Abstract
We investigated whether glyphosate influences the cellular toxicity of the surfactants TN-20 and LN-10 on the mouse fibroblast-like cells, alveolar epithelial cells, and a heart cell line. The cytotoxicity of TN-20 and LN-10 (0.4-100 µM), in the presence or absence of glyphosate was determined by assessing membrane integrity. TN-20 toxicity was significantly lower in the presence of 50 µM glyphosate for the fibroblast-like cell (6.25 µM; 3.9% ± 3.4% vs -4.8% ± 0.7%), for the alveolar cells (0.78 µM; 5.7% ± 0.9% vs 0.1% ± 0.6%), and for the heart cell line (25.0 µM; 7.9% ± 3.0% vs 19.4% ± 0.7%) compared to that of TN-20 alone. The cellular toxicity of LN-10 towards the fibroblast-like cells was found to be increased in the presence of 50 µM glyphosate when LN-10 concentrations of 50 µM (31.3% ± 3.9% vs 19.2% ± 0.9%) and 100 µM (62.1% ± 3.4% vs 39.0% ± 0.7%) were compared to that of LN-10 alone. These results suggest that the mixture toxicity may be a factor in glyphosate-surfactant toxicity in patients with acute glyphosate herbicide intoxication.
Keywords: Glyphosate, Surfactants, Complex Mixtures, Toxicity, Herbicides
INTRODUCTION
Glyphosate (N-[phosphonomethyl]glycine) is one of the most commonly used herbicides worldwide. It has an oral acute LD50 of 4,230 mg/kg in rats and has been categorized by the World Health Organization (WHO) as "unlikely to present acute hazard in normal use". the ingestion of large amounts of glyphosate herbicide results in grave clinical outcomes (1-3). In severe intoxicated cases, patients suffer severe toxic effects, such as respiratory distress necessitating positive pressure ventilation, metabolic acidosis, arrhythmia, renal failure, electrolyte imbalance, or death (3-5). We have also found that patients who ingest large volumes of glyphosate herbicides are also at risk of hypotension, mental deterioration, respiratory failure, and arrhythmia, particularly when the calculated surfactant volume in herbicide product is over 8 mL (6). Indeed, it has been proposed that the surfactant in the formula, and not the glyphosate, is the essential element of these mixtures responsible for human toxicity (7, 8). Surfactants comprise a large group of surface-active substances that have a large number of applications, (9) including its use in herbicides, although the particular surfactant ingredients used in herbicide formulations may vary depending on the manufacturer.
Over the last 2 decades, much research has been carried out to investigate the nature of glyphosate-surfactant toxicity in clinical toxicology (3-5), and several possible mechanisms for this phenomenon have been proposed. For instance, in addition to the individual toxicity of these agents, a mixture of surfactants and glyphosate may not only cause direct toxicity, but could result in synergistic effects on the toxicities of either or both of these compounds. The term mixture toxicity refers to the unwanted adverse effects that may occur when chemicals are mixed. Although significant advances in mixture toxicology have been made over the last few decades (10, 11), a majority of the investigations that have examined the toxicity of herbicide mixtures have been carried out using a herbicide and an insecticide or fungicide (12). We therefore recently assessed the cellular toxicity of surfactants that are commonly included in herbicide formulations (13). Of these, polyoxyethylene tallow amine (TN-20) and polyoxyethylene lauryl amine ether (LN-10) are commonly found in glyphosate herbicides in Korea. We have now extended upon this by investigating the mixture toxicity of glyphosate and the TN-20 and LN-10 surfactants (Table 1).
Table 1.
Summary of TN-20 and LN-10 surfactants
*Molecular weight, average molecular weight of herbicide product; †Hydrophilic, hydrophilic part of the surfactant.
MATERIAL AND METHODS
Cell culture
The cellular cytotoxicity and cell viability of mouse fibroblast-like cells (fibroblast-like cell line, L-929; American Type Culture Collection [ATCC], CCL-1), alveolar cells (alveolar epithelial cell line, A549; ATCC, CCL-185), and heart cells (heart cell line, H9C2; ATCC, CRL-1446) were assessed. Cells were maintained and cultured in RPMI media (RPMI-1640; HyClone, Logan, UT, USA) containing 10% heat inactivated fetal bovine serum (FBS, HyClone), 2 mM L-glutamine (Sigma, St. Louis, MO, USA), and 100 U/mL penicillin (Sigma, St. Louis, MO, USA). Cells were grown in 75-cm2 tissue culture flasks at 37℃ in a 5% CO2 humidified incubator.
