Abstract
The aim of this study was to analyze the natural history of atopic dermatitis (AD) and the risk factors associated with the remission of AD in Korean children. We enrolled 597 children with AD that occurred in the first year of life. A variety of factors influencing the prognosis were assessed by medical records and telephone interviews. Their outcome was classified into complete remission, intermittent, and persistent AD. AD had completely disappeared in 422 cases (70.6%), while 149 (25%) and 26 cases (4.4%) showed intermittent and persistent skin symptoms, respectively. The average healing time was 29.6 months in complete remission group and expected healing time of the AD was 60 months. None of risk factors were significant by multivariate analysis. But, in moderate-to-severe AD group, maternal diet restriction during lactation (P = 0.046) and no sensitization to cow's milk (P = 0.017) were significantly associated with remission of AD in the multivariate analysis. In conclusion, AD occurring in the first year of life disappears in a significant proportion of patients. In addition, in Korean children with moderate-to-severe AD, maternal diet restriction of allergenic food during lactation and sensitization to cow's milk seem to predict the prognosis of AD.
Keywords: Dermatitis, Atopic; Natural History; Risk Factors
INTRODUCTION
Atopic dermatitis (AD) is a common allergic disease that often develops in early childhood, beginning in the first year of life in half of the cases (1). According to the nationwide International Study of Asthma and Allergies in Childhood (ISAAC) survey in Korea, the life time prevalence of physician-diagnosed AD increased from 1995 to 2000 in 6-12 yr olds (16.6% in 1995 and 24.9% in 2000) and in 12-15 yr olds (7.3% in 1995 and 12.8% in 2000) (2). This increasing prevalence of AD is a consistent trend in the developing countries shown by ISAAC Phase One and Phase Three Study Groups (3).
Recently, loss-of-function mutations of the epidermal barrier protein, filaggrin, have been demonstrated to cause skin barrier dysfunction and predisposed to AD (4). These mutations were reported in European Caucasians as well as Asians (5, 6), and may be associated with early onset, severe and persistent AD (7, 8). However, the mutations of filaggrin gene represent a subset of AD and the clinical symptoms in a number of AD patients appear to improve as they grow older with varying rates (9-13).
Understanding natural history of AD would be helpful to predict the prognosis and to establish appropriate management strategy for each patient. Considering the AD is caused by genetic susceptibility, environmental factors or in combination, the natural history might depend upon the pathogenesis, ethnicity, geographical differences and so on. The analysis of this variation requires long-term studies during the course of AD in each region with presumably similar environment or in each group of susceptible individuals. However, there is a lack of reports about the course of AD in Korean children, although several reports are available from other countries (14-16). In this study, we aimed at analyzing the natural history of AD in Korean children occurring in their first year of life as well as the risk factors associated with persistent clinical symptoms.
MATERIALS AND METHODS
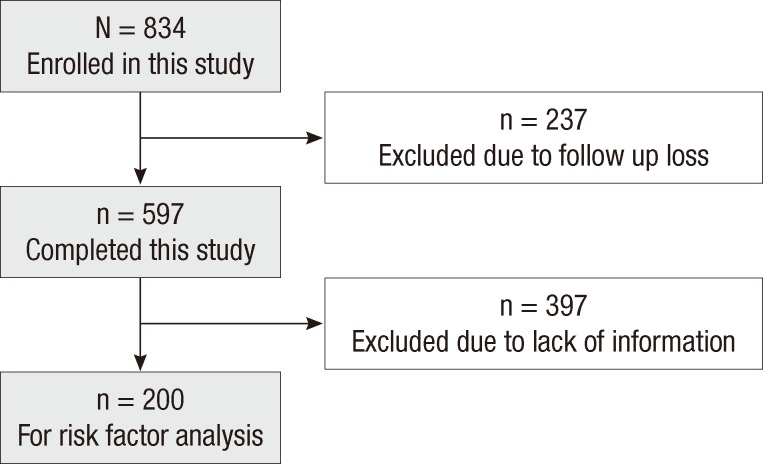
Among the patients with AD who visited Department of Pediatrics, Samsung Medical Center, Seoul, Korea between September 1995 and March 2006, those who manifested skin symptoms in the first year of life were selected in this study. The age at their first visit to our clinic was less than 36 months, and the diagnosis of AD was based on the criteria of Hanifin and Rajka (17). For this study, their medical records were reviewed and telephone survey using preformed questionnaire was done if the patients did not visit our clinic for more than one year. Out of 834 children 237 patients were excluded because of loss of follow-up after first visit. A total of 597 children who were regularly followed at least once a year at the clinic or by telephone were finally enrolled in this study (Fig. 1).
Fig. 1.
Patient enrollment.
