Abstract
Combination treatment of trans-catheter arterial chemoembolization (TACE) and conformal radiation therapy (RT) reported promising results in patients with hepatocellular carcinoma (HCC), but, optimal interval was not determined. We hypothesized that a two-week interval between TACE and RT would be optimal. Therefore, we designed this study to evaluate the safety and efficacy of scheduled interval TACE followed by RT. HCC patients who were not eligible for standard therapies were enrolled for scheduled interval TACE followed by RT (START). Patients received TACE on the first day of treatment, and then RT was delivered after 14 days. The entire course of treatment took between four and five weeks. In 81 patients (96.4%), START was completed in the planned treatment period. RT was delayed in the remaining three patients because of decreased liver function or poor performance status after TACE. Of the 81 patients, objective response was observed in 57 patients (70.4%). One unexpected death occurred after START due to hepatic failure. Other toxicities were manageable. The median survival was 14.7 months. There was a significant difference in overall survival according to the response to START (P < 0.001). In conclusion, START is safe and feasible.
Keywords: Carcinoma, Hepatocellular; Radiationtherapy; Trans-Catheter Arterial Chemoembolization; Combination Treatment
INTRODUCTION
Trans-catheter arterial chemoembolization (TACE) is a primary and complementary measure to treat unresectable HCC (1). However, TACE alone is not sufficient to control unresectable HCC because of the existence of portal vein tumor thrombosis (PVTT), viable tumor cells around the capsule, and little effect on daughter nodules (2).
Combined treatment with TACE and three-dimensional conformal radiation therapy (3D-CRT) has some rational advantages (3). RT can eliminate residual tumor cells after TACE, and tumor shrinkage after TACE makes the radiation field smaller, which permits higher tumor doses and improves normal liver tolerance. Moreover, lipiodol uptake by the tumor aids in delineating the target volume, and anti-cancer drugs retained in the tumor after TACE may have a radio-sensitizing effect. Several publications have reported promising results using a combination of TACE and conformal RT (3-5). However, there is no consensus on how to combine those two modalities to achieve optimal results in patients with unresectable HCC; in particular, the optimal interval between TACE and RT has yet to be determined.
We hypothesized that a 2-week interval between TACE and RT would be optimal for the treatment of unresectable HCC. Based on this hypothesis, we performed the trial described in this study at our institution. This study might indicate the safety and efficacy of scheduled interval TACE followed by RT (START).
MATERIALS AND METHODS
Eligibility
Eligibility criteria included unresectable HCC for which TACE alone was expected to be ineffective, clinical liver function with Child-Pugh classification A or B, a life expectancy of more than 12 weeks, and no previous history or treatment for any other malignancy. Patients who had an Eastern Cooperative Oncology Group (ECOG) performance status of more than 3 or complete obstruction of main PVTT were also excluded.
Patients for whom TACE was expected to be ineffective were defined as those patients who had more than one of the following characteristics: tumor size greater than 10 cm, portal vein (main, left, or right lobar portal vein) tumor thrombosis (PVTT), or a previous history of ineffective TACE (twice or more).
Treatment protocol
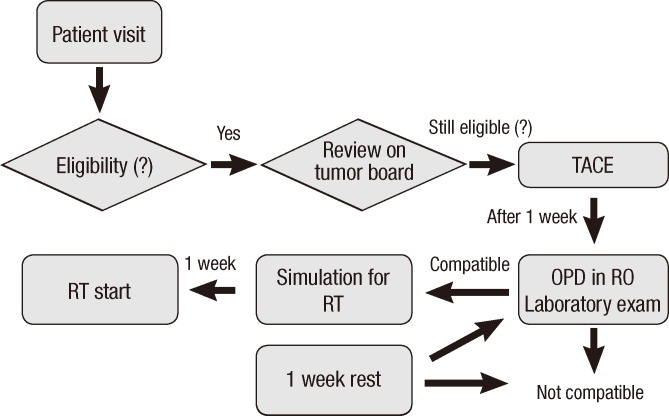
Fig. 1 shows the summary of study design. All eligible patients were first treated by TACE. On day 8 after TACE, all patients underwent pre-RT clinical examinations, including a physical examination, complete blood counts, and biochemical profiles with liver function tests. If the results of the pre-RT clinical examinations indicated that the patient was fit to undergo RT, RT simulation was performed on the same day. RT was initiated on day 14 after TACE.
