Abstract
We investigated serotype distribution and antimicrobial resistance of pneumococcal carriage isolates from children after optional immunization with the 7-valent pneumococcal conjugate vaccine (PCV7) in Korea. From June 2009 to June 2010, 205 (16.5%) pneumococcal isolates were obtained from 1,243 nasopharyngeal aspirates of infants and children at Seoul National University Children's Hospital, Korea. Serotype was determined by Quellung reaction and antibiotic susceptibility was tested by E-test. The results were compared to previous studies done in the pre-PCV7 period. In this study, the most common serotypes were 6A (15.3%), 19A (14.7%), 19F (10.2%), 35B (7.3%), and 6D (5.6%). The proportion of PCV7 serotypes decreased from 61.9% to 23.8% (P < 0.001). The overall penicillin nonsusceptibility rate increased from 83.5% to 95.4% (P = 0.001). This study demonstrates the impact of optional PCV7 vaccination in Korea; the proportion of all PCV7 serotypes except 19F decreased while antimicrobial resistant serotypes 6A and 19A further increased.
Keywords: Streptococcus pneumoniae, Serotype, Drug Resistance, Pneumococcal Vaccines
INTRODUCTION
Streptococcus pneumoniae is a major cause of invasive infections in young infants and children. S. pneumoniae is part of the commensal flora of the upper respiratory tract, colonizing the nasopharyngeal (NP) niche. Sometimes NP carriage can progress to respiratory or even systemic pneumococcal disease in the host and also serves as a reservoir for the horizontal spread of pneumococcus within the community (1). Since the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7), the incidence of invasive pneumococcal diseases (IPD) has been decreasing; however, an increase of IPD caused by non-vaccine serotypes is being observed (2, 3). There were several studies concerning the impact of PCV7 on pneumococcal NP carriage rate, showing no change in the overall carriage rate, unlike IPD. In most carriage studies performed in countries with routine PCV7 immunization, carriage of vaccine serotypes almost disappeared and replacement with non-vaccine serotypes were clearly observed (4-7).
In Korea, PCV7 was introduced in November 2003 as an optional vaccine. In 2009, the assumed PCV7 coverage rate of infants younger than two years was about 50%-70%, depending on the region (data given by sales volume and annual birth rate collected by the pharmaceutical company). PCV7 is no longer in use, ever since two newly developed extended-valency pneumococcal conjugate vaccines were introduced in Korea in 2010. Before the introduction of PCV7 in Korea, there were two studies done on pneumococcal serotypes of NP carriage isolates, each performed in 1997-1998 and 2001-2002, respectively (8, 9).
The aim of this study is to investigate the distribution of pneumococcal serotypes and antimicrobial susceptibilities of NP carriage isolates from children after optional immunization of PCV7 in Korea, thereby analyzing its impact on the NP carriage and providing a basis for further establishment of appropriate immunization strategy.
MATERIALS AND METHODS
Pneumococcal isolates
NP aspirates were obtained from infants and children aged 18 yr or less who presented with respiratory symptoms at Seoul National University Children's Hospital from June 2009 to June 2010.
Once the NP specimen was obtained, it was refrigerated for less than three days before pneumococcal culture. After vortexing the NP specimen, 10 µL of NP aspirate was inoculated onto a 5% defibrinated sheep blood agar plate, then incubated overnight at 37℃ in a 5% CO2 chamber. Identification of S. pneumoniae was based on the presence of alpha-hemolysis and inhibition by optochin. For subsequent isolates collected from the same child, repeated serotypes were excluded from the analysis and only initial isolates were included in the study. Different serotypes from the same child were included in the analysis.
The results of serotype distribution and antimicrobial resistance of isolates recovered from children aged < 5 yr in 2009-2010 (post-PCV7 period) were compared to previous pneumococcal NP carriage studies done on Korean children before the introduction of PCV7 (pre-PCV7 period). For the pre-PCV7 period, data of a total 139 isolates from two different studies were used: 72 NP isolates from healthy children aged 1-5 yr in 1997-1998 (8) and 67 NP isolates from children aged < 5 yr who visited pediatric clinics in 2001-2002 (9). Nontypeable pneumococci were excluded from the analysis for comparison of serotype distribution between pre-PCV7 period and post-PCV7 period.
Serotype determination
Serotype determination of all isolates was done by Quellung reaction using antiserum (Statens Serum Institute, Copenhagen, Denmark). To assign serotypes 6C and 6D, all serogroup 6 strains were screened for wciNβ and wciP6B genes by using 2 simplex PCRs and subsequent sequencing analyses as previously described (10). Serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F were classified as PCV7 serotypes. Non-PCV7 serotypes included all other serotypes.
Antimicrobial drug susceptibility testing
All pneumococcal isolates obtained from children aged < 5 yr except non-typeable strains were tested for minimal inhibitory concentrations (MICs) of 8 antimicrobial drugs (penicillin, cefotaxime, chloramphenicol, tetracycline, clindamycin, erythromycin, trimethoprim-sulfamethoxazole, and levofloxacin) by E-test (AB Biodisk, Solna, Sweden). For penicillin, susceptibility was analyzed by epidemiological breakpoints (same as oral penicillin breakpoints of the Clinical and Laboratory Standards Institute 2008 Guideline) (11, 12) for comparison with previous studies (8, 9). Multidrug resistance was defined as nonsusceptibility to ≥ 3 antimicrobial drug classes.