All children were divided into 3 groups: a group with complete remission, intermittent and persistent AD, by medical records and parental telephone interviews. The "complete remission group" was comprised of children who showed no AD symptoms for over 1 yr without any medication including topical steroid. The "persistent group" included children whose skin symptoms persisted and required medical management at least once a month. The remaining patients whose symptoms were between "complete remission group" and "persistent group" were classified as "intermittent group". In the "complete remission" group, the average healing time of AD was calculated, and the expected healing time of AD, i.e., the expected duration of disease from the disease onset to remission in the case of AD occurring in the first year of life, was also estimated by Kaplan-Meier survival analysis.
Of the 597 children with AD, we analyzed the risk factors of 200 patients whose information was completely obtained by their medical records, parental telephone interviews and blood tests. Those risk factors were gender, family history of allergic diseases, parental AD, feeding patterns during infancy, the age at introduction of solid foods, pet ownership, parental smoking, hospitalization history before 1 yr of age, serum specific IgE level to food allergens before 1 yr of age, and the disease severity at the first examination.
The family history of allergic diseases was determined as positive when at least one family member has been diagnosed with AD, asthma or allergic rhinitis by a physician. Parental AD was considered positive when either mother or father or both have AD. For the feeding patterns during infancy, duration of breast milk feeding (BMF) and/or formula feeding were evaluated. In case of BMF, maternal restriction of allergenic diet during lactation such as egg white, dairy products or peanuts was assessed. The presence of hospitalization history was determined when the patients were admitted due to serious febrile illnesses before 1 yr of age. The level of serum specific IgE against two common food allergens, egg white and cow's milk were measured by ImmunoCAP (Phadia, Uppsala, Sweden), and concentrations above 0.7 kU/L were regarded as positive. The severity of AD was evaluated on the basis of the SASSAD (Six Area, Six Sign Atopic Dermatitis) score (18), and "moderate-to-severe AD" was arbitrarily defined when the disease score at the first visit was over 20.
