Abstract
Purpose
The aim of this study was to develop and validate the Trust in Oncologist Scale (TiOS), which aims to measure cancer patients' trust in their oncologist. Structure, reliability and validity were examined.
Methods
Construction of the TiOS was based on a multidimensional theoretical framework. Cancer patients were surveyed within a week after their consultation. Trust, satisfaction, trust in health care, self-reported health and background variables were assessed. Dimensionality, internal consistency, test–retest reliability and construct validity were investigated.
Results
Data of 423 patients were included (response rate = 65%). After item reduction, the TiOS included 18 items. Trust scores were high. Exploratory factor analysis suggested one-dimensionality. Confirmatory factor analysis nevertheless indicated a reasonable fit of our four-dimensional theoretical model, distinguishing competence, fidelity, honesty and caring. Internal consistency and test–retest reliabilities were high. Good construct validity was indicated by moderate correlations of trust (TiOS) with satisfaction, trust in health care, willingness to recommend and number of consultations with the oncologist. Exploratory analyses suggested significant correlations of trust with ethnicity and age.
Conclusions
The TiOS reliably and validly assesses cancer patients' trust in their oncologist. The questionnaire can be employed in both clinical practice and future research of cancer patients' trust.
Keywords: Trust, Cancer, Oncology, Communication, Doctor–patient relationships, Psychometrics
Introduction
Patients' trust in their physician is considered essential for good quality and effective medical care [1]. Several definitions of trust exist, but they invariably include the aspect of patients' vulnerability [2, 3]. Hall et al. [4] contend that to trust, patients have to optimistically accept their vulnerable situation and believe that the physician will care for their interests. The significance of trust is reflected in an abundance of analytical essays published on the topic (e.g. [5–8]). Yet, empirical research has long lagged behind in both quantity and quality [7]. Over the past decade, increased empirical attention has resulted in more knowledge on patients' trust. Evidence suggests that trusting patients are more satisfied [9, 10], more adherent [11–13], more involved in decision making [11] and less inclined to request a second opinion [10, 14]. Research papers reporting that trust is not associated with presumed beneficial effects, or that strong trust is associated with harmful outcomes, are very scarce. This might, however, be the result of a publication bias [15].
Most of the present evidence on patients' trust originates from correlational research performed in primary care. Within this setting, three different questionnaires have been developed to assess patients' interpersonal trust in their physician [10, 16, 17].
The ‘Trust in Physician Scale’ [16] was developed first, and measures patients' trust in their primary care provider. It assesses patients' perception of the physician's (1) ‘dependability’, i.e. looking out for the patient's best interest, (2) skills and knowledge and (3) provision of reliable information. The ‘Patient Trust Scale’ [17] was constructed subsequently to incorporate changes in the healthcare system, specifically the transition to managed care. Although based on the ‘Trust in Physician Scale’, the scale additionally took into account cost restraints faced by the physician.
The ‘Physician Trust Scale’ [10] was developed most recently and is currently the most widely used instrument to assess patients' trust [2]. The original theoretical model by Hall et al. encompassed five trust dimensions: (1) Fidelity, the physician's pursuit of the patients' interests; (2) Competence, the physician's medical and interpersonal skills; (3) Honesty, telling the truth and avoiding intentional falsehoods; (4) Confidentiality, the physician's adequate handling of sensitive information and (5) Global trust, ‘the irreducible soul of trust, or aspects that combine elements from some or all of the separate dimensions’ ([10]; p.298). The ‘Confidentiality’ dimension was removed after pilot testing, because it lacked relevance to patients. Moreover, empirical data did not support the multidimensional model, but rather suggested that patients' trust was best regarded as one-dimensional.
As all three existing scales were developed in the primary care setting, it is unclear to what extent they accurately assess trust in specific patient populations. Aspects of trust irrelevant to primary care patients could be significant to patients with specific diseases or vice versa [10]. Confidentiality, for example might become more significant for trust in more severe or sensitive circumstances, such as HIV/AIDS or genetics.
For cancer patients, different elements might be relevant for trust than for primary care patients. When confronted with a cancer diagnosis, trust in the oncologist appears to be essential [8, 18]. This necessity may be stronger than in most other conditions, because of the life-threatening nature of cancer. Patients need to cope with a severe diagnosis and intensive treatment which strongly impacts on their daily lives and future. Moreover, they need to grasp complex information and make difficult medical decisions. Consequently, cancer patients' constructions and explanations of trust might differ from trust in other patient populations.
Presently, no measure of trust for oncology patients exists, preventing advances in systematic research of predictors and correlates of cancer patients' trust. Therefore, the aim of this study was to develop a questionnaire which reliably captures cancer patients' trust in their oncologist, the Trust in Oncologist Scale (TiOS), and to assess its psychometric properties, i.e. dimensionality, reliability and validity.
