Abstract
This work evaluates the enzymatic hydrolysis of starch from cassava using pectinase, α-amylase, and amyloglucosidase. A central composite rotational design (CCRD) was carried out to evaluate the effects of amyloglucosidase, pectinase, reaction time, and solid to liquid ratio. All the experiments were carried out in a bioreactor with working volume of 2 L. Approximately 98% efficiency hydrolysis was obtained, resulting in a concentration of total reducing sugar released of 160 g/L. It was concluded that pectinase improved the hydrolysis of starch from cassava. Reaction time was found to be significant until 7 h of reaction. A solid to liquid ratio of 1.0 was considered suitable for hydrolysis of starch from cassava. Amyloglucosidase was a significant variable in the process: after its addition to the reaction media, a 30%–50% increase in the amount of total reducing sugar released was observed. At optimal conditions the maximum productivity obtained was 22.9 g/(L·h).
Keywords: Cassava, Enzymatic hydrolysis, Enzyme reactor, Starch
1. Introduction
In recent years, there has been an increasing trend towards efficient utilization of agro-industrial by-products aimed at obtaining value-added bio-products, including biofuels, biochemicals, and biomaterials. Bioprocessing of agro-industrial residues minimizes environmental problems associated with their disposal and reduces dependence on petroleum resources when they are used to produce biofuels. The exploration of novel, efficient bioprocesses for underused biomasses is thus at the forefront of biotechnological research and industrial application (Rattanachomsri et al., 2009; Pan et al., 2011).
Cassava (Manihot esculenta Cranz) is the world’s sixth most important crop and is grown in many African, Asian, and Latin American countries. Cassava is a starch-containing root crop and is one of the most important sources of calories in the tropics. Cassava is also widely employed as a raw material for many industrial applications in the animal feed industry and starch industry, and more recently for production of ethanol. Cassava can be cultivated on arid and semiarid land where other crops, such as corn, do not thrive (Lin et al., 2011). Cassava is a root crop that produces high yield with little input. Yield as high as 45 t/ha has been reported (Ogbonna and Okoli, 2010). Cassava pulp contains about 50%–70% starch on a dry weight basis and 20%–30% fibers, which are composed mainly of cellulose and other non-starch polysaccharides (Rattanachomsri et al., 2009).
Cassava starch has several characteristics which favor its industrial use, including as a raw material in ethanol production. Some characteristics of cassava starch are its high purity, neutral flavor, and solubility; it is easily swollen, is highly viscous, and has a low tendency to retrograde compared with other starches such as potato, rice, and corn (Zamora et al., 2010). Different treatments and pretreatments have been studied with the aim of significantly improving the overall ethanol yield from cassava starch, including ultrasonic pretreatment (Nitayavardhana et al., 2008; 2010), wet oxidation pretreatment (Martín and Thomsen, 2007), and combined heat treatment and acid hydrolysis (Agu et al., 1997).
Improved starch recovery from cassava can be achieved by using multi-enzyme preparations. A number of reports suggest the benefits of using such enzyme systems. Cassava starch contaminated with fibers was more efficiently saccharified after treatment with fungal cellulases (de Menezes et al., 1978). Ethanol production increased using hydrolyzed cassava starch residues with a multi-enzymatic preparation consisting of cellulase, D-xylanase, β-D-glucosidase, α-amylase, amyloglucosidase, and pectinase (Shamala and Sreekantiah, 1986). Raw starch digestion from cassava roots was improved by using amyloglucosidase in combination with pectinase (Chitradon et al., 1996a; 1996b). Cassava pulp was treated either by sonication or incubation with a multi-enzyme mixture of cellulase and pectinase. Both methods improved the efficiency of starch extraction by disrupting the complex structure of polysaccharides associated with and entrapping starch granules. Use of either cellulase or pectinase alone failed to effectively improve starch extraction (Sriroth et al., 2000).
