Abstract
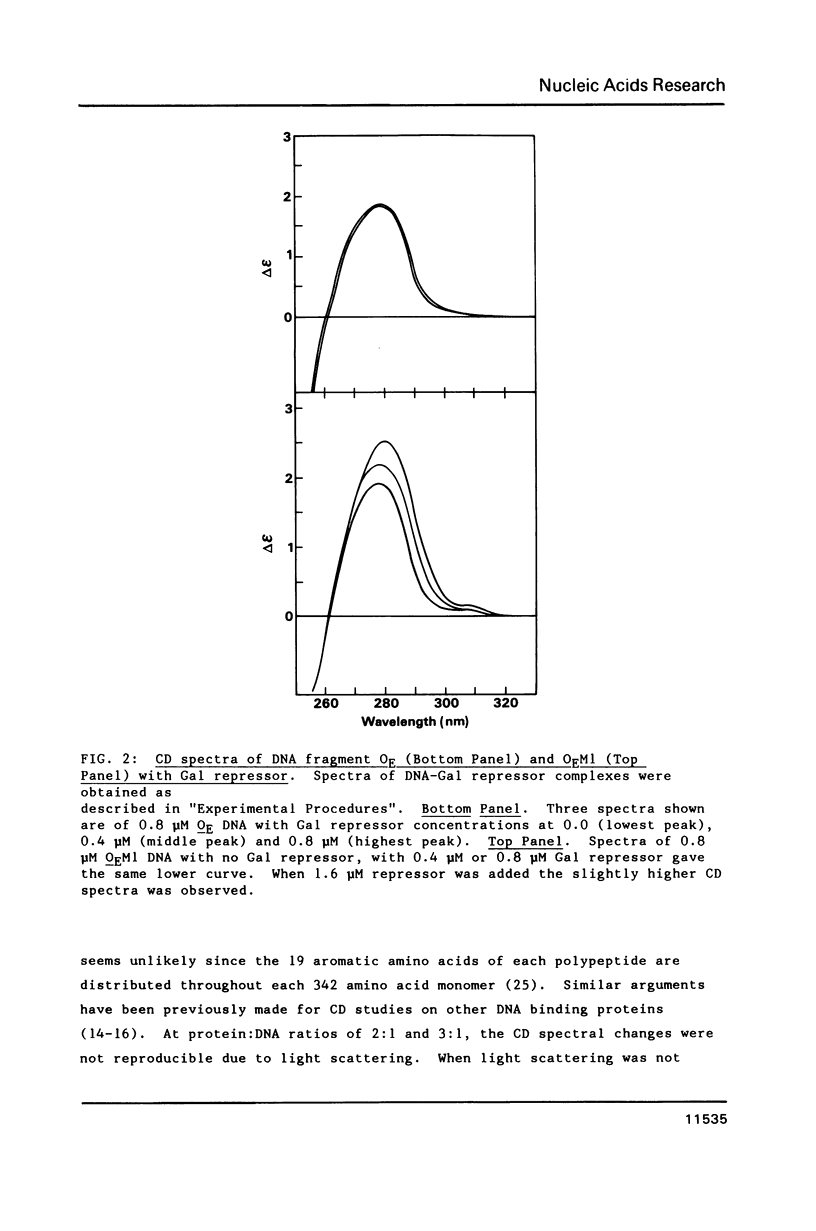
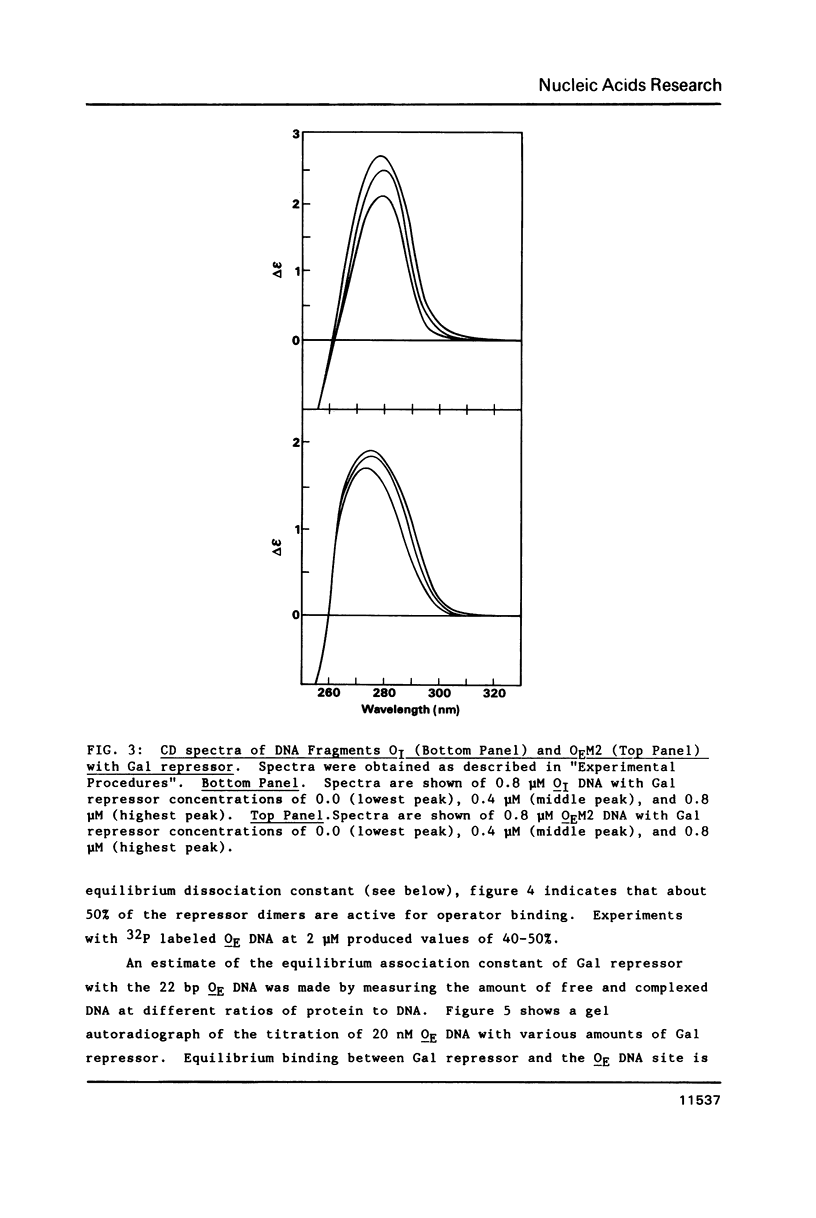
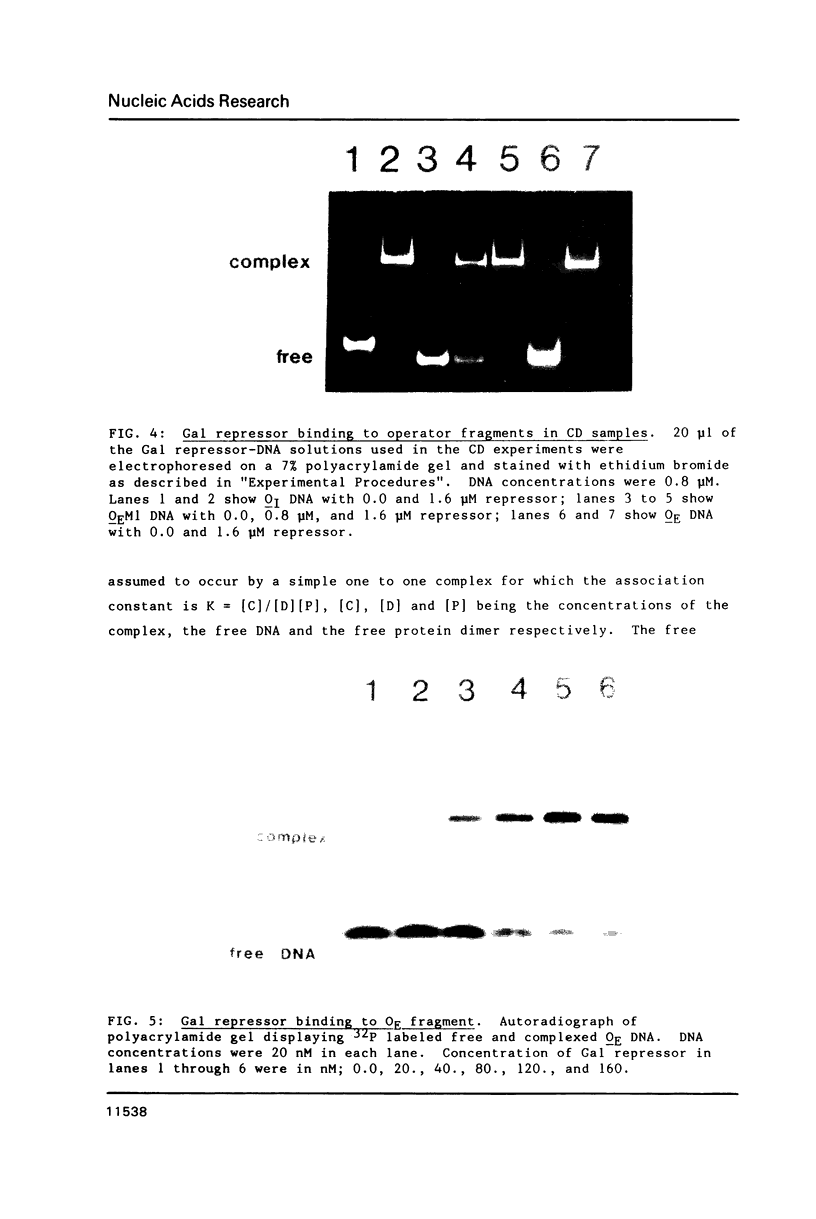
Gal repressor dimer binds to two gal operator sites, OE and OI, which are 16 bp long similar sequences with hyphenated dyad symmetries (11,12). Repressor occupation hinders the reactivity of the N7 atoms in the major groups of guanines, located at positions 1, 3 and 8, and the rotational 1', 3' and 8' of the symmetries. We have shown that Gal repressor binding to OE or OI DNA fragments increases the circular dichroism (CD) spectral peak in the 270 to 300 nm range. The CD change is similar to that observed for Lac repressor binding to its operator site (14). It is consistent with a DNA conformational change during complex formation between Gal repressor and OE and OI DNA. The CD spectral change was not observed when the central 8,8' G-C base pairs in the DNA-protein complex were replaced by A-T base pairs, whereas substitution of the 1,1' G-C base pairs do show the accompanying increase in the spectra during repressor binding. The absence of CD change of the Gal repressor complex with DNA mutated at the 8,8' base pairs suggest that the central G-C base pairs are required for the repressor induced conformational change.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Echols H. Glucose effect and the galactose enzymes of Escherichia coli: correlation between glucose inhibition of induction and inducer transport. J Bacteriol. 