Statistical analysis
Statistical analysis was performed by using the SPSS software version 19.0 (SPSS, Chicago, IL, USA). Serotype proportion in each period (pre-PCV7 and post-PCV7) was compared using the chi-squared or Fisher exact test, as appropriate. A P value < 0.05 was considered statistically significant.
Ethics statements
The study protocol was approved by the institutional review board of Seoul National University Hospital (IRB registration number-H-0912-034-303). Informed consent was exempted, since NP aspirates were obtained as a standard of patient care to identify etiologic agents of respiratory tract infections.
RESULTS
Pneumococcal isolates
A total of 1,243 NP aspirates were collected from June 2009 through June 2010. Sixty-two percent of the subjects (774/1,243) were male and the median age of children was 2.28 yr (range: 0.01-18.88 yr). Two hundred five pneumococcal isolates were recovered, with a detection rate of 16.5%. The male:female ratio was 1:0.64 (125:80) and the median age of children who were pneumococcal carriers was 2.18 yr (range: 0.11-15.66 yr). The percentages of the carriers by age group were 45.9% (< 2 yr), 24.9% (2-4 yr), and 29.2% (≥ 5 yr), respectively. The pneumococcal detection rate of each age group was 16.3% (93/571) among children aged < 2 yr, 24.9% (77/309) among children aged 2-4 yr, and 9.6% (35/363) among children aged ≥ 5 yr. The detection rate was significantly high among children aged between 2-4 yr (by chi-squared test, P < 0.001). Vaccination history of the children was not available.
Serotype distributions in post-PCV7 period (2009-2010)
Among the 205 pneumococcal isolates, 28 (13.7%) isolates were nontypeable, which means no capsules for those pneumococci. Serotype distribution of 205 pneumococci demonstrated that PCV7 serotypes accounted for 16.6% (34/205), and most common serotypes 6A, 19A and 19F accounted for 13.2%, 12.7%, and 8.8%, respectively.
A total of 177 isolates for which specific serotypes were determined were included for comparison of serotype distribution between the pre-PCV7 period and post-PCV7 period. Table 1 shows the overall serotype distribution of the 177 nasopharyngeal pneumococcal isolates obtained from 175 children. A single pneumococcal isolate was recovered from each child, with the exception of two children, from whom two different isolates were recovered from different specimens. Among the 177 pneumococcal isolates obtained during 2009-2010, the most common serotypes were 6A (n = 27, 15.3%), 19A (n = 26, 14.7%), 19F (n = 18, 10.2%), 35B (n = 13, 7.3%), 6D (n = 10, 5.6%), and 34 (n = 10, 5.6%). The proportion of PCV7 serotypes accounted for 19.2% (34 isolates). In subgroup analysis by age groups, serotype 6A occupied a constant proportion of the isolates regardless of age (14.7-15.7%). On the other hand, serotypes 19A and 19F occupied a large proportion of the isolates recovered from children aged < 2 yr (18.1% and 12.0%, respectively), while the proportion was smaller in children aged ≥ 5 yr (7.7% and 3.8%, respectively).