Materials and methods
Construction
In open-ended qualitative interviews with cancer patients [18], we identified aspects defining patients' trust in their oncologist. This resulted in the development of an initial conceptual five-dimensional model of cancer patients' trust, reflecting four of the dimensions of Hall et al. [10], i.e. Competence, Fidelity, Confidentiality and Honesty. In addition, patients' accounts of trust obtained in our qualitative study resulted in the construction of a fifth dimension, which we labelled ‘Caring’. This dimension, added because the existing dimensions did not fully capture patients' constructions of trust, refers to the oncologist's expression of involvement, sympathy and attention to the patient [18].
Item collection and development were performed by two of the authors (MH and ES, both researchers), based on the five-dimensional model. In addition to all ten items of the original ‘Physician Trust Scale’, appropriate items were selected from other trust measures [10, 16, 19, 20] so that at least three items per dimension were included. When not enough items were available, new items were constructed by the first author, based on the results of our qualitative study. Where possible, patients' original wording was used. Newly constructed items were discussed among two of the authors (MH and ES, both researchers), following guidelines for item construction [21]. Existing items phrased in English were translated into Dutch following a forward–backward procedure, involving two forward and two backward translators. The two most general items of Hall et al.'s ‘Global Trust’ dimension were added to the new scale, as overall trust assessments. To allow for variation in responses to the two items, they were modelled as single indicator variables. Item collection resulted in 33 candidate items1.
Wording, relevance and difficulty of items were tested in a pilot sample of 12 patients visiting the Department of Medical Oncology of an academic hospital, using guidelines for cognitive interviewing [22]. Patients commented upon the wording and relevance for trust (content validity) of each item, and on possible relevant additions to the questionnaire. Consequently, minor adjustments were made. No items were removed or added.
Patients and procedure
Cancer patients were recruited from three departments of an academic hospital: Medical Oncology (MO), Radiation Oncology (RO) and Surgery (SG) and from the Department of Surgery at a regional hospital (SR). Inclusion criteria were (1) >18 years of age, (2) a diagnosis of malignant disease and (3) sufficient mastery of the Dutch language. Patients were either in treatment or received regular checkups. According to rules of thumb, a sample size of 10–15 participants per variable is required to perform Confirmatory Factor Analysis (CFA) [23]. After item reduction, we expected to include approximately 20 items in the definitive scale. Because of our intention to split the data into a training and a test set, a minimum of 400 patients (200 for each data set) was required. Two different recruitment procedures were adopted, depending on department policies and preferences: the researcher approached patients either directly by mail within 3 days after their consultation with the oncologist (RO and SG) or after consent was obtained by their attending oncologist (SR and MO). All patients received an introductory letter and the questionnaire, along with a form to indicate refusal to participate. Reminders were sent to non-responders after 3–4 weeks if possible (RO and SR). A subset of 77 patients was asked to complete the questionnaire again 3–4 weeks after the first time, to assess test–retest reliability. This time interval was expected to be long enough to avoid memory bias and short enough to prevent changes in patients' levels of trust (e.g. as a result of new consultations with their oncologist).
Materials
Trust in Oncologist Scale
The initial version of the TiOS contained 33 candidate items. All items consisted of a proposition in the third-person singular, to be answered on a 5-point Likert scale (‘strongly disagree’ = 1 to ‘strongly agree’ = 5). Ten items were negatively phrased. An example of a question is “Your doctor strongly cares about your health”. An overall trust score (range 1–5), was obtained by averaging the responses, with higher scores indicating higher trust.
Background characteristics
Socio-demographics assessed were patients' age, gender, marital status, education level, ethnicity and religion (‘do you consider yourself religious?’). Disease characteristics included time since diagnosis, cancer site and treatments undergone. Questions about the relationship with the caregivers included the number of different oncologists patients had seen, and the number of consultations with their present oncologist.
Satisfaction with physician
Satisfaction with the previous three consultations with the oncologist was measured by the five-item Patient Satisfaction Questionnaire (PSQ) [24]. Responses were marked on a visual analogue scale (‘not at all satisfied’ = 1 to ‘extremely satisfied’ = 10). Internal consistency for the PSQ was strong (Cronbach's α = 0.89). Additionally, one question assessed whether patients would recommend their oncologist to others, rated on a 5-point Likert scale (‘strongly disagree’ = 1, to ‘strongly agree’ = 5).
Trust in Dutch health care system
Patients' general trust in the Dutch health care system was assessed with one item, adapted from a trust in Dutch health care questionnaire [19]. Patients were asked: ‘How much trust do you have in the current Dutch health-care system’, rated on a 5-point Likert scale (‘very little trust’ = 1 to ‘a great deal of trust’ = 5).
Self-reported health status
Self-reported physical and mental health status were measured using a Dutch translation [25] of the 12-item short-form (SF-12) of the Medical Outcomes Study [26]. Internal consistency was strong for both the physical (α = 0.89) and the mental (α = 0.89) component.
Analyses
Unless otherwise indicated, all analyses were performed using SPSS 16 (SPSS Inc., Chicago, IL, USA). Data of participants with more than 25% missing overall or on the TiOS specifically were completely removed. Remaining missing values on the TiOS were replaced using expectation maximization (EM) [27]. Regression analysis was performed to check whether the different acquisition procedures, i.e. via the researcher or via the oncologist, might have induced bias.
