss of old DNA strands in this mutant (Fig. 3B) is mostly confined to the hybrid duplexes. The recA mutant result also shows that recombination limits degradation of the new DNA (most probably, via double-strand break repair (Fig. 3A)).
We would like to point out that the results of the two assays (Fig. 3, B versus C) should not be compared quantitatively. Whereas the total chromosome was uniformly labeled in B, only approximately half of it (according to Fig. 1E) was labeled in C, and preferentially on one strand. Therefore, the sensitivity of the DNA loss detection in the first assay is about three times lower than in the second assay, this is indirectly confirmed by comparison of the DNA loss values between the two assays in the recA mutant (22 versus 65%). Thus, we could expect detecting ∼8% old DNA strand loss in the wild type cells (Fig. 3B), this would still be a detectable difference, considering our low error margins. Overall, we conclude that the DNA synthesized during T-starvation is partially unstable during the RED phase, whereas the DNA strands synthesized before T-starvation are mostly not affected.
Double-strand Breaks during T-starvation Preferentially Target New DNA
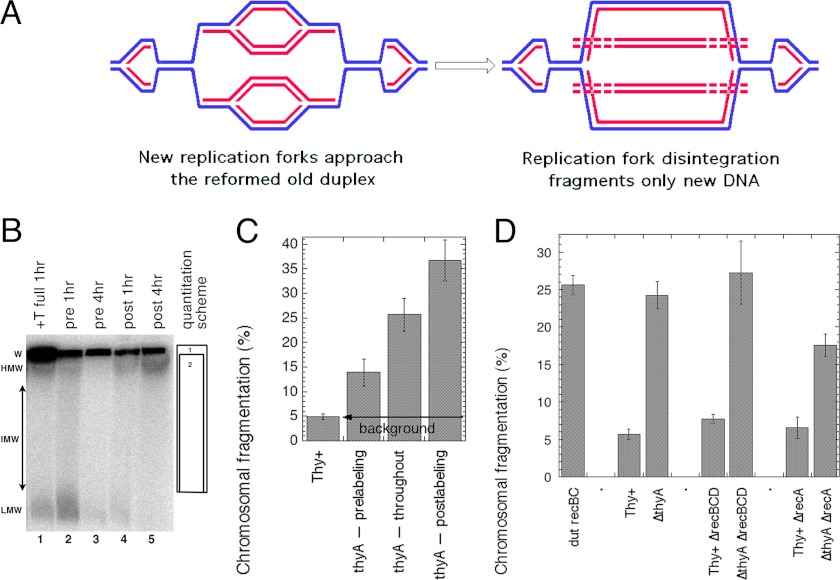
An important outcome of the model of DNA degradation in T-starved cells (Fig. 3A) was the reformation of the parental DNA duplex behind the T-starved replication forks, generating fork structures made only of the template strands. Whereas both pre-existing and new replication forks are likely to progress to the same extent during T-starvation, only the new forks are expected to run into these fork structures in the reformed parental duplexes and disintegrate. Disintegration of the new replication bubbles upon reaching these re-formed old duplexes would release linear subchromosomal fragments that were entirely made of, or at the least enriched for, new DNA strands (Fig. 4A). We and others have demonstrated previously that T-starvation induces chromosomal fragmentation (10, 21). According to the model in Fig. 4A, this chromosomal fragmentation during T-starvation should mostly affect the newly replicated DNA (also contributing to its preferential degradation), whereas affecting less the DNA synthesized before T-starvation.
FIGURE 4.
DNA synthesized during T-starvation is preferentially fragmented. A, the model of new DNA fragmentation in T-starved cells, when new replication bubbles run into the reformed parental duplexes behind previous replication forks. As a result of replication fork disintegration, linear DNA duplexes made of new (red) DNA strands are released as subchromosomal fragments (and degraded). B, a representative pulsed-field gel of pre- or postlabeled chromosomal DNA during T-starvation. The strain is KKW58. Chromosomal DNA was labeled with [32P]orthophosphate. After either 1 or 4 h of growth without thymidine cells were lysed in agarose plugs, and the chromosomal DNA was separated in pulsed-field gels. Prelabeling: the 32P label was removed together with cold thymidine. Postlabeling: the label was added upon removal of thymidine. Throughout (=full): the label was re-added upon thymidine removal or was present during whole incubation (if thymidine was not removed). W, wells; HMW, IMW, and LMW, high (>2,000 kbp), intermediate (200- 2,000 kbp), and low molecular weight (>200 kbp) subchromosomal fragments, respectively. The quantification scheme is shown on the right: the percentage of fragmentation is calculated by dividing total counts in box 2 (lane) by total counts in box 1 (well + lane) × 100. Lanes are: 1, growth in the presence of thymidine, with labeling throughout (Thy+ background), DNA isolated 1 h after dT readdition; 2, prelabeling, DNA isolated 1 h after dT removal; 3, prelabeling, DNA isolated 4 h after dT removal; 4, postlabeling, DNA isolated 1 h after dT removal; 5, postlabeling, DNA isolated 4 h after dT removal. C, the nature of chromosomal fragmentation during thymine starvation, revealed by quantification of 4-h samples from several gels like in B. In the chromosomal DNA isolated 4 h after dT removal, fragmentation preferentially affects the DNA synthesized during thymine starvation (compare lanes 3 and 5 in B). D, partial RecA dependence and RecBCD independence of T-starvation-induced fragmentation. The label was present throughout, and the fragmentation was measured after 4 h of T-starvation. The dut recBC(Ts) mutant was used as a positive control for chromosomal fragmentation. The strains are: Rec+, KKW58; ΔrecA, KJK61; ΔrecBCD, KJK63; dut recBC(Ts), AK107.
To establish whether this chromosomal fragmentation preferentially affects old or new DNA strands, we used the pre- and postlabeling protocol in combination with pulsed-field gel electrophoresis to measure fragmentation levels (Fig. 4B). We observed the abundant formation of low molecular weight DNase-sensitive species, which are especially prominent in the prelabeling protocol if T-starvation was for 1 h only (Fig. 4B, lane 2). Because the same species form in nonstarved cells (Fig. 4B, lane 1) and because they are shorter than 100 kbp and form equally in Rec+ and Rec− cells, suggesting constrained ends, we decided to exclude them from quantification of chromosomal fragmentation until we understand their nature. If these low molecular weight species are excluded, fragmentation after 1 h of thymine starvation is close to the background of nonstarved cells (data not shown).