1966 Sep;92(3):601–608. doi: 10.1128/jb.92.3.601-608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Miller W. Modulation of the two promoters of the galactose operon of Escherichia coli. Nature. 1979 Jun 7;279(5713):492–494. doi: 10.1038/279492a0. [DOI] [PubMed] [Google Scholar]
- Altschmied L., Hillen W. TET repressor.tet operator complex formation induces conformational changes in the tet operator DNA. Nucleic Acids Res. 1984 Feb 24;12(4):2171–2180. doi: 10.1093/nar/12.4.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. I. LA BIOSYNTH'ESE INDUITE DE LA GALACTOKINASE ET L'INDUCTION SIMULTAN'EE DE LA S'EQUENCE ENZYMATIQUE. J Mol Biol. 1963 Aug;7:164–182. doi: 10.1016/s0022-2836(63)80044-3. [DOI] [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. II. LE D'ETERMINISME G'EN'ETIQUE DE LA R'EGULATION. J Mol Biol. 1963 Aug;7:183–205. doi: 10.1016/s0022-2836(63)80045-5. [DOI] [PubMed] [Google Scholar]
- Blazy B., Culard F., Maurizot J. C. Interaction between the cyclic AMP receptor protein and DNA. Conformational studies. J Mol Biol. 1987 May 5;195(1):175–183. doi: 10.1016/0022-2836(87)90334-2. [DOI] [PubMed] [Google Scholar]
- Culard F., Maurizot J. C. Lac repressor - lac operator interaction. Circular dichroism study. Nucleic Acids Res. 1981 Oct 10;9(19):5175–5184. doi: 10.1093/nar/9.19.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiethen L., Starlinger P. Mutations in the galactose-operator. Mol Gen Genet. 1970;108(4):322–330. doi: 10.1007/BF00267769. [DOI] [PubMed] [Google Scholar]