The main objective of this work was to investigate the enzymatic hydrolysis of starch from cassava using pectinase, α-amylase, and amyloglucosidase. A central composite rotational design (CCRD) was carried out to evaluate the effects of amyloglucosidase, pectinase, reaction time, and solid to liquid ratio (R S/L).
2. Materials and methods
2.1. Raw materials and enzymes
Fresh cassava samples used were obtained in a local market, which were planted in September 2009 and harvested in July 2010. The samples as received were washed, milled, and maintained at −18 °C until the experiments. The composition of raw material used, considering the presence of peels, is presented in Table 1. The enzymes used in this work were pectinase, α-amylase, and amyloglucosidase. Pectinase from Aspergillus niger was purchased from Sigma-Aldrich, while α-amylase (Starmax TG 120) and amyloglucosidase (Starmax GA 400) were donated by Prozyn (Brazil).
Table 1.
Composition of raw material used in this work
| Composition | Wet basis (%)* | Dry basis (%)* |
| Moisture | 62.4 | |
| Dry matter | 37.8 | 100.0 |
| Nitrogen free extracts | 34.3 | 91.4 |
| Etheric extracts | 0.3 | 0.7 |
| Crude fiber | 1.2 | 3.1 |
| Ash | 0.7 | 1.8 |
| Crude protein | 1.1 | 3.0 |
| Total sugars | 3.2 | 8.6 |
| Starch | 29.3 | 77.9 |
In mass
2.2. Experimental apparatus and procedures
The bioreactor used in this work was purchased from Metalquim Ltda (São Paulo, Brazil) and consists of a jacketed vessel of stainless steel with a volume of 5 L, which is equipped with a device to control agitation, temperature, and pressure.
The reactions were carried out considering a working volume of 2 L. Initially (Step 1), an amount of raw material was added to the bioreactor, taking into account the R S/L employed. The pH of the solution was adjusted to 5.5 using 0.1 mol/L HCl and the temperature increased to 45 °C. After the temperature stabilized, an amount of pectinase was added according to experimental design (Table 2) and the reaction was carried out for 60 min under agitation of 200 r/min. Next (Step 2), the temperature was increased to 95 °C and, after stabilization, 0.8 μl/g starch of α-amylase was added as suggested by Apar and Ozbek (2004) and confirmed in preliminary tests. The reaction was carried out for 60 min under agitation of 200 r/min. The temperature was then decreased to 60 °C and the pH of the solution was adjusted to 4.0 using 0.1 mol/L HCl. The total time of Steps 1 and 2 was 3 h, including the time required for heating and cooling the reaction media. In Step 3, amyloglucosidase was added and the reaction was carried out for a defined time (Table 2) under agitation of 200 r/min. At the end of reaction, the samples were withdrawn from the bioreactor and the solids were removed by filtration. The amount of total reducing sugar (TRS) released by the hydrolysis was measured in the supernatant. The temperature and pH of each step were adjusted according to the enzyme manufacturers’ recommendations.
Table 2.
Levels of independent variables investigated in the central composite rotational design
| Coded level | x 1 (mg/g) | x 2 (μl/g) | x 3 (h) | x 4 (g/g) |
| −2 | 0.36 | 0.00 | 4 | 0.33 |
| −1 | 0.52 | 2.35 | 21 | 0.50 |
| 0 | 0.68 | 4.70 | 38 | 0.67 |
| 1 | 0.84 | 7.05 | 55 | 0.83 |
| 2 | 1.00 | 9.40 | 72 | 1.00 |
x 1: amyloglucosidase (mg/g starch); x 2: pectinase (μl/g dry matter); x 3: time (h); x 4: solid to liquid ratio (g dry matter/g water)
2.3. Central composite rotational design
The influences of pectinase, amyloglucosidade, R S/L, and reaction time counted from the addition of enzyme amyloglucosidase were assessed by means of a CCRD for four independent variables, for a total of 28 experiments. The ranges of variables investigated are listed in Table 2. The results were analyzed using the software Statistica 7.0 (Stasoft Inc., Tulsa, USA), considering a significance level of 95%.