Kaplan-Meier survival analysis was used to analyze the natural history of AD and the expected healing time of AD. Breslow and Log Rank tests were performed to analyze the risk factors associated with AD remission, and Cox's proportional hazard regression was used for multivariate analysis. The SAS software 9.1.3 program (SAS Institute Inc., Cary, NC, USA) was used for all statistical analysis. A P value < 0.05 was considered significant.
Ethics statement
This study protocol was reviewed and approved by the institutional review board (IRB) of Samsung Medical Center, Seoul (IRB number 2012-03-081). Informed consent was exempted by the board.
RESULTS
Demographic data and Natural course of AD
Of the 597 children, 337 were boys and 260 were girls. At the first visit to our clinic, 449 children were before 1 yr of age, 98 between 13 and 24 months, and 50 between 25 and 36 months. Forty percent of children (n = 207) had a family history of allergic diseases and 7.8% (n = 47) had parental AD. Regarding AD severity, the mild group (SASSAD score < 20) consisted of 238 children (73.9%), and the moderate-to-severe group (SASSAD score ≥ 20) had 84 children (26.1%) (Table 1). Sensitization to either one of egg white and cow's milk was found in 176 children (54%), although only 328 patients out of 597 performed a blood test. We did not conduct the blood test in the patients with mild AD unless the aggravation of skin symptoms after ingestion of food was clear in history.
Table 1.
Characteristics of 597 children with atopic dermatitis
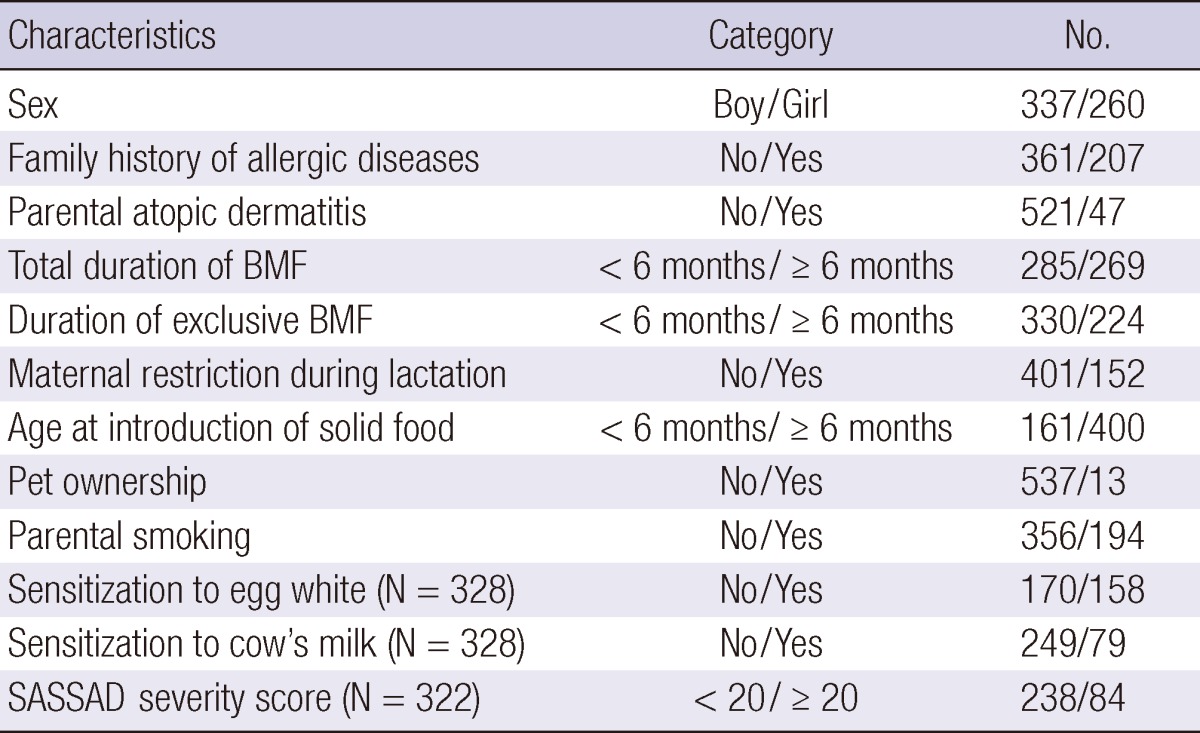
BMF, Breast milk feeding; SASSAD, Six Area Six Sign in Atopic Dermatitis.
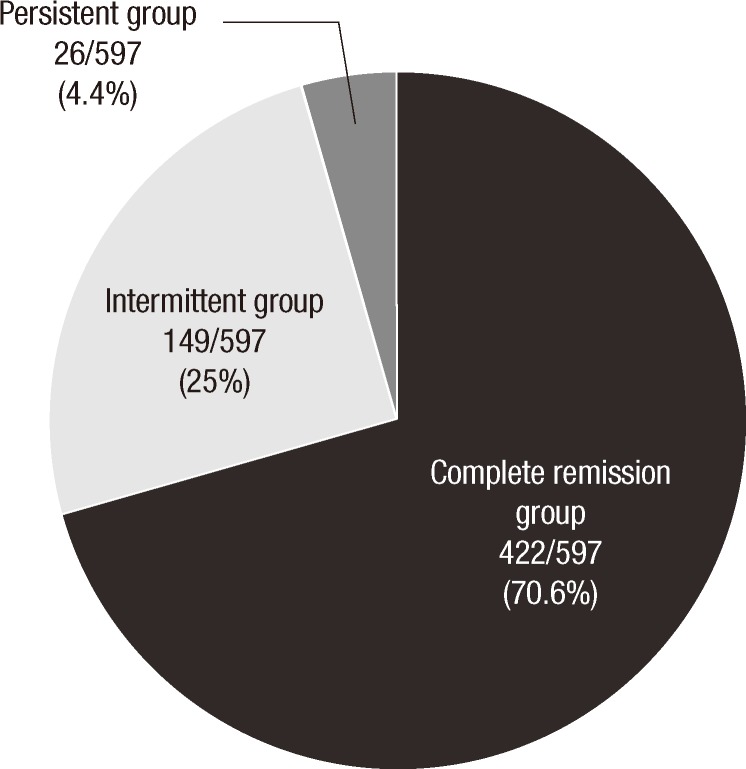
Of the 597 children, AD disappeared completely in 422 (70.6 %), while 149 (25%) and 26 (4.4%) patients had an intermittent and persistent symptoms (Fig. 2). The mean duration of follow-up was 60 months (range 12-137 months) and 60, 62, 58 months in each group, respectively. The mean age at the last visit to our clinic was 70 months (range 38-148 months).
Fig. 2.
Natural history of atopic dermatitis in 597 children with age of onset within 1 yr after birth.