To analyse dimensionality, the data was randomly split in two halves: a training set and a test set. First, the original 33-item 5-factor model was tested on the training set with confirmatory factor analysis (CFA), using Lisrel 8.5 (Scientific Software International, Lincolnwood, IL, USA). We calculated χ 2, Root Mean Square Error of Approximation (RMSEA) and Comparative Fit Index (CFI) to test the fit of the model, using Robust Maximum Likelihood estimation. An acceptable model fit would be indicated by χ 2 of >0.05, RMSEA of <0.06 and CFI of >0.95. Second, in case of unsatisfactory fit of the initial model and item set, item reduction would be performed, based on preliminary descriptive characteristics of all items. Items deemed inadequate because of high rates of non-response, low-item scale and test–retest correlations and high skewness were removed. If two similar items had equivalent content and psychometric properties, two authors (MH and ES) selected the most appropriate alternative based on additional criteria, i.e. avoiding confusing or lengthy wording, and patients' additional comments indicating an item's relevance for trust. Third, to examine alternative models of dimensionality, exploratory factor analysis (EFA) was performed on the training set, using the definitive item selection. Outcomes were used to adapt the theoretical dimensional model. Based on the EFA findings, CFA was performed to test the final model, using the test set. Fourth, internal consistency of the TiOS overall and its subscales was calculated using Crohnbach's α, within-item-variance and between-items-covariance. Inter-item correlations between 0.10 and 0.50 were considered acceptable. Item-scale correlations should be >0.20. Correlations between test and retest scores were calculated to assess test–retest reliability. Both internal consistency and test–retest reliability were expected to be high.
Construct validity was assessed by calculating Spearman's correlations between trust (TiOS) and satisfaction (PSQ), trust in health care, willingness to recommend the oncologist to others and number of previous consultations with the oncologist. Moderately positive associations between trust and these correlates would indicate good construct validity [4, 9, 10, 28]. Exploratory analyses were performed to assess Spearman's correlations between trust and patients' age, gender, education, ethnicity, religion, time since diagnosis and physical and mental health.
Results
Descriptives and missing data
Of all 675 patients who received the questionnaires, 506 (75%) responded, 70 (10%) of whom declined participation, resulting in a response rate of 65% (n = 436). Most frequent reasons to decline were (1) too much of a burden (n = 28), (2) not enough contact with the oncologist (n = 12) and (3) poor health (n = 10).
Data of 13 participants with high rates of missing data were completely excluded. For the remaining 0.74% of missing data on the TiOS, EM was performed. Patients' socio-demographic data are displayed in Table 1. The distribution was left-skewed, with high Kurtosis. Most patients currently had one (45%) or two (36%) oncologists presently involved in their cancer care. Regression analysis indicated that the type of recruitment, i.e. by the researcher or the oncologist explained no significant variation in trust scores (0.8%, F(1, 421) = 3.26, p = 0.07).
Table 1.
Demographic, health and relationship characteristics of the sample (n = 423)
| Median (Range) | SD | |
|---|---|---|
| Age (n = 413) | 63 (19–90) | 13 |
| N | % | |
| Gender (n = 423) | ||
| Male | 239 | 57 |
| Female | 184 | 43 |
| Educational level (n = 420) | ||
| None/primary school | 47 | 11 |
| Secondary/lower level vocational school | 248 | 59 |
| College/university | 125 | 30 |
| Ethnicity (n = 421) | ||
| Dutch | 400 | 95 |
| Other, Europe or USA | 7 | 2 |
| Surinamese | 4 | 1 |
| Turkish | 5 | 1 |
| Moroccan | 3 | 1 |
| Other, non-Western country | 2 | 0 |
| Religious (n = 421) | ||
| Yes | 209 | 50 |
| No | 212 | 50 |
| Time since diagnosis (n = 422) | ||
| <1 month | 5 | 1 |
| 1–6 months | 77 | 18 |
| 6–24 months | 148 | 35 |
| >24 months | 192 | 46 |
| Specialist about whom reported (n = 423) | ||
| Radiation oncologist | 148 | 35 |
| Medical oncologist | 133 | 31 |
| Surgeon/urologist | 142 | 34 |
| Self-reported cancer site (n = 419) | ||
| Gastrointestinal | 182 | 43 |
| Genitourinary | 85 | 20 |
| Breast | 84 | 20 |
| Gynecologic | 15 | 4 |
| Bone | 13 | 3 |
| Head/neck | 13 | 3 |
| Lung | 12 | 3 |
| Other | 15 | 4 |
| Number of consultations with present oncologist (n = 419) | ||
| <3 Consultations | 40 | 10 |
| 3–5 Consultations | 112 | 27 |
| 6–10 Consultations | 123 | 29 |
| 11–15 Consultations | 57 | 14 |
| >15 Consultations | 87 | 21 |
Dimensionality and item reduction
-
Step 1
Confirmatory factor analysis of initial model
To address problems of under-identification due to empty cells, response categories 1 (completely disagree) and 2 (disagree) were collapsed, leaving four Likert response categories. Our original theoretically driven 5-factor model did not result in a satisfactory model fit: Satorra-Bentler Scaled χ 2 (476, N = 211) = 1,283.81 (p < 0.001), RMSEA = 0.09 and CFI = 0.94.