All statistical interpretation was based on the generation of a quadratic model for each one of the responses (concentration of TRS released and efficiency of hydrolysis) according to Eq. (1):
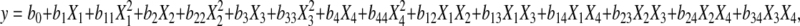 |
(1) |
where y is the response (concentration of TRS released or efficiency of hydrolysis), X is the coded independent variable (Table 2), and b is the coefficient. After validation by analysis of variance (ANOVA), the model was used as process estimator.
The optimum values for the independent variables were determined by using the response/desirability profiling function of Statistica 7.0, in a way that high amount of TRS and maximum efficiency of hydrolysis simultaneously were obtained. The desirability function enables analysis of the response surface produced, by fitting the observed responses using the above equation based on levels of the independent variables. The equation was used to predict values for both responses (amount of TRS released and efficiency of hydrolysis) at different combinations of levels of the independent variables, specify desirability functions for the dependent variables, and search for the levels of the independent variables that produce the most desirable responses on the dependent variables.
2.4. Analytical methods
Moisture content was determined by drying at 105 °C to constant weight (AOAC, 1995). The contents of protein in cassava pulp (total N×6.25, by Kjeldahl method), crude fiber (by acid and alkali treatment), etheric extract (by Soxhlet extraction with petroleum ether), and ash (temperature 600 °C for 3 h) were quantified as described by AOAC (1995) methods. The starch content of raw cassava was measured by acid hydrolysis of samples according to the procedure reported by Leonel (1998). According to this procedure, the samples were treated with 2 mol/L HCl in a boiling water bath for 15 min. The pH of the mixture was neutralized with 2 mol/L NaOH after it cooled down to room temperature. The amount of TRS released was measured by the 3,5-dinitrosalicylic acid method (Miller, 1959), and the starch content was determined by multiplying the content of TRS by a correction factor of 0.9 (Leonel, 1998).
3. Results and discussion
Table 3 presents the experimental results obtained in the CCRD during the hydrolysis of cassava in terms of TRS released and efficiency of hydrolysis. The final concentrations of TRS ranged from 46.8 to 157.5 g/L in Runs 1 and 24, respectively. In the same conditions, the efficiencies of hydrolysis were 51.7% and 97.0%, respectively. Table 3 shows that the concentration of TRS released increased in the experiments with a high R S/L. In terms of efficiency of hydrolysis, values higher than 90% were obtained in Runs 6 (92.2%), 10 (94.7%), 12 (98.1%), 16 (97.3%), and 24 (97%).
Table 3.
Matrix of the experimental results obtained in the central composite rotational design
| Run | X 1 | X 2 | X 3 | X 4 | TRS (g/L) | Efficiency (%) |
| 1 | −1 | −1 | −1 | −1 | 46.8 | 51.7 |
| 2 | 1 | −1 | −1 | −1 | 69.5 | 76.8 |