Fig. 1.
Study design. All eligible patients were treated by trans-catheter arterial chemoembolization and underwent standard clinical and laboratory studies on day 7 after TACE. If the patient was considered suitable for RT, RT simulation was performed that day and radiation therapy, was delivered at day 14 after TACE. OPD, outpatient department; RO, radiation oncology.
If aspartate aminotransferase (AST)/alanine aminotransferase (ALT) levels were elevated three-fold or more above normal levels and/or the Child-Pugh score was elevated two or more one week after TACE, RT was delayed by one week. If patients had pre-RT clinical examination results that were not good consistently, they were excluded from the study.
Trans-catheter arterial chemoembolization
After catheterization of the celiac artery, arteriography was initially performed to assess patient anatomy, tumor burden, vascularity, and portal vein patency. Then, superior mesenteric artery, or common hepatic artery with a 5-french (F) catheter, selective angiography was performed. Then, a coaxial superselective micro-catheter was inserted through the 5-F catheter as close to the tumor as possible. After the micro-catheter was positioned in the target branch, a mixture of doxorubicin hydrochloride (Adriamycin; Ildong, Seoul, Korea) and iodized oil (Lipiodol; Guerbet, Aulnay-sous-Bois, France) was slowly injected through the catheter. The mixture of iodized oil and doxorubicin hydrochloride was prepared by vigorously pumping the solutions 10 to 20 times between two syringes interconnected with a three-way stopcock. The doses of adriamycin and lipiodol were determined based on the size and vascularity of the tumor, though we limited the maximum doses of adriamycin and lipiodol for one session of TACE to 70 mg and 25 mL, respectively. Infusion of the lipiodol mixture was followed by particulate embolization with 1- to 2-mm-diameter gelatin sponge pledgets (Cutanplast; MasciaBrunelli, Milan, Italy). The end-point of TACE was fluoroscopic observation of complete lipiodol uptake by the tumor or stagnation of blood flow in the tumor-feeding arteries, or the injection of the maximum dose of the lipiodol mixture allowed per session.
Radiation therapy
Before simulation, patients were educated and trained in the shallow breathing used during the procedures. Patients wore video goggles and audio coaching was used to improve the periodicity of respiration.
On simulation day (usually one or two days after training), all patients underwent CT scans. CT images were acquired using a GE Light Speed plus 16 scanner (General Electric, Milwaukee, WI, USA). After free breathing, non-contrast CT images for dose calculation were obtained. Intravenous (IV) contrast media (Visa-paque 270, Amersham Health; 2 mL/kg to a maximum of 200 mL) was delivered at a rate of 5 mL/second, and exhale breath-hold CT scans were repeated at 25 to 30 sec intervals (arterial phase) and 50 to 60 sec after injection (portal phase). Then, 4D-CT scans were acquired by a retrospective 4D-CT scanning technique with visual prompting goggles and a Real-time Position Management system (Varian Medical Systems, Palo Alto, CA, USA).
The gross tumor volume (GTV) was defined as lipiodolized lesions and the radiographically abnormal areas noted on the CT images. GTV was delineated on all phases of the 4D-CT as well as triple-phase CT images. A 7-mm margin around the GTV within liver and nonenhancing thromboses was included within the clinical target volume (CTV). The planning target volume (PTV) was chosen by summing all phases of CTVs with an additional 5-mm setup margin. Conformal planning was done with 5-mm block margin from PVT using three to 4 coplanar or noncoplanar beams of 6 to 10 MV Radiography. Intensity modulated RT was not allowed. A dose-volume histogram (DVH) was also generated.