-
Step 2
Item reduction and model adjustment
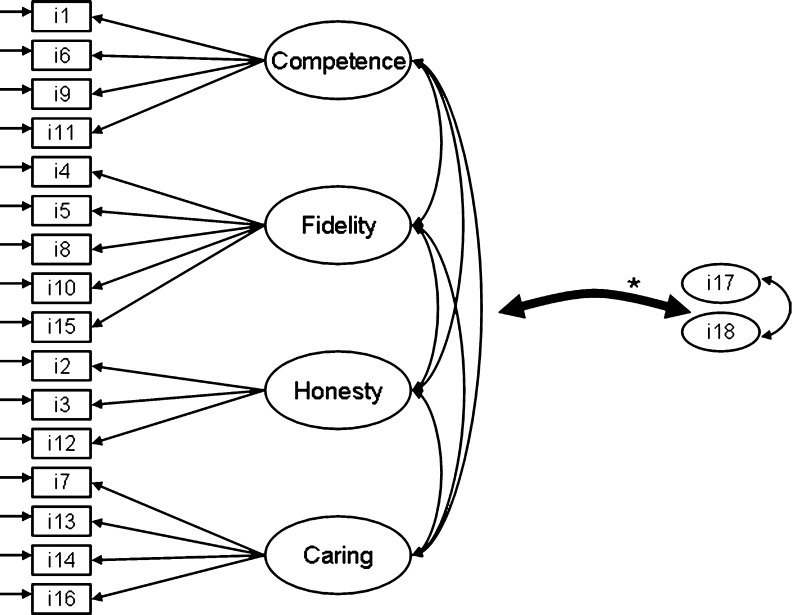
In total, 15 items were removed, resulting in a definitive sample of 18 items, displayed in Table 2 along with their origin and psychometric properties. All three items of the ‘Confidentiality’ dimension were removed because of insufficient psychometric quality, and because patients, in their additional comments to the questionnaire, indicated these items to be irrelevant to trust. Consequently, we re-specified our final model to be four-dimensional (see Fig. 1). The mean overall trust score for the definitive item set (TiOS) was 4.56 (SD = 0.51, range 2.44–5.00).
-
Step 3
Exploratory factor analysis
EFA with direct oblimin rotation (∆ = 0) was conducted for the definitive set of items. Three components were extracted with eigenvalues >1, jointly explaining 61.51% of the variance. Item loadings suggested that component 1, on which all items loaded strongly, represented a general measure of ‘trust’ (eigenvalue = 8.42). Component 2 comprehended negatively phrased items (eigenvalue = 1.48), and component 3 was the least coherent, containing a variety of items (eigenvalue = 1.18). These results suggest that meaningful dimensions of trust could not be distinguished, and that a one-dimensional model of trust might be the most appropriate.
-
Step 4
Testing of definitive model
Based on the EFA outcomes, CFA was used to test a one-dimensional model. This model did not fit the data satisfactory (Satorra-Bentler Scaled χ 2 (134, N = 212) = 210.64 (p < 0.001), RMSEA = 0.05 and CFI = 0.93). Additionally, we tested our definitive, theoretically driven 4-factor model (see Fig. 1). This model fit the data reasonably well (Satorra-Bentler Scaled χ 2 (122, N = 212) = 154.58 (p = 0.03), RMSEA = 0.04 and CFI = 0.95). Standardized factor loadings of the items were adequate (see Table 3). However, correlations between common factors were extremely strong (see Table 4). This, and issues indicating persistent multi-collinearity encountered when running the model, suggest that conceptually, a one-dimensional model best explains our findings.
Table 2.