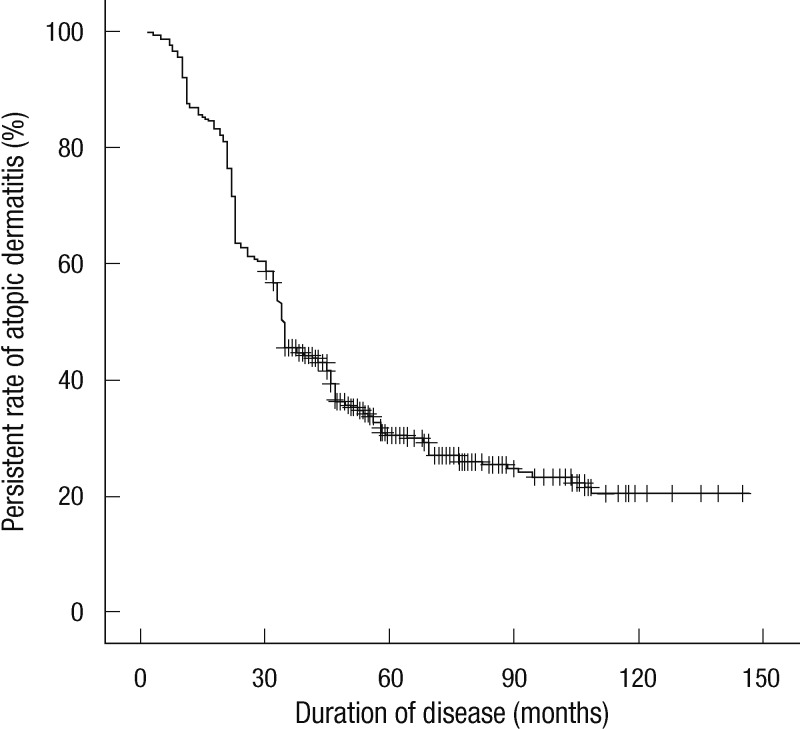
The average duration required for outgrowing AD in the complete remission group was 29.6 months. The expected healing time in the case of early AD was estimated by survival analysis, showing that 50% could have remission 34 months after its onset and 70% could have remission after 60 months (Fig. 3).
Fig. 3.
Kaplan-Meier survival analysis of persistence of atopic dermatitis with age of onset within 1 yr.
Risk factors associated with the remission of AD
Of the 200 children, 123 (61.5%) were boys and 77 (38.5%) were girls. Seventy children (35%) had a family history of allergic diseases and 17 children (8.5%) had parental AD. One hundred seventy-four children (87%) were breastfed, and breast milkfeeding for 6 months or longer was found in 108 patients (62%). Ingestion of allergenic food during lactation was restricted in 73 mothers (42%). Sensitization to egg white and cow's milk was found in 106 (53%) and 54 (27%), respectively. Regarding AD severity, the mild group (SASSAD score < 20) consisted of 151 children (75.5%), and the moderate-to-severe group (SASSAD score ≥ 20) had 49 children (24.5%) (Table 2). When we compared the characteristics between original 597 patients group and 200 patients group in risk factor analysis by using chi-square test, we could not find statistical difference in most of the variables between two groups. However, the proportion of the patients with maternal restriction during lactation was higher in 200 patients group than in 597 patients group (P < 0.05).
Table 2.
Variables predictive of remission of atopic dermatitis by univariate analysis
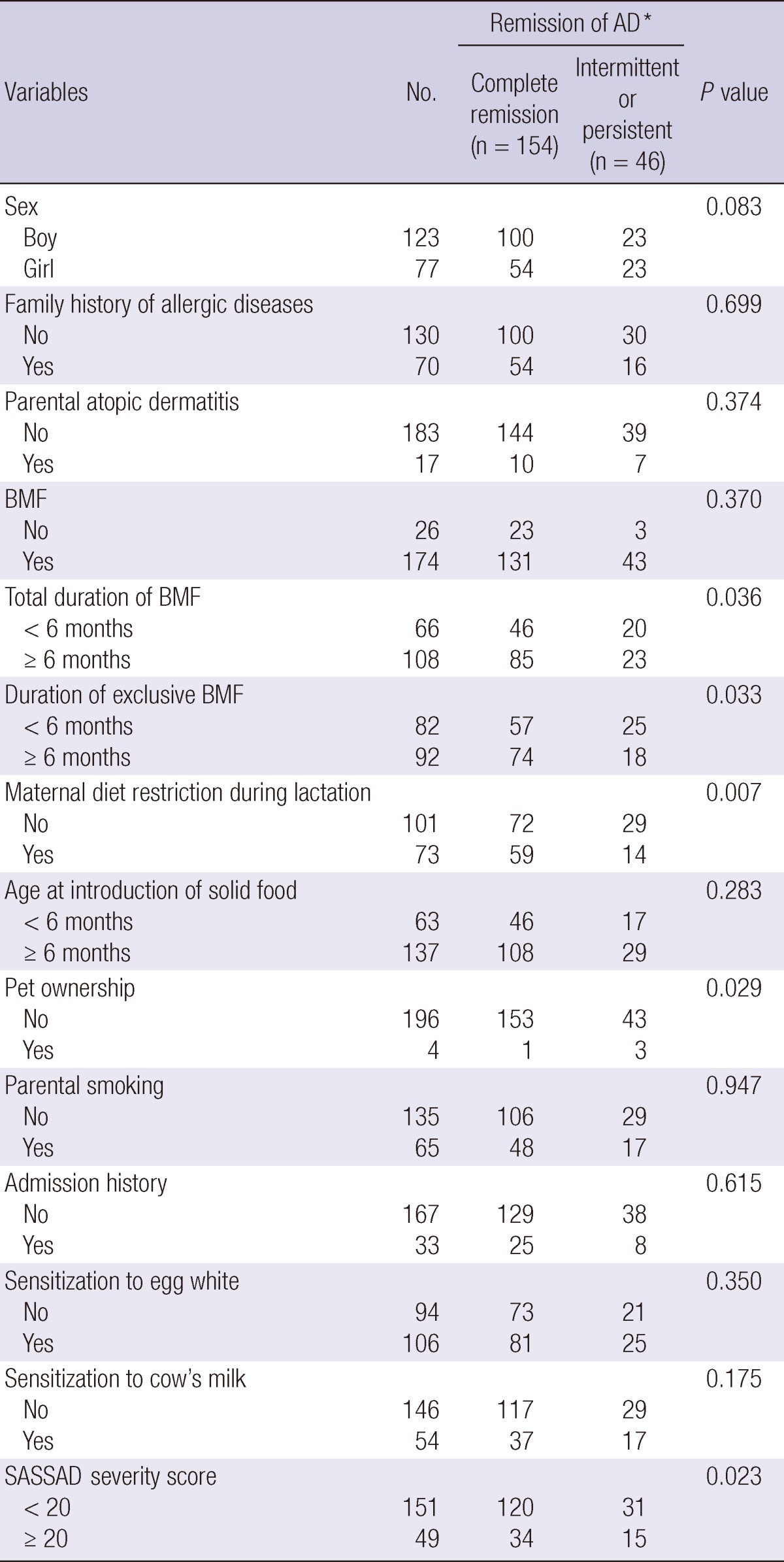