Determination of suitable ranges of glyphosate, TN-20, and LN-10 concentrations to be used in further experiments
KPX Green Chemical Co. (Seoul, Korea) provided the LN-10 and TN-20 (Table 1). The range of each chemical concentration used in the cytotoxicity test was derived from the inhibitory concentration 50 (IC50) based on relative cell viability (%) (14). The cellular viability following exposure to glyphosate, TN-20, or LN-10 was determined using an MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay. Briefly, previously sub-cultured cells (1 × 104 cells/mL) were exposed to various concentrations of the test compounds in a 24-well plate and incubated for 72 hr at 37℃ in 5% CO2. Following this, 5 mg/mL MTT solution (Sigma) was added to the wells, and cells were incubated for a further 4 hr. The supernatant was then removed and 1 mL of dimethyl sulfoxide (DMSO) added to each well. Immediately after purple formazan crystals had formed and dissolved, the solution was collected and pipetted into a 96-well plate. The absorbance was measured at a wavelength of 595 nm using an enzyme-linked immunosorbent assay reader (VICTOR X3, Perkin Elmer). Cell viability was measured as the ratio of the optical density in the medium containing the extract solution to the optical density in fresh medium.
Assays of TN-20 and LN-10 cytotoxicity in the presence or absence of glyphosate
The cytotoxicity of the surfactants in the presence or absence of glyphosate was examined using a commercial LDH (lactate dehydrogenase) assay kit to spectrophotometrically assess membrane integrity (Cytotoxicity Kit, Xenometrix AG, Allschwil, Switzerland). Previously sub-cultured mouse fibroblast-like cells, alveolar epithelial cells, and heart cells (1 × 104 cells/mL) were incubated in a 5% CO2 incubator at 37℃ for 72 hr with TN-20 and LN-10 at concentrations ranging from 0.4 µM to100 µM, in the presence or absence of 50 µM or 100 µM glyphosate. The membrane integrity is presented relative to the toxicity of 1% Triton X-100.
Statistics
Each experiment was carried out 4 times, and the data were presented as mean ± standard error. Statistical analyses were performed using SPSS software (Version 14.0; SPSS Inc., Chicago, IL, USA). Data between groups were compared using the Mann-Whitey U test, and P < 0.05 was considered significant.
RESULTS
Cytotoxicity of glyphosate, TN-20, and LN-10
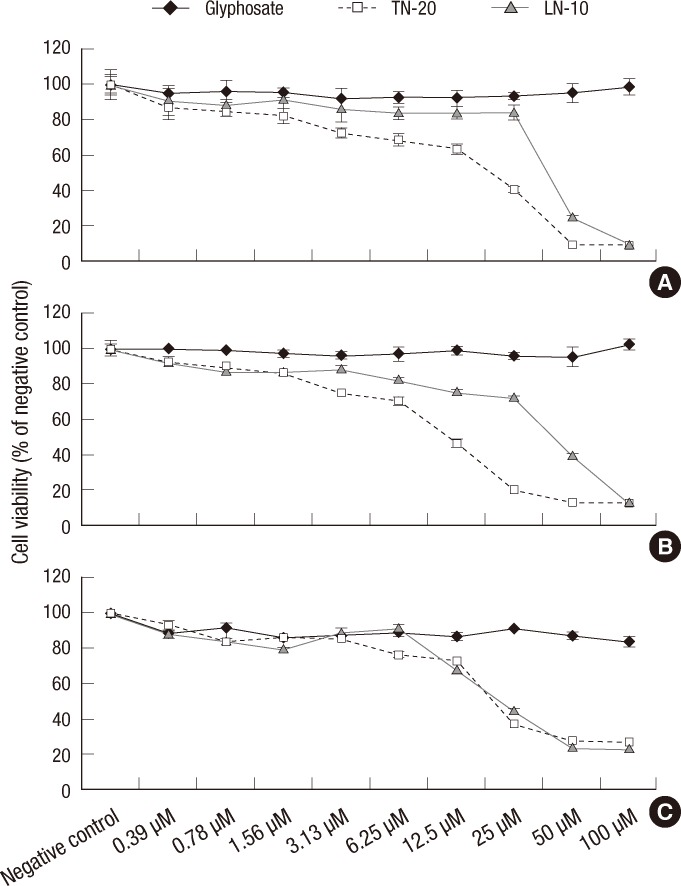
The cellular toxicity of TN-20 and LN-10 was observed to be dose-dependent (0.4-100 µM) in all the 3 cell lines (Fig. 1). The toxicity graph of concentration vs cell viability was found to have a point of inflection at 25 µM, whereby the slope was gentle when the concentrations of the chemicals were below 25 µM and steep when they were greater than 25 µM. Although glyphosate was not observed to elicit cellular toxicity at concentrations of 0-100 µM in any of the 3 cell lines examined, this concentration range was used in further experiments.
