Abstract
We cloned the complete coding sequences of porcine Gpr3, Gpr6, and Gpr12 genes. Further, on the basis of their high levels of sequence similarity, these genes are identified as a subfamily of G protein-coupled receptors. These putative protein sequences also showed high sequence identity with other mammalian orthologs, including several highly conserved motifs. A wide expression of the Gpr3 gene in pigs was observed through tissue distribution analysis by reverse transcriptase-polymerase chain reaction (RT-PCR) and real-time PCR, specially in the brain, pituitary, fat, liver and oocyte, where its strong expression was observed. The Gpr3 gene was found to be located on chromosome 6 and a single exon coded for the entire open-reading frame. Expression of porcine Gpr3 in HEK293 cells resulted in constitutive activation of adenylate cyclase (AC) similar in amplitude to that produced by fully stimulated Gs-coupled receptors. Moreover, sphingosine 1-phosphate (S1P) could increase AC activation via the constitutively active Gpr3 receptor. When a Gpr3-green fluorescent protein (GFP) construct was expressed in HEK293 cells, GFP-labeled Gpr3 protein was shown to be localized in the plasmalemma and subcellular membranes. After S1P treatment, agonist-mediated internalization could be visualized by confocal microscopy. In short, our findings suggest the porcine Gpr3, Gpr6, and Gpr12 genes as a subfamily of G protein-coupled receptors, and porcine Gpr3 was a constitutively active G protein-coupled receptor. Constitutive activation of AC and agonist-mediated internalization of Gpr3 receptor could be modulated by the S1P, suggesting that S1P might act as an activator for porcine Gpr3 receptor.
Keywords: G protein-coupled receptor, Constitutive activity, Sphingosine 1-phosphate, Receptor internalization, Porcine Gpr3, Molecular cloning
1. Introduction
G protein-coupled receptors (GPCRs), as the largest family of cell-surface receptors, respond to a diverse array of ligands and mediate cellular responses to hormones, peptides, lipids, and neurotransmitters, as well as the senses of smell and taste (Whorton et al., 2007). Identification of novel GPCRs and their endogenous ligands can help to get insight into different physiological processes and pathophysiological disorders (Howard et al., 2001). The GPCR Gpr3 is a constitutive activator of intracellular cyclic adenosine monophosphate (cAMP) (Hinckley et al., 2005; Tanaka et al., 2007), which is predominantly expressed in mammalian brain areas and gonads related to promoting neurite outgrowth, emotional-like behaviors (Tanaka et al., 2007; Valverde et al., 2009), and maintenance of meiotic prophase arrest in oocytes (Hinckley et al., 2005). Devoid of a natural ligand, it is currently classified as orphan GPCRs. It is reported that the closest relatives of Gpr3 are lipid and peptide receptors (Joost and Methner, 2002). Moreover, sphingosine 1-phosphate (S1P) and sphingosylphosphorylcholine (SPC) have been respectively identified as the high-affinity ligands for Gpr6 and Gpr12 (Uhlenbrock et al., 2002; Ignatov et al., 2003a; 2003b), which share high levels of sequence identity with Gpr3. S1P and SPC are the members of lysophospholipids.
The lysophospholipids were originally described as intracellular second messengers, but, recently, they had been recognized as extracellular mediators for GPCRs (Pyne and Pyne, 2000). S1P is an important bioactive lysophospholipid mediator generated from membrane phospholipids or in a de novo anabolic sequence of reactions. S1P also induces a broad range of biological responses, including cell growth, differentiation, migration, apoptosis (Clemens et al., 2003), and angiogenesis (Murakami et al., 2010). Its production is catalyzed by sphingosine kinase, while degradation is either via cleavage to produce palmitaldehyde and phosphoethanolamine or by dephosphorylation (Pyne and Pyne, 2000). So far, a specific subset of the endothelial differentiation gene (Edg) family, including the S1P1 (formerly Edg-1), S1P2 (Edg-5), S1P3 (Edg-3), S1P4 (Edg-6), and S1P5 (Edg-8) receptors, has been certified to be activated by the S1P (Clemens et al., 2003). Recently, some additional GPCRs responding to lipid-like mediators have been identified. It was found that ovarian cancer GPCR1 (OGR1) and Gpr12 were activated by SPC (Xu et al., 2000), Gpr6 was stimulated by S1P (Ignatov et al., 2003b), and G2A receptor responded to lysophosphatidylcholine (LPC) (Kabarowski et al., 2001). Vast numbers of biologically active lipids and the recent pairings of such lipids with orphan GPCRs suggest that several additional, not yet identified, orphan lipid GPCRs may exist.
In this study, we first cloned the complete coding sequences of Gpr3, Gpr6, and Gpr12 genes from porcine ovary using reverse transcriptase-polymerase chain reaction (RT-PCR) method, and their putative protein sequences were analyzed by bioinformatic methods. We reported the tissue distribution and subcellular localization of porcine Gpr3 receptor, and showed that the heterologous expression of porcine Gpr3 in HEK293 cells resulted in constitutive activation of adenylyl cyclase which was further activated by S1P. In addition, S1P treatment of transfected cells also internalized the Gpr3 receptor to the intracellular compartments.
2. Materials and methods
2.1. Cell culture
Human embryonic kidney cells (HEK293) were cultured at 37 °C in a humidified 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Shanghai Invitrogen Biotechnology Co., Ltd., China) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco), 100 U/ml penicillin, and 100 U/ml streptomycin (Sigma, Shanghai, China).