Overview of all items included in the TiOS, their source, dimension, mean, standard deviation, skewedness and item-scale correlation
| Item | Content | Sourcea | Dimension | Mb | SD | Skewness | Item-scale correlation |
|---|---|---|---|---|---|---|---|
| 1 | Your doctor is very careful and precisec | PTS | Competence | 4.65 | 0.67 | −2.65 | 0.53 |
| 2 | Your doctor is totally honest in telling you about all the different treatment options available for your condition | PTS | Honesty | 4.68 | 0.63 | −2.31 | 0.63 |
| 3 | Your doctor always gives you honest information about your prospects | New | Honesty | 4.58 | 0.77 | −2.28 | 0.56 |
| 4 | Your doctor strongly cares about your healthc | IP PTS | Fidelity | 4.51 | 0.79 | −1.97 | 0.69 |
| 5 | Your doctor always tells you everything you want to know about your illness | MISS | Fidelity | 4.63 | 0.72 | −2.33 | 0.64 |
| 6 | You think your doctor can handle any medical situation, even a very serious onec | IP PTS | Competence | 4.53 | 0.76 | −1.77 | 0.65 |
| 7 | Your doctor always takes his/her time with you | New | Caring | 4.61 | 0.70 | −2.24 | 0.58 |
| 8 | Your doctor explains everything so that you can consent to medical decisions | New | Fidelity | 4.64 | 0.64 | −2.04 | 0.71 |
| 9 | Sometimes you worry that your doctor's medical decisions are wrongd | IP PTS | Competence | 4.37 | 1.09 | −1.87 | 0.54 |
| 10 | Your doctor only thinks about what is best for you | PTS | Fidelity | 4.62 | 0.69 | −2.35 | 0.63 |
| 11 | Sometimes your doctor does not pay full attention to what you are trying to tell him/herd | PTS | Competence | 4.24 | 1.10 | −1.48 | 0.61 |
| 12 | Your doctor would always tell you the truth about your health, even if there was bad newsc | PCAS | Honesty | 4.67 | 0.65 | −2.65 | 0.51 |
| 13 | You have doubts whether your doctor really cares about you as a personc,d | TiPS | Caring | 4.47 | 0.98 | −2.03 | 0.61 |
| 14 | Your doctor listens with care and concern to all the problems you havec | IP PTS | Caring | 4.57 | 0.72 | −1.94 | 0.63 |
| 15 | Your doctor will do whatever it takes to get you all the care you need | PTS | Fidelity | 4.70 | 0.56 | −1.87 | 0.75 |
| 16 | Your doctor is available for you whenever you need him/her | New | Caring | 4.46 | 0.78 | −1.37 | 0.62 |
| 17 | You have no worries about putting your life in your doctor's hands | PTS | Global item | 4.39 | 0.99 | −1.91 | 0.51 |
| 18 | All in all, you have complete trust in your doctor | PTS | Global item | 4.39 | 0.60 | −2.21 | 0.72 |
a PTS Patient Trust Scale, IP PTS Item Pool for PTS, MISS Medical Interview Satisfaction Scale, PCAS Primary Care Assessment Survey, TiPS Trust in Physician Scale
bFive-point likert scale: 1 = totally disagree, 2 = disagree, 3 = as much agree as disagree, 4 = agree, 5 = strongly agree
cMinor modifications to original wording
dReverse-scored items
Fig. 1.
Final measurement model of the definitive four-dimensional theoretical model tested in confirmatory factor analysis. Items 17 and 18 are modelled as single indicator exogenous variables. Asterisk represents all possible correlations between item 17 and item 18 and the common factors
Table 3.
Confirmatory factor analysis of the 4-dimensional conceptual model for the TiOS: standardized factor loadings
| Standardized factor loading | ||||
|---|---|---|---|---|
| Item | 1a | 2 | 3 | 4 |
| Your doctor is very careful and precise (item 1) | 0.77 | |||
| You think your doctor can handle any medical situation, even a very serious one (item 6) | 0.80 | |||
| Sometimes you worry that your doctor's medical decisions are wrong (item 9) | 0.65 | |||
| Sometimes your doctor does not pay full attention to what you are trying to tell him/her (item 11) | 0.69 | |||
| Your doctor strongly cares about your health (item 4) | 0.85 | |||
| Your doctor always tells you everything you want to know about your illness (item 5) | 0.80 | |||
| Your doctor explains everything so that you can consent to medical decisions (item 8) | 0.87 | |||
| Your doctor only thinks about what is best for you (item 10) | 0.83 | |||
| Your doctor will do whatever it takes to get you all the care you need (item 15) | 0.93 | |||
| Your doctor is totally honest in telling you about all the different treatment options available for your condition (item 2) | 0.87 | |||
| Your doctor always gives you honest information about your prospects (item 3) | 0.87 | |||
| Your doctor would always tell you the truth about your health, even if there was bad news (item 12) | 0.72 | |||
| Your doctor always takes his/her time with you (item 7) | 0.83 | |||
| You have doubts whether your doctor really cares about you as a person (item 13) | 0.66 | |||
| Your doctor listens with care and concern to all the problems you have (item 14) | 0.86 | |||
| Your doctor is available for you whenever you need him/her (item 16) | 0.84 | |||
a Factor 1 Competence, factor 2 Fidelity, factor 3 Honesty, factor 4 Caring
Table 4.
Confirmatory factor analysis of the four-dimensional conceptual model for the TiOS: common factor loadings
| Factor | ||||||
|---|---|---|---|---|---|---|
| Competence | Fidelity | Honesty | Caring | Global 1 (item 17) | Global 2 (item 18) | |
| Competence | 1 | |||||
| Fidelity | 1 | 1 | ||||
| Honesty | 0.96 | 0.88 | 1 | |||
| Caring | 0.91 | 0.98 | 0.76 | 1 | ||
| Global 1 (item 17) | 0.86 | 0.78 | 0.80 | 0.74 | 1 | |
| Global 2 (item 18) | 0.93 | 0.92 | 0.82 | 0.92 | 0.85 | 1 |
Reliability
Reliability of the TiOS overall was high (α = 0.92) and ranged from acceptable to good for the subscales: Competence (α = 0.65), Fidelity (α = 0.87), Honesty (α = 0.75) and Caring (α = 0.73). Correlations between all four dimensions and the two global validation items ranged from 0.48 to 0.67. Item–scale correlations ranged from 0.51 to 0.71. Inter-item correlations were acceptable, ranging between 0.2 and 0.6, mostly. Test–retest reliability for the total score was high, r s = 0.93, and ranged between 0.43 and 0.92 for individual items. We found equally high test–retest reliability, ranging between 0.77 and 0.82, for all four dimensions of the TiOS.
