Abstract
More than 95% of the thyroid carcinomas are well differentiated types showing favorable prognosis. However, only a few therapeutic options are available to treat the patients with undifferentiated thyroid carcinomas, especially with refractory thyroid carcinomas that are not amenable to surgery or radioiodine ablation. We investigated the anticancer effects of 20 chemotherapy and hormonal therapy drugs on 8 thyroid carcinoma cell lines. In vitro chemosensitivity was tested using the adenosine-triphosphate-based chemotherapy response assay (ATP-CRA). The tumor inhibition rate (TIR; or cell death rate) or half maximal inhibitory concentration (IC50) was analyzed to interpret the results. Of the 12 chemotherapy drugs, etoposide (178.9 index value in follicular carcinoma cell line) and vincristine (211.7 in Hürthle cell carcinoma cell line) were the most active drugs showing the highest chemosensitivity, and of the 8 additional drugs, trichostatin A (0.03 µg/mL IC50 in follicular carcinoma cell line) showed favorable outcome having the anticancer effect. In our study, the result of etoposide and vincristine show evidence as active anticancer drugs in thyroid carcinoma cell lines and trichostatin A seems be the next promising drug. These drugs may become an innovative therapy for refractory thyroid carcinomas in near future.
Keywords: Thyroid Carcinoma Cell Lines, Refractory Thyroid Carcinoma, Chemotherapy Drugs, Hormonal Therapy Drugs, ATP-CRA
INTRODUCTION
In general, thyroid carcinomas can be classified as well differentiated thyroid carcinomas having favorable prognoses, and undifferentiated thyroid carcinomas having poor prognoses (1). Fortunately, more than 95% of the thyroid carcinomas are well differentiated types, including papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), and their related variant types. However, the remaining 5% which includes anaplastic thyroid carcinoma (ATC), poorly differentiated types, and parafollicular C cell origin medullary thyroid carcinoma (MTC), show unfavorable disease processes despite an aggressive therapy (2).
For thyroid carcinomas, surgery with thyroid stimulating hormone (TSH) suppression and radioiodine ablation (RIA) is the most effective therapy. In general, well differentiated thyroid carcinomas such as PTC and FTC show excellent prognoses after the surgery with the combination of RIA according to the disease stage and many patients experience a curative or indolent clinical course (3). Also, the prognosis after a local recurrence or distant metastasis for most of the well differentiated thyroid carcinomas are well controlled under the adequate re-operation or RIA (4).
About 5% of the thyroid carcinoma is classified as undifferentiated type and although rare, ATC and MTC are usually resistant to the standard thyroid carcinoma treatment. At the time of diagnosis, ATC is usually unresectable, RIA resistant; and external radiation therapy has been used with no definite clinical survival benefits (5, 6). Postoperative RIA has no effect on MTC which is parafollicular C cell origin (7, 8). Likewise, such lack of treatment modality is even worse especially with patients having refractory thyroid carcinomas. The refractory thyroid carcinomas could be defined as not amenable to surgery or RIA, iodine-refractory metastatic thyroid carcinomas, or which RIA is not an appropriate therapy from the beginning (9, 10). To overcome these problems, many institutions are investigating to find a new treatment modality for refractory thyroid carcinoma focused on the combination of drug chemotherapy, hormonal therapy and molecular targeted therapy (9, 11).
Previous studies about chemotherapy drugs for refractory thyroid carcinomas include doxorubicin, cisplatin, vincristine, cyclophosphamide, epirubicin and 5-fluorouracil (7, 12-14). Doxorubicin has been the most widely investigated drug analyzed for anticancer effect either by itself or in combination regimens (15). However, such studies were performed only on few patients and yielded unsatisfactory results, with best of the results showing either limited partial response or stable disease status (8).
Due to these unsatisfactory outcomes, many studies about the etiology and oncogenic mutations of refractory thyroid carcinomas are underway, with the latter including mutations in the BRAF, RAS, and RET/PTC genes (16, 17). In addition, drugs with different mechanisms have been developed mostly targeted at vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and tyrosine-kinase inhibitors (15, 18, 19). To test these targeted drugs and to find an innovative drug therapy for refractory thyroid carcinomas, we have tested the effects of 20 drugs on 8 thyroid carcinoma cell lines.