Table 1.
Distribution of serotypes among 177 pneumococcal isolates from nasopharyngeal aspirates of children in Korea by age group, 2009-2010*
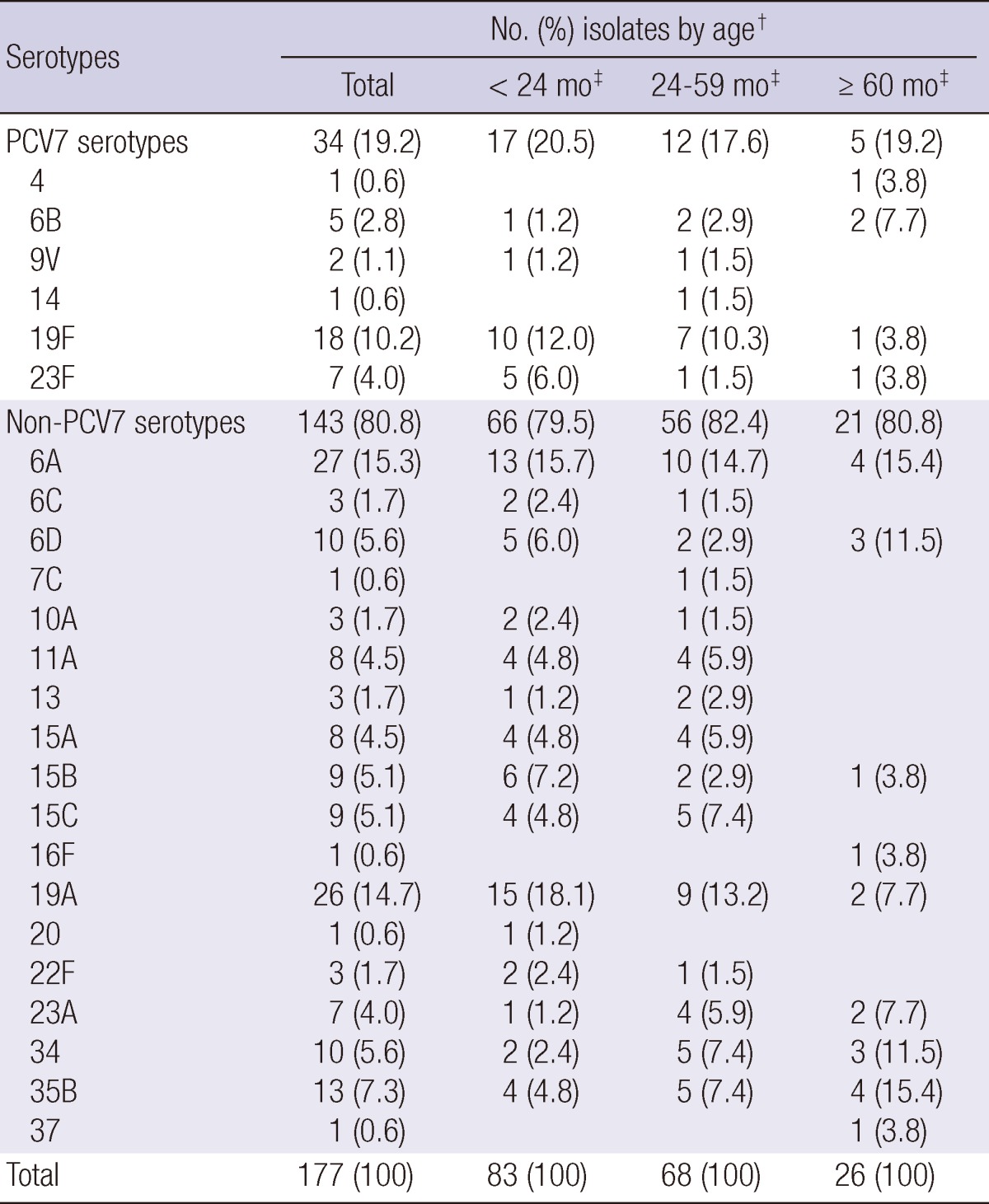
*PCV7, 7-valent conjugate vaccine; †Percentages have been rounded; ‡mo, months.
Changes in serotype distributions, pre-versus post-PCV7 period
For the pre-PCV7 period, data of 139 pneumococcal isolates from two studies were used; 72 NP isolates from healthy children aged 1-5 yr in 1997-1998 and 67 NP isolates from children < 5 yr who visited pediatric clinics in 2001-2002. Serotypes were determined by Quellung reaction in these two studies. The most common serotypes among these isolates were 19F (n = 30, 21.6 %), 23F (n = 24, 17.3%), 14 (n = 17, 12.2%), 6A (n = 14, 10.1%), 19A (n = 11, 7.9%), 6B (n = 10, 7.2%) and 11A (n = 10, 7.2%). PCV7 serotypes accounted for 61.9% (86 isolates) (8, 9).
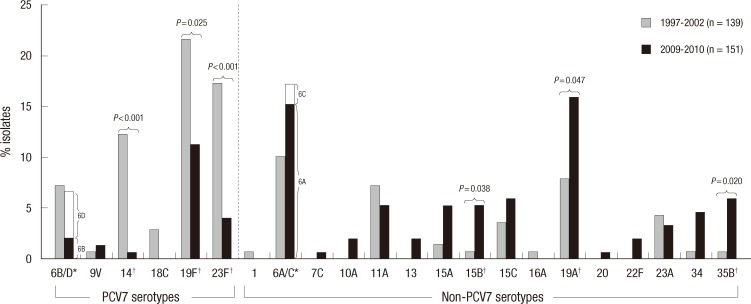
To compare changes in serotype distribution among pneumococcal carriage isolates between pre-and post-PCV7 period, the serotype distribution in children aged < 5 yr of age is shown in Fig. 1. In this comparison, since the differentiation of 6C/6D from 6A/6B was not done in the 1997-2002 studies, serotypes 6C/6D in the 2009-2010 results were regarded as 6A/6B, and 6D was regarded as a PCV7 serotype.
Fig. 1.
Distribution of serotypes with regard to 7-valent conjugate vaccine (PCV7) among pneumococcal isolates from nasopharyngeal aspirates of children < 5 yr of age during 1997-2002 and 2009-2010 periods in Korea. *Differentiation of 6C/6D from 6A/6B was not done in 1997-2002 studies; †Statistically significant (P < 0.05, by Fisher exact test) increase or decrease in the proportion of each serotypes are marked.
The percentage of PCV7 serotypes significantly decreased from 61.9% in the pre-PCV period to 23.8% in the post-PCV period (by Fisher exact test, P < 0.001). This reduction was mostly due to the decrease of serotypes 14 (from 12.2% to 0.7%, P < 0.001), 18C (from 2.9% to 0%, P = 0.052), 19F (from 21.6% to 11.3%, P = 0.025), and 23F (from 17.3% to 4.0%, P < 0.001), while other PCV7 serotypes 6B/6D (from 7.2% to 6.6%) and 9V (from 0.7% to 1.3%) showed no significant change in incidence. Despite the decrease in carriage of PCV7 serotypes, 19F remained the third most common serotype in the post-PCV7 period as well.