2.2. Molecular cloning and plasmid constructions
By searching the porcine genomic database (GenBank) with the human Gpr3, Gpr6, and Gpr12 sequences (accession Nos. NM_005281, NM_005284.3, and NM_005288.3), the porcine counterparts of Gpr3, Gpr6, and Gpr12 were identified, respectively. To verify and clone the cDNA sequences of the three receptors, the fragments of porcine Gpr3 (P31, P32), Gpr6 (P61, P62), and Gpr12 (P121, P122), including their complete coding sequences, were amplified by PCR from porcine ovarian complementary DNA (cDNA) and genomic DNA extracted with TIANamp Genomic DNA kit (TIANGEN, Beijing, China) following manufacturer’s instructions. The predominant PCR product was purified, subsequently ligated into pMD19-T vector (TaKaRa, Dalian, China), and then was sequenced on an ABI 7300 sequencer (Invitrogen, Shanghai, China). Primers were listed in Table 1. After the subsequent bioinformatic analysis, the full-length coding region of Gpr3 amplified by using the primers 5′-GCCGGTACCATGATGTGGGGTGCAG-3′ (a KpnI site is underlined) and 5′-GCCCTCGAGCTAGACGTCACTGGGAGAAC-3′ (an XhoI site is underlined) was subcloned into pcDNA 3.1 (5′-KpnI and 3′-XhoI sites) for its expression in mammalian cells, namely pcDNA-Gpr3. To obtain a C-terminal Gpr3-green fluorescent protein (GFP) construct, the coding region of Gpr3 was amplified from pcDNA-Gpr3 plasmid DNA using the primers 5′-GCCCTCGAGATGATGTGGGGTGCAG-3′ (an XhoI site is underlined) and 5′-GCGGGATCCAAGACGTCACTGGGAGAAC-3′ (a BamHI site is underlined), simultaneously excluding the stop codon (LA Taq polymerase, TaKaRa, China). The PCR product was subcloned into the fusion vector pEGFP-N1 (5′-XhoI and 3′-BamHI sites), namely pEGFP-Gpr3. All constructs were sequenced to ensure correct insertion and exclude frame shifts or point mutations.
Table 1.
Primer sequences of Gpr3, Gpr6, Gpr12, and GAPDH in pigs
| Primer | Sequence | AT (°C) | PS (bp) |
| P31 | 5′-ATGATGTGGGGTGCAGGCA-3′ | 55.0 | 1283 |
| P32 | 5′-AGGGACTTGGAATGGCACTGTA-3′ | ||
| P61 | 5′-ACACCTGACCCTTGTCCTCCCT-3′ | 59.5 | 1399 |
| P62 | 5′-TGCCACATCCTGAACACCTCC-3′ | ||
| P121 | 5′-GGGGTTAAAATGAATGAAGAC-3′ | 56.3 | 1157 |
| P122 | 5′-CAAATGAACGCTTCTCCAA-3′ | ||
| P33 | 5′-CCTTGTGTCCTGTGAGAATG-3′ | 57.0 | 738 |
| P34 | 5′-GCGTAGATAATGGGGTTGA-3′ | ||
| qP31 | 5′-CTTTGCTGCTGTCTTCTG-3′ | 58.0 | 159 |
| qP32 | 5′-GTGGTCTCTGAGTAGTAGG-3′ | ||
| qPG1 | 5′-GGACTCATGACCACGGTCCAT-3′ | 58.0 | 220 |
| qPG2 | 5′-TCAGATCCACAACCGACACGT-3′ |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; AT: annealing temperature; PS: product size
2.3. Tissue distribution of porcine Gpr3 gene
Tissue samples, including the cerebellum, hypothalamus, cerebrum, pituitary, heart, spleen, lung, kidney, fat, liver, ovary, testis, uterus, and oocytes, were obtained from three adult commercial pigs provided by Changxing Ecological Agriculture Co., Ltd., China. Total RNA was extracted from the samples using TRIzol reagent (Invitrogen, California, USA) and treated with DNase I (RNase-free) (TaKaRa, Dalian, China) to remove genomic DNA. The RNA samples from the same tissues were mixed into a RNA-pool and reverse-transcribed using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, Madison, USA) and oligo(dT)18. The primers for amplifying a 738-bp Gpr3 cDNA fragment were P33 and P34 (listed in Table 1). The PCR was performed as follows: denaturation at 95 °C for 5 min, followed by 29 cycles at 94 °C for 30 s, 57 °C for 30 s, 72 °C for 45 s, and a final extension of 72 °C for 7 min. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), served as a loading control, was amplified with the primers of qPG1 and qPG2 (Table 1). The resulting PCR products were analyzed by agarose gel electrophoresis to determine the tissue distribution of porcine Gpr3 gene.
To further examine the tissue expression levels of porcine Gpr3 gene, real-time PCR was employed according to our previous studies (Zhang et al., 2012). The primer sequences (qP31/qP32 and qPG1/qPG2) were commercially synthesized (Invitrogen, Shanghai, China), as listed in Table 1.
2.4. Transfection and subcellular localization of porcine Gpr3 gene
HEK293 cells were grown on glass coverslips coated with polyamino acids in six-well plates to 40%‒50% confluence, and then transiently transfected with 2 μg indicated plasmids (pEGPF-N1 or pEGFP-Gpr3) using the Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s instructions. Forty-eight hours after transfection, cells were washed with phosphate buffered saline (PBS) three times, fixed in 4% (v/v) paraformaldehyde for 20 min, washed three times again with PBS, and then were preincubated with PBS supplemented with 0.05% (v/v) Triton X-100 for 30 min at room temperature to permeabilize the cells in order to allow 4′,6′-diamidino-2-phenylindole (DAPI, Sigma, China) penetration for the detection of nucleus. After three times washing with cold PBS, slides were incubated with 0.1 μg/ml DAPI for 15 min at room temperature in dark. Then, the slide was washed with cold PBS three times again and mounted using Vectashield. GFP/DAPI fluorescence was visualized using a Zeiss LSM510 confocal microscope (Zeiss, Jena, Germany) with a digital camera using the fluorescein isothiocyanate (FITC) filter (488 nm)/DAPI filter (405 nm) and the 40× water immersion objective lens.