MATERIALS AND METHODS
Cell culture
We cultured 8 thyroid carcinoma cell lines, 1 papillary (TPC-1), 4 follicular (WRO, FTC133, FTC236 and FTC238), 1 Hürthle cell (XTC-1), 1 medullary (TT) and 1 anaplastic (FRO), all of which were purchased from American Type Culture Collection (Manassas, VA, USA) and all were originated from known tumors without laboratory cross-contamination (20). TPC-1, FTC133, FTC236, FTC238, XTC-1, and TT cells were cultured by Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 µg/mL streptomycin and D-glucose. WRO and FRO cells were cultured by Roswell Park Memorial Institute (RPMI) medium supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, 25 mM HEPES, and L-glutamine.
Chemotherapy and hormonal therapy drugs
The 12 well acknowledged chemotherapy drugs tested were 5-fluorouracil (5-FU), bleomycin, carboplatin, cisplatin, cyclophosphamide, doxorubicin, epirubicin, etoposide, methotrexate, oxaliplatin, paclitaxel, and vincristine. They were selected for their known anticancer effect and cytotoxicity for other malignancies, and that they have been previously studied on thyroid carcinomas.
The 8 additional chemotherapy and hormonal therapy drugs tested were quercetin, resveratrol, rosiglitazone, sunitinib, tamoxifen, trichostatin A, valproic acid, and vandetanib. They were selected because their anticancer effect on other malignancies, especially breast carcinoma, has been reported but not clearly tested for thyroid carcinoma and some, not yet in clinical trial. The vandetanib drug was donated donated by AstraZeneca UK Limited and all other drugs were obtained from ISU ABXIS Co., Ltd. in Yonsei University Medical Center, Korea.
Evaluation of in vitro chemosensitivity
The cells were plated at 6000 cells per well in 96 well plates (Nunclon, Roskilde, Denmark) and cultured for 24 hr. Drugs were added to each well and incubated with the cells for 48 hr before the chemosensitivity test. The assay used to test the chemosensitivity was adenosine-triphosphate-based chemotherapy response assay (ATP-CRA) (21).
ATP is the basic energy source of all live cells and rapidly decreases with cell death. Cells from the treated and untreated control groups were lysed and the amount of ATP in the lysated cell was measured using flash type luminescence measurements on a Victor 3 multilabel counter (PerkinElmer, Boston, MA, USA). The cell death rate for each drug was defined as the rate of ATP luminescence reduction in the treated group compared with the untreated control (21). The increase in ATP reduction would mean better anti-cancer effect. By doing so, the tumor growth inhibition or cell death was compared between the drug treated cells and untreated control cells.
For the 12 well acknowledged chemotherapy drugs, we performed 3 test drug concentrations (TDC) (21-23) at 20%, 100%, and 500% to calculate the chemosensitivity (23) and the application continued for a maximum of 6 cycles or until the reduction was reproducible within the error range. Tumor inhibition rate (TIR; or cell death rate) was calculated by measuring the reduction in ATP luminescence and compare with untreated control cell; and the drug was defined as an active drug when TDC resulted in more than 30% TIR (21). To calculate the TIR index value, the sum of TIR (20%-500% TDC) was subtracted from 300, and this index value was used to rank the tested drugs which a lower value indicated a more active drug. The results of the 8 index values for each drug were averaged to calculate the mean index values. The cut-off mean TIR index value for determining more active drug was set at 100 for our study.
To evaluate the chemosensitivity of additional 8 drugs, we used the half maximal inhibitory concentration (IC50) (24). The IC50 is a function antagonist assay which measures the effectiveness of a compound in inhibiting the biological or biochemical function. The IC50 of each drug was determined using the regression equation (Sigma Plot, San Jose, CA, USA), y = min + (maxmin)/(1 + (x/EC50)Hill slope) and the lower value indicated a more active drug. Since the optimal drug doses for a recognizable activity against the thyroid carcinoma cell lines have not been clarified, the inhibition dependent dose ranges were obtained from nine different dose response curve, using cells treated with 1.875-300 µg/mL quercetin (25, 26), 0.39-800 µg/mL resveratrol (27), 0.75-192 µg/mL rosiglitazone (28), 0.0002-200 µg/mL sunitinib (29), 0.11-30 µg/mL tamoxifen (30), 0.00469-1.2 µg/mL trichostatin A (31), 777.6-2000 µg/mL valproic acid (32), and 0.039-60 µg/mL vandetanib (33). Recently, the studies about sunitinib and vandetanib are actively investigated by many other institutions (34).