| 3 | −1 | 1 | −1 | −1 | 68.8 | 76.1 |
| 4 | 1 | 1 | −1 | −1 | 78.7 | 87.0 |
| 5 | −1 | −1 | 1 | −1 | 59.1 | 65.4 |
| 6 | 1 | −1 | 1 | −1 | 83.4 | 92.2 |
| 7 | −1 | 1 | 1 | −1 | 64.8 | 71.6 |
| 8 | 1 | 1 | 1 | −1 | 81.1 | 89.6 |
| 9 | −1 | −1 | −1 | 1 | 114.6 | 85.0 |
| 10 | 1 | −1 | −1 | 1 | 127.8 | 94.7 |
| 11 | −1 | 1 | −1 | 1 | 118.2 | 87.7 |
| 12 | 1 | 1 | −1 | 1 | 132.3 | 98.1 |
| 13 | −1 | −1 | 1 | 1 | 107.3 | 79.6 |
| 14 | 1 | −1 | 1 | 1 | 115.1 | 85.3 |
| 15 | −1 | 1 | 1 | 1 | 108.4 | 80.4 |
| 16 | 1 | 1 | 1 | 1 | 131.1 | 97.3 |
| 17 | −2 | 0 | 0 | 0 | 72.3 | 66.7 |
| 18 | 2 | 0 | 0 | 0 | 81.5 | 75.2 |
| 19 | 0 | −2 | 0 | 0 | 68.1 | 62.9 |
| 20 | 0 | 2 | 0 | 0 | 93.0 | 85.8 |
| 21 | 0 | 0 | −2 | 0 | 94.4 | 87.1 |
| 22 | 0 | 0 | 2 | 0 | 87.4 | 80.7 |
| 23 | 0 | 0 | 0 | −2 | 61.8 | 76.1 |
| 24 | 0 | 0 | 0 | 2 | 157.5 | 97.0 |
| 25 | 0 | 0 | 0 | 0 | 87.5 | 80.8 |
| 26 | 0 | 0 | 0 | 0 | 90.0 | 82.9 |
| 27 | 0 | 0 | 0 | 0 | 85.3 | 78.7 |
| 28 | 0 | 0 | 0 | 0 | 86.9 | 80.2 |
X 1, X 2, X 3, and X 4 are coded values of amyloglucosidase, pectinase, time, and solid to liquid ratio, respectively
Data of Table 3 were used to estimate the parameters of the quadratic model (Eq. (1)), whose parameters represent the effects of independent variables on the responses studied (concentration of TRS released and efficiency of hydrolysis). The model parameters that predict the concentration of TRS released are listed in Table 4, whereas those referring to efficiency of hydrolysis are listed in Table 5. Regarding the concentration of TRS released, the linear terms for amyloglucosidase, pectinase, and R S/L showed a significant positive effect, whereas the reaction time was not significant in the investigated range. Only the quadratic effect of R S/L presented a positive significant effect (P<0.05). This indicates that at low values of R S/L, there is the formation of a minimum point, and that increasing their values would increase the amount of TRS released within the range investigated in this work. Similar results were obtained for efficiency of hydrolysis. However, the quadratic and interaction terms did not present significant effects in the investigated range.
Table 4.
Estimated parameters for the quadratic model predicting the amount of TRS released from cassava hydrolysis
| Terms | Coefficient | Standard error | t(13) | P-value |
| Mean | 87.45 | 3.29 | 26.60 | <0.0001 |
| X 1 (1L) | 6.23 | 1.34 | 4.64 | 0.0005 |
| X 1 (Q) | −1.85 | 1.34 | −1.38 | 0.1912 |
| X 2 (2L) | 4.57 | 1.34 | 3.40 | 0.0047 |
| X 2 (Q) | −0.94 | 1.34 | −0.70 | 0.4970 |
| X 3 (3L) | −0.85 | 1.34 | −0.63 | 0.5373 |
| X 3 (Q) | 1.65 | 1.34 | 1.23 | 0.2405 |
| X 4 (4L) | 24.75 | 1.34 | 18.45 | <0.0001 |
| X 4 (Q) | 6.34 | 1.34 | 4.72 | 0.0004 |
| 1L by 2L | −0.31 | 1.64 | −0.19 | 0.8521 |
| 1L by 3L | 0.70 | 1.64 | 0.43 | 0.6771 |
| 1L by 4L | −0.96 | 1.64 | −0.59 | 0.5681 |
| 2L by 3L | −1.18 | 1.64 | −0.72 | 0.4872 |
| 2L by 4L | −0.59 | 1.64 | −0.36 | 0.7264 |
| 3L by 4L | −3.48 | 1.64 | −2.11 | 0.0543 |
L: linear term; Q: quadratic term
Table 5.