Each number in 'complete remission group' and 'intermittent or persistent group' indicates the number of event and censored cases, respectively, because the follow-up duration is different in each patient. *The remission of AD (Atopic dermatitis) was estimated using Breslow or Log rank test for time to analysis. BMF, Breast milk feeding; SASSAD, Six Area Six Sign in Atopic Dermatitis;
Of 200 patients, 154 children (77%) were in complete remission, 40 (20%) had an intermittent pattern of disease, and 6 (3%) had persistent AD symptoms. The follow-up duration of each group was 50, 54, and 51 months, respectively, with an average of 51 months (range 28-75 months). In the univariate analysis, duration of total and exclusive breast milk feeding for 6 months or more (P = 0.036, P = 0.033), maternal restriction of allergenic food during lactation (P = 0.007), no pet ownership (P = 0.029), and mild severity (P = 0.023) were associated with complete remission of AD in late childhood (Table 2). However, none of these factors were significant by multivariate analysis (data not shown).
We performed risk factor analysis after the patients were divided into mild and moderate-to-severe group according to the SASSAD score in the first year of life. In mild group, none of the risk factors showed a significant association with remission of AD (data not shown). In contrast, in moderate-to-severe group, total duration of BMF for more than 6 months (P = 0.007) and maternal restriction of allergenic food during lactation (P = 0.002) were good prognostic factors by univariate analysis (Table 3). In the multivariate analysis, maternal diet restriction during lactation (P = 0.046) and no sensitization to cow's milk (P = 0.017) were significantly associated with complete remission of AD (Table 4).
Table 3.