RESULTS
Well acknowledged chemotherapy drugs
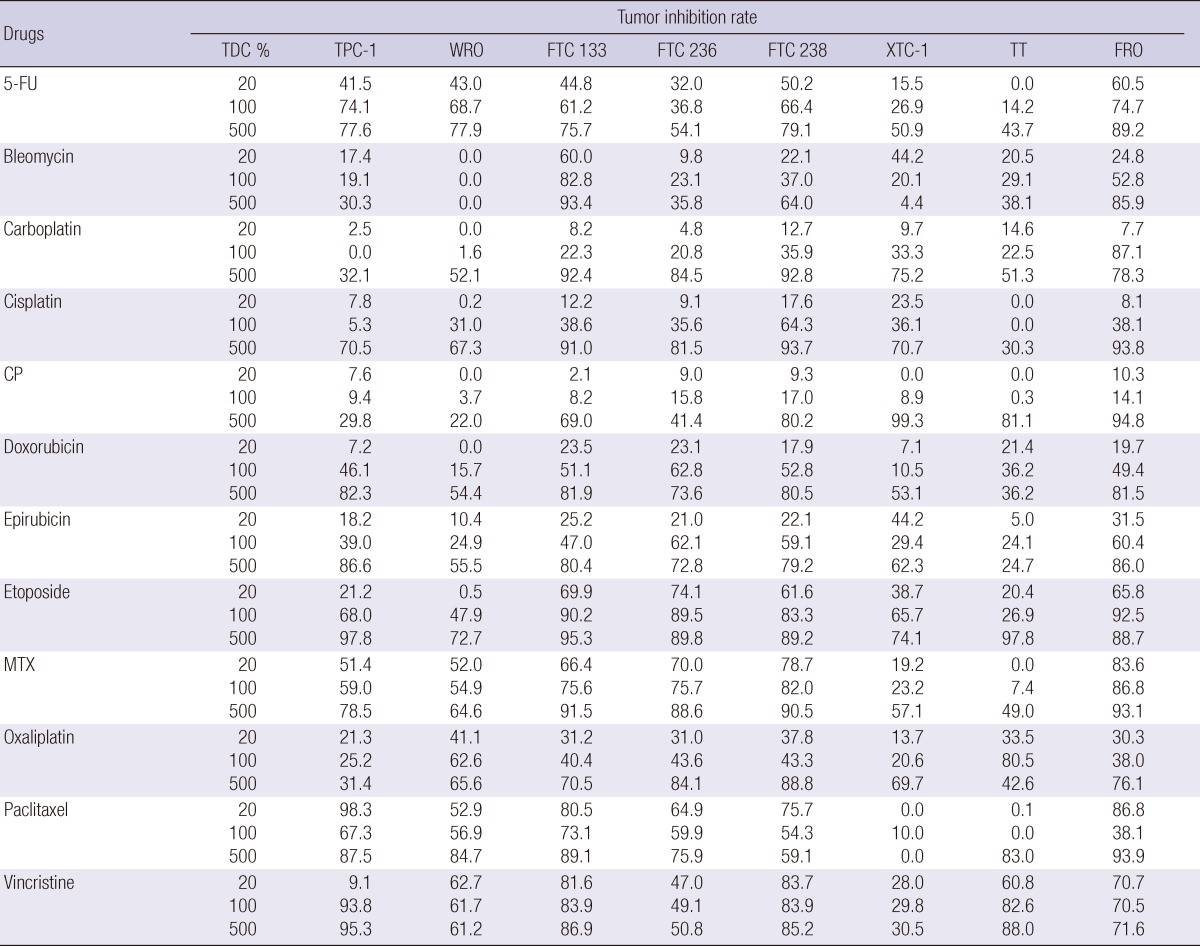
Three TDCs (20%, 100%, and 500%) of 12 well acknowledged chemotherapy drugs were individually applied to 8 thyroid carcinoma cell lines to measure the TIR (Table 1). The mean TIR was calculated to analyze those drugs showing more than 30% TIR in cell lines (data not shown). From 100% TDC results, we found that etoposide, vincristine and methotrexate, and 5-FU were the most active drugs, whereas cyclophosphamide the least active. Although drugs had a more than 30% TIR at 500% TDC, this concentration would be beyond clinical application. Even though all TIR did not increase in uniform pattern according to the TDC, we found that 5-FU, etoposide, methotrexate and vincristine showed a linear increase in TIR.