Estimated parameters for the quadratic model predicting the efficiency of hydrolysis of starch from cassava
| Terms | Coefficient | Standard error | t(13) | P-value |
| Mean | 80.65 | 3.01 | 26.75 | <0.0001 |
| X 1 (1L) | 5.85 | 1.23 | 4.76 | 0.0004 |
| X 1 (Q) | −1.85 | 1.23 | −1.50 | 0.1573 |
| X 2 (2L) | 4.29 | 1.23 | 3.48 | 0.0040 |
| X 2 (Q) | −1.00 | 1.23 | −0.81 | 0.4325 |
| X 3 (3L) | −0.35 | 1.23 | −0.29 | 0.7781 |
| X 3 (Q) | 1.39 | 1.23 | 1.13 | 0.2789 |
| X 4 (4L) | 5.81 | 1.23 | 4.72 | 0.0004 |
| X 4 (Q) | 2.05 | 1.23 | 1.67 | 0.1192 |
| 1L by 2L | −0.69 | 1.51 | −0.46 | 0.6529 |
| 1L by 3L | 0.71 | 1.51 | 0.47 | 0.6472 |
| 1L by 4L | −2.38 | 1.51 | −1.58 | 0.1382 |
| 2L by 3L | −1.52 | 1.51 | −1.01 | 0.3320 |
| 2L by 4L | −1.21 | 1.51 | −0.80 | 0.4379 |
| 3L by 4L | −3.13 | 1.51 | −2.08 | 0.0581 |
L: linear term; Q: quadratic term
ANOVA was applied to validate the model parameters referring to the concentration of TRS released (Table 4) and efficiency of hydrolysis (Table 5). The calculated F-value for the model referring to the concentration of TRS released was 29.4; this value is approximately 12 times higher than the listed one. The regression coefficient R 2 was 0.9694, indicating that about 96% of the total variations in the experimental data are explained by the model. The calculated F-value for the model referring to the efficiency of hydrolysis was 5.4; this value is approximately 2 times higher than the listed one. The regression coefficient R 2 was 0.8528. Based on the ANOVA, it is concluded that both models are statistically significant, providing reliable responses as functions of independent variables, and they are therefore useful tools for statistical optimization of the process.
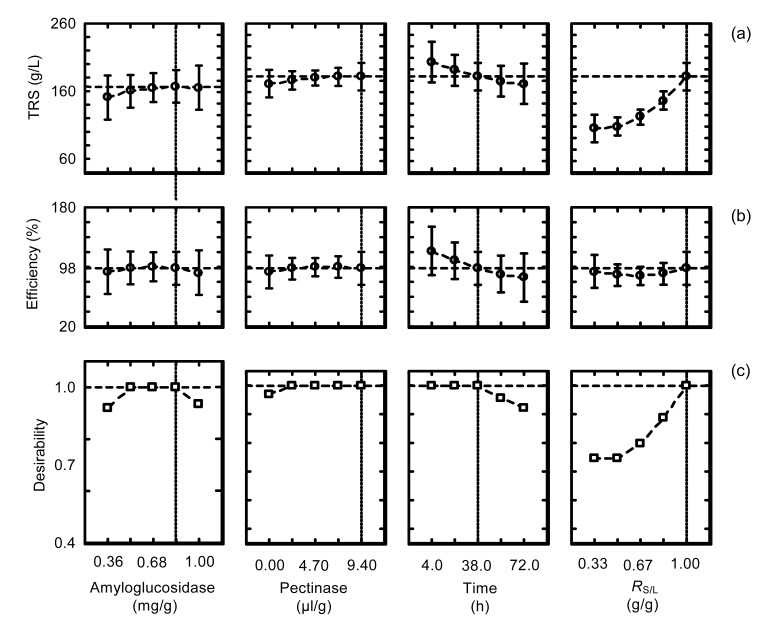
Fig. 1 presents the desirability profiling function obtained after the use of the models referring to the concentration of TRS released (Table 4) and efficiency of hydrolysis (Table 5). With respect to the significance level of model predictions that are represented by the error bars, it is seen that ranging the concentration of amyloglucosidase from 0.36 to 1.00 mg/g starch (at constant time, concentration of pectinase, and R S/L) has little effect on the maximum concentration of TRS released. Similar results are obtained for concentration of pectinase and reaction time. However, the variation of the RS/L from 0.33 to 1.00 results in a pronounced effect on the amount of TRS released. This is expected, since at high RS/L the amount of starch in the reaction media increases, favoring the release of TRS.