2.5. Radioimmunoassay (RIA) of cAMP
HEK293 cells were initially seeded into twelve-well plates, then transiently transfected with pcDNA 3.1, pcDNA-Gpr3, and pEGFP-Gpr3 (50% confluence, 2 μg/well, Lipofectamine 2000), respectively. Forty-eight hours after transfection, cells were cultured in serum-free medium for 1 h, and incubated for 30 min at 37 °C in 2 ml Hanks’ balanced salt solution (HBSS)/4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (55.17 mmol/L NaCl, 2.13 mmol/L KCl, 1.67 mmol/L NaHCO3, 0.18 mmol/L KH2PO4, 0.14 mmol/L Na2HPO4, 2.22 mmol/L D-glucose, 30 mmol/L HEPES, containing 0.5 mmol/L 3-isobutyl-1-methylxanthine (IBMX) and 0.1% bovine serum albumin (BSA)). Then, the cells in one well of the plate were incubated in 0, 0.1, 1, and 10 μmol/L S1P (Tocris, Shanghai, China), 1 μmol/L sphingosine (Sph, Sigma, China), and 1 μmol/L sphingomyelin (Sphi, Sigma, China) for several hours (37 °C, 5% CO2) to detect its activation effect. A concentration of 0.1 mmol/L forskolin in 2 ml HBSS/HEPES buffer was used to incubate the wild-type cells as positive control. Discarding the buffer, 1 ml of 1 mol/L cold HClO4 was added to stop the accumulation of cAMP, and then 200 μl of 2 mol/L K2CO3 was added to neutralize the reaction, and then bathed on ice for 10 min. Transfected cells were scraped into a fresh centrifuge tube, centrifuged at 2 000×g for 10 min at 4 °C. The supernatant was used to measure the cAMP levels using [125I] cAMP RIA kits (Shanghai University of Traditional Chinese Medicine, China) by RIA, according to the manufacturer’s instructions. The amount of bound radioactivity was determined by a γ-counter.
2.6. Internalization studies
The protocol for receptor internalization studies was similar to the method used in previous reports (Uhlenbrock et al., 2002). Briefly, HEK293 cells were seeded on glass coverslips in six-well plates, and then were transiently transfected with pEGPF-N1 and pEGFP-Gpr3 (50% confluence, 2 μg/well, Lipofectamine 2000), respectively. Six hours after transfection, cells were incubated in the medium with 10% charcoal-stripped FBS, which is deficient in serum-borne lipids. Forty-eight hours after transfection, cells were set to fresh serum-free medium for 60 min and were treated with 1 μmol/L S1P, Sph, and Sphi for several minutes (37 °C, 5% CO2). After the treatment, cells were washed three times with PBS, fixed in 4% paraformaldehyde, counterstained with DAPI, and then were examined by a Zeiss confocal microscope.
2.7. Statistical analysis
All data were expressed as mean±standard deviation (SD). The independent-samples t-test was used to evaluate the statistical significance of the difference between two groups, and one-way analysis of variance (ANOVA) and Tukey’s post-hoc tests were used to evaluate the statistical significance of the differences among more than two groups. SPSS Version 18.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Differences at P<0.05 were considered significant. Each experiment was repeated at least three times.
3. Results
3.1. Cloning and bioinformatic analysis
We cloned and sequenced the fragments of the three receptors, and then did sequence alignments with the porcine genomic DNA sequences from GenBank (accession Nos. CU469239.2, FP102331.2, and CU468407.2). We found that the sequences obtained from porcine ovarian cDNA of three receptors are all fully consistent with the one obtained from genomic DNA, suggesting that all three fragments, including the entire open-reading frame (ORF), are located in a single exon. Analyses of the partial cDNA sequences of the porcine Gpr3, Gpr6, and Gpr12 revealed the following: (1) the partial cDNA of porcine Gpr3 consists of 1 283 bp that contained an ORF of 993 bp encoding a protein of 331 residues with an isoelectric point of 7.70 and a calculated molecular mass of 35.2 kDa, having a 3′-untranslated region (3′-UTR) of 290 bp (Fig. 1a). The newly cloned porcine Gpr3 exhibits 94.9% and 90% identity to its human and mouse orthologs at amino acid levels. Porcine genomic database searches revealed that the porcine Gpr3 gene is located on chromosome 6. The short amino terminus of porcine Gpr3 contained no signal-peptide sequence. PSORTII prediction analysis (Nakai and Horton, 1999) indicated that the Gpr3 protein resides existed predominantly in the plasma membrane. (2) The partial cDNA of porcine Gpr6 consists of 1 399 bp that contains a 1 101-bp ORF flanked by a 51-bp 5′-UTR and 247-bp 3′-UTR, which encodes a 367-amino acid polypeptide with isoelectric point of 7.63 and molecular weight of 38.4 kDa. The putative amino acid of porcine orthologs shared 95.1% and 93.7% homology with those of human and mouse, respectively. Porcine genomic database searches revealed that the porcine Gpr6 gene is located on chromosome 1. (3) The partial cDNA of porcine Gpr12 consists of 1 157 bp; computer analysis revealed a 1 005-bp ORF flanked by a 9-bp 5′-UTR and 143-bp 3′-UTR. The porcine Gpr12 gene is predicted to encode a polypeptide of 335 amino acids with isoelectric point of 7.22 and molecular mass of 36.7 kDa. The putative amino acid of porcine orthologs shared 95.8% and 93.8% homology with those of human and mouse, respectively. Porcine genomic database searches revealed that porcine Gpr12 gene is located on chromosome 11. The nucleotide sequence data of porcine Gpr3, Gpr6, and Gpr12 were submitted to GenBank (accession Nos. HM777009, HM777010, and HM77711, respectively).
Fig. 1.
Bioinformatic analysis of porcine Gpr3, Gpr6, and Gpr12
(a) Nucleotide and putative amino acid sequences of porcine Gpr3. The start (ATG) and stop (TAG) codons are in bold. Regions corresponding to the putative transmembrane domains TM1–TM7 are underlined. (b) Multiple primary sequence alignment of Gpr3, Gpr6, and Gpr12 including consensus sequence and putative transmembrane domains (in the box) indicating 57%–58% sequence identity
A primary sequence alignment (Fig. 1b) of the respective coding region of three receptors revealed 57%–58% sequence identity to each other at the amino acid levels. Transmembrane region predictions made by the TMHMM program (Moller et al., 2001) showed that the similar seven putative hydrophobic transmembrane domains (boxed in Fig. 1b) were present in the three receptors, a characteristic feature of members of the GPCR family. In addition, the DRY (Asp-Arg-Tyr) motif located at the interface of transmembrane domain III and the second intracellular loop of the three receptors represented the most highly conserved sequence element in the family of GPCRs (Uhlenbrock et al., 2002), which was also considered as a mutation target for rendering receptors constitutively active (Alewijnse et al., 2000).