- Fried M. G., Crothers D. M. Equilibrium studies of the cyclic AMP receptor protein-DNA interaction. J Mol Biol. 1984 Jan 25;172(3):241–262. doi: 10.1016/s0022-2836(84)80025-x. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H. J., Bicknäse H., Gleumes B., Heibach C., Rosahl S., Ehring R. Characterization of two mutations in the Escherichia coli galE gene inactivating the second galactose operator and comparative studies of repressor binding. EMBO J. 1983;2(12):2129–2135. doi: 10.1002/j.1460-2075.1983.tb01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- Irani M., Orosz L., Busby S., Taniguchi T., Adhya S. Cyclic AMP-dependent constitutive expression of gal operon: use of repressor titration to isolate operator mutations. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4775–4779. doi: 10.1073/pnas.80.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpichnikov M. P., Yartzev A. P., Minchenkova L. E., Chernov B. K., Ivanov V. I. The absence of non-local conformational changes in OR3 operator DNA on complexing with the cro repressor. J Biomol Struct Dyn. 1985 Dec;3(3):529–536. doi: 10.1080/07391102.1985.10508440. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Federici M. M. Quantitation of aromatic residues in proteins: model compounds for second-derivative spectroscopy. Biochemistry. 1982 May 25;21(11):2600–2606. doi: 10.1021/bi00540a004. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Adhya S. Demonstration of two operator elements in gal: in vitro repressor binding studies. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6100–6104. doi: 10.1073/pnas.81.19.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A., Adhya S. Probing the structure of gal operator-repressor complexes. Conformation change in DNA. J Biol Chem. 1987 Sep 25;262(27):13258–13262. [PubMed] [Google Scholar]
- Majumdar A., Rudikoff S., Adhya S. Purification and properties of Gal repressor:pL-galR fusion in pKC31 plasmid vector. J Biol Chem. 1987 Feb 15;262(5):2326–2331. [PubMed] [Google Scholar]
- Matthews B. W., Ohlendorf D. H., Anderson W. F., Takeda Y. Structure of the DNA-binding region of lac repressor inferred from its homology with cro repressor. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1428–1432. doi: 10.1073/pnas.79.5.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Saedler H., Gullon A., Fiethen L., Starlinger P. Negative control of the galactose operon in E. coli. Mol Gen Genet. 1968;102(1):79–88. doi: 10.1007/BF00341872. [DOI] [PubMed] [Google Scholar]
- Saxe S. A., Revzin A. Cooperative binding to DNA of catabolite activator protein of Escherichia coli. Biochemistry. 1979 Jan 23;18(2):255–263. doi: 10.1021/bi00569a003. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- von Wilcken-Bergmann B., Müller-Hill B. Sequence of galR gene indicates a common evolutionary origin of lac and gal repressor in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2427–2431. doi: 10.1073/pnas.79.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]