In contrast, fragmentation was robust at 4 h of T-starvation (Fig. 4, B and C), where, after subtraction of the background, it was ∼8% in the “prelabeled” DNA strands (synthesized before T-starvation), ∼20% in DNA that was labeled throughout both growth and T-starvation, and ∼31% in the “postlabeled” DNA synthesized during T-starvation only (Fig. 4C). Thus, during the RED phase of T-starvation (explained in Fig. 1C), fragmentation indeed preferentially affected DNA that was synthesized in the absence of thymidine, whereas the old DNA strands were fragmented to a significantly lesser degree.
Genetics of the overall fragmentation was also interesting. Both the RecBCD+ and ΔrecBCD mutant cells showed high levels of chromosomal fragmentation after 4 h of T-starvation ((Fig. 4D), although the interim kinetics of fragmentation in the two strains are different (10)), suggesting that thymine starvation induces irreparable double-strand breaks, and that these breaks may be the ultimate cause of thymineless death itself. These results also suggest that repair of double-strand breaks in T-starved cells (presumably possible after thymidine readdition) is mostly blocked in the absence of normal replication (before thymidine is readded). Interestingly, we found that (once the background fragmentation in Thy+ controls was taken into account) the recA defect decreased the level of fragmentation in T-starved cells by about half (Fig. 4D), indicating that half of the double-strand breaks in wild type cells do not require any RecA involvement, but the other half is caused by recombinational misrepair. Alternatively, it is possible that the initial level of fragmentation in RecA+ and RecA− mutants is similar, but the final level of detected fragmentation is lower in recA mutants due to linear DNA degradation in this mutant (Fig. 3, B and C). Overall, we conclude that replication continues in T-starved cells, but the newly synthesized DNA is unstable against fragmentation and degradation, triggered in part by RecA-promoted misrepair, which is consistent with double-strand breaks at persistent gaps and re-formation of the old DNA duplex behind the stalling replication forks (Fig. 3A).
T-starvation Triggers Replication Initiations, followed by Origin Destruction
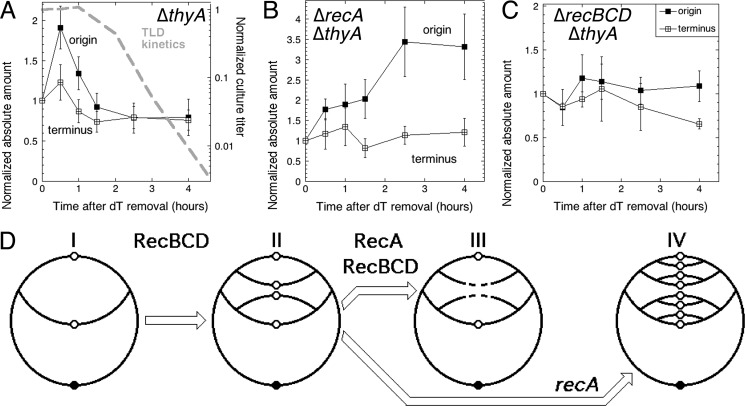
The instability of the new DNA in T-starvation conditions suggests that a continuous availability of DNA degradation-derived thymidine may allow cells to initiate new replication rounds. To determine how many replication rounds T-starved cells actually initiate, we measured the kinetics of the absolute amount of origin DNA versus terminus DNA in T-starved cells. We found that T-starvation causes the origin DNA to increase 2-fold, but then to decrease sharply below the prestarvation level (Fig. 5A). The terminus DNA goes through a similar evolution, but the differences between values in this case are not statistically significant (Fig. 5A). Thus, T-starvation conditions cause formation of new replication bubbles that cannot reach the terminus, which is reflected in the increased ori/ter ratio (supplemental Fig. S1A). Remarkably, this regular initiation from the origin is followed by the origin DNA disappearance, which clearly precedes the RED phase (Fig. 5A), so this origin loss might itself be a trigger of TLD.
FIGURE 5.
The stability of the chromosomal origin and terminus during thymine starvation. Total DNA was isolated at the indicated times from the same volume of culture, the amount of the origin or terminus DNA was determined by dot hybridization and is presented as absolute amounts, normalized to the amounts at time 0. A, the evolution of the absolute amount of the origin and the terminus DNA in the ΔthyA mutant (KKW58). The TLD kinetics (10) are overlaid in a dashed gray line. B, same as in A, but in the ΔrecA ΔthyA double mutant (KJK61). C, same as in A, but in the ΔrecBCD ΔthyA double mutant (KJK63). D, a scheme of chromosomal origin and terminus behavior in T-starved cells. I, normal replication in the presence of thymidine. II, T-starvation triggers RecBCD-dependent initiation of a replication round. III, in RecA+ cells, at least half of the replication origin copies are degraded (this degradation has to be RecBCD-dependent). IV, in recA mutant cells, new initiations continue.
Continuous Initiations Depend on RecBCD, Whereas Origin Destruction Depends on RecA
In the recBCD thyA mutant cells, both the absolute amounts of the origin and terminus DNA (Fig. 5C) and the ori/ter ratio (supplemental Fig. S1C) stay more-or-less constant during T-starvation, as if the bulk of new initiations at the origin depends on RecBCD. This surprising RecBCD dependence of new initiations is consistent with the idea that degradation of the broken chromosome by RecBCD provides enough thymine to support new initiations (although our own measurements (Fig. 3C) argue against any significant RecBCD-dependent DNA loss). In contrast, T-starvation in the recA mutants causes an increased ori/ter ratio (supplemental Fig. S1B), due to new initiations without loss of origin DNA (Fig. 5B). Because recA mutants degrade more DNA than RecA+ cells (Fig. 3), the increased initiation in the recA mutant cells is consistent with the idea that it is DNA degradation that sustains new replication bubbles in T-starved conditions. Remarkably, the origin DNA is somehow spared destruction in the recA mutants.