The radiation dose was escalated in four strata designed under tentative guidelines. The total radiation dose was determined according to the percentage of the normal liver volume irradiated at more than 50% of the prescribed dose. If less than 20% of the normal liver volume would be exposed to more than 50% of the prescribed dose, a total dose of 5 Gy daily for ten fractions was selected. A dose of 4.5 Gy, 4.0 Gy, or 3.5 Gy for ten fractions was chosen when 20%-35%, 35%-50%, 50%-75% of the liver was irradiated, respectively. In exceptional cases where full dose exposure to the stomach or duodenum was inevitable, a daily dose of 3.0 Gy for 11 fractions was prescribed to avoid gastro-duodenal toxicity.
RT was initiated on the 14th or 21st day (± 2 days) after TACE. All patients were treated with a linear accelerator equipped with a kV imager (Varian, Medical Systems). To verify the target position, exhalation phase gated on-board images (OBIs) were obtained daily before each treatment and compared to dynamic reconstruction and rendering (DRR) images reconstructed with 50% phase CT images. Liver dome or lipiodol deposits were compared as fiducials. If the fiducial positions were shifted more than 0.5 cm, which was considered to be the critical margin, OBIs were re-taken and the target position was verified again. If shifts greater than 0.5 cm were again detected after this, the isocenter was replaced using an automatic adjustment function.
Follow-up
We examined all patients at least once a week during treatment. Then, patients were followed-up 1 month after RT completion and at 2-3 months intervals thereafter. Treatment response was assessed using CT scans one month after completing RT using the Response Evaluation Criteria in Solid Tumors (RECIST) was used to measure tumor response.
Acute morbidity was evaluated weekly during treatment and one month after the treatment. Late morbidity was defined as that occurring after three months. If patients complained of melena or upper abdominal pain persisting for longer than two weeks during the follow-up period, fiberoptic gastro-duodenoscopy was conducted to evaluate gastrointestinal (GI) toxicity. Toxicity was scored using the Common Terminology Criteria for Adverse Events (CTCAE; version 3.0) at every visit.
Statistical analysis
The chi-square test or Fisher's exact test was used to compare the other clinical variables according to START interval and treatment response. Overall survival (OS) was estimated using the Kaplan-Meier product-limit method. OS was measured from the date that RT was initiated to the date of death or the last follow-up visit. Univariate logistic regression analysis was used to evaluate the association between OS and various parameters. To evaluate the relationships between OS and various parameters using multivariate analysis, a stepwise procedure was performed using a logistic regression model containing all variables that attained or had a trend toward univariate statistical significance. P < 0.05 was considered statistically significant. All calculations were performed using PASW 17.0 software for Windows (SPSS, Chicago, IL, USA).
Ethics statement
This study was approved and exempted of permission by the institutional review board of Samsung Medical Center, Sungkyunkwan University School of Medicine (IRB No. 2011-03-081). Informed consent was obtained from all enrolled patients.
RESULTS
Patient characteristics
Between July 2008 and December 2009, 141 patients with unresectable HCC were referred to the Department of Radiation Oncology. Among them, 84 patients met our inclusion criteria. Of these patients, 65 received TACE and RT separated by a two-week interval, 16 patients received TACE and RT separated by a three week interval, while the remaining 3 patients were excluded from the study because the interval between TACE and RT was longer than three weeks. The main PVTT (P = 0.036) is the only significant factor related with START interval and Child-Pugh class (P = 0.062) is marginal. However, T stage (P = 0.723), age (P = 0.793), and sex (P = 0.502) are not related factors.
Remaining 81 patients who had received treatments as per protocol were analyzed. The median patient age was 54 yr (range 26-76 yr). Among the 81 patients, 42 patients had received TACE more than once (median two times) before START. Detailed characteristics of the patients are provided in Table 1.
Table 1.