Fig. 1.
Desirability function for concentration of TRS released and efficiency of hydrolysis of cassava starch
(a) The simulated data for the concentration of TRS released from cassava as a function of each one of the independent variables. (b) The simulated data for the efficiency of hydrolysis of cassava as a function of each one of the independent variables. (c) The desirability function of the process. The dotted horizontal lines refer to the predicted values when the independent variables are maintained at the specified levels, whose levels are defined by the dotted vertical lines. The deviations presented in the figure are due to model predictions
Regarding efficiency of hydrolysis, different profiles were observed from those obtained for the concentration of TRS released. Ranging a determined independent variable from the low to the high level of the CCRD and maintaining the other variables at the specified levels did not produce significant variations in the efficiency of hydrolysis; this result is consistent with Fig. 1b. The alternative method to obtain a high concentration of TRS released and a high efficiency of hydrolysis simultaneously is to compute the process desirability, presented in Fig. 1c.
Based on the analysis of Fig. 1, it is concluded that the concentration of amyloglucosidase in the range of 0.52 to 0.84 mg/g starch leads to the maximum concentration of TRS released and maximum efficiency of hydrolysis since at this range the desirability function is at the maximum value. The use of pectinase improved the efficiency of hydrolysis of cassava and the releasing of TRS, since at the lowest level of pectinase (0.0 μl/g dry matter) a decreasing value of the desirability function was observed. This result is confirmed by analyzing Runs 17 and 18, where increasing the concentration of pectinase from 0.0 to 9.4 μl/g dry matter and maintaining other variables at the central point of CCRD increased the releasing of TRS from 72.3 to 81.5 g/L and the efficiency of hydrolysis from 66.7% to 75.2 %, respectively. Reaction time (considered after the addition of amyloglucosidase) in the range of 4‒38 h leads to the maximum value of the desirability function when the other variables are maintained at the specified values. R S/L at the highest level led to the optimum value of the desirability function.
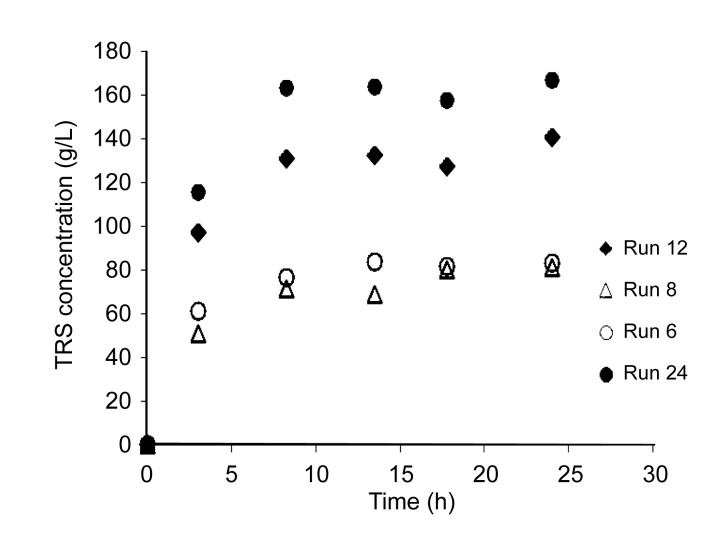
Some results discussed in Fig. 1 are confirmed in Fig. 2, which presents the kinetic evaluation of the concentration of TRS of selected experimental conditions with high efficiency of hydrolysis for low level of R S/L (Runs 6 and 8) and high level (Runs 12 and 24). From kinetics results it is seen that after 7 h of reaction (3 h after the action of α-amylase and pectinase and 4 h after the action of amyloglucosidase), the amount of TRS released stabilized, confirming the result obtained in the desirability function. Increasing the R S/L led to an increased amount of TRS released to the reaction media. In addition, after 3 h of reaction, 50%–70% of the amount of TRS was released due to the actions of α-amylase and pectinase. The amyloglucosidase was effective at hydrolyzing the cassava, since the amount of TRS released increased from 30% to 50%. This result is due to the fact that pectinase helps disrupt the complex structure of polysaccharides associated with and entrapping starch granules, increasing the efficiency of hydrolysis of starch. In addition, an amount of TRS released remains adsorbed in the fibers, decreasing the yield (Leonel and Cereda, 1999).