Validity
Good construct validity was indicated by significant Spearman's correlations between overall trust and satisfaction (r s (420) = 0.63, p < 0.001), willingness to recommend the oncologist to others (r s (408) = 0.62, p < 0.001), trust in healthcare (r s (423) = 0.24, p < 0.001) and number of consultations (r s (419) = 0.16, p < 0.001) (see Table 5).
Table 5.
Construct validity of the TiOS: means, standard deviations and Spearman's correlation of physician satisfaction, willingness to recommend, trust in health care and number of consultations with overall TiOS score
| Measure (possible range) | M | SD | r s |
|---|---|---|---|
| Trust in oncologist (1–5) | 4.56 | 0.51 | |
| Satisfaction with oncologist (0–500) | 407 | 74 | 0.63a |
| Trust in healthcare (1–5) | 3.93 | 0.77 | 0.24a |
| Willingness to recommend oncologist (1–5) | 4.64 | 0.67 | 0.62a |
| Number of physician consultations | 3.09 | 1.27 | 0.16a |
aSignificant at the 0.001 level
Exploratory analyses
Trust was significantly stronger among older patients (r s (413) = 0.10, p < 0.05) and patients with better mental health (r s (287) = 0.17, p < 0.01). Dutch patients (M = 4.57, SD = 0.51) reported stronger trust than non-Dutch patients (M = 4.34, SD = 0.49), F(421) = 0.90, p < 0.05. Correlations between trust and all other exploratory variables were non-significant (p > 0.05).
Discussion
In this study, the TiOS, an 18-item questionnaire assessing cancer patients' trust in their oncologist, was developed and empirically validated. Our findings suggest that the TiOS reliably and validly assesses cancer patients' trust in their oncologist. In addition to the dimensions distinguished previously, i.e. Fidelity, Competence and Honesty, a new dimension of cancer patients' trust, labelled ‘Caring’, was distinguished and empirically validated in this study. Internal consistency, test–retest reliability and construct validity of the TiOS were good, as indicated by moderate correlations between trust and satisfaction, willingness to recommend the oncologist to others, trust in the health care system and the number of consultations with the oncologist. Exploratory analyses indicated higher trust among older, Dutch patients with better mental health.
Dimensionality analyses lead us to conclude that at least in this heterogeneous sample of cancer patients, trust is very coherent and can therefore be regarded as one-dimensional. At the same time, testing of our theoretical model suggests that patients do distinguish between different aspects contributing to trust, i.e. Competence, Fidelity, Honesty and Caring. This distinction allows for the separate use or investigation of one of these four specific aspects of trust. Depending on the researcher's aims, trust can therefore be represented and used both as a broad, general construct or as a combination of separate dimensions each representing an aspect of trust [29]. Possibly, multi-dimensionality is more pronounced among cancer patients with stronger variations in trust levels, e.g. in palliative care.
The skewed distribution of trust scores is in line with findings in other populations [9, 10, 16, 17]. Indeed, the high trust levels among cancer patients reported here possibly even exceed trust levels reported in the primary care setting [10, 14, 30]. Possibly, such strong trust is induced by the life-threatening nature of cancer. Previous qualitative findings indeed suggest that for these patients, the need to trust their oncologist is strong [18]. Even in a sub-optimal relation with the oncologist, patients might refrain from questioning their trust, thus preventing them from experiencing cognitive dissonance that could arise when they remain with an oncologist they do not trust completely. Salmon and Young [31] suggest that patients with more threatening diseases, such as cancer, might create more positive images of their physicians, reasoning that ‘patients subjectively construct their image of the practitioner to meet their own safety and dependency needs and in line with their own mental models of self and other’ (p. 33). However, this theory is not completely in line with our exploratory finding of stronger trust among patients with better mental health. Research would need to confirm whether trust levels are indeed higher for patients with severe conditions like cancer by comparing different patient populations and with varying health status.
The finding that trust is less strong among patients with non-Dutch ethnicities has been reported previously, both in primary care and in the oncology setting [28, 30, 32–34]. Previous studies were mostly performed in the USA, and report lower trust among African American than among Caucasian patients. In the present study, trust was lower among non-Dutch patients from both Western and non-Western countries. However, the relative scarcity of non-Dutch patients in our sample and the exploratory nature of this analysis warrant further investigation of the relationship between trust and ethnicity. Such research should preferably not only focus on trust levels, but also on differences in constructions and explanations of trust.