Table 1.
The tumor inhibition rate of 12 well acknowledged chemotherapy drugs at 3 test drug concentrations
TDC, test drug concentration; 5-FU, 5-fluorouracil; CP, cyclophosphamide; MTX, methotrexate.
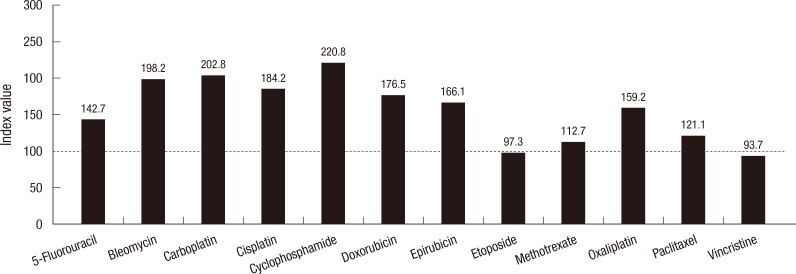
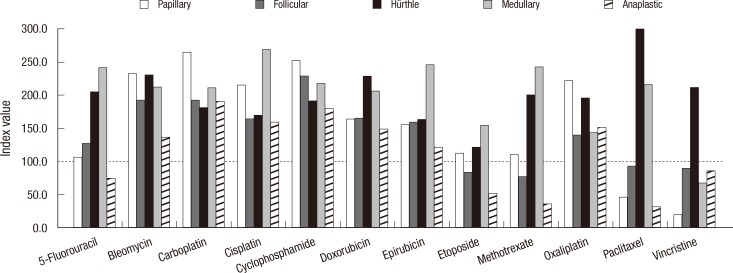
Then, we determined the TIR index value for each drug at 3 TDCs (Fig. 1). The mean TIR index values of 3 TDCs showed that vincristine and etoposide had the lowest values than the other drugs suggesting that these 2 drugs were more active in inhibiting thyroid carcinoma all growth. In contrast, cyclophosphamide was observed as inactive. Next, we compared the mean TIR index values according to the types of thyroid carcinoma cell lines: papillary, follicular, Hürthle cell, medullary and anaplastic (Fig. 2). In this figure, paclitaxel and vincristine showed as most active drugs when cut-off mean index value set at 100. Etoposide, methotrexate, paclitaxel, and vincristine showed similar index values for follicular cell lines in general.
Fig. 1.
The mean tumor inhibition rate index values of 12 well acknowledged chemotherapy drugs. The lowest mean index values were seen in etoposide and vincristine suggesting that these drugs are more active than others.
Fig. 2.
The mean tumor inhibition rate index values of 12 well acknowledged chemotherapy drugs applied to 8 thyroid carcinoma cell lines analyzed according to the types of thyroid carcinoma cell lines. Follicular cell lines data shown are the mean value of 4 cell lines.