Our findings about the origin events in T-starved cells before the onset of rapid exponential death are schematically illustrated in Fig. 5D. We conclude that 1) both the Rec+ and recA mutant cells initiate new replication rounds during T-starvation, perhaps fueled by the RecBCD-dependent linear DNA degradation, which may supply enough thymine for the observed continued DNA synthesis and replication initiation; 2) new initiations are shortly followed by origin DNA destruction in Rec+ cells; and 3) this RecA-dependent origin DNA disappearance precedes TLD and, therefore, may cause TLD. We envision that the existing fork disintegration, via DNA degradation, provides enough thymidine to trick the cell into initiating new replication bubbles and thus shifting the focus of thymineless poisoning from the existing replication forks to the replication origin.
Genome Profiles Document Origin Replication and Destruction
Evidence was accumulating over the years that the most affected chromosomal location during thymine starvation is the replication origin, as the origin DNA disappearance was detected by the classic marker frequency analysis (in Bacillus subtilis) (43) and in E. coli (Fig. 5A of this study), by direct visualization with fluorescent in situ hybridization (23) and by gene arrays (44). To examine to what extent the overall chromosome is affected beyond the origin itself, we visualized both the location and extent of chromosome replication and degradation in cells undergoing TLD using the marker frequency profiling with gene arrays (36, 45). To make various profiles directly and quantitatively comparable, we normalized them to the signals from the corresponding terminus of the chromosome, which is relatively constant throughout T-starvation (Fig. 5, A–C).
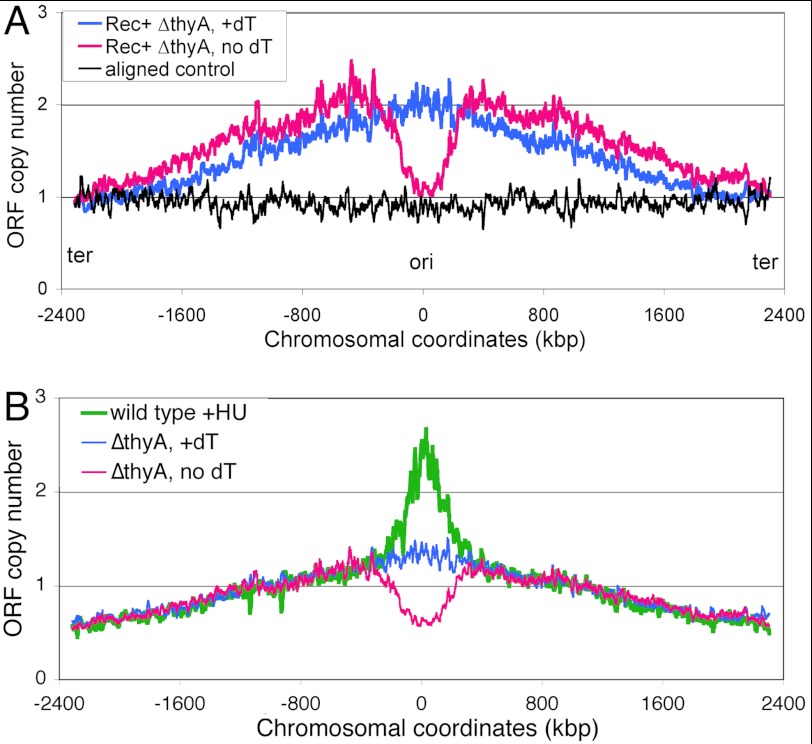
The genome profile of a nonreplicating chromosome control shows a flat line (Fig. 6A, black profile), whereas the profile of the normally replicating cells (Thy+ conditions) shows the classic slopes initiating at the terminus and converging at the origin with the maximum, which is twice the terminus minimum (Fig. 6A, blue profile). After 4 h of thymine starvation, the Rec+ cells' profile (normalized to the terminus) becomes somewhat elevated over the nonstarved one, indicating slowing down of replication forks. Remarkably, there is a dramatic loss of DNA in the broad ∼600-kbp segment centered on the replication origin (Fig. 6A, magenta profile). The origin signal does not fall below “1,” consistent with our direct measurements of the origin DNA (Fig. 5A). Such a loss of the origin-centered chromosomal segment, although incomplete, indicates formation of double-strand DNA gaps of enormous size (up to 0.6 Mbp) that could be irreparable, explaining cell death during T-starvation (44).
FIGURE 6.
The chromosomal marker frequency profiles in Rec+ cells undergoing thymineless death. For clarity, only the 15-point average trend lines are shown. In both panels, the blue profile comes from the normally replicating cells (dT supplementation), whereas the magenta profile comes from the same cells, but starved without thymine for 4 h. A, the Rec+ ΔthyA cells (KKW 58). The black trend line, wild type cells, incubated in the presence of 40 μg/ml of chloramphenicol for 3 h in growing conditions, to prevent new replication initiations, whereas allowing the existing replication round to finish, so that ori/ter = 1. The profiles are normalized to the average value of 76 CDS covering roughly 100 kbp in the terminus region around dif. B, the Rec+ ΔthyA cells (KKW58). The green profile are Thy+ cells (KKW59) treated with 80 mm HU for 4 h, as before (26). Normalization in this case is to the combined signal from the area of the chromosome between 450 and 500 kbp, as in Fig. 9.
To test whether the disappearance of the origin signal is a general consequence of DNA synthesis inhibition or is unique to TLD, we determined the chromosomal profile of wild type cells treated with hydroxyurea (HU), a specific inhibitor of the biosynthesis of all four DNA precursors, which in vivo inhibits DNA synthesis similar to T-starvation (26). In contrast to T-starvation, treatment with HU for 4 h is bacteriostatic, rather than bactericidal (26), therefore providing a useful reference of the same magnitude of replication inhibition that, however, does not lead to lethality. We found that the marker frequency profile of HU-treated cells is the inversion of the TLD profile: the same chromosomal segment that is being degraded in the Rec+ T-starved cells is elevated in the HU-treated cells (Fig. 6B). This remarkable coincidence confirms the link between continued replication of the origin-containing chromosomal segment (HU) and its degradation (T-starvation) on the one hand, and between death and origin segment disappearance during T-starvation on the other.