Fig. 1.
The cytotoxicity of glyphosate, TN-20, and LN-10. Cytotoxicity was determined by assessing the number of metabolically active (A) fibroblast, (B) alveolar, and (C) heart cells using an MTT assay. Cells were cultured with various concentrations of glyphosate, TN-20, and LN-10, ranging from 0 to 100 µM. The cytotoxicity of TN-20 and LN-10 is found to be dose dependent in all the 3 cell lines, whereas no cellular toxicity due to any concentration of glyphosate is observed.
Cellular toxicity of TN-20 in the presence or absence of glyphosate
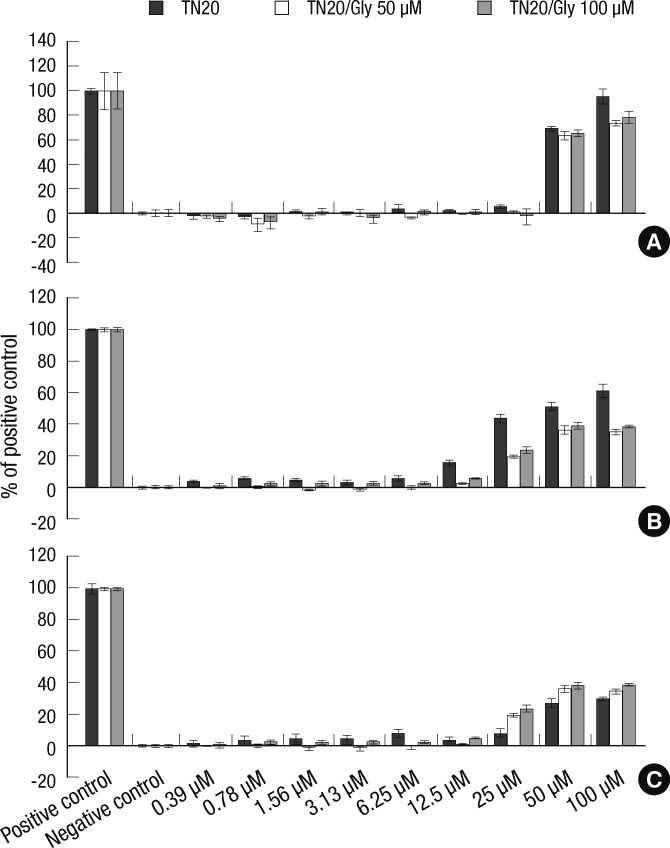
The LDH assay examining the toxicity of various concentrations of TN-20 in the presence or absence of glyphosate in the fibroblast-like cell line demonstrated that TN-20 toxicity was significantly lower in the presence of 50 µM glyphosate compared to that of TN-20 alone. In contrast, there was no significant difference between the cellular toxicity of TN-20 with or without 100 µM glyphosate (Fig. 2A). In the alveolar cells, the cellular toxicity of TN-20 was also lower when it was mixed with 50 µM of glyphosate compared to that of TN-20 alone. This was also observed when higher concentrations of TN-20 were mixed with 100 µM of glyphosate (Fig. 2B). In the heart cell line, the cellular toxicity of select concentrations of TN-20 was found to be higher when it was mixed with 50 µM of glyphosate. This was also observed when certain TN-20 concentrations were mixed with 100 µM of glyphosate (Fig. 2C).
Fig. 2.