While dramatic reductions in PCV7 serotypes were observed, 6A/6C (from 10.1% to 17.2%, P = 0.089) and 19A (from 7.9% to 15.9%, P = 0.047) serotypes, which already made up large proportions of the isolates before PCV7 introduction, further increased after PCV7 use. The proportion of non-PCV7 serotypes also increased; especially 15B (from 0.7% to 5.3%, P = 0.038), and 35B (from 0.7% to 6.0%, P = 0.020). While serotypes 15A (from 1.4% to 5.3%, P = 0.106), 15C (from 3.6% to 6.0%, P = 0.418), and 34 (from 0.7% to 4.6%, P = 0.068) also increased, but did not reach a statistically significant level.
Antimicrobial drug susceptibility testing in post-PCV7 period (2009-2010)
The MIC was tested for 151 isolates collected from NP aspirates of children < 5 yr of age during 2009-2010. The susceptibility data are shown in Table 2. Nonsusceptibility rates for penicillin (oral) and erythromycin were 95.4% (144 isolates) and 92.7% (140 isolates), respectively.
Table 2.
In vitro activity of antimicrobial agents against 151 pneumococcal isolates from nasopharyngeal aspirates of Korean children < 5 yr of age, 2009-2010
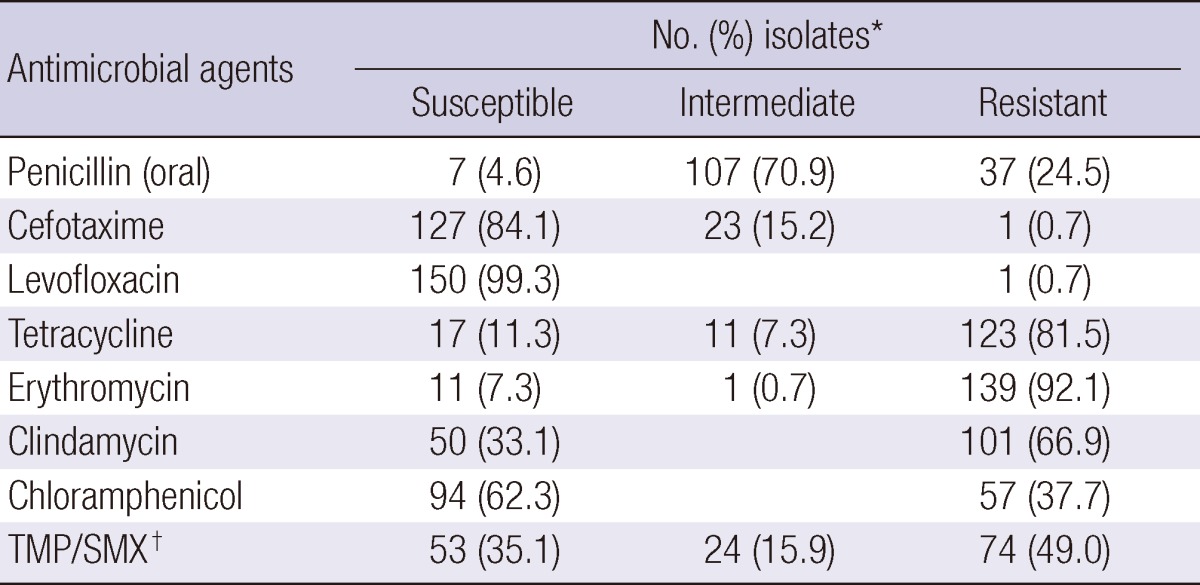
MIC interpretive standard was based on Clinical and Laboratory Standards Institute (2008). *Percentages have been rounded; †TMP/SMX, trimethoprim/sulfamethoxazole.
A total of 139 (92.1%) isolates showed multidrug resistance (MDR), nonsusceptible to ≥ 3 antimicrobial drug classes. All PCV7 serotype isolates (n = 29, 19.2%) were MDR. Serotype 19A (n = 24, 15.9%) was the most common serotype that had penicillin nonsusceptibility and MDR, followed by 6A (n = 23, 15.2%), 19F (n = 17, 11.3%), 35B (n = 9, 6.0%), 15C (n = 9, 6.0%), 15A (n = 8, 5.3%), and 15B (n = 8, 5.3%). Also, all of the serogroup 6 isolates including serotypes 6C (n = 3, 2.0%) and 6D (n = 7, 4.6%) were MDR.
Changes of penicillin susceptibility, pre-versus post-PCV7 period
In the post-PCV7 period, penicillin nonsusceptibility rate increased to 95.4% compared to 83.5% of the pre-PCV7 period (by Fisher exact test, P = 0.001). Penicillin nonsusceptibility rate increased in both PCV7 serotype (from 83.7% to 100%, P = 0.010) and non-PCV7 serotype (from 83.0% to 93.9%, P = 0.044) groups. The changes in penicillin susceptibility for each serotype are shown in Table 3.
Table 3.