The skewed distribution of trust scores raises the question whether these high scores accurately reflect very strong levels of trust or result from a measurement limitation. It would be worthwhile to first investigate whether, among specific groups of cancer patients, TiOS scores are less skewed. Such research could be conducted in samples where lower trust might reasonably be expected, e.g. immigrants or patients requesting second opinions. A lack of more variability in TiOS scores among such samples would warrant further explorations of possibly more sensitive research methods. More variability in trust levels could possibly be registered with different item wordings or other response formats (e.g. asymmetric answer formats). Alternatively, researchers might resort to different methods, such as unobtrusive measures, in order to avoid patients' conscious processes and cognitions [35]. Presently, the quantitative, self-report, nature of the TiOS prohibits conclusions about patients' underlying processes during questionnaire completion. As such, the TiOS might be assessing patients' determination to trust the oncologist, rather than their actual basic trust.
In addition to these considerations about skewness, this study has some limitations. First, although construct validity of the TiOS was addressed, more research would be needed to assess how TiOS scores relate to relevant clinical and/or patient-reported outcomes, thus establishing predictive validity. Second, the TiOS was presently only validated in a sample of Dutch cancer patients. Further validation should include administration of the scale among patients in other countries. A final important consideration is the scope of the questionnaire. As the oncology setting is becoming increasingly multidisciplinary, patients are confronted with treatment by an interdisciplinary team of health care professionals. The oncologist is no longer by definition the central carer. Indeed, in patients' perceptions, there might not be a central care provider at all [36]. Patients' additional comments in the survey suggest that, consequently, some patients find it easier to report on the care delivered by the entire team, than by the oncologist specifically. It is worth investigating whether and how increased multi-disciplinarity in oncology care impacts on patients' interpersonal and more general trust.
In conclusion, the current study has contributed to the study of cancer patients' trust in their oncologist by enabling the accurate assessment of trust in the cancer patient population. We think that the TiOS improves further expansion of this field of study, resulting in better insight into the nature, predictors and consequences of these patients' trust, which could ultimately improve patient care.
Acknowledgements
We would like to acknowledge all the physicians of the Departments of Clinical Oncology, Radiation Oncology and Surgery, as well as the nurses of the Department of Surgery at the AMC and Mariëtte Weterman for their assistance in the recruitment of patients. We are grateful to Phyllis Butow for her constructive comments during the analysis and reporting phase. This study was supported by the Dutch Cancer Society [grant number: UVA 2008-4015].
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Readers interested in all candidate items, please contact the authors.
References
- 1.Mechanic D, Meyer S. Concepts of trust among patients with serious illness. Soc Sci Med. 2000;51(5):657–668. doi: 10.1016/S0277-9536(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 2.Goudge J, Gilson L. How can trust be investigated? Drawing lessons from past experience. Soc Sci Med. 2005;61:1439–1451. doi: 10.1016/j.socscimed.2004.11.071. [DOI] [PubMed] [Google Scholar]
- 3.McKinstry B, Ashcroft RE, Car J, Freeman GK, Sheikh A (2006) Interventions for improving patients' trust in doctors and groups of doctors. Cochrane Database Syst Rev 3: Art. No.: CD004134 [DOI] [PubMed]
- 4.Hall MA, Dugan E, Zheng BY, Mishra AK. Trust in physicians and medical institutions: what is it, can it be measured, and does it matter? Milbank Q. 2001;79(4):613–639. doi: 10.1111/1468-0009.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baier A. Trust and antitrust. Ethics. 1986;96:231–260. doi: 10.1086/292745. [DOI] [Google Scholar]
- 6.Goold SD. Trust, distrust and trustworthiness. J Gen Intern Med. 2002;17(1):79–81. doi: 10.1046/j.1525-1497.2002.11132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson SD, Raeke LH. Patients' trust in physicians: many theories, few measures, and little data. J Gen Intern Med. 2000;15(7):509–513. doi: 10.1046/j.1525-1497.2000.11002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seetharamu N, Iqbal U, Weiner JS. Determinants of trust in the patient-oncologist relationship. Palliat Support Care. 2007;5(4):405–409. doi: 10.1017/S1478951507000600. [DOI] [PubMed] [Google Scholar]
- 9.Thom DH, Ribisl KM, Stewart AL, Luke DA. Further validation and reliability testing of the Trust in Physician Scale. Med Care. 1999;37(5):510–517. doi: 10.1097/00005650-199905000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Hall MA, Zheng BY, Dugan E, et al. Measuring patients' trust in their primary care providers. Med Care Res Rev. 2002;59(3):293–318. doi: 10.1177/1077558702059003004. [DOI] [PubMed] [Google Scholar]