Fig. 2.
Kinetics of concentration of TRS obtained from the hydrolysis of cassava for some selected experimental conditions of CCRD
Paolucci-Jeanjean et al. (2000) investigated the hydrolysis of raw cassava starch using only α-amylase and verified maximum TRS released after 1 h of reaction, where the amount produced was about 25 g/L. Rattanachomsri et al. (2009) evaluated the direct hydrolysis and saccharification of cassava pulp without a pre-gelatinization step, which was developed using the simultaneous action of multi non-starch polysaccharide hydrolyzing enzymes and raw starch degrading amylolytic activity. The composite enzyme activities were analyzed, and the reaction parameters were investigated with the multi-enzyme preparation from a selected strain of A. niger. The authors obtained a maximum efficiency of hydrolysis of about 90% after 30 h of reaction. Sriroth et al. (2000) investigated the effects of physical disruption through sonication and enzymatic treatment using cellulase, pectinase, and α-amylase to hydrolyze starch from cassava and obtained a maximum efficiency of 40%.
4. Conclusions
This work evaluated the enzymatic hydrolysis of starch from cassava using pectinase, α-amylase, and amyloglucosidase. A maximum efficiency of hydrolysis about 98% was obtained, resulting in a concentration of TRS released of 160 g/L. The optimal experimental conditions for hydrolysis of starch from cassava were 0.52 g/g starch of amyloglucosidase, 2.35 μl/g dry matter of pectinase, 4 h of reaction time after the addition of amyloglucosidase, and 1.0 of R S/L. Using these parameters, the maximum productivity was 22.9 g/(L·h).
Footnotes
Project supported by the National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Level Personnel (CAPES), Brazil
References
- 1.Agu RC, Amadife AE, Ude CM, Onyia A, Ogu EO, Okafor M, Ezejiofor E. Combined heat treatment and acid hydrolysis of cassava grate waste (CGW) biomass for ethanol production. Waste Manage. 1997;17(1):91–96. doi: 10.1016/S0956-053X(97)00027-5. [DOI] [Google Scholar]
- 2.AOAC (Association of Official Analytical Chemists) Official Method of Analysis. 16th Ed. Virginia: AOAC; 1995. [Google Scholar]
- 3.Apar DK, Ozbek B. α-Amilase inactivation during corn starch hydrolysis process. Process Biochem. 2004;39(12):1877–1892. doi: 10.1016/j.procbio.2003.09.014. [DOI] [Google Scholar]
- 4.Chitradon L, Poonpairoj P, Mahakhan P, et al. Pectinases from Rhizopus sp. Efficient in Enhancing the Hydrolysis of Raw Cassava Starch: Purification and Characterization. In: Visser J, Voragen AGJ, editors. Pectins and Pectinases: Proceedings of an International Symposium. Amsterdam: Elsevier; 1996. pp. 715–722. [DOI] [Google Scholar]
- 5.Chitradon L, Mahakhan P, Poonpairoj P, et al. Production of Pectinases from Rhizopus sp. in Solid Substrates. In: Visser J, Voragen AGJ, editors. Pectins and Pectinases: Proceedings of an International Symposium. Amsterdam: Elsevier; 1996. pp. 853–860. [DOI] [Google Scholar]
- 6.de Menezes TJB, Arakaki T, DeLamo PR, Sales AM. Fungal cellulases as an aid for the saccharification of cassava. Biotechnol Bioeng. 1978;20(4):555–565. doi: 10.1002/bit.260200407. [DOI] [Google Scholar]
- 7.Leonel M. Evaluation of Complementary Enzymes in the Production of Ethanol from Cassava Meal. Botucatu, Brazil: Universidade Federal Paulista, Faculdade de Ciências Agronômicas; 1998. PhD Thesis. (in Portuguese) [Google Scholar]