3.2. Tissue distribution of the porcine Gpr3 gene
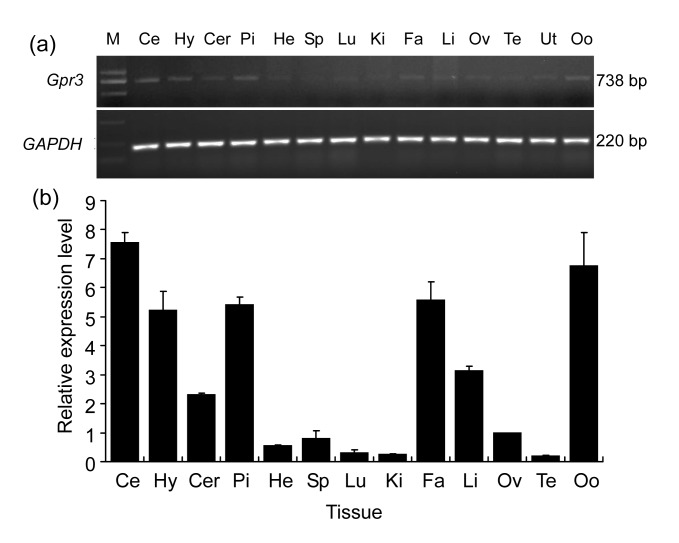
To investigate the tissue distribution of porcine Gpr3 gene, we performed RT-PCR and real-time PCR on a panel of porcine-tissue cDNAs. The housekeeping gene, GAPDH, was used for endogenous control. As shown in Fig. 2a, the transcript of Gpr3 was detectable in all the samples (cerebellum, hypothalamus, cerebrum, pituitary, heart, spleen, lung, kidney, fat, liver, ovary, testis, uterus, and oocyte), whereas was only strongly expressed in the brain (including cerebellum, hypothalamus, cerebrum), pituitary, fat, liver, and oocyte (Fig. 2b).
Fig. 2.
Expression profiles of Gpr3 mRNA in different porcine tissues
(a) Tissue distribution of Gpr3 mRNA was analyzed by RT-PCR. Amplification of GAPDH served as loading control. (b) Expression level of Gpr3 mRNA was analyzed by real-time PCR (Zhang et al., 2012). Mean value of three experiments in ovary was set as 1. Graph shows mean±SD (n=3). Ce: cerebellum; Hy: hypothalamus; Cer: cerebrum; Pi: pituitary; He: heart; Sp: spleen; Lu: lung; Ki: kidney; Fa: fat; Li: liver; Ov: ovary; Te: testis; Ut: uterus; Oo: oocyte
3.3. Subcellular localization of porcine Gpr3 protein
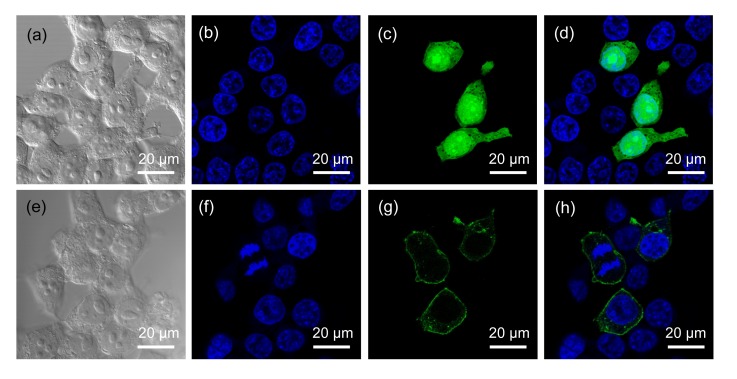
To determine the subcellular localization of porcine Gpr3 protein, we transfected the pEGFP-Gpr3/pEGFP-N1 plasmids into HEK293 cells and examined them under a confocal fluorescence microscope. HEK293 cells expressing the control plasmid pEGFP-N1 exhibited a strong green fluorescent signal that could be seen throughout the transfected cells, and there was no specific subcellular localization (Figs. 3c and 3d in p.563). In contrast, transfecting the cells with the pEGFP-Gpr3 construct showed that the green fluorescent product Gpr3-GFP was predominantly localized in the plasma membrane (Figs. 3g and 3h), corresponding to the result of bioinformatic analysis. However, a significant fraction of Gpr3-GFP fluorescence was also observed in punctate, intracellular vesicles. This pattern of receptor localization is similar to that of wild-type Gpr3 in mouse oocytes, as determined by indirect immunofluorescence analysis (Ledent et al., 2005).
Fig. 3.