- 11.Trachtenberg F, Dugan E, Hall MA. How patients' trust relates to their involvement in medical care. J Fam Pract. 2005;54(4):344–352. [PubMed] [Google Scholar]
- 12.Safran DG, Taira DA, Rogers WH, et al. Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220. [PubMed] [Google Scholar]
- 13.O'Malley AS, Sheppard VB, Schwartz M, Mandelblatt J. The role of trust in use of preventive services among low-income African-American women. Prev Med. 2004;38(6):777–785. doi: 10.1016/j.ypmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Balkrishnan R, Dugan E, Camacho FT, Hall MA. Trust and satisfaction with physicians, insurers, and the medical profession. Med Care. 2003;41(9):1058–1064. doi: 10.1097/01.MLR.0000083743.15238.9F. [DOI] [PubMed] [Google Scholar]
- 15.Hillen MA, de Haes HC, Smets EM. Cancer patients' trust in their physician—a review. Psychooncology. 2011;20:227–241. doi: 10.1002/pon.1745. [DOI] [PubMed] [Google Scholar]
- 16.Anderson AA, Dedrick FD. Development of the trust in physician scale: a measure to assess interpersonal trust in patient–physician relationships. Psychol Rep. 1990;67:1091–1100. doi: 10.2466/pr0.1990.67.3f.1091. [DOI] [PubMed] [Google Scholar]
- 17.Kao AC, Green DC, Zaslavsky AM, Koplan JP, Cleary PD. The relationship between method of physician payment and patient trust. JAMA. 1998;280(19):1708–1714. doi: 10.1001/jama.280.19.1708. [DOI] [PubMed] [Google Scholar]
- 18.Hillen MA, Onderwater AT, Van Zwieten MCB, de Haes JC, Smets EM (2011) Disentangling cancer patients’ trust in their oncologist: a qualitative study. Psychooncology (in press) [DOI] [PubMed]
- 19.Wolf MH, Putnam SM, James SA, Stiles WB. The medical interview satisfaction scale: development of a scale to measure patient perceptions of physician behavior. J Behav Med. 1978;1(4):391–401. doi: 10.1007/BF00846695. [DOI] [PubMed] [Google Scholar]
- 20.Safran DG, Kosinski M, Tarlov AR, et al. The primary care assessment survey—tests of data quality and measurement performance. Med Care. 1998;36(5):728–739. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Oosterveld P, Vorst HCM (1998) Constructie van meetinstrumenten. Testleer en testconstructie, In: Van Den Brink WP and Mellenbergh GJ (Eds), Amsterdam, Boom pp 305–337
- 22.Willis G (1999) Cognitive Interviewing. A ‘How To’ Guide. Research Triangle Park, NC, Research Triangle Institute
- 23.Tabachnick BG, Fidell LS. Using multivariate statistics. 4. Allyn and Bacon: Boston; 2001. [Google Scholar]
- 24.Ong LML, Visser MRM, Lammes FB, de Haes JCJM. Doctor-patient communication and cancer patients' quality of life and satisfaction. Patient Educ Couns. 2000;41:145–156. doi: 10.1016/S0738-3991(99)00108-1. [DOI] [PubMed] [Google Scholar]
- 25.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. J Clin Epidemiol. 1998;51(11):1171–1178. doi: 10.1016/S0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary test of reliability and validity. Med Care. 1996;34:220–226. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Tabachnick BG, Fidell LS (2000) Cleaning up your act: screening data prior to analysis. In Using multivariate statistics, 4th ed. Allyn and Bacon pp 56–110
- 28.Carpenter WR, Godley PA, Clark JA, et al. Racial differences in trust and regular source of patient care and the implications for prostate cancer screening use. Cancer. 2009;115(21):5048–5059. doi: 10.1002/cncr.24539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall GN, Hays RD, Sherbourne CD, Wells KD. The structure of patient satisfaction with outpatient medical care. Psychol Assess. 1993;5(4):477–483. doi: 10.1037/1040-3590.5.4.477. [DOI] [Google Scholar]
- 30.Doescher MP, Saver BG, Franks P, Fiscella K. Racial and ethnic disparities in perceptions of physician style and trust. Arch Fam Med. 2000;9:1156–1163. doi: 10.1001/archfami.9.10.1156. [DOI] [PubMed] [Google Scholar]
- 31.Salmon P, Young B. Dependence and caring in clinical communication: the relevance of attachment and other theories. Patient Educ Couns. 2009;74(3):331–338. doi: 10.1016/j.pec.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berrios-Rivera JP, Street RL, Popa-Lisseanu MGG, et al. Trust in physicians and elements of the medical interaction in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 2006;55(3):385–393. doi: 10.1002/art.21988. [DOI] [PubMed] [Google Scholar]
- 33.Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Rep. 2003;118:358–365. doi: 10.1016/S0033-3549(04)50262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon HS, Street RL, Jr, Sharf BF, Kelly PA, Souchek J. Racial differences in trust and lung cancer patients' perceptions of physician communication. J Clin Oncol. 2006;24(6):904–909. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 35.De Houwer J, Teige-Mocigemba S, Spruyt A, Moors A. Implicit measures: a normative analysis and review. Psychol Bull. 2009;135(3):347–368. doi: 10.1037/a0014211. [DOI] [PubMed] [Google Scholar]
- 36.Epner DE, Ravi V, Baile WF (2010) “When patients and families feel abandoned”. Support Care Cancer doi:10.1007/s00520-010-1007-6 [DOI] [PubMed]