Penicillin susceptibility results of each pneumococcal serotype isolated from nasopharyngeal aspirates of children < 5 yr of age during 1997-2002 and 2009-2010 periods in Korea*,†
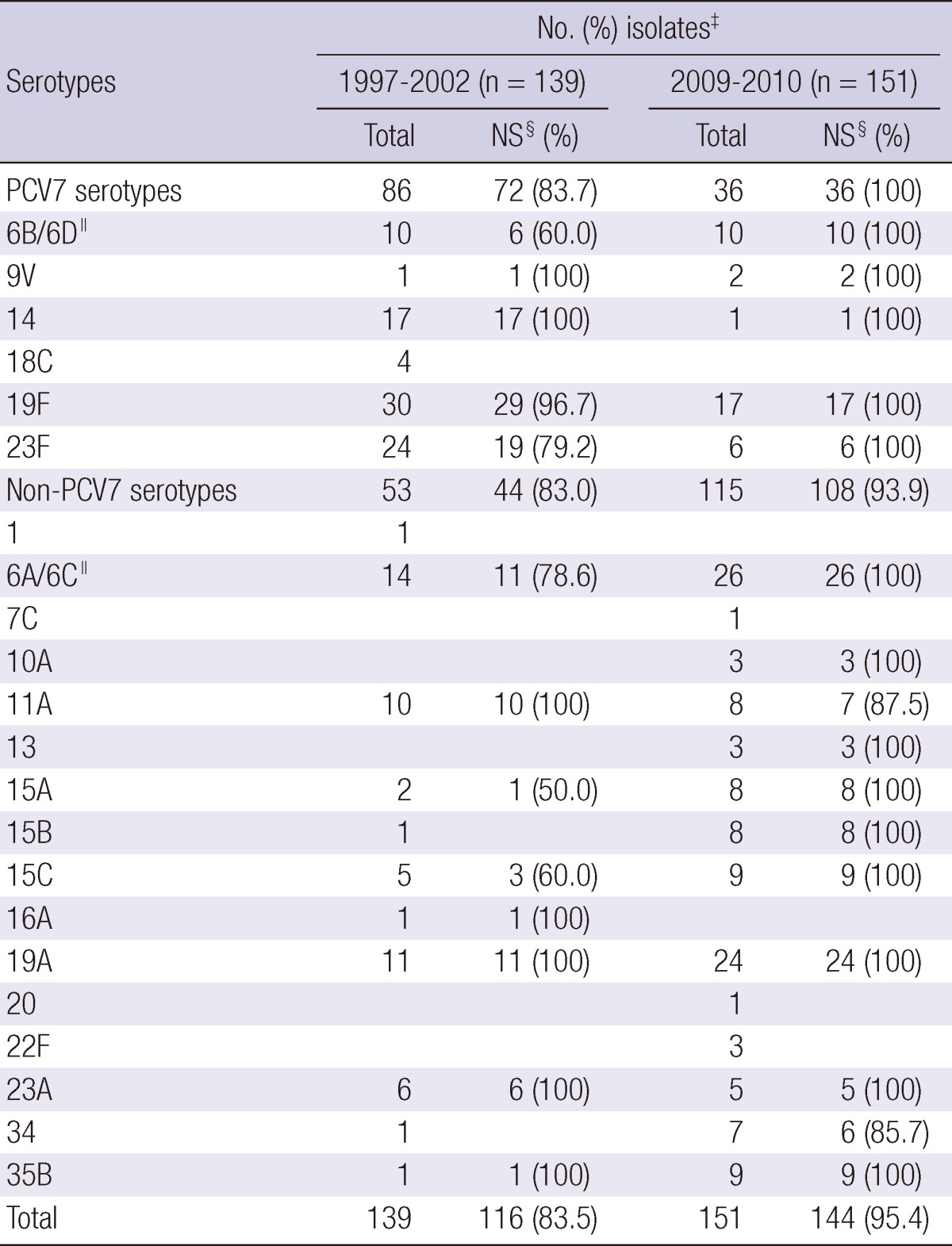
*For 1997-2002 period, data of 139 pneumococcal isolates from two studies were used; 72 isolates in 1997-1998 and 67 isolates in 2001-2002 (8, 9); †PCV7, 7-valent conjugate vaccine; ‡Percentages have been rounded; §NS, Pneumococci nonsusceptible to penicillin; ∥Differentiation of serotype 6C/6D from 6A/6B was not done in 1997-2002 studies. 6C isolates are included in 6A and 6D isolates are included in 6B. During 2009-2010 period, number of serotype 6A, 6B, 6C, and 6D were 23, 3, 3, and 7, respectively.
PCV7 serotypes 19F (21.6%), 23F (17.3%) and 14 (12.2%) were the most common penicillin nonsusceptible serotypes in the pre-PCV7 period. In the post-PCV7 period, the proportion of PCV7 serotype isolates decreased, although serotype 19F (11.3%) remained the third most common penicillin nonsusceptible serotype, whereas serotype 23F (4.0%) and 14 (0.7%) nearly vanished. Serotypes 19A and 6A were the most common penicillin nonsusceptible serotypes in the post-PCV7 period. Serotype 19A was 100% nonsusceptible to penicillin in both the pre- and post-PCV7 period. For serotype 6A/6C, the nonsusceptibility rate increased from 78.6% in the pre-PCV7 period to 100% in the post-PCV7 period (P = 0.037). The increasing serotypes (34, 35B, and serogroup 15) in the post-PCV7 period also showed high percentages of nonsusceptibility.