Characteristics of patients and tumor
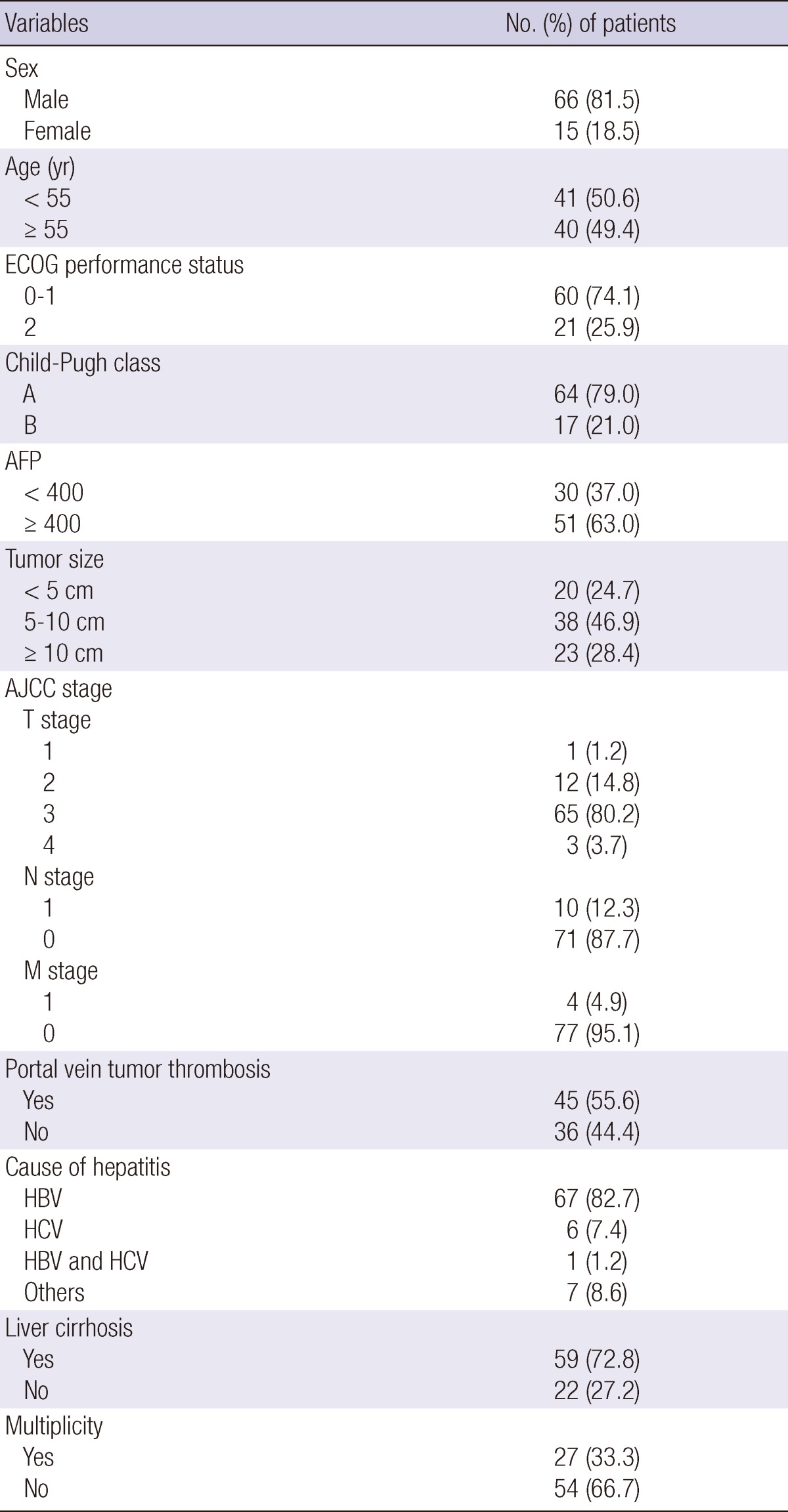
ECOG, Eastern Cooperative Oncology Group; AJCC, American Joint Committee on Cancer; AFP, Alpha feto-protein; HBV, hepatitis B virus; HCV, hepatitis C virus.
Treatment responses
About four weeks after the completion of START, complete response (CR) was observed in 7 patients (8.6%), partial response (PR) in 50 patients (61.7%). Therefore, an objective response rate was 70.4%.
Prognostic factors for response
The relationships between the objective response and various parameters are summarized in Table 2. Significant prognostic factors for the objective response rate were ECOG performance status (P = 0.002), Child-Pugh classification (P = 0.034), tumor multiplicity (P = 0.043), a primary tumor size greater than 10 cm (P = 0.008), PVTT (P = 0.028), and RT dose (biological effective dose, α/β = 10).