Variables predictive of remission of moderate-to-severe atopic dermatitis (SASSAD ≥ 20) by univariate analysis
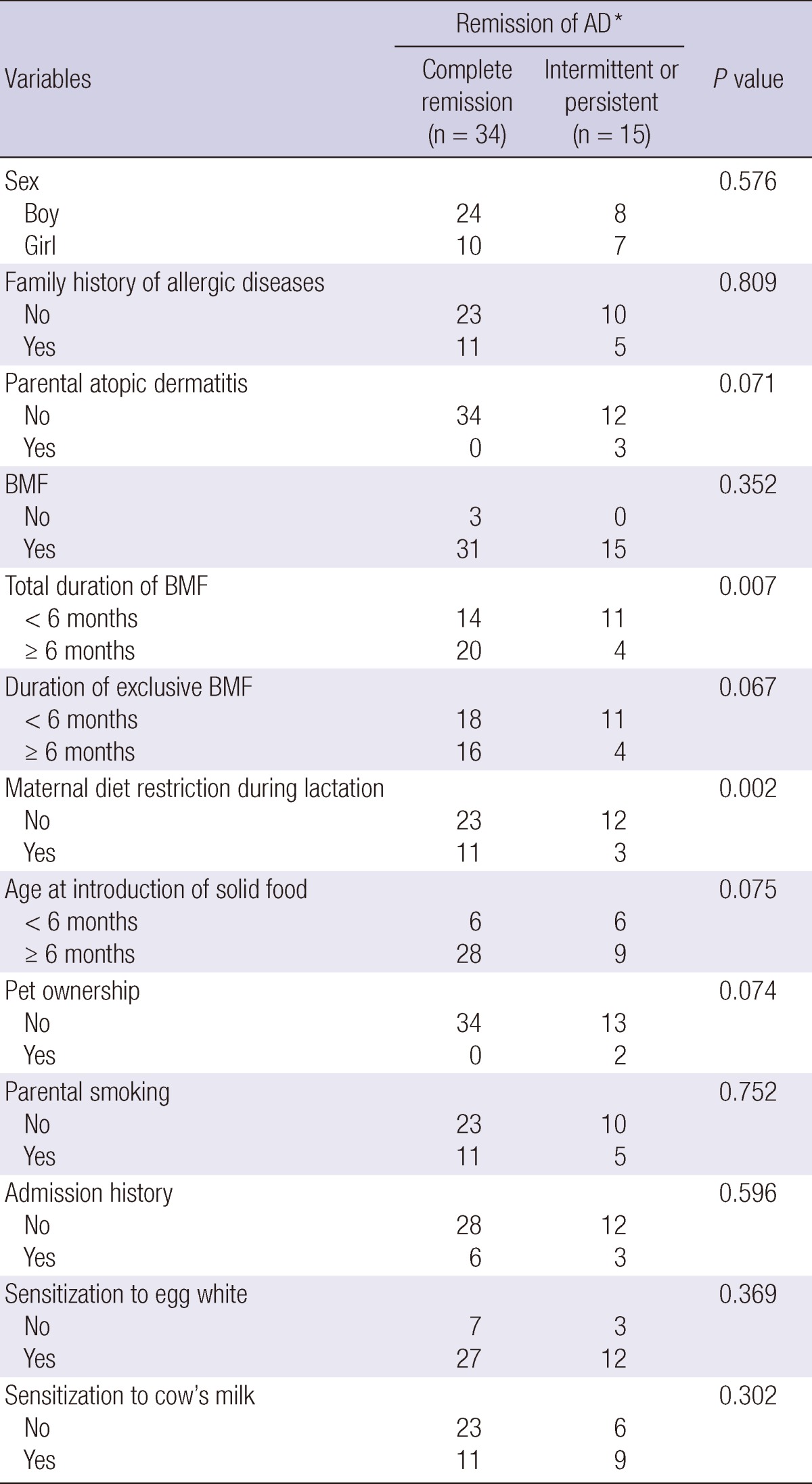
Each number in 'complete remission group' and 'intermittent or persistent group' indicates the number of event and censored cases, respectively, because the follow-up duration is different in each patient. *The remission of AD (Atopic dermatitis) was estimated using Breslow or Log rank test for time to analysis. BMF, Breast milk feeding; SASSAD, Six Area Six Sign in Atopic Dermatitis.
Table 4.
Variables predictive of remission of moderate-to-severe atopic dermatitis (SASSAD ≥ 20) by multivariate analysis
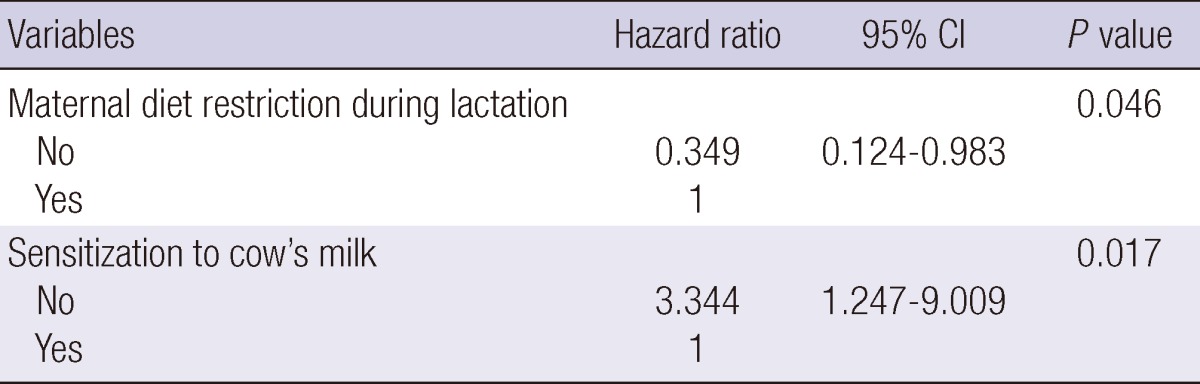
Adjusted for sex, family history of allergic disease, BMF duration, maternal diet restriction during lactation, age at introduction of solid food, parental smoking, admission history, sensitization to egg white and cow's milk. CI, confidence interval; SASSAD, Six Area Six Sign in Atopic Dermatitis.