- 8.Leonel M, Cereda MP. Evaluation of the cellulase and pectinase by complementary enzymes in the process of hydrolysis-saccharification of cassava fibrous waste for alcohol production. Ciênc Tecnol Aliment. 1999;19(1):113–117. doi: 10.1590/S0101-20611999000100020. (in Portuguese) [DOI] [Google Scholar]
- 9.Lin HJ, Xian L, Zhang QJ, Luo XM, Xu QS, Yang Q, Duan CJ, Liu JL, Tang JL, Feng JX. Production of raw cassava starch-degrading enzyme by Penicillium and its use in conversion of raw cassava flour to ethanol. J Ind Microbiol Biotechnol. 2011;38(6):733–742. doi: 10.1007/s10295-010-0910-7. [DOI] [PubMed] [Google Scholar]
- 10.Martín C, Thomsen AB. Wet oxidation pretreatment of lignocellulosic residues of sugarcane, rice, cassava and peanuts for ethanol production. J Chem Technol Biotechnol. 2007;82(2):174–181. doi: 10.1002/jctb.1648. [DOI] [Google Scholar]
- 11.Miller GL. Use of dinitrosalisylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 12.Nitayavardhana S, Rakshit SK, Grewell D, van Leeuwen J, Khanal SK. Ultrasound pretreatment of cassava chip slurry to enhance sugar release for subsequent ethanol production. Biotechnol Bioeng. 2008;101(3):487–496. doi: 10.1002/bit.21922. [DOI] [PubMed] [Google Scholar]
- 13.Nitayavardhana S, Shrestha P, Rasmussen ML, Lamsal BP, van Leeuwen J, Khanal SK. Ultrasound improved ethanol fermentation from cassava chips slurry in cassava-based ethanol plants. Bioresource Technol. 2010;101(8):2741–2747. doi: 10.1016/j.biortech.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 14.Ogbonna CN, Okoli EC. Conversion of cassava flour to fuel ethanol by sequential solid state and submerged cultures. Process Biochem. 2010;45(7):1196–1200. doi: 10.1016/j.procbio.2010.04.004. [DOI] [Google Scholar]
- 15.Pan SK, Wu SJ, Kim JM. Preparation of glucosamine by hydrolysis of chitosan with commercial α-amylase and glucoamylase. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(11):931–934. doi: 10.1631/jzus.B1100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paolucci-Jeanjean D, Belleville MP, Zakhia N, Rios GM. Kinetics of cassava starch hydrolysis with Termamyl® enzyme. Biotechnol Bioeng. 2000;68(1):71–77. doi: 10.1002/(SICI)1097-0290(20000405)68:1<71::AID-BIT8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Rattanachomsri U, Tanapongpipat S, Eurwilaichitr L, Champreda V. Simultaneous non-thermal saccharification of cassava pulp by multi-enzyme activity and ethanol fermentation by Candida tropicalis. J Biosci Bioeng. 2009;107(5):488–493. doi: 10.1016/j.jbiosc.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Shamala TR, Sreekantiah KR. Saccharification of tapioca starch residue with a multienzyme preparation of Aspergillus ustus . Starch. 1986;38(12):428–432. doi: 10.1002/star.19860381208. [DOI] [Google Scholar]
- 19.Sriroth K, Chollakup R, Chotineeranat S, Piyachomkwan K, Oates CG. Processing of cassava waste for improved biomass utilization. Bioresour Technol. 2000;71(1):63–69. doi: 10.1016/S0960-8524(99)00051-6. [DOI] [Google Scholar]
- 20.Zamora LL, Calderón JAG, Vázquez ET, Reynoso EB. Optimization of ethanol production process from cassava starch by surface response. J Mex Chem Soc. 2010;54(4):198–203. [Google Scholar]