RecA-dependent Double-strand Breaks Around the Origin Lead to Its RecBCD-dependent Degradation
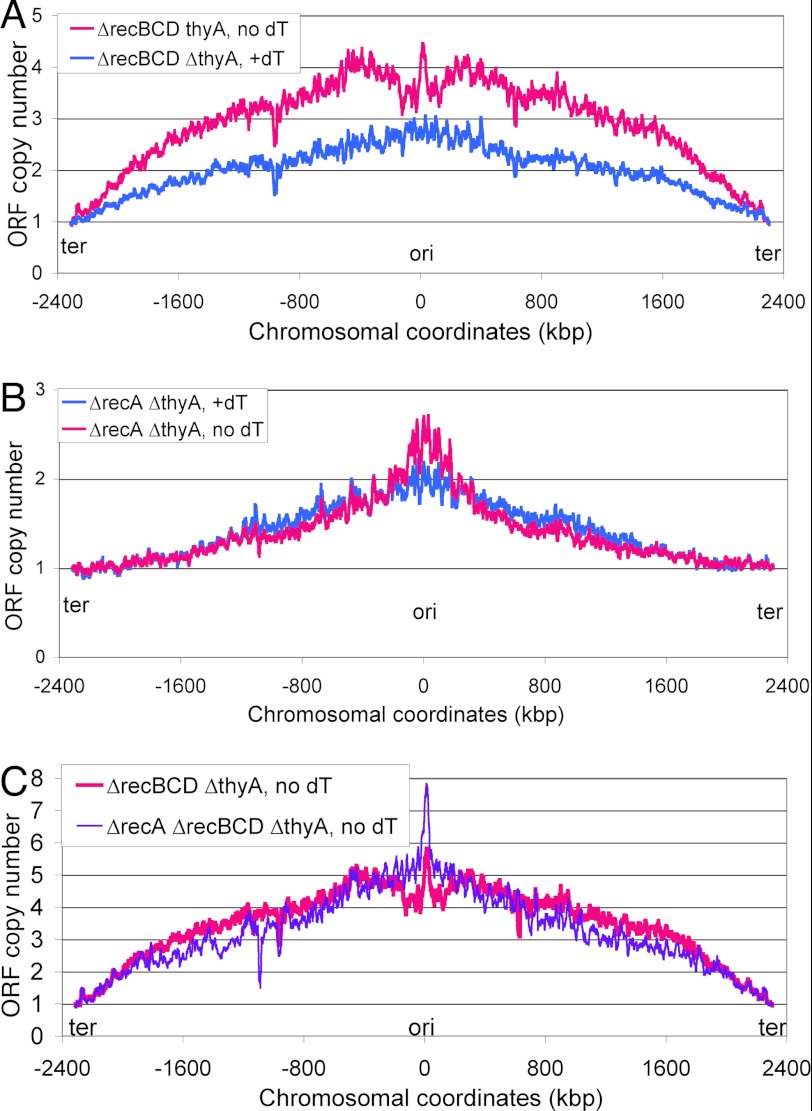
Khodursky and colleagues (44) have reported that genome profiles of recF and recO mutants undergoing thymineless death reveal much reduced disappearance of the origin region, consistent with the idea that origin degradation is triggered by a RecA-promoted recombinational misrepair. To reveal the contribution of RecA and RecBCD to the origin degradation, we determined the chromosomal profiles in the corresponding mutant cells undergoing TLD. The recBCD mutant is deficient in linear DNA degradation, thus, the profile of this mutant undergoing TLD should identify the starting point of the chromosomal degradation that leads to the origin region loss in the Rec+ cells (Fig. 6). The chromosomal profile in the recBCD thyA mutant after 4 h of T-starvation shows even steeper slopes, indicating severe inhibition of replication forks, especially closer to the terminus (Fig. 7A). Interestingly, in contrast to the Rec+ cells, there is only a mild trough in the origin-centered segment in the recBCD mutant, with the origin itself still surviving in a micropeak (Fig. 7A), indicating that the loss of this region in the RecBCD+ cells is due to chromosomal DNA degradation starting from double-strand breaks around the origin (the left minimum is located ∼126 kbp from the origin in rfaY, the right minimum is ∼118 kbp from the origin, in yihF).
FIGURE 7.
The chromosomal marker frequency profiles in rec mutant cells undergoing thymineless death. In all panels, the blue profile comes from the normally replicating cells (dT supplementation), whereas the magenta profile comes from the same cells, but starved without thymine for 4 h. All the profiles are normalized to the average value of 76 CDS covering roughly 100 kbp in the terminus region around dif. A, the ΔrecBCD ΔthyA cells (KJK63). B, the ΔrecA ΔthyA cells (KJK61). C, the ΔrecA ΔrecBCD ΔthyA cells (KJK102).
The chromosomal profile of the recA thyA mutant (Fig. 7B) is even more interesting, being similar to the chromosomal profile of the HU-treated cells (Fig. 6B) in that it is an inversion of the RecA+ profile: the overall chromosome, elevated in the RecA+ cells, is decreased in the recA mutant, suggesting overall degradation, whereas the segment around the replication origin, missing in the Rec+ cells, is overreplicated in the recA mutant (Fig. 7B), consistent with the elevated ori/ter ratio in response to T-starvation in the absence of RecA (Fig. 5B and supplemental Fig. S1B). Apparently, the double-strand breaks around the replication origin, which are detectable in the recBCD mutant (Fig. 7A) and trigger the loss of the origin region in Rec+ cells (Fig. 6A), do not form in the recA mutant (Fig. 7B).