GFP directed by the Gpr3 N-terminus to the plasmalemma
HEK293 cells were plated at a density of 2×105 cells/ml on glass coverslips coated with polyamino acids. The cells were transfected with 1 μg/ml pEGFP-N1 (a‒d) and pEGFP-Gpr3 (e‒h) by using the transfection reagent Lipofectamine 2000 according to the manufacturer’s protocol. (b, f) After 48 h of transfection, the cells were fixed in 4% paraformaldehyde and then counterstained with DAPI (blue). (c, g) The cells were examined by confocal microscopy to verify expression of the GFP protein (green). (a, e) The images were visualized by phase-contrast microscopy and clearly show the nuclei and cytoplasm of the cells. (d, h) Composite images, respectively, of (b, c) and (f, g)
3.4. Effect of plasmids on Gpr3 expression
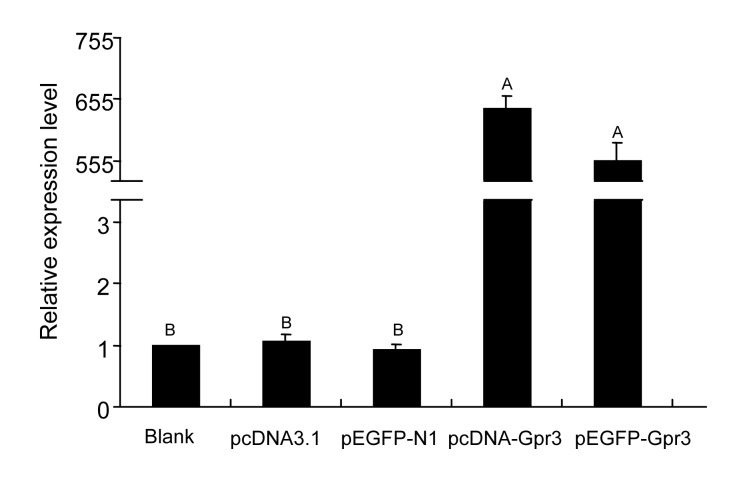
In order to build the model of Gpr3 receptor overexpression in HEK293 cells, the plasmids including pcDNA3.1, pcDNA-Gpr3, pEGFP-N1, and pEGFP-Gpr3 were transfected into the cells. Expression level of Gpr3 mRNA was analyzed by real-time RT-PCR at post-transfection, as described in Fig. 4. The results in Fig. 4 showed that the Gpr3 mRNA overexpressed after transfection with pcDNA-Gpr3 and pEGFP-Gpr3, the Gpr3 transcript level being increased by approximately 600-fold compared with that in the control cultures (blank, pcDNA-Gpr3, and pEGFP-N1).
Fig. 4.
Effect of plasmids on the level of Gpr3 mRNA detected after transfection for 48 h by real-time RT-PCR
Relative mRNA expression analyses were performed using the 2−ΔΔCt method. The expression levels of Gpr3 mRNA were normalized to the blank group. The data are presented as mean±SD of three independent experiments. Bar with different uppercase is very significantly different (P<0.01)
3.5. Effect of exogenous S1P on adenylate cyclase (AC) activation via the constitutively active porcine Gpr3 receptor
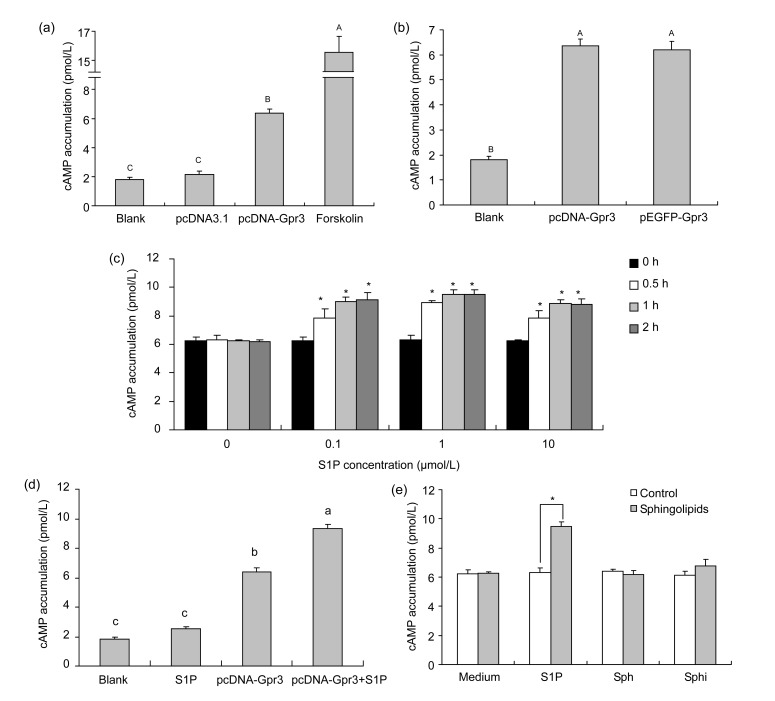
To see if porcine Gpr3 was a constitutively active GPCR, we assayed the functional cAMP in HEK293 cells after transfection. Transfection of empty vector pcDNA3.1 did not significantly change the level of cAMP as compared with the wild-type cell group (P>0.05), suggesting that the transfection process itself did not affect cAMP accumulation (Fig. 5a). In the absence of any ligands, the cAMP level in pcDNA-Gpr3 transfected cells was dramatically elevated (P<0.01) as compared with empty vector transfected cells, indicating that the Gpr3 receptor was constitutively coupled to Gs (Fig. 5a).
Fig. 5.
Increased cAMP levels in HEK293 cells expressing Gpr3
(a) HEK293 cells transiently transfected with pcDNA-Gpr3 vector showed constitutive activation of AC. Empty vector pcDNA3.1 was used as a control. (b) Constitutive activation of AC via the Gpr3-GFP fusion protein, proving functionality of the construct similar to that of pcDNA-Gpr3. (c) Gpr3-transfected cells were incubated for additional hours in the absence or presence of S1P and were collected as above for cAMP measurement (* P<0.05). (d) Effect of exogenous S1P (1 μmol/L) on AC activation in wild-type and Gpr3-transfected HEK293 cells. (e) Gpr3-transfected HEK293 cells were treated with medium or 1 μmol/L S1P, Sph, and Sphi for additional 1 h, and were collected as above for cAMP assay (* P<0.05). Bar with different lowercase is significantly different (P<0.05), while bar with different uppercase is very significantly different (P<0.01). Graph shows mean±SD. Each experiment was repeated thrice at least
To confirm whether addition of GFP polypeptide to the extreme COOH terminal of Gpr3 molecule affects functionality of the receptor and/or obscures the features of constitutive activity previously associated with this mutation, HEK293 cells expressing the fusion Gpr3-GFP construct were used to assay cAMP accumulation without addition of any ligands. As displayed in Fig. 5b, compared with the pcDNA-Gpr3 vector, similar findings (P>0.05) were obtained with the vector expressing the Gpr3-GFP fused protein, indicating that the addition of the GFP tag at the C-terminus of Gpr3 does not affect its functions.