Table 2.
Prognostic factors for RT response
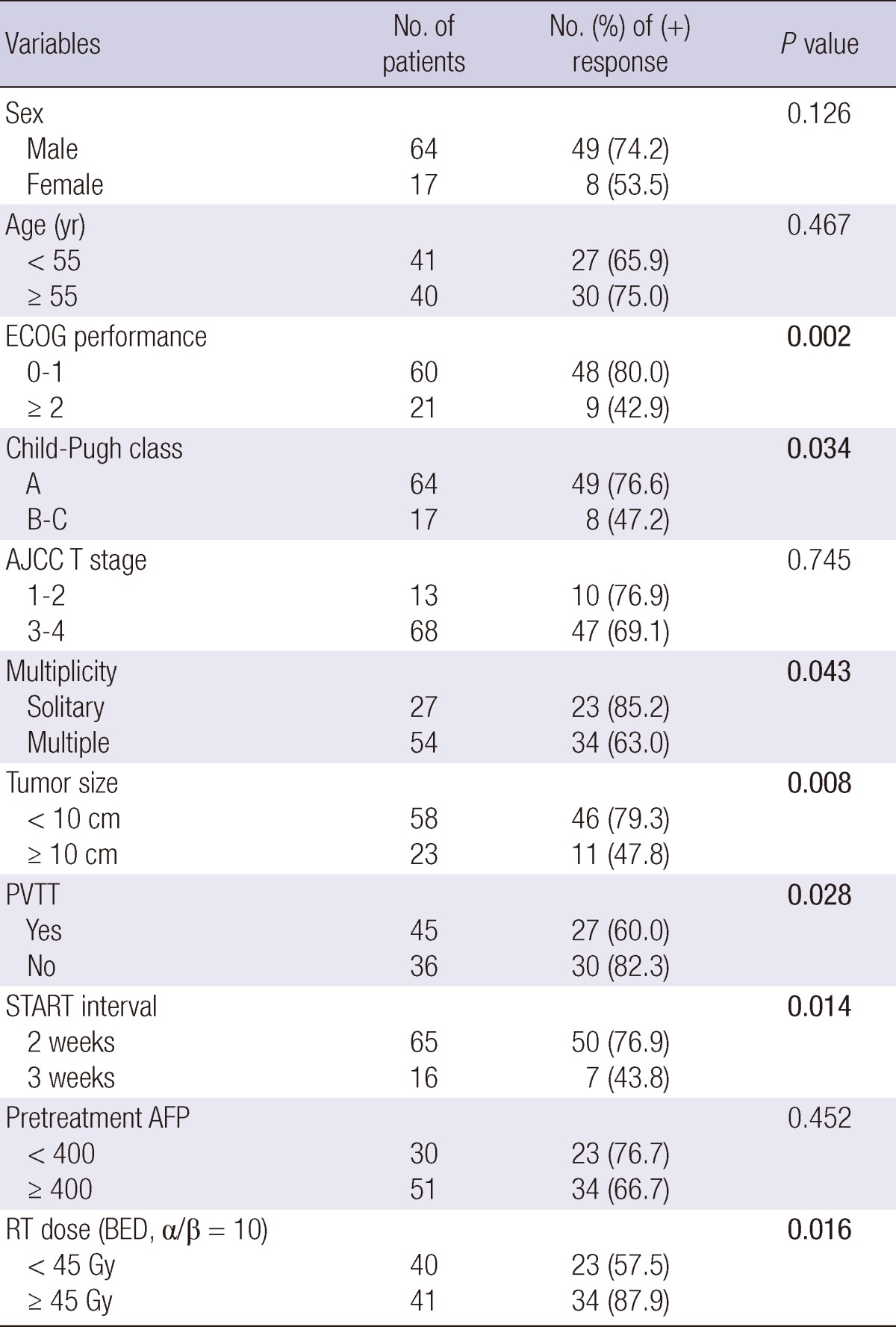
ECOG, Eastern Cooperative Oncology Group; AJCC, American Joint Committee on Cancer; PVTT, Portal vein tumor thrombosis; AFP, Alpha feto-protein; RT, radiation therapy; BED, biologically effective dose.