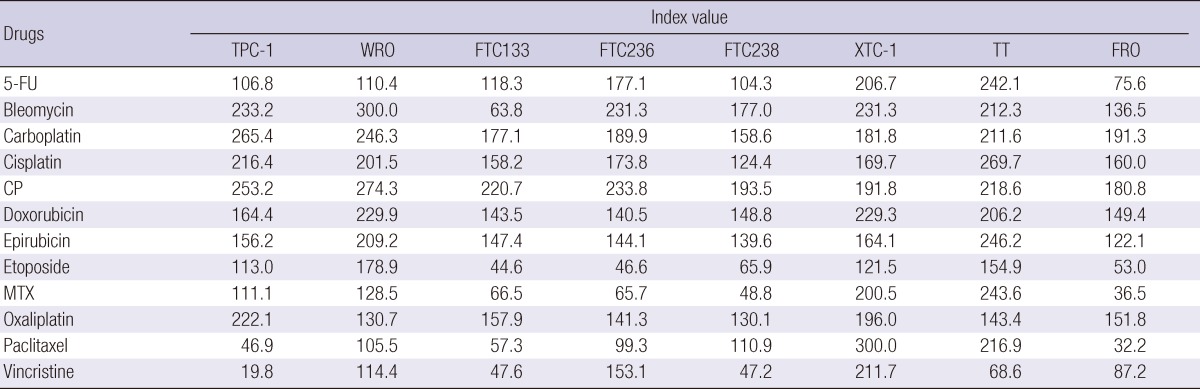
When these results were individualized by each cell lines, vincristine had the lowest index value of 19.8 in papillary TPC-1 cell. Also, paclitaxel showed favorable activity of 46.9. In follicular cell lines, etoposide and methotrexate showed potent activity in FTC133, FTC236, and FTC238. Vincristine was the only active drug in medullary TT cell; and 5-FU, etoposide, methotrexate, paclitaxel, and vincristine showed activity in anaplastic FRO cell. Unfortunately, none of the tested drugs inhibited the growth of Hürthle cell line, XTC-1 (Table 2).
Table 2.
The tumor inhibition rate index values of 12 well acknowledged chemotherapy drugs at 3 concentrations applied to 8 thyroid carcinoma cell lines analyzed according to the types of thyroid carcinoma cell lines
Index value, 300 - [sum of tumor inhibition rate at 3 test drug concentrations]; 5-FU, 5-fluorouracil; CP, cyclophosphamide; MTX, methotrexate.
Additional chemotherapy and hormonal therapy drugs
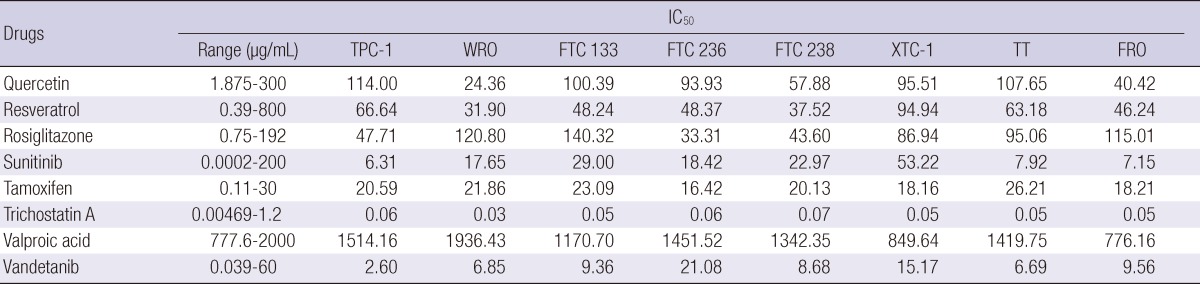
We tested the chemosensitivity of the 8 additional chemotherapy and hormonal therapy drugs which were not well tested for thyroid carcinoma cell lines previously. The drugs were tested by determining their IC50 values to each cell line and we found that trichostatin A was the most active drug in all cell lines tested. The least active drug was valproic acid (Table 3).
Table 3.
The half maximal inhibitory concentration (IC50) of 8 additional chemotherapy and hormonal therapy drugs
IC50, half maximal inhibitory concentration.
When we pair the results of 4 drugs having similar cytotoxic mechanisms; quercetin and resveratrol which are derived from grape-polyphenols, and sunitinib and vandetanib as tyrosine kinase inhibitors; resveratrol and vandetanib showed lower IC50 values than quercetin and sunitinib, respectively.