Finally, the marker frequency profile of the recA recBCD double mutant undergoing TLD (Fig. 7C, purple profile) clearly shows that the loss of DNA on both sides of the replication origin in the recBCD single mutant is dependent on RecA. Interestingly, the very limited progress of replication forks away from the origin in the recA recBCD double mutant, compared with the recA single mutant (Fig. 7B, magenta profile, versus C, purple profile), suggests that RecBCD-catalyzed linear DNA degradation generates thymidine to fuel the progress of new replication bubbles. Because the recA recBCD double mutant dies during T-starvation as fast as the recBCD single mutant (10), yet there is no origin DNA loss in the double mutant, this argues that the loss of the origin-containing chromosomal segment in the RecABCD+ cells is a reflection of irreparable chromosomal lesions (for example, the proposed double-strand breaks behind thymine-starved replication forks), rather than a mechanistic prerequisite for TLD. In other words, although the origin DNA is not lost in thymine-starved recA and recBCD mutants, a massive number of double-strand breaks in their chromosomes during thymine-starvation (Fig. 4) ensures that these mutants die anyway as a result of their inability to repair these double-strand breaks. At the same time, the Rec+ cells, although being in a position to repair this fragmentation around the chromosome, lose the replication origin region due to the RecA-dependent breaks in it and die for this other reason. Overall, the marker frequency analysis supports the idea that continued initiation at the origin during T-starvation is not dependent on RecA, but the subsequent origin destruction is.
DISCUSSION
To understand the nature of chromosomal lesions leading to TLD in E. coli, we used physical techniques to characterize the chromosomal replication and degradation patterns during T-starvation in Rec+ cells, as well as in the recA and recBCD mutants. We found that the T-starved cells incorporate label into DNA at 10% rate of the nonstarved cells, leading to a significant overall DNA accumulation during the resistance phase of T-starvation, but this newly synthesized DNA becomes fragmented and is partially lost during the rapid exponential death phase. These results are consistent with formation of double-strand breaks at persistent single-strand gaps behind the T-starved replication forks. We also found that new replication rounds are initiated during the resistance phase (confirming one old report (46)), but this somehow makes the origin DNA unstable in RecA+ cells during the RED phase. Thus, at least some poisoning events during T-starvation happen around the replication origin and result directly from new initiations, rather than being associated with pre-existing replication forks, as was imagined before (10). Marker frequency analysis with gene arrays in Rec+, recA, and recBCD mutant cells demonstrated the RecA- and RecBCD-dependent degradation of the origin-containing chromosomal segment between two chromosomal regions ∼600 kbp apart.
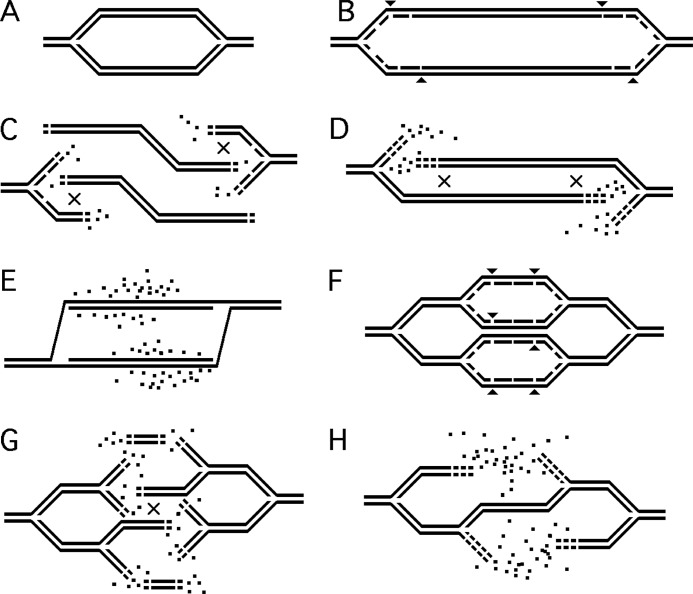
We propose that the instability of ss-gaps and the resulting DNA degradation behind the existing T-starved replication forks (Fig. 8, B → C → D) temporarily relieves the deficit of thymidine, fueling initiation of new replication bubbles (Fig. 8F). According to this logic, the origin-containing segment is eventually destroyed as a result of double-strand breaks formed at persistent ss-gaps behind the replication forks in these nascent replication bubbles that cannot progress away from the origin (Fig. 8, G → H). We suggest that the detrimental role of RecA(F)-catalyzed strand exchange is 2-fold: 1) it interferes with the filling in of persistent ss-gaps by tying them up in futile recombination intermediates; and 2) it dissociates the replisomes, which are trying to close these ss-gaps (in the process known as “replication fork restart” (47)), making them available for initiation of new Okazaki fragments and new replication bubbles, that likewise cannot be finished due to the deficit of dTTP. Upon re-addition of thymidine, these gaps must be filled by DNA pol I (48).
FIGURE 8.
A scheme of the proposed chromosomal events during T-starvation leading to thymineless death. “×” signs in C, D, and G show the position of recombinational repair of double-strand breaks between overlapping ends. Small arrowheads in B and F mark the position of future double-strand breaks. Dots, DNA precursors, released by degradation. A, normal replication. B, T-starvation leads to formation of persistent single-strand gaps behind replication forks. C, some of the persistent ss-gaps are eventually broken, leading to replication bubble disintegration. Recombinational repair of double-strand ends begins. D, restoration of the replication bubble by recombinational repair continues. E, the original replication bubble is restored, whereas T-starvation continues. F, a new replication round is started at the origin. For clarity, the existing replication forks are shown inactive. G, disintegration of the new replication bubbles due to breaks at persistent gaps behind replication forks. H, because the new disintegrated bubbles were small, the ensuing degradation from open DNA ends restores only one duplex by recombinatonal repair.
Replication Origin Loss during T-starvation
Our results tie together two phenomena associated with T-starvation, (i) the proposed double-strand breaks at persistent ss-gaps behind replication forks and (ii) the demonstrated disappearance of replication origins, into a possible mechanism of thymineless death. The link between the two phenomena is the fact that, during T-starvation, because of the overall inhibition of DNA synthesis, yet active initiations of the new replication rounds, new replication forks cannot progress far away from the origin, and the proposed double-strand breaks at persistent ss-gaps form in the vicinity of the origin.
The critical role of the DNA destruction behind the replication forks and the vulnerability of replication origin during T-starvation has been crystallizing over the years. In 1970, Ramareddy and Reiter (49, 50) reported sequential loss of chromosomal markers in thyA mutant B. subtilis cells, synchronized by germinating spores and switched to a medium without thymine. The loss of markers started at the position of replication forks and went toward the replication origin. Although these authors did not specifically look at the disappearance of markers around the replication origin, the known initiation of new replication rounds during T-starvation (46), together with severe inhibition of the replication fork progress, should make the origins susceptible to degradation due to disintegration of new rounds of replication forks. For example, Nakayama and colleagues (20) reported in E. coli that the chromosomal fragments carrying replication origin are unable to enter pulsed-field gels after 1 h of T-starvation, perhaps due to initiation events generating replication bubbles at the origin that could not progress out of the region, making DNA nonmigrating under the pulsed-field gel electrophoresis conditions.