To examine whether S1P possessed agonistic properties on Gpr3-mediated stimulation of AC, we measured the cAMP accumulation induced by transient expression of a Gpr3 plasmid in HEK293 cells in presence of a graded series of S1P. HEK293 cells transiently transfected with pcDNA-Gpr3 vector were incubated in HBSS/HEPES buffer (containing 0.5 mmol/L IBMX and 0.1% BSA) for 30 min and then stimulated with 0, 0.1, 1, and 10 μmol/L S1P for 0.5, 1, and 2 h. After the treatment of the transfected cells with a graded series of S1P for 0.5 h, a small but significant increase in cAMP accumulation was observed (P<0.05), and the increase was unremitting until 2 h, suggesting that S1P indeed exerts agonistic effects on AC stimulation (Fig. 5c). In addition, the optimal condition for test of the Gpr3-mediated AC stimulation in HEK293 cells was incubated in 1 μmol/L S1P for 1 h.
Due to low levels of endogenous S1P receptors (S1P1, S1P2, S1P3, and S1P5) present in the HEK293 cells (Uhlenbrock et al., 2002), we checked the effect of exogenous S1P on AC activation in wild-type and Gpr3-transfected HEK293 cells. As shown in Fig. 5d, there was no significant difference between S1P incubating group and wild-type group, although the cAMP accumulation was slightly elevated after the S1P treatment for 1 h (P>0.05). Moreover, compared with the cAMP level of Gpr3-transfected or S1P-incubated HEK293 cells, a significant increase of cAMP accumulation was observed for the Gpr3-transfected and S1P incubating group (P<0.05), suggesting that S1P played a role in AC activation mainly through Gpr3 receptor.
In order to know if other sphingolipids also had a similar ability to stimulate AC via Gpr3-mediated signal pathway, we examined the cAMP accumulation induced by transient expression of a Gpr3 plasmid in HEK293 cells in presence of different sphingolipids: S1P (as an positive control), Sph, and Sphi in concentrations up to 1 μmol/L for 1 h. After the treatment of the Gpr3-transfected cells with Sph or Sphi, no significant increase of cAMP accumulation was detected (P>0.05), whereas S1P caused a significant increase in Gpr3-transfected cells (P<0.05) (Fig. 5e).
3.6. Effect of exogenous S1P on internalization of GFP-labeled porcine Gpr3 receptor
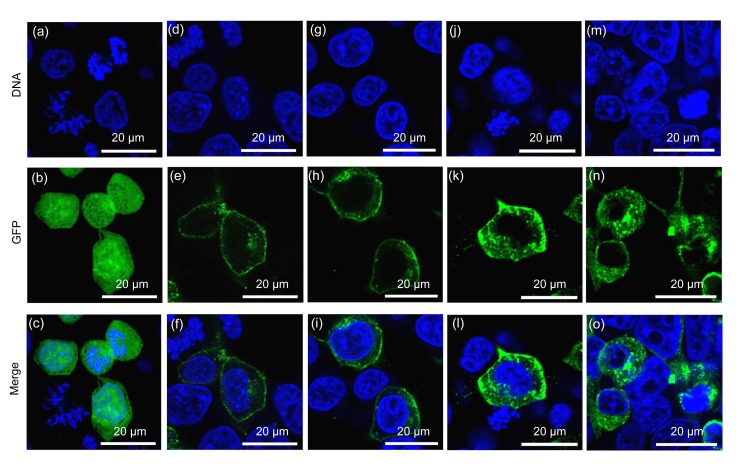
Due to the fact that the Gpr3-GFP construct plays a role as a Gpr3 receptor, it can be used to investigate whether S1P has an ability to induce the Gpr3 receptor internalization in transiently transfected HEK293 cells. As shown in Fig. 6, while GFP is expressed throughout the cells (Figs. 6a–6c), Gpr3-GFP (Figs. 6d–6o) could be mostly localized in the plasma membrane of S1P-untreated cells (Figs. 6d–6f). After incubation of Gpr3-GFP expressing cells in 1 μmol/L S1P for 30 min, the fusion proteins began to be translocated to intracellular compartments (Figs. 6g–6i). Exposure to S1P for 60 min (Figs. 6j–6l) and 120 min (Figs. 6m–6o) resulted in increased translocations to punctate intracellular structures, which are consistent with the processes of receptor internalization, indicating that S1P might be a potential and functional Gpr3 activator. In addition, exposure to Sph or Sphi for 60 min did not lead to significantly increased translocations, which is consistent with functional inactivity of the respective sphingolipids.
Fig. 6.
GFP-labeled porcine Gpr3 receptor internalization induced by S1P
HEK293 cells, transiently transfected with pEGFP-N1 (a‒c) or pEGFP-Gpr3 (d–o), were treated with 1 μmol/L S1P for 0 (d‒f), 30 (g‒i), 60 (j‒l), and 120 min (m‒o) at 37 °C. (c, f, i, l, and o) Composite images, respectively, of (a, b), (d, e), (g, h), (j, k), and (m, n). Cells were fixed and imaged using confocal microscopy. The experiments were repeated thrice at least
4. Discussion
Based on sequence similarity, the superfamily of GPCRs can be subdivided into several subfamilies, members of which often share similar ligands (Wittenberger et al., 2002). We cloned and identified the porcine GPCRs Gpr3, Gpr6, and Gpr12 as a subfamily of receptors, sharing 57%–58% sequence identity with each other at the amino acid levels. The RNAs encoding Gpr3, Gpr6, and Gpr12 were all detectable in the porcine ovary. However, Gpr6 was not detected in human ovaries, although it was actually expressed in the brain (DiLuigi et al., 2008), suggesting that the subfamily of three receptors might play different roles among species. To date, receptors in this family have been reported to constitutively stimulate cAMP production via a Gs protein-mediated pathway to regulate oocyte maturation (Mehlmann et al., 2004; Hinckley et al., 2005) and the nervous system development (Tanaka et al., 2007; Ruiz-Medina et al., 2011). In our present and previous studies (Zhang et al., 2012), we found that Gpr3 was a broadly expressed gene in pigs (Fig. 2), but its mRNA and protein were primarily expressed in the brain, pituitary, and oocyte (Zhang et al., 2012) like its human and mouse counterparts (Saeki et al., 1993; Iismaa et al., 1994; Mehlmann et al., 2004; Valverde et al., 2009), indicating a similar role in pigs.