To analyze serotype-related resistance, we compared the proportion of each serotypes in penicillin nonsusceptible isolates. In the pre-PCV7 period, PCV7 serotypes accounted for 62.1% (72/116) of the total penicillin nonsusceptible isolates. However in the post-PCV7 period, PCV7 serotypes accounted for only 25.0% (36/144), showing significant decrease in PCV7 serotypes as penicillin nonsusceptible pneumococci (P < 0.001). Among non-PCV7 serotypes, serotype 6A/6C increased from 9.5% to 18.1% (P = 0.052) and serotype 19A also increased from 9.5% to 16.7% (P = 0.102), though statistically insignificant.
DISCUSSION
This study highlights changes in serotype distribution of nasopharyngeal carriage in children before and after the introduction of PCV7 in a country where PCV7 has been used as an optional vaccine for 7 yr, since 2003. In the pre-PCV7 period, PCV7 serotypes 19F, 23F, and 14 were the most common serotypes, but in the post-PCV7 period, all PCV7 serotypes except 19F nearly vanished and serotypes 6A, 19A and 19F become dominant. In addition, the substantial increase in serotypes 35B and 6D was interesting.
Change in serotype distribution among penicillin nonsusceptible isolates was noteworthy. In the pre-PCV7 period, penicillin nonsusceptible isolates were mostly PCV7 serotypes, but in the post-PCV7 period, they were mostly non-PCV7 serotypes like 6A, 19A. The only remaining PCV7 serotype was 19F, making non-PCV7 serotypes more responsible for the increase in the penicillin nonsusceptibility rate.
Previously, many studies on pneumococcal NP carriage were conducted in countries with routine PCV7 immunization. However, not all countries have PCV in their national immunization program. Korea is a country with optional PCV immunization, with a gradual increase in coverage rate after the introduction of PCV7 in 2003, from 14% in 2004 to 42% in 2006 and 72% in 2009 (data given by sales volume and annual birth rate collected by the pharmaceutical company), creating a unique ecology for pneumococci.
In studies that investigated NP colonizing pneumococci after routine PCV7 immunization, PCV7 reduced the carriage of PCV7 serotype pneumococci, which was replaced with non-PCV7 serotypes, making only a slight decrease or even no change in the overall pneumococcal carriage (4-7, 13). These results seem to be consistent with the results in our study, where the proportion of PCV7 serotype isolates decreased and non-PCV7 serotypes increased. However, the detailed percentages of each serotype were different.
We found that most PCV7 serotypes except 19F decreased after optional PCV7 use in Korea, but serotype 19F remained a major serotype. Many previous carriage studies showed a decrease in serotype 19F along with other PCV7 serotypes (5, 6, 13), but some studies done in European countries showed delayed reduction of serotype 19F (14-16). This finding may be due to the differences in effect that PCV7 has on each serotype. In clinical trials that evaluated the immunogenicity of PCV13 compared to PCV7, post-PCV7 ELISA antibody geometric mean concentration (GMC) for serotype 19F was similar to other PCV7 serotypes but opsonophagocytic assay (OPA) of PCV7 for serotype 19F showed the lowest geometric mean titer (GMT) among PCV7 serotypes (17, 18). Since OPA better predicts functional serotype-specific effectiveness than ELISA (19), vaccine-induced mucosal immunity against serotype 19F may actually be weaker, making 19F the only remaining PCV7 serotype among carriage isolates in modest PCV7 coverage regions like Korea.
It is well known that PCV7 has no cross-protection to serotype 19A (20), and in Korea, serotype 19A has been an increasing strain found in IPD isolates even before the introduction of PCV7 (21). On the other hand, PCV7 has a cross-protection effect for serotype 6A and many studies showed a reduction of serotype 6A in NP carriage, while an increase of serotype 19A was noticed at the same time (5, 6, 13). However in our result, serotype 6A shared the lead with serotype 19A as the most common NP carriage isolate of the post-PCV7 period. This is consistent with a study performed in Hong Kong, a region with 20%-40% PCV7 coverage rate, where serotypes 6A and 19A both were increasing carriage isolates (22). These differences may also be related to the functional serotype-specific effectiveness of PCV7. After PCV7 immunization, OPA GMT was very low for serotype 19A, reflecting no functional effect of PCV7 to serotype 19A. But for serotype 6A, OPA GMT reached similar levels with that of serotype 19F, the lowest in PCV7 serotypes (17, 18). As a result, serotype 6A may present delayed reduction in NP carriage like serotype 19F in modest PCV7 coverage regions. In 2010, PCV13, the new extended-valency vaccine including serotype 6A and 19A was introduced in Korea and we are anticipating changes in both serotypes during the post-PCV13 period.