Looking again at the thyA mutants spores of B. subtilis, germinating into a thymineless medium, Wake and colleagues (43) have noticed premature formation of the cell constriction assembly and traced the reason to the diminishing amounts of the replication origin DNA. The disappearance was quite specific and sharp in that the loci even 20 kbp away from the origin were not affected (43). Recently, Rosenberg and colleagues (23) reported that T-starvation causes a shift from cells containing mostly 2 origins and 1 terminus (the normal 2:1 ratio) to cells containing 1 origin and 1 terminus (meaning that one origin is lost). Interestingly, the same authors also reported a substantial increase in cells with one origin and no termini (meaning that the terminus is lost?); it should be noted, although, that almost 30% of cells in their normally replicating cultures lack either origin or terminus, suggesting that the “no signal” background in this experimental system is rather high (23). Finally, Khodursky and colleagues (44) reported the dramatic decrease of a broad zone centered at the replication origin, detected by marker frequency profiling of the entire chromosome, the observation that we independently confirmed (Fig. 6A). As a result of this decrease, which affects a region of ∼1 Mbp, the ori/ter ratio becomes 1:1 in their T-starved cultures (44), the magnitude of the decrease that we also observed (Fig. 6A and supplemental Fig. S1A).
Although T-starvation-triggered decrease of the broad origin-containing chromosomal segment can be considered established at this point, and it happens with the same kinetics as TLD (44), this phenomenon by itself cannot be the sole explanation of thymineless death, as Fig. 8H clarifies. Indeed, the detected loss of the origin DNA is always partial (Refs. 23, 43, and 44, and this work), specifically, it only comes down to the level of the terminus DNA, making the ori/ter ratio = 1:1. If this 1:1 ratio refers to the whole chromosome, and this population average indeed reflects the situation in most individual cells, the chromosome simply becomes aligned (basically circular, with some linear branches still to be degraded), and no killing is expected. Something else must be happening with the chromosomal DNA besides this origin-centered chromosomal DNA disappearance for the cells to lose viability. For example, the remaining DNA may be entangled in multiple unresolved single-strand gap repair intermediates, which makes it unusable as a template for DNA synthesis. Alternatively, the population average may not reflect events in individual cells, with most chromosomes in the population carrying short double-strand gaps (that are, of course, lethal) that do not overlap among cells. A similar idea was entertained by Khodursky and colleagues (44). Other regions of instability, such as the terminus (23), are also possible (see Fig. 5A and Fig. 9), which can cause addition toxicity. The current methods of detection also miss any rearrangements and mutations that may cause further toxicity.
FIGURE 9.
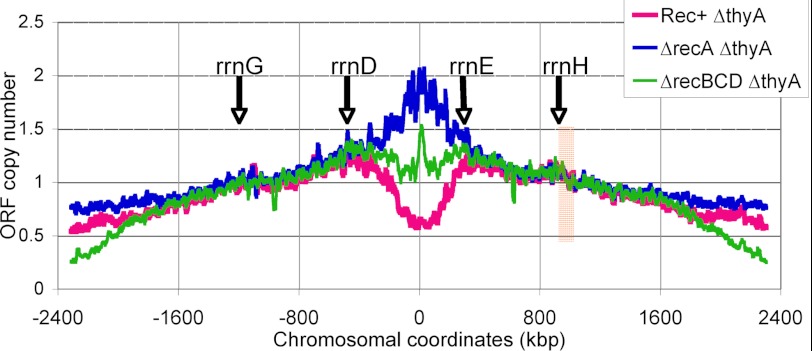
The three TLD profiles normalized to the middle of the right replichore. These three 15-point average trend lines are generated from the same data as profiles in Figs. 6 and 7, but normalization is done by the combined signal from the area of the chromosome (comprising 100 CDS in the right replichore), marked by the vertical light brown band. Positions of the four origin-distal rRNA operons are indicated by arrows.
Interpretation of the Marker Frequency Chromosomal Profiles
Marker frequency analysis at the scale of the chromosome is becoming the standard method of analysis of the replication patterns in bacteria and archaea (36, 51–53), as the profiles of replication waves in synchronized cultures of wild type cells, rolling from the origin toward the terminus in an orderly fashion, are not only highly visual, but are also straightforward to interpret (40, 45). Indeed, the terminus signal in these synchronized cells can be taken as “one” by definition (such as the black profile in Fig. 6A). However, interpretation of the results of marker frequency analysis in growing asynchronous populations is not trivial as it depends on the normalization point. In our analysis, because we showed that the terminus is stable throughout T-starvation (Fig. 5), we took it for normalization for most of the profiles in Figs. 6 and 7. As a result, the profiles from the starving cells looked quite different over the entire chromosome length (for example, at the point of “−1,100 kb,” the wild type profile shows values in the 1.8–2.0 range, the recBCD profile shows 3.2, whereas the recA profile shows on the average the value of 1.3, suggesting the slowest replication in the recBCD mutants and the fastest in the recA mutants. However, if a point in the middle of a replichores is taken for 1, all three T-starvation profiles look surprisingly similar over a total of 2.8 Mbp (∼64% of the entire chromosome), excluding the two ∼800-kbp zones, one centered on the replication origin, the other on the terminus (Fig. 9). Such representation of marker frequency profiles does not affect the results of our analysis, but it does affect the conclusion about the behavior of the terminus during T-starvation, which seems to be replicating in the recA mutants, whereas disappearing from the recBCD mutants. This shows the importance of rooting this analysis in data on the replication terminus dynamics, obtained by independent methods.