The cellular toxicity of TN-20 in the presence or absence of glyphosate. (A) LDH assays examining the toxicity of TN-20 in fibroblast-like cells revealed that there was significantly lower toxicity in the mixtures of 50 µM glyphosate and 6.25, 12.5, 25, and 100 µM TN-20. No significant differences in the cellular toxicity of TN-20 in the presence or absence of 100 µM of glyphosate were observed. (B) The cellular toxicity of TN-20 toward alveolar cells is lower when TN-20 was mixed with 50 µM of glyphosate at concentrations of 0.78, 1.56, 3.13, 6.25, 12.5, 25, 50, and 100 µM. It is also lower when TN-20 was mixed with 100 µM of glyphosate at concentrations of 12.5, 25, 50, and 100 µM. (C) In heart cells, the cellular toxicity of TN-20 is greater when mixed with 50 µM of glyphosate at concentrations of 25 and 50 µM, as well as when TN-20 was mixed with 100 µM of glyphosate at concentrations of 25, 50, and 100 µM.
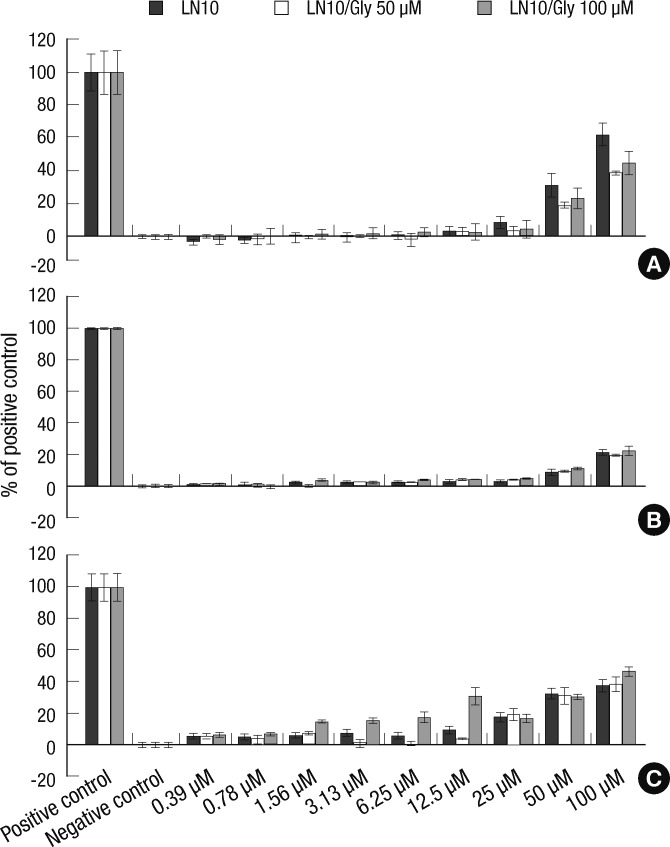
The cellular toxicity of LN-10 in the presence or absence of glyphosate
The cellular toxicity of LN-10 towards the fibroblast-like cells was found to be increased in the presence of 50 µM glyphosate when LN-10 concentrations of 50 µM and 100 µM were examined. Similarly, the cellular toxicity of 100 µM of LN-10 was also greater in the presence of 100 µM of glyphosate, when compared to that of LN-10 alone (Fig. 3A). In the alveolar cells, no significant difference in the cellular toxicity of LN-10 was observed between samples in the presence or absence of glyphosate (Fig. 3B). Although no significant difference between the cellular toxicity of LN-10 towards the heart cells was observed in the presence of 50 µM of glyphosate, it was found to be greater when select concentrations of LN-10 were mixed with 100 µM of glyphosate (Fig. 3C).
Fig. 3.
Cellular toxicity of LN-10 in the presence or absence of glyphosate. (A) LDH assays examining the toxicity of TN-20 in the fibroblast-like cells revealed that there was significantly greater toxicity in the mixtures containing 50 µM glyphosate and 50 µM and 100 µM LN-10 than LN-10 alone. The cellular toxicity of LN-10 is also greater in a mixture of 100 µM of both LN-10 and glyphosate. (B) In alveolar cells, no significant difference in the cellular toxicity of LN-10, with or without glyphosate is observed. (C) The cellular toxicity of LN-10 toward heart cells is greater than LN-10 alone when mixed with 100 µM of glyphosate at concentrations of 1.56, 3.13, 6.25, and 12.5 µM, although no significant differences in cellular toxicity were observed when LN-10 was mixed with 50 µM of glyphosate.