DISCUSSION
Patients with intractable thyroid carcinoma typically have an unfavorable prognosis (2), with refractory thyroid carcinoma having the most limited therapeutic options (9, 10). Recently, there have been studies of chemotherapy drugs to overcome such thyroid carcinomas (11). We therefore tested 20 chemotherapy and hormonal therapy drugs in 8 thyroid carcinoma cell lines, all of which originated from refractory thyroid carcinomas. We found that 5-FU, etoposide, methotrexate, and vincristine showed favorable results in mean TIR each resulting in more than 30%. In addition, etoposide and vincristine had favorable mean index values for tumor growth inhibition. The previous studies focused on the combination chemotherapy indicated that doxorubicin, etoposide, paclitaxel, and vincristine were the most active drugs warranting further test (6, 7). In most of these studies, however, drugs were tested only against medullary or anaplastic thyroid carcinomas (8). Fortunately, we yield a uniform result for etoposide and vincristine having favorable outcomes.
Molecular targeted therapy and angiogenesis targeted therapies are also being investigated in patients with refractory thyroid carcinomas (9, 15, 18). We found that trichostatin A had cytotoxic effect against refractory thyroid carcinoma cells. In another study, trichostatin A has been shown to increase the effect of RIA in advanced thyroid carcinomas (35). We also found that quercetin, resveratrol, sunitinib and vandetanib showed favorable results. Quercetin and resveratrol are grape-polyphenols that act as antioxidants, antiangiogenic agents and selective estrogen receptor modifiers. These drugs had beneficial effects in breast carcinomas and gynecologic diseases (36). Sunitinib and vandetanib are multi targeted receptor tyrosine kinase inhibitors. These drugs, which block the VEGF receptors, thereby inhibiting tumor angiogenesis and proliferation, are currently being investigated in patients with refractory thyroid carcinomas (15, 37, 38). Between the drugs having similar cytotoxic mechanisms, resveratrol and vandetanib were better than the counter drugs. In addition to these drugs, several other VEGF related drugs are being tested, including sorafenib (37), as well as tamoxifen, rosiglitazone and valproic acid (32, 39, 40). Unfortunately, our study showed that cyclophosphamide and valproic acid were the least active drugs in tumor growth inhibition.
The limitation of this study is that the cell lines were cultured and tested without the prior information about gene mutation differences such as BRAF or RAS. Also, such drugs were tested in in vitro study and the results may differ in in vivo study. The toxicity control in in vivo study will be a challenge as well. Also, selecting the patients to benefit from such therapy and deciding the primary end point as either overall survival of progression-free survival of such application would be another dilemma to solve. Further investigation of the above drugs and combination test of the active drugs for their interaction effect should be continued for clinical application.
In conclusion, etoposide, vincristine, trichostatin A, resveratrol, and vandetanib show prior favorable outcomes as the anticancer drugs in thyroid carcinoma cell lines. These drugs may be used for an innovative therapy for refractory thyroid carcinomas.
References
- 1.DeLellis RA. Pathology and genetics of thyroid carcinoma. J Surg Oncol. 2006;94:662–669. doi: 10.1002/jso.20700. [DOI] [PubMed] [Google Scholar]
- 2.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–511. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 4.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 5.Heron DE, Karimpour S, Grigsby PW. Anaplastic thyroid carcinoma: comparison of conventional radiotherapy and hyperfractionation chemoradiotherapy in two groups. Am J Clin Oncol. 2002;25:442–446. doi: 10.1097/01.COC.0000023060.34146.B3. [DOI] [PubMed] [Google Scholar]