DISCUSSION
In clinical practice, one of the misunderstandings of the parents of children with AD is that AD is a life-long persistent disease, although this is true only in a small number of patients. As a consequence, they tend to try something specific for cure, instead of maintaining conventional treatment such as bathing, application of moisturizer, avoidance of aggravating factors, and appropriate use of topical corticosteroid. Therefore, reassurance about the natural history of the disease is very important to keep this proper management, as spontaneous remission of AD over time has been observed in a majority of patients (11, 12). The prognosis of AD may be different between the countries, because clinical characteristics could be affected by both genetic and environmental factors. In this regard, our study could be a useful data in Korea since there are few reports about natural history of AD in Korean children.
Illi et al. (11) found that in children with AD occurring in the first 2 yr of life, 43.2% were in complete remission by age 3 yr, 38.3% had an intermittent pattern of disease, and 18.7% had symptoms of AD every year during 7 yr follow-up in their prospective birth cohort. In a long-term, retrospective follow-up study of 252 children with AD occurring before 3 yr of life (12), AD had completely disappeared in 124 cases (60.5%), and a longer period is required in moderate-to-severe AD. Our study showed that 70.6% were in complete remission, 25% had an intermittent pattern of disease, and 4.4% had persistent AD symptoms. This result indicates a higher remission rate and better prognosis of AD, compared with previous studies (11, 12). This might be explained by several factors including difference in genetic predisposition, selection of study population, and duration of follow-up. It has been reported that filaggrin gene (FLG) mutations are associated with persistent and more severe eczema (19, 20). Although FLG null alleles were identified in 8.8% and 12.0% in approximately 7,000 English birth cohort (19) and in the Danish birth cohort (4), respectively, there is no report on the prevalence of FLG mutations in Korea, and might be lower, like in Japanese population (5). In addition, by selection of AD patients whose clinical symptoms occurred in the first year of life, a large number of mild cases with a good prognosis could be included in our study. Furthermore, the length of follow-up might influence on the results. In this study, the total follow-up duration was relatively short, so we might miss the cases of potential relapse or late-onset AD.
Significant predictors for a poor prognosis of AD occurring during the first year of life were found to be severity of disease, atopic sensitization (11, 12). In a population-based cohort study where 2,857 children with 9 to 11 yr of age were followed up to 8 yr prospectively to investigate the course of AD over puberty, high socioeconomic status, female sex, atopic asthma, parental history of allergic diseases and having worked in a high-risk job seem to be significant predictors for the course of disease (21). Our study showed that breast milk feeding, maternal restriction of allergenic food during lactation, pet ownership and severity of AD were associated with prognosis in univariate analysis, but this significance was lost in multivariate analysis. However, we found that prognostic factors are different according to the severity of the disease. In other words, in mild cases no prognostic factors were found, while good prognosis was expected in those who were not sensitized to cow's milk and whose mother restrict ingestion of allergenic food during lactation in moderate-tosevere AD, although the patients whose mother restricted their diet during lactation seem to be included more in our risk factor analysis. Our result is consistent with the previous studies (11, 12) and suggests that food allergy might be associated with the prognosis in Korean children with moderate-to-severe AD. In our study, there was no significant difference in severity, partly because severity was assessed at one time point when the patients were first brought to our clinic. Our study also failed to show that family history of allergic diseases is a predictor for prognosis. There is concern of diagnostic accuracy as data on family history were not confirmed by objective methods such physical examination or laboratory tests.
Some limitations have to be taken into account. First, this study was done in a single institute, so selection bias might occur. In our study, the majority of patients had mild AD, which was related to good prognosis. Second, assessment of related risk factors was relied on questionnaires. Risk factor analysis was not very well controlled, and could be affected by recall bias. Third, the remission state was determined by telephone survey in some patients, not by physical examination. There is a possibility that severe persistent patients were selectively lost, although telephone survey was done in every parent or caretaker who could be contacted. Fourth, we classified our patients into mild AD and moderate-to-severe AD according to the severity at first visit. However, as the AD severity changes from time to time according to the various environmental factors, it is clear that SASSAD at first visit does not represent the AD severity of each patient during the disease course. Lastly, unintended selection bias due to retrospective study design might exist. In other words, we analyzed the risk factors only in 200 patients, not in 597 patients, so missing data of each risk factor might be distributed differently between 200 patients group and 597 patients group.
In conclusion, AD occurring in the first year of life disappears or improves in a significant proportion of children in Korea as they grow older. These data will support the parents or caretakers of infants with AD in reassuring spontaneous regression over time in most cases. In addition, in Korean children with moderate-to-severe AD, maternal diet restriction of allergenic food during lactation and sensitization to cow's milk seem to be predictive of prognosis of AD.