DISCUSSION
The principal finding of the present study is that the effects of glyphosate on the cellular toxicity of TN-20 and LN-10 surfactants, which have comparable toxicities alone, vary according to the cell line, and can be additive, antagonistic, or result in potentiation. Additivity refers to a situation where the combined effect of two substances is equal to the sum of their individual effects, whereas antagonism occurs if two substances counteract each other's action, and/or if one substance, which is in itself non-toxic, suppresses the effect of the other substance. Potentiation is the phenomenon whereby a substance that does not exert a toxic effect on its own strengthens the toxicity of another toxic substance (15). In our study, it was found that the mixture toxicity of glyphosate with both the surfactants was antagonistic in the fibroblast-like cells. The TN-20 and glyphosate mixture was found to be antagonistic in the alveolar cells, whereas the LN-10 and glyphosate mixture was found to be additive. Finally, in the heart cell line, mixtures of glyphosate with both TN-20 and LN-10 resulted in potentiation. As this study was not designed to assess the qualitative aspects of the combined action, we cannot explain the mechanisms of the potentiation and/or antagonism; however, there is strong evidence those chemicals with common specific modes of action work together synergistically to produce combination effects that are greater than the effects of each single mixture component. Bearing in mind that this is an in vitro experiment, we believe our findings also suggest that the mixture toxicity of surfactants and glyphosate would likely be cell and/or organ specific in vivo. Therefore, further toxicokinetic studies should be carried out in vivo.
The quantitative aspect of the toxicity of mixtures is often expressed in toxic unit (TA). The dose (DA) is then related to the effective dose (EDA) of the substance, hence: TA = DA/EDA toxic units (16). For a mixture of 2 substances, results can be presented as the quantity of substance A in the mixture against the quantity of substance B. The easiest way to compare results is therefore to express both quantities as effective doses (concentration) and toxic units: TA = DA/EDA and TB = DB/EDB. In our experimental setting, the 2 surfactants manifested cytotoxicity at concentrations between 1 µM and 100 µM, whereas glyphosate was not found to be cytotoxic in that range. In order to observe any mixture toxicity, a relatively high, but comparable concentration of glyphosate (50 or 100 µM) was therefore added to the surfactants. These two glyphosate concentrations were chosen because, first, we believed that if mixture toxicity between glyphosate and surfactants occurred, the reaction would proceed based on a mole to mole ratio, and the concentration of chemicals would therefore be comparable to each other. Second, we wanted the concentrations of the chemicals used in our vitro experiment to be comparable to potential chemical concentrations in vivo. In general, glyphosate herbicide products are composed of 25%-40% glyphosate and 10%-20% surfactants, by volume. Due to the lack of toxicokinetic studies using TN-20 and LN-10 in vivo, we cannot determine the in vitro equivalent concentration of these surfactants to the blood concentration that cause glyphosate-surfactant toxicity. However, in a previous case report, surfactant concentrations of 100 ppm in the brain, 550 ppm in the blood, 60 ppm in the liver, and 3,650 ppm in the kidney were reported upon post-mortem analysis of tissue samples by HPLC/PCR (10).
We chose three different cell lines for our experiment (fibroblast-like cells, alveolar cells, and heart cells) based on our assumption that each in vitro cell line would have biological similarity to in vivo cells. Although we ideally would have also included a nerve cell line in this study, as central nervous system deterioration is a common complication of glyphosate-surfactant toxicity, nerve cell lines were found in preliminary studies to grow far too slowly for inclusion. Our results with regard to mixture toxicity in the heart cell line would, however, potentially be relevant to the cardiovascular instability that is frequently observed in patients with acute glyphosate herbicide intoxication(6), including hypotension and arrythmia.
Our study has a limit to extend the result of in vitro experiment to the clinical practice. First of all, the concentration of glyphosate and surfactants used in the vitro experiment may not be that of the serum levels of the patients with acute glyphosate intoxication. Furthermore, the relative concentration (glyphosate-surfactant ratio) used in our experiment may not be that of in vivo. Even with these limitation, our findings provide in vitro evidence of mixture toxicity between glyphosate and both TN-20 and LN-10 surfactants. It also demonstrates that these effects may be additive, antagonistic, or result in potentiation, depending on the cell line being examined. With this, we believe that mixture toxicity is likely involved in glyphosate-surfactant toxicity in patients with acute glyphosate herbicide intoxication.