- 6.Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006;13:453–464. doi: 10.1245/ASO.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 7.Stein R, Chen S, Reed L, Richel H, Goldenberg DM. Combining radioimmunotherapy and chemotherapy for treatment of medullary thyroid carcinoma: effectiveness of dacarbazine. Cancer. 2002;94:51–61. doi: 10.1002/cncr.10157. [DOI] [PubMed] [Google Scholar]
- 8.Moley JF, Fialkowski EA. Evidence-based approach to the management of sporadic medullary thyroid carcinoma. World J Surg. 2007;31:946–956. doi: 10.1007/s00268-006-0846-2. [DOI] [PubMed] [Google Scholar]
- 9.Pfister DG, Fagin JA. Refractory thyroid cancer: a paradigm shift in treatment is not far off. J Clin Oncol. 2008;26:4701–4704. doi: 10.1200/JCO.2008.17.3682. [DOI] [PubMed] [Google Scholar]
- 10.Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, Robbins RJ. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000;10:261–268. doi: 10.1089/thy.2000.10.261. [DOI] [PubMed] [Google Scholar]
- 11.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 12.Williams SD, Birch R, Einhorn LH. Phase II evaluation of doxorubicin plus cisplatin in advanced thyroid cancer: a Southeastern Cancer Study Group Trial. Cancer Treat Rep. 1986;70:405–407. [PubMed] [Google Scholar]
- 13.Pudney D, Lau H, Ruether JD, Falck V. Clinical experience of the multimodality management of anaplastic thyroid cancer and literature review. Thyroid. 2007;17:1243–1250. doi: 10.1089/thy.2007.0026. [DOI] [PubMed] [Google Scholar]
- 14.Bajetta E, Rimassa L, Carnaghi C, Seregni E, Ferrari L, Di Bartolomeo M, Regalia E, Cassata A, Procopio G, Mariani L. 5-Fluorouracil, dacarbazine, and epirubicin in the treatment of patients with neuroendocrine tumors. Cancer. 1998;83:372–378. doi: 10.1002/(sici)1097-0142(19980715)83:2<372::aid-cncr23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Sherman SI. Early clinical studies of novel therapies for thyroid cancers. Endocrinol Metab Clin North Am. 2008;37:511–524. doi: 10.1016/j.ecl.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Fagin JA. How thyroid tumors start and why it matters: kinase mutants as targets for solid cancer pharmacotherapy. J Endocrinol. 2004;183:249–256. doi: 10.1677/joe.1.05895. [DOI] [PubMed] [Google Scholar]
- 17.Laird AD, Cherrington JM. Small molecule tyrosine kinase inhibitors: clinical development of anticancer agents. Expert Opin Investig Drugs. 2003;12:51–64. doi: 10.1517/13543784.12.1.51. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 19.Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plénat F, Leclère J, Duprez A, Weryha G. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:656–658. doi: 10.1210/jcem.86.2.7226. [DOI] [PubMed] [Google Scholar]
- 20.Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon YW, Choi SH, Kim YT, Sohn JH, Chang J, Kim SK, Park MS, Chung KY, Lee HJ, Kim JH. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided platinum-based 2-drug chemotherapy for unresectable nonsmall-cell lung cancer. Cancer. 2007;109:1829–1835. doi: 10.1002/cncr.22601. [DOI] [PubMed] [Google Scholar]
- 22.Kim HA, Yom CK, Moon BI, Choe KJ, Sung SH, Han WS, Choi HY, Kim HK, Park HK, Choi SH, et al. The use of an in vitro adenosine triphosphate-based chemotherapy response assay to predict chemotherapeutic response in breast cancer. Breast. 2008;17:19–26. doi: 10.1016/j.breast.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Han SS, Choi SH, Lee YK, Kim JW, Park NH, Song YS, Lee HP, Kang SB. Predictive value of individualized tumor response testing by ATP-based chemotherapy response assay in ovarian cancer. Cancer Invest. 2008;26:426–430. doi: 10.1080/07357900701813250. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 25.Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ, Kandaswami C, Middleton E, Jr, Lee MT. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol. 1999;128:999–1010. doi: 10.1038/sj.bjp.0702879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang HJ, Youn YK, Hong MK, Kim LS. Antiproliferation and redifferentiation in thyroid cancer cell lines by polyphenol phytochemicals. J Korean Med Sci. 2011;26:893–899. doi: 10.3346/jkms.2011.26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun CY, Hu Y, Guo T, Wang HF, Zhang XP, He WJ, Tan H. Resveratrol as a novel agent for treatment of multiple myeloma with matrix metalloproteinase inhibitory activity. Acta Pharmacol Sin. 2006;27:1447–1452. doi: 10.1111/j.1745-7254.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 28.Antonelli A, Ferrari SM, Fallahi P, Berti P, Materazzi G, Minuto M, Giannini R, Marchetti I, Barani L, Basolo F, et al. Thiazolidinediones and antiblastics in primary human anaplastic thyroid cancer cells. Clin Endocrinol (Oxf) 2009;70:946–953. doi: 10.1111/j.1365-2265.2008.03415.x. [DOI] [PubMed] [Google Scholar]
- 29.Yee KW, Schittenhelm M, O'Farrell AM, Town AR, McGreevey L, Bainbridge T, Cherrington JM, Heinrich MC. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood. 2004;104:4202–4209. doi: 10.1182/blood-2003-10-3381. [DOI] [PubMed] [Google Scholar]
- 30.Petinari L, Kohn LK, de Carvalho JE, Genari SC. Cytotoxicity of tamoxifen in normal and tumoral cell lines and its ability to induce cellular transformation in vitro. Cell Biol Int. 2004;28:531–539. doi: 10.1016/j.cellbi.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Tuncel M, Aydin D, Yaman E, Tazebay UH, Güç D, Doğan AL, Taşbasan B, Uğur O. The comparative effects of gene modulators on thyroid-specific genes and radioiodine uptake. Cancer Biother Radiopharm. 2007;22:443–449. doi: 10.1089/cbr.2006.319.A. [DOI] [PubMed] [Google Scholar]
- 32.Greenblatt DY, Cayo MA, Adler JT, Ning L, Haymart MR, Kunnimalaiyaan M, Chen H. Valproic acid activates Notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Ann Surg. 2008;247:1036–1040. doi: 10.1097/SLA.0b013e3181758d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann S, Gläser S, Wunderlich A, Lingelbach S, Dietrich C, Burchert A, Müller H, Rothmund M, Zielke A. Targeting the EGF/VEGF-R system by tyrosine-kinase inhibitors: a novel antiproliferative/antiangiogenic strategy in thyroid cancer. Langenbecks Arch Surg. 2006;391:589–596. doi: 10.1007/s00423-006-0104-y. [DOI] [PubMed] [Google Scholar]
- 34.Sherman SI. Targeted therapies for thyroid tumors. Mod Pathol. 2011;24:S44–S52. doi: 10.1038/modpathol.2010.165. [DOI] [PubMed] [Google Scholar]
- 35.Zarnegar R, Brunaud L, Kanauchi H, Wong M, Fung M, Ginzinger D, Duh QY, Clark OH. Increasing the effectiveness of radioactive iodine therapy in the treatment of thyroid cancer using Trichostatin A, a histone deacetylase inhibitor. Surgery. 2002;132:984–990. doi: 10.1067/msy.2002.128690. [DOI] [PubMed] [Google Scholar]
- 36.Schlachterman A, Valle F, Wall KM, Azios NG, Castillo L, Morell L, Washington AV, Cubano LA, Dharmawardhane SF. Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl Oncol. 2008;1:19–27. doi: 10.1593/tlo.07100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DW, Jo YS, Jung HS, Chung HK, Song JH, Park KC, Park SH, Hwang JH, Rha SY, Kweon GR, et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab. 2006;91:4070–4076. doi: 10.1210/jc.2005-2845. [DOI] [PubMed] [Google Scholar]
- 38.Roman S, Mehta P, Sosa JA. Medullary thyroid cancer: early detection and novel treatments. Curr Opin Oncol. 2009;21:5–10. doi: 10.1097/CCO.0b013e32831ba0b3. [DOI] [PubMed] [Google Scholar]
- 39.Aljada A, O'Connor L, Fu YY, Mousa SA. PPAR gamma ligands, rosiglitazone and pioglitazone, inhibit bFGF- and VEGF-mediated angiogenesis. Angiogenesis. 2008;11:361–367. doi: 10.1007/s10456-008-9118-0. [DOI] [PubMed] [Google Scholar]
- 40.Hoelting T, Siperstein AE, Duh QY, Clark OH. Tamoxifen inhibits growth, migration, and invasion of human follicular and papillary thyroid cancer cells in vitro and in vivo. J Clin Endocrinol Metab. 1995;80:308–313. doi: 10.1210/jcem.80.1.7829632. [DOI] [PubMed] [Google Scholar]