Complications
The complications encountered during RT are described in Table 3. The most common adverse event was elevation of liver enzyme levels. However, liver enzyme levels were generally less than five-fold elevated compared to the upper normal limits, with the exception of AST levels in five patients, ALT levels in four patients, and ALP levels in one patient. These effects were transient and most patients recovered within one or two weeks.
Table 3.
Acute and chronic complication after radiation therapy
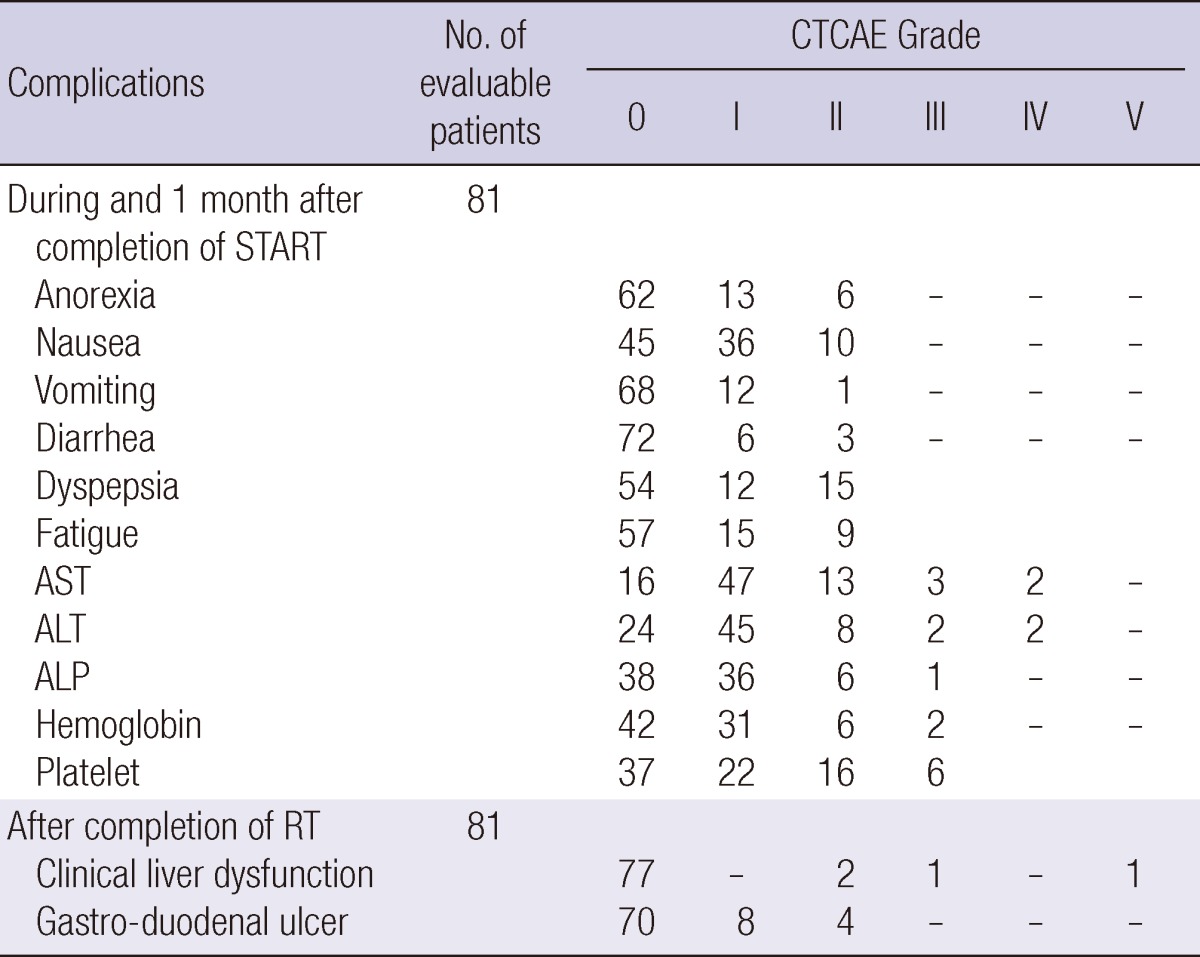
CTCAE, Common Terminology Criteria for Adverse Events; AST, Aspartate transaminase; ALT, Alanine transaminase; ALP, Alkaline phosphatase; RT, Radiation therapy.