Footnotes
This work was supported by "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ008246)" Rural Development Administration, Republic of Korea.
References
- 1.Chen YJ, Wu ML, Deng JF, Yang CC. The epidemiology of glyphosate-surfactant herbicide poisoning in Taiwan, 1986-2007: a poison center study. Clin Toxicol (Phila) 2009;47:670–677. doi: 10.1080/15563650903140399. [DOI] [PubMed] [Google Scholar]
- 2.Roberts DM, Buckley NA, Mohamed F, Eddleston M, Goldstein DA, Mehrsheikh A, Bleeke MS, Dawson AH. A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin Toxicol (Phila) 2010;48:129–136. doi: 10.3109/15563650903476491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradberry SM, Proudfoot AT, Vale JA. Glyphosate poisoning. Toxicol Rev. 2004;23:159–167. doi: 10.2165/00139709-200423030-00003. [DOI] [PubMed] [Google Scholar]
- 4.Moon JM, Chun BJ. Predicting acute complicated glyphosate intoxication in the emergency department. Clin Toxicol (Phila) 2010;48:718–724. doi: 10.3109/15563650.2010.488640. [DOI] [PubMed] [Google Scholar]
- 5.Sawada Y, Nagai Y, Ueyama M, Yamamoto I. Probable toxicity of surface-active agent in commercial herbicide containing glyphosate. Lancet. 1988;1:299. doi: 10.1016/s0140-6736(88)90379-0. [DOI] [PubMed] [Google Scholar]
- 6.Seok SJ, Park JS, Hong JR, Gil HW, Yang JO, Lee EY, Song HY, Hong SY. Surfactant volume is an essential element in human toxicity in acute glyphosate herbicide intoxication. Clin Toxicol (Phila) 2011;49:892–899. doi: 10.3109/15563650.2011.626422. [DOI] [PubMed] [Google Scholar]
- 7.Lee HL, Kan CD, Tsai CL, Liou MJ, Guo HR. Comparative effects of the formulation of glyphosate-surfactant herbicides on hemodynamics in swine. Clin Toxicol (Phila) 2009;47:651–658. doi: 10.1080/15563650903158862. [DOI] [PubMed] [Google Scholar]
- 8.Tai T, Yamashita M, Wakimori H. Hemodynamic effects of Roundup, glyphosate and surfactant in dogs. Jpn J Toxicol. 1990;3:63–68. [Google Scholar]
- 9.Baeurle SA, Kroener J. Modeling effective interactions of micellar aggregates of ionic surfactants with the Gauss-core potential. J Math Chem. 2004;36:409–421. [Google Scholar]
- 10.Hermens J, Canton H, Janssen P, De Jong R. Quantitative structure-activity relationships and toxicity studies of mixtures of chemicals with anaesthetic potency: acute lethal and sublethal toxicity to Daphnia magna. Aquat Toxicol. 1984;5:143–154. [Google Scholar]
- 11.Junghans M, Backhaus T, Faust M, Scholze M, Grimme L. Application and validation of approaches for the predictive hazard assessment of realistic pesticide mixtures. Aquat Toxicol. 2006;76:93–110. doi: 10.1016/j.aquatox.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Deneer JW. Toxicity of mixtures of pesticides in aquatic systems. Pest Manag Sci. 2000;56:516–520. [Google Scholar]
- 13.Song HY, Kim YH, Seok SJ, Gil HW, Yang JO, Lee EY, Hong SY. Cellular toxicity of surfactants used as herbicide additives. J Korean Med Sci. 2012;27:3–9. doi: 10.3346/jkms.2012.27.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini GE. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ Health Perspect. 2005;113:716–720. doi: 10.1289/ehp.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phoa FK, Xu H, Wong WK. The use of nonregular fractional factorial designs in combination toxicity studies. Food Chem Toxicol. 2009;47:2183–2188. doi: 10.1016/j.fct.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown VM. The calculation of the acute toxicity of mixtures of poisons to rainbow trout. Water Res. 1968;2:723–733. [Google Scholar]