The three centrally overlapping profiles (Fig. 9) also highlight the four chromosomal regions at which profiles either converge into a single line or diverge into three separate lines. In the left replichore, the two regions are “−2,000–1,800” and “−450–350” kbp, whereas in the right replichore, the two regions are 350–400 and 1600–1800 kbp. Of the four, the two origin-proximal regions contain the obvious “replication/degradation barriers.” It is intriguing that these barriers are in the vicinity of the rrnD and rrnE ribosomal RNA operons (Fig. 9). Moreover, the outermost rRNA operons, rrnG and rrnH, also coincide with irregularities on the profiles, suggesting some changes in replication in the vicinity of rRNA operons. The surprising well delineated zones in which new replication forks disintegrate during T-starvation (the minima around the origin in the recBCD mutant), as well as the zones of inhibition of replication forks (in HU-treated cells and in the recA mutant T-starved cells) or of the chromosomal DNA destruction (in the Rec+ T-starved cells), represent an interesting topic for future TLD studies. Specifically, whether the limitation of the chromosomal DNA destruction or replication is caused by specific rRNA operons, rrnD in the left replichore and rrnE in the right replichore (Fig. 9), deserves experimental testing.
Acknowledgments
We thank Steve Kowalczykowski and John Roth (both University of California, Davis), as well as Sharik Khan and Elena Kouzminova (both this lab) for valuable discussion of the project and for commenting on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM 073115.

This article contains supplemental Fig. S1.
- T-starvation
- thymine starvation
- TLD
- thymineless death
- RED
- rapid exponential death
- CDS
- coding sequences
- HU
- hydroxyurea
- ss
- single strand.
REFERENCES
- 1. Ahmad S. I., Kirk S. H., Eisenstark A. (1998) Thymine metabolism and thymineless death in prokaryotes and eukaryotes. Annu. Rev. Microbiol. 52, 591–625 [DOI] [PubMed] [Google Scholar]
- 2. Cohen S. S. (1971) On the nature of thymineless death. Ann. N.Y. Acad. Sci. 186, 292–301 [DOI] [PubMed] [Google Scholar]
- 3. Seno T., Ayusawa D., Shimizu K., Koyama H., Takeishi K., Hori T. (1985) Thymineless death and genetic events in mammalian cells. Basic Life Sci. 31, 241–263 [DOI] [PubMed] [Google Scholar]
- 4. Chernyshev A., Fleischmann T., Kohen A. (2007) Thymidyl biosynthesis enzymes as antibiotic targets. Appl. Microbiol. Biotechnol. 74, 282–289 [DOI] [PubMed] [Google Scholar]
- 5. Van Triest B., Peters G. J. (1999) Thymidylate synthase. A target for combination therapy and determinant of chemotherapeutic response in colorectal cancer. Oncology 57, 179–194 [DOI] [PubMed] [Google Scholar]
- 6. Breitman T. R., Maury P. B., Toal J. N. (1972) Loss of deoxyribonucleic acid-thymine during thymine starvation of Escherichia coli. J. Bacteriol. 112, 646–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kunz B. A., Haynes R. H. (1982) DNA repair and the genetic effects of thymidylate stress in yeast. Mutat. Res. 93, 353–375 [Google Scholar]
- 8. Ladner R. D. (2001) The role of dUTPase and uracil-DNA repair in cancer chemotherapy. Curr. Protein Pept. Sci. 2, 361–370 [DOI] [PubMed] [Google Scholar]
- 9. Nakayama H. (2005) Escherichia coli RecQ helicase. A player in thymineless death. Mutat. Res. 577, 228–236 [DOI] [PubMed] [Google Scholar]
- 10. Kuong K. J., Kuzminov A. (2010) Stalled replication fork repair and misrepair during thymineless death in Escherichia coli. Genes Cells 15, 619–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morganroth P. A., Hanawalt P. C. (2006) Role of DNA replication and repair in thymineless death in Escherichia coli. J. Bacteriol. 188, 5286–5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fonville N. C., Vaksman Z., DeNapoli J., Hastings P. J., Rosenberg S. M. (2011) Pathways of resistance to thymineless death in Escherichia coli and the function of UvrD. Genetics 189, 23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rupp W. D., Howard-Flanders P. (1968) Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J. Mol. Biol. 31, 291–304 [DOI] [PubMed] [Google Scholar]
- 14. Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. (1971) Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J. Mol. Biol. 61, 25–44 [DOI] [PubMed] [Google Scholar]
- 15. Kuzminov A. (1999) Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol. Mol. Biol. Rev. 63, 751–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakayama H., Nakayama K., Nakayama R., Nakayama Y. (1982) Recombination-deficient mutations and thymineless death in Escherichia coli K-12. Reciprocal effects of recBC and recF and indifference of recA mutations. Can. J. Microbiol. 28, 425–430 [DOI] [PubMed] [Google Scholar]
- 17. Nakayama K., Shiota S., Nakayama H. (1988) Thymineless death in Escherichia coli mutants deficient in the RecF recombination pathway. Can. J. Microbiol. 34, 905–907 [DOI] [PubMed] [Google Scholar]
- 18. Gallant J., Suskind S. R. (1961) Relationship between thymineless death and ultraviolet inactivation in Escherichia coli. J. Bacteriol. 82, 187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukherjee P., Mukherjee N., Bhattacharjee S. B. (1971) Effect of UV irradiation and 32P decay on thymineless death in E. coli 15. Mutat. Res. 12, 341–347 [DOI] [PubMed] [Google Scholar]
- 20. Nakayama K., Kusano K., Irino N., Nakayama H. (1994) Thymine starvation-induced structural changes in Escherichia coli DNA. Detection by pulsed field gel electrophoresis and evidence for involvement of homologous recombination. J. Mol. Biol. 243, 611–620 [DOI] [PubMed] [Google Scholar]
- 21. Guarino E., Salguero I., Jiménez-Sánchez A., Guzmán E. C. (2007) Double-strand break generation under deoxyribonucleotide starvation in Escherichia coli. J. Bacteriol. 189, 5782–5786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahmad S. I. (1980) Thymineless death in recombination deficient mutants of Escherichia coli K-12. Z. Naturforsch. C 35, 279–283 [DOI] [PubMed] [Google Scholar]