Footnotes
This study was financially supported by the Ministry of Environment, Republic of Korea Government.
References
- 1.Clark RA, Kristal L. Atopic dermatitis. In: Sams WM, Lynch PJ, editors. Principles and practice of dermatology. New York: Churchill Livingston; 1996. pp. 403–418. [Google Scholar]
- 2.Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, Son JA, Lee SY, Lee SI. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004;19:716–723. doi: 10.3346/jkms.2004.19.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947–954. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 5.Nomura T, Sandilands A, Akiyama M, Liao H, Evans AT, Sakai K, Ota M, Sugiura H, Yamamoto K, Sato H, et al. Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J Allergy Clin Immunol. 2007;119:434–440. doi: 10.1016/j.jaci.2006.12.646. [DOI] [PubMed] [Google Scholar]
- 6.Kang TW, Lee JS, Oh SW, Kim SC. Filaggrin mutation c.3321delA in a Korean patient with ichthyosis vulgaris and atopic dermatitis. Dermatology. 2009;218:186–187. doi: 10.1159/000163083. [DOI] [PubMed] [Google Scholar]
- 7.Barker JN, Palmer CN, Zhao Y, Liao H, Hull PR, Lee SP, Allen MH, Meggitt SJ, Reynolds NJ, Trembath RC, et al. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. J Invest Dermatol. 2007;127:564–567. doi: 10.1038/sj.jid.5700587. [DOI] [PubMed] [Google Scholar]
- 8.Sandilands A, Smith FJ, Irvine AD, McLean WH. Filaggrin's fuller figure: a glimpse into the genetic architecture of atopic dermatitis. J Invest Dermatol. 2007;127:1282–1284. doi: 10.1038/sj.jid.5700876. [DOI] [PubMed] [Google Scholar]
- 9.Wüthrich B. Clinical aspects, epidemiology, and prognosis of atopic dermatitis. Ann Allergy Asthma Immunol. 1999;83:464–470. doi: 10.1016/S1081-1206(10)62852-9. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson D, Sjöberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis: a prospective follow-up to 7 years of age. Allergy. 2000;55:240–245. doi: 10.1034/j.1398-9995.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 11.Illi S, von Mutius E, Lau S, Nickel R, Grüber C, Niggemann B, Wahn U Multicenter Allergy Study Group. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113:925–931. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 12.Ricci G, Patrizi A, Baldi E, Menna G, Tabanelli M, Masi M. Long-term follow-up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol. 2006;55:765–771. doi: 10.1016/j.jaad.2006.04.064. [DOI] [PubMed] [Google Scholar]
- 13.Ohshima Y, Yamada A, Hiraoka M, Katamura K, Ito S, Hirao T, Akutagawa H, Kondo N, Morikawa A, Mayumi M. Early sensitization to house dust mite is a major risk factor for subsequent development of bronchial asthma in Japanese infants with atopic dermatitis: results of a 4-year followup study. Ann Allergy Asthma Immunol. 2002;89:265–270. doi: 10.1016/S1081-1206(10)61953-9. [DOI] [PubMed] [Google Scholar]
- 14.Kissling S, Wüthrich B. Follow-up of atopic dermatitis after early childhood. Hautarzt. 1993;44:569–573. [PubMed] [Google Scholar]
- 15.Musgrove K, Morgan JK. Infantile eczema: a long-term follow-up study. Br J Dermatol. 1976;95:365–372. doi: 10.1111/j.1365-2133.1976.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 16.Roth HL, Kierland RR. The natural history of atopic dermatitis. A 20-year follow-up study. Arch Dermatol. 1964;89:209–214. doi: 10.1001/archderm.1964.01590260047008. [DOI] [PubMed] [Google Scholar]
- 17.Hanifin JM, Rajka G. Diagnostic feature of atopic dermatitis. Acta Derm Venereol (Stockholm) 1980;92:44–47. [Google Scholar]
- 18.Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996;135:25–30. doi: 10.1111/j.1365-2133.1996.tb00706.x. [DOI] [PubMed] [Google Scholar]
- 19.Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, Mukhopadhyay S, Smith GD, Palmer CN, McLean WH, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872–877. doi: 10.1016/j.jaci.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, Irvine AD, Weidinger S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123:1361–1370.e7. doi: 10.1016/j.jaci.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 21.Peters AS, Kellberger J, Vogelberg C, Dressel H, Windstetter D, Weinmayr G, Genuneit J, Nowak D, von Mutius E, Radon K. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2010;126:590–595. doi: 10.1016/j.jaci.2010.06.020. [DOI] [PubMed] [Google Scholar]