Thrombocytopenia (platelets < 50,000/µL) was developed in three patients. Two patients showed thrombocytopenia before TACE and one had not (86,000/µL). Anemia (hemoglobin < 8 g/dL) occurred in two patients who had more than 10 g/dL hemoglobin level, initially. The other common adverse effects were anorexia (19 patients, 23.5%) and nausea (46 patients, 56.8%), but the severity of these complications during RT was confined to CTCAE grade 1 or 2.
Subacute clinical liver dysfunction was observed in four patients (4.9%), with resolution in three patients during follow-up. However, the fourth patient died from liver failure with jaundice two weeks after the completion of START. Although the symptoms of this patient were not consistent with the definition of classic radiation induced liver disease (RILD), we suspect that our combined treatment protocol caused liver function deterioration in this patient. Gastro-duodenal ulcers inside the RT field were present in 12 patients, and 4 of these patients required medical interventions.
Patterns of failure
All failures were diagnosed by radiological examination. Combined recurrences as initial failures were counted separately. A total of 62 treatment failures (76.5%) occurred. Local progression or recurrence within the RT field developed in 12 patients (14.8%). Intrahepatic metastasis developed in 43 patients (53.1%), and extrahepatic metastasis was found in 28 patients (34.6%).
Survival outcomes
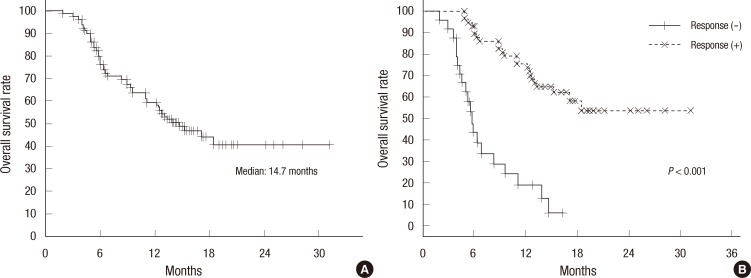
The median follow-up duration was 13 months, ranging from 1 to 31 months. Thirty-eight (46.9%) patients were still alive at the time of the last follow-up. The median survival time was 14.7 months (Fig. 2A). There was a significant difference in survival statistics according to treatment response (P < 0.001, Fig. 2B).
Fig. 2.