- 23. Fonville N. C., Bates D., Hastings P. J., Hanawalt P. C., Rosenberg S. M. (2010) Role of RecA and the SOS response in thymineless death in Escherichia coli. PLoS Genet. 6, e1000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inouye M. (1971) Pleiotropic effect of the recA gene of Escherichia coli. Uncoupling of cell division from deoxyribonucleic acid replication. J. Bacteriol. 106, 539–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martín C. M., Guzmán E. C. (2011) DNA replication initiation as a key element in thymineless death. DNA Repair 10, 94–101 [DOI] [PubMed] [Google Scholar]
- 26. Kuong K. J., Kuzminov A. (2009) Cyanide, peroxide, and nitric oxide formation in solutions of hydroxyurea causes cellular toxicity and may contribute to its therapeutic potency. J. Mol. Biol. 390, 845–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kouzminova E. A., Kuzminov A. (2004) Chromosomal fragmentation in dUTPase-deficient mutants of Escherichia coli and its recombinational repair. Mol. Microbiol. 51, 1279–1295 [DOI] [PubMed] [Google Scholar]
- 28. Miller J. H. (1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 29. Miranda A., Kuzminov A. (2003) Chromosomal lesion suppression and removal in Escherichia coli via linear DNA degradation. Genetics 163, 1255–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Datsenko K. A., Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zalkin H., Nygaard P. (1996) in Escherichia coli and Salmonella, Cellular and Molecular Biology (Neidhardt F. C., ed) pp. 561–579, ASM Press, Washington, D. C [Google Scholar]
- 32. Kuzminov A., Stahl F. W. (2005) in The Bacterial Chromosome (Higgins N. P., ed) pp. 349–367, ASM Press, Washington, D.C [Google Scholar]
- 33. Silverstein J. L., Goldberg E. B. (1976) T4 DNA injection. II. Protection of entering DNA from host exonuclease V. Virology 72, 212–223 [DOI] [PubMed] [Google Scholar]
- 34. Neidhardt F. C., Bloch P. L., Smith D. F. (1974) Culture medium for enterobacteria. J. Bacteriol. 119, 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Drlica K., Engle E. C., Manes S. H. (1980) DNA gyrase on the bacterial chromosome. Possibility of two levels of action. Proc. Natl. Acad. Sci. U.S.A. 77, 6879–6883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kouzminova E. A., Kuzminov A. (2008) Patterns of chromosomal fragmentation due to uracil-DNA incorporation reveal a novel mechanism of replication-dependent double-stranded breaks. Mol. Microbiol. 68, 202–215 [DOI] [PubMed] [Google Scholar]
- 37. Kouzminova E. A., Kuzminov A. (2006) Fragmentation of replicating chromosomes triggered by uracil in DNA. J. Mol. Biol. 355, 20–33 [DOI] [PubMed] [Google Scholar]
- 38. Wachsman J. T., Kemp S., Kogg L. (1964) Thymineless death in Bacillus megaterium. J. Bacteriol. 87, 1079–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cummings D. J., Mondale L. (1967) Thymineless death in Escherichia coli. Strain specificity. J. Bacteriol. 93, 1917–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kouzminova E. A., Kuzminov A. (2012) Chromosome demise in the wake of ligase-deficient replication. Mol. Microbiol. 84, 1079–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clark A. J., Chamberlin M. (1966) Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K-12. J. Mol. Biol. 19, 442–454 [DOI] [PubMed] [Google Scholar]
- 42. Khan S. R., Kuzminov A. (2012) Replication forks stalled at ultraviolet lesions are rescued via RecA and RuvABC protein-catalyzed disintegration in Escherichia coli. J. Biol. Chem. 287, 6250–6265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Regamey A., Harry E. J., Wake R. G. (2000) Mid-cell Z ring assembly in the absence of entry into the elongation phase of the round of replication in bacteria. Coordinating chromosome replication with cell division. Mol. Microbiol. 38, 423–434 [DOI] [PubMed] [Google Scholar]
- 44. Sangurdekar D. P., Hamann B. L., Smirnov D., Srienc F., Hanawalt P. C., Khodursky A. B. (2010) Mol. Micorbiol. 75, 1455–1467 [DOI] [PubMed] [Google Scholar]
- 45. Khodursky A. B., Peter B. J., Schmid M. B., DeRisi J., Botstein D., Brown P. O., Cozzarelli N. R. (2000) Analysis of topoisomerase function in bacterial replication fork movement. Use of DNA microarrays. Proc. Natl. Acad. Sci. U.S.A. 97, 9419–9424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pritchard R. H., Lark K. G. (1964) Induction of replication by thymine starvation at the chromosome origin in Escherichia coli. J. Mol. Biol. 9, 288–307 [DOI] [PubMed] [Google Scholar]
- 47. Khidhir M. A., Casaregola S., Holland I. B. (1985) Mechanism of transient inhibition of DNA synthesis in ultraviolet-irradiated E. coli. Inhibition is independent of recA while recovery requires RecA protein itself and an additional, inducible SOS function. Mol. Gen. Genet. 199, 133–140 [DOI] [PubMed] [Google Scholar]
- 48. Sharma R. C., Smith K. C. (1987) Role of DNA polymerase I in postreplication repair. A re-examination with Escherichia coli δpolA. J. Bacteriol. 169, 4559–4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramareddy G., Reiter H. (1970) Sequential loss of loci in thymine-starved Bacillus subtilis 168 cells. Evidence for a circular chromosome. J. Mol. Biol. 50, 525–532 [DOI] [PubMed] [Google Scholar]
- 50. Reiter H., Ramareddy G. (1970) Loss of DNA behind the growing point of thymine-starved Bacillus subtilis 168. J. Mol. Biol. 50, 533–548 [DOI] [PubMed] [Google Scholar]
- 51. Duggin I. G., McCallum S. A., Bell S. D. (2008) Chromosome replication dynamics in the archaeon Sulfolobus acidocaldarius. Proc. Natl. Acad. Sci. U.S.A. 105, 16737–16742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tehranchi A. K., Blankschien M. D., Zhang Y., Halliday J. A., Srivatsan A., Peng J., Herman C., Wang J. D. (2010) The transcription factor DksA prevents conflicts between DNA replication and transcription machinery. Cell 141, 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang J. D., Berkmen M. B., Grossman A. D. (2007) Genome-wide coorientation of replication and transcription reduces adverse effects on replication in Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A. 104, 5608–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]