We also found that newly recognized serotypes 6C and 6D accounted for 7.3% of the isolates in 2009-2010. In Korea, serotypes 6C and 6D were found from pneumococcal isolates in the 1990s and 6D occupied 10.4% of serogroup 6 (10). Since 6C and 6D were unidentifiable by standard methods, they may have been masked as serotypes 6A/6B in the pre-PCV7 results used in our study for comparison. The proportion of serotype 6A (and 6C) and 6B (and 6D) were 10.1% and 7.2% before the introduction of PCV7, then changed to 15.3% : 2.8% : 1.7% : 5.6% (6A : 6B : 6C : 6D) in the post-PCV7 period. Although serotype 6D has similar capsular characteristics with 6B, which is the only serotype in serogroup 6 included in PCV7, 6D occupied a large portion of pneumococcal isolates in spite of PCV7 use. After the introduction of PCV13, which includes serotypes 6A and 6B, continuous monitoring is required for the distribution of these two closely related serotypes 6C and 6D among nasopharyngeal carriage and IPD.
As for antimicrobial resistance, nonsusceptibility to penicillin remained steady in many of the previous studies, mostly due to an increased proportion of penicillin nonsusceptible non-PCV7 type pneumococci (4-7, 22). In this study, the very high and still increasing penicillin nonsusceptibility was remarkable. The high nonsusceptiblilty was most likely the result of antibiotic pressure and not related to PCV7, especially as Korea is one of many countries with high antimicrobial pressure. The decreasing proportion of PCV7 serotypes in penicillin nonsusceptible pneumococci despite the overall increase in penicillin nonsusceptibility means that non-PCV7 serotypes, especially common serotypes 6A and 19A, were increasing in antimicrobial resistance. The high penicillin nonsusceptibility rates in dominantly increasing serotypes were also remarkable: all serogroup 15 and serotype 35B were penicillin nonsusceptible. In addition, newly recognized serotypes 6C and 6D were all penicillin nonsusceptible.
Hanage et al. (23) explained the phenomenon of changing serotypes in the aspect of antimicrobial susceptibility by the contribution of clonal expansion and serotype switching. They found that previously PCV7-type (4, 9V, 19F) related clones with high-level penicillin resistance changed to serotype 15B/C, 19A, and 35B. Since the overall carriage of pneumococci continues to be prevalent despite vaccination, the continued exposure of NP colonized pneumococci to antibiotics may result in the selection of resistance (12). These findings suggest that highly resistant strains can continue to circulate or even increase after the elimination of vaccine serotypes and as new extended-valency vaccines are introduced.
This study has several limitations. First, because pneumococcal carriage isolates were collected at a single center in a large city, this sample may not represent the whole national data. The data from pre-PCV7 period was mixed with isolates from different location and time, therefore caution should be practiced when making direct comparisons. However, the lack of better results allowed for a rough estimation of the current epidemiologic data on pneumococcal carriage in Korea. Multicenter-based data collection is needed to investigate the change of pneumococcal carriage to evaluate the impact of newly introduced pneumococcal conjugate vaccines on serotype distribution. Second, this study did not include the individual's vaccination status and previous antibiotic history. PCV7 is an optional vaccine in Korea; therefore there was no accurate data on the coverage rate of the targeted vaccination group. Also, there is no surveillance system of antimicrobial prescriptions in Korea, making it hard to evaluate the exact impact of vaccine or antibiotic use on pneumococcal serotype distribution and antimicrobial susceptibility.
Despite these limitations, this study demonstrates the impact of optional PCV7 vaccination on serotype distribution and antimicrobial susceptibilities of NP carriage isolates from children in Korea. The proportion of all PCV7 serotypes except 19F decreased, possibly relating to the functional serotype specific effectiveness of PCV7, while antimicrobial resistant serotypes 6A and 19A further increased.
This present study has several implications for future pneumococcal immunization programs. In particular, this study demonstrates that a large proportion of highly resistant serotypes 6A and 19A still remained common after the use of PCV7, making us aware of possible changes after the introduction of extended-valency vaccines. However, there are concerns about the serotype-specific effectiveness of PCVs and the replacement of nasopharyngeal carriage with other non-vaccine related high antimicrobial resistant serotypes, creating concerns about the reduced effect of new vaccines. Future surveillance studies will be required to evaluate the impact of newly introduced vaccines on serotype distribution, in efforts to develop more extended-valent and more effective vaccines. In addition, to reduce antibiotic pressure in Korea, further strategies are needed to encourage judicious antibiotic use.
ACKNOWLEDGMENTS
We thank Sun Jung Kim and Seong Yeon Lee for their excellent technical assistance.
Footnotes
This study was supported in part by the Seoul National University Hospital grant (06-2010-0190) which was underwritten by Wyeth Research.