In the present study, transfection of the HEK293 cells with the pEGFP-Gpr3 construct showed the GFP-labeled receptor protein to be localized in the plasmalemma and subcellular membranes (Figs. 3g and 3h), corresponding to the pattern of wild-type Gpr3 in mouse oocytes (Ledent et al., 2005). Moreover, most of the red fluorescent protein tagged mouse Gpr3 receptor proteins were highly expressed and localized along the cytoplasmic membrane as well as in a perinuclear compartment of the transfected Neuro2a cells (Tanaka et al., 2007). Both of them indicated the constructs of Gpr3 function in a manner similar to that of wild-type Gpr3. Interestingly, one GPCR of the Gpr3 subfamily, the rat Gpr6, was recently shown to be located in the intracellular compartments of the transfected HEK293 cells and striatal neurons (Padmanabhan et al., 2009). It would be of interest to investigate the reason for the different subcellular localization in the Gpr3 subfamily.
In an attempt to examine the relationship between sphingolipids and Gpr3, we investigated the role of sphingolipids (including S1P, Sph, and Sphi) related to the features of Gpr3 receptor. One distinctive feature of Gpr3 is that it shows constitutive activity towards Gs when expressed in several heterologous systems (Uhlenbrock et al., 2002; Tanaka et al., 2007). As expected, this property of the receptor was confirmed by the expression of porcine Gpr3 in HEK293 cells. It is important to note that cAMP release induced by Gpr3 reached levels similar to that of other Gαs-linked GPCRs fully stimulated by their respective ligands. Similar findings were also obtained with the vector expressing the GFP-fused receptors, indicating that addition of the GFP tag at the COOH-terminus does not affect its functions. Recently, a few studies have shown that sphingolipids are endogenous agonists for this receptor subfamily (Uhlenbrock et al., 2002; Ignatov et al., 2003b). When we added the S1P into the medium for culture of the transfected HEK293 cells, the cAMP accumulation was small, but significantly elevated, consistent with the results of previous studies (Uhlenbrock et al., 2002). It was suggested that S1P might be a potential activator for porcine Gpr3 receptor. However, Valverde et al. (2009) recently reported that S1P did not have the ability to affect Gpr3 constitutive basal activity in CHO cell transient expression of a Gpr3 plasmid, suggesting that the cell-specific role of S1P related to Gpr3 might exist. In addition, when we incubated the Gpr3-transfected HEK293 cells in 1 μmol/L Sph or Sphi medium for 1 h, no significant increase of cAMP accumulation was detected, indicating that Sph and Sphi might not be as the candidate ligands for Gpr3 receptor.
To further confirm the relationship between sphingolipids and Gpr3, ligand-induced receptor internalization, the other feature of the GPCRs (including Gpr3 receptor), was performed. As Uhlenbrock et al. (2002) recently showed that agonist (S1P)-induced trafficking and internalization of Gpr6 existed in HEK293 cells, we reported here regarding about S1P-induced translocation of the Gpr3-GFP fusion protein to subcellular compartments of HEK293 cells. Although Gpr3-GFP is initially localized in the plasmalemma, it is internalized rapidly after the addition of exogenous S1P, confirming the efficiency of S1P to Gpr3 internalization (Fig. 6). However, exposure to Sph or Sphi did not lead to a significant increase of Gpr3 internalization, which is similar with the result of the previous studies (Uhlenbrock et al., 2002). So far, the function of receptor internalization in signal transduction is still controversial. It is widely presumed that receptor internalization is a mechanism of ligand-induced desensitization (Conway et al., 1999). In contrast, recent data indicated that activation of specific signaling pathways requires receptor internalization (Xiao et al., 2009). According to the later opinion, it is likely that internalization of Gpr3 may transport S1P to a specific subcellular location (such as the perinuclear or lysosomal compartment) to activate other biological responses. The present studies provided clear evidence that Gpr3 activates Gs-linked signaling pathways (Mehlmann et al., 2004; Tanaka et al., 2009). Further studies are necessary to define how the S1P activates the Gpr3 receptor internalization, and the role of Gpr3 internalization in receptor signaling.
In conclusion, we cloned and identified the complete coding sequence (CDS) of porcine GPCRs Gpr3, Gpr6, and Gpr12. Subsequent investigation of mRNA expression patterns in different tissues of porcine Gpr3 gene was conducted by RT-PCR and real-time PCR. The porcine Gpr3 receptor has been identified as a constitutively active GPCR, localized to the plasmalemma and subcellular membranes. The lipid mediator S1P has been identified as an activator of porcine Gpr3 via functional cAMP assay and internalization studies.