References
- 1.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196:1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction: eight states, 1998-2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–148. [PubMed] [Google Scholar]
- 4.Moore MR, Hyde TB, Hennessy TW, Parks DJ, Reasonover AL, Harker-Jones M, Gove J, Bruden DL, Rudolph K, Parkinson A, et al. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J Infect Dis. 2004;190:2031–2038. doi: 10.1086/425422. [DOI] [PubMed] [Google Scholar]
- 5.Park SY, Moore MR, Bruden DL, Hyde TB, Reasonover AL, Harker-Jones M, Rudolph KM, Hurlburt DA, Parks DJ, Parkinson AJ, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr Infect Dis J. 2008;27:335–340. doi: 10.1097/INF.0b013e318161434d. [DOI] [PubMed] [Google Scholar]
- 6.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein J. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–e11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivea IN, Panagiotou M, Tsantouli AG, Syrogiannopoulos GA. Impact of heptavalent pneumococcal conjugate vaccine on nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae among day-care center attendees in central Greece. Pediatr Infect Dis J. 2008;27:519–525. doi: 10.1097/INF.0b013e318168d28a. [DOI] [PubMed] [Google Scholar]
- 8.Lee JA, Kim NH, Kim DH, Park KW, Kim YK, Kim KH, Park JY, Choi EH, Lee HJ. Serotypes and penicillin susceptibility of Streptococcus pneumoniae isolated from clinical specimens and healthy carriers of Korean children. J Korean Pediatr Soc. 2003;46:846–853. [Google Scholar]
- 9.Kim SM, Hur JK, Lee KY, Shin YK, Park SE, Ma SH, Min AY, Kang JH. Epidemiological study of pneumococcal nasal carriage and serotypes among Korean children. Korean J Pediatr. 2004;47:611–616. [Google Scholar]
- 10.Choi EH, Lee HJ, Cho EY, Oh CE, Eun BW, Lee J, Kim MJ. Prevalence and genetic structures of Streptococcus pneumoniae serotype 6D, South Korea. Emerg Infect Dis. 2010;16:1751–1753. doi: 10.3201/eid1611.100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 18th information supplement. Wayne PA: National Committee for Clinical and Laboratory Standards; 2008. CLSI document M100-S18. [Google Scholar]
- 12.Dagan R, Klugman KP. Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infect Dis. 2008;8:785–795. doi: 10.1016/S1473-3099(08)70281-0. [DOI] [PubMed] [Google Scholar]
- 13.Dunais B, Bruno-Bazureault P, Carsenti-Dellamonica H, Touboul P, Pradier C. A decade-long surveillance of nasopharyngeal colonisation with Streptococcus pneumoniae among children attending day-care centres in south-eastern France: 1999-2008. Eur J Clin Microbiol Infect Dis. 2011;30:837–843. doi: 10.1007/s10096-011-1154-9. [DOI] [PubMed] [Google Scholar]
- 14.Grivea IN, Tsantouli AG, Michoula AN, Syrogiannopoulos GA. Dynamics of Streptococcus pneumoniae nasopharyngeal carriage with high heptavalent pneumococcal conjugate vaccine coverage in Central Greece. Vaccine. 2011;29:8882–8887. doi: 10.1016/j.vaccine.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues F, Nunes S, Sá-Leão R, Gonçalves G, Lemos L, de Lencastre H. Streptococcus pneumoniae nasopharyngeal carriage in children attending day-care centers in the central region of Portugal, in the era of 7-valent pneumococcal conjugate vaccine. Microb Drug Resist. 2009;15:269–277. doi: 10.1089/mdr.2009.0043. [DOI] [PubMed] [Google Scholar]
- 16.Vestrheim DF, Høiby EA, Aaberge IS, Caugant DA. Impact of a pneumococcal conjugate vaccination program on carriage among children in Norway. Clin Vaccine Immunol. 2010;17:325–334. doi: 10.1128/CVI.00435-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryant KA, Block SL, Baker SA, Gruber WC, Scott DA PCV13 Infant Study Group. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010;125:866–875. doi: 10.1542/peds.2009-1405. [DOI] [PubMed] [Google Scholar]
- 18.Kieninger DM, Kueper K, Steul K, Juergens C, Ahlers N, Baker S, Jansen KU, Devlin C, Gruber WC, Emini EA, et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine. 2010;28:4192–4203. doi: 10.1016/j.vaccine.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Schuerman L, Wysocki J, Tejedor JC, Knuf M, Kim KH, Poolman J. Prediction of pneumococcal conjugate vaccine effectiveness against invasive pneumococcal disease using opsonophagocytic activity and antibody concentrations determined by enzyme-linked immunosorbent assay with 22F adsorption. Clin Vaccine Immunol. 2011;18:2161–2167. doi: 10.1128/CVI.05313-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 21.Choi EH, Kim SH, Eun BW, Kim SJ, Kim NH, Lee J, Lee HJ. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis. 2008;14:275–281. doi: 10.3201/eid1402.070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho PL, Chiu SS, Chan MY, Ang I, Chow KH, Lau YL. Changes in nasopharyngeal carriage and serotype distribution of antibiotic-resistant Streptococcus pneumoniae before and after the introduction of 7-valent pneumococcal conjugate vaccine in Hong Kong. Diagn Microbiol Infect Dis. 2011;71:327–334. doi: 10.1016/j.diagmicrobio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Hanage WP, Bishop CJ, Huang SS, Stevenson AE, Pelton SI, Lipsitch M, Finkelstein JA. Carried pneumococci in Massachusetts children: the contribution of clonal expansion and serotype switching. Pediatr Infect Dis J. 2011;30:302–308. doi: 10.1097/INF.0b013e318201a154. [DOI] [PMC free article] [PubMed] [Google Scholar]