Footnotes
Project supported by the National High-Tech R&D Program (863) of China (No. 2006AA10Z136) and a Grant-in-Aid for Innovative Training of Doctoral Students in Jiangsu Province of China (No. CXLX11-0701)
References
- 1.Alewijnse AE, Timmerman H, Jacobs EH, Smit MJ, Roovers E, Cotecchia S, Leurs R. The effect of mutations in the DRY motif on the constitutive activity and structural instability of the histamine H2 receptor. Mol Pharmacol. 2000;57(5):890–898. [PubMed] [Google Scholar]
- 2.Clemens JJ, Davis MD, Lynch KR, Macdonald TL. Synthesis of para-alkyl aryl amide analogues of sphingosine-1-phosphate: discovery of potent S1P receptor agonists. Bioorg Med Chem Lett. 2003;13(20):3401–3404. doi: 10.1016/S0960-894X(03)00812-6. [DOI] [PubMed] [Google Scholar]
- 3.Conway BR, Minor LK, Xu JZ, Gunnet JW, DeBiasio R, D′Andrea MR, Rubin R, Giuliano K, DeBiasio L, Demarest KT. Quantification of G-protein coupled receptor internatilization using G-protein coupled receptor-green fluorescent protein conjugates with the ArrayScantrade mark high-content screening system. J Biomol Screen. 1999;4(2):75–86. doi: 10.1177/108705719900400207. [DOI] [PubMed] [Google Scholar]
- 4.DiLuigi A, Weitzman VN, Pace MC, Siano LJ, Maier D, Mehlmann LM. Meiotic arrest in human oocytes is maintained by a Gs signaling pathway. Biol Reprod. 2008;78(4):667–672. doi: 10.1095/biolreprod.107.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287(2):249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Howard AD, McAllister G, Feighner SD, Liu Q, Nargund RP, van der Ploeg LH, Patchett AA. Orphan G-protein-coupled receptors and natural ligand discovery. Trends Pharmacol Sci. 2001;22(3):132–140. doi: 10.1016/S0165-6147(00)01636-9. [DOI] [PubMed] [Google Scholar]
- 7.Ignatov A, Lintzel J, Hermans-Borgmeyer I, Kreienkamp HJ, Joost P, Thomsen S, Methner A, Schaller HC. Role of the G-protein-coupled receptor GPR12 as high-affinity receptor for sphingosylphosphorylcholine and its expression and function in brain development. J Neurosci. 2003;23(3):907–914. doi: 10.1523/JNEUROSCI.23-03-00907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ignatov A, Lintzel J, Kreienkamp HJ, Schaller HC. Sphingosine-1-phosphate is a high-affinity ligand for the G protein-coupled receptor GPR6 from mouse and induces intracellular Ca2+ release by activating the sphingosine-kinase pathway. Biochem Biophys Res Commun. 2003;311(2):329–336. doi: 10.1016/j.bbrc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Iismaa TP, Kiefer J, Liu ML, Baker E, Sutherland GR, Shine J. Isolation and chromosomal localization of a novel human G-protein-coupled receptor (GPR3) expressed predominantly in the central nervous system. Genomics. 1994;24(2):391–394. doi: 10.1006/geno.1994.1635. [DOI] [PubMed] [Google Scholar]
- 10.Joost P, Methner A. Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol. 2002;3(11):RESEARCH0063. doi: 10.1186/gb-2002-3-11-research0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293(5530):702–705. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- 12.Ledent C, Demeestere I, Blum D, Petermans J, Hamalainen T, Smits G, Vassart G. Premature ovarian aging in mice deficient for Gpr3. PNAS. 2005;102(25):8922–8926. doi: 10.1073/pnas.0503840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306(5703):1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 14.Moller S, Croning MD, Apweiler R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics. 2001;17(7):646–653. doi: 10.1093/bioinformatics/17.7.646. [DOI] [PubMed] [Google Scholar]
- 15.Murakami A, Takasugi H, Ohnuma S, Koide Y, Sakurai A, Takeda S, Hasegawa T, Sasamori J, Konno T, Hayashi K, et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: investigation based on a new S1P3 receptor antagonist. Mol Pharmacol. 2010;77(4):704–713. doi: 10.1124/mol.109.061481. [DOI] [PubMed] [Google Scholar]
- 16.Nakai K, Horton P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24(1):34–36. doi: 10.1016/S0968-0004(98)01336-X. [DOI] [PubMed] [Google Scholar]
- 17.Padmanabhan S, Myers AG, Prasad BM. Constitutively active GPR6 is located in the intracellular compartments. FEBS Lett. 2009;583(1):107–112. doi: 10.1016/j.febslet.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 18.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349(Pt 2):385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Medina J, Ledent C, Valverde O. GPR3 orphan receptor is involved in neuropathic pain after peripheral nerve injury and regulates morphine-induced antinociception. Neuropharmacology. 2011;61(1-2):43–50. doi: 10.1016/j.neuropharm.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Saeki Y, Ueno S, Mizuno R, Nishimura T, Fujimura H, Nagai Y, Yanagihara T. Molecular cloning of a novel putative G protein-coupled receptor (GPCR21) which is expressed predominantly in mouse central nervous system. FEBS Lett. 1993;336(2):317–322. doi: 10.1016/0014-5793(93)80828-I. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka S, Ishii K, Kasai K, Yoon SO, Saeki Y. Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 up-regulates cyclic AMP levels and promotes neurite outgrowth. J Biol Chem. 2007;282(14):10506–10515. doi: 10.1074/jbc.M700911200. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka S, Shaikh IM, Chiocca EA, Saeki Y. The Gs-linked receptor GPR3 inhibits the proliferation of cerebellar granule cells during postnatal development. PLoS One. 2009;4(6):e5922. doi: 10.1371/journal.pone.0005922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhlenbrock K, Gassenhuber H, Kostenis E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell Signal. 2002;14(11):941–953. doi: 10.1016/S0898-6568(02)00041-4. [DOI] [PubMed] [Google Scholar]
- 24.Valverde O, Celerier E, Baranyi M, Vanderhaeghen P, Maldonado R, Sperlagh B, Vassart G, Ledent C. GPR3 receptor, a novel actor in the emotional-like responses. PLoS One. 2009;4(3):e4704. doi: 10.1371/journal.pone.0004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. PNAS. 2007;104(18):7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittenberger T, Hellebrand S, Munck A, Kreienkamp HJ, Schaller HC, Hampe W. GPR99, a new G protein-coupled receptor with homology to a new subgroup of nucleotide receptors. BMC Genomics. 2002;3(1):17. doi: 10.1186/1471-2164-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao MF, Xu JC, Tereshchenko Y, Novak D, Schachner M, Kleene R. Neural cell adhesion molecule modulates dopaminergic signaling and behavior by regulating dopamine D2 receptor internalization. J Neurosci. 2009;29(47):14752–14763. doi: 10.1523/JNEUROSCI.4860-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Zhu K, Hong G, Wu W, Baudhuin LM, Xiao Y, Damron DS. Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nat Cell Biol. 2000;2(5):261–267. doi: 10.1038/35010529. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Ding J, Li Y, Wang J, Zhao Y, Wang W, Shi S, Dong F, Zhang Z, Shi F, et al. The porcine Gpr3 gene: molecular cloning, characterization and expression level in tissues and cumulus-oocyte complexes during in vitro maturation. Mol Biol Rep. 2012;39(5):5831–5839. doi: 10.1007/s11033-011-1393-y. [DOI] [PubMed] [Google Scholar]