Overall survival of all 81 patients with HCC treated by START: Kaplan-Meier curves for overall survival (A) and overall survival according to tumor response after START (B).
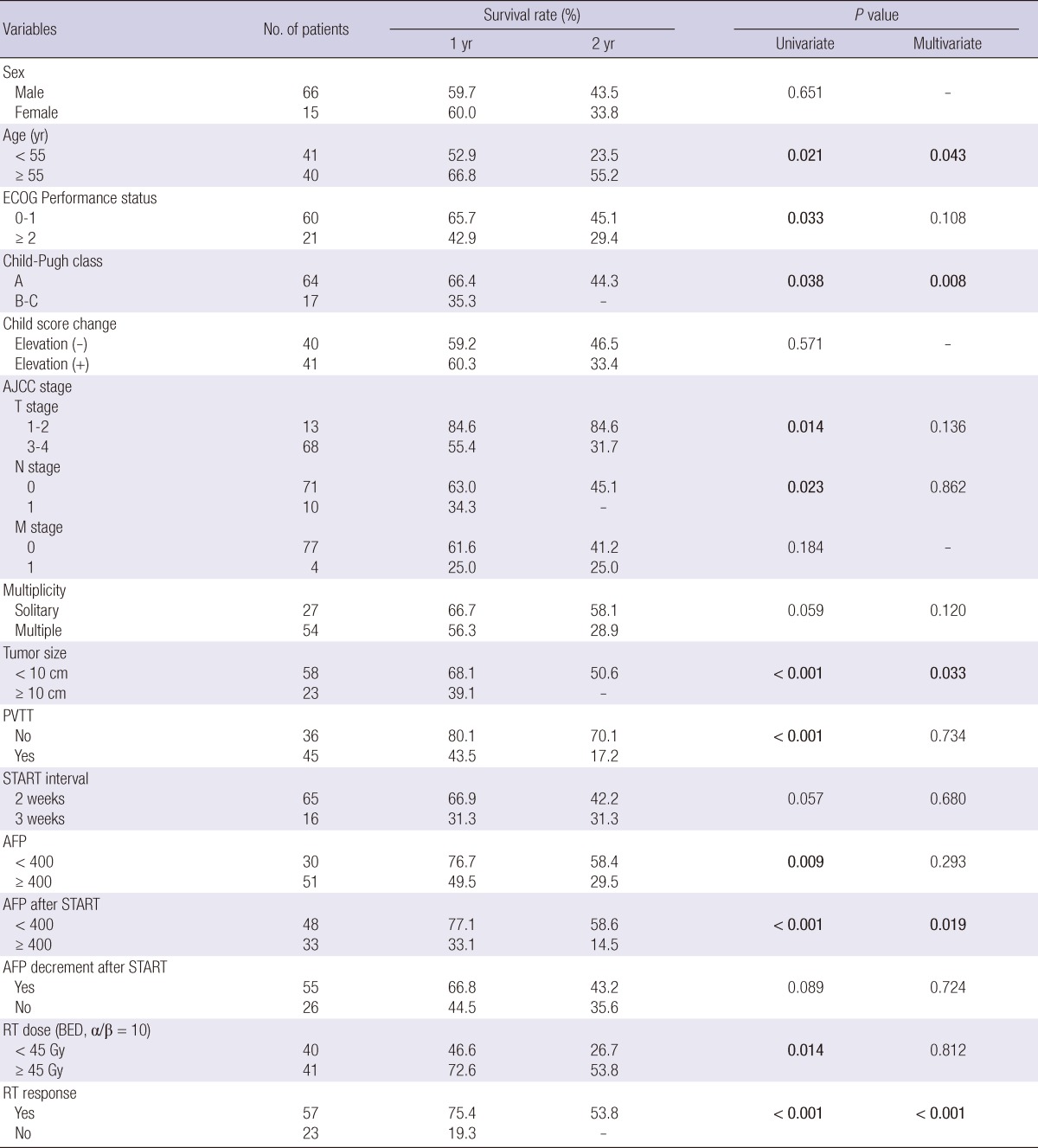
Prognostic factors for overall survival
The results of the relationships between OS and various parameters are summarized in Table 4. Significant prognostic factors for OS in multivariate analysis were age (less than 55), Child-Pugh classification, tumor size (more than 10 cm), AFP decrement after START, and the response to the START.
Table 4.
Prognostic factors for overall survival rate
ECOG, Eastern Cooperative Oncology Group; AJCC, American Joint Committee on Cancer; PVTT, Portal vein tumor thrombosis; AFP, Alpha feto-protein; START, Scheduled interval TACE followed by RT; RT, radiation therapy; BED, biologically effective dose.
DISCUSSION
TACE is currently used to treat unresectable HCC (1). Tumor necrosis can be achieved by the combined effects of antitumoral chemotherapy and selective ischemia of tumor tissues (6). Unfortunately, however, TACE is often associated with life-threatening toxicities and complications, which frequently necessitate anti-cancer drug dosage reduction and interruption or discontinuation of TACE (7). TACE has well-documented limitations even when used for local control only. Pathologic evidence from patients who later underwent resection after TACE revealed that tumors remain viable after TACE, particularly in and around the capsule (2).
Additional local therapy for unresectable HCC after TACE failure is justified because patients usually die of liver failure due to intrahepatic tumor progression. Delivery of an effective local therapy could impact the course of the disease even in patients who are refractory to repeated TACE (8, 9). The introduction of 3D-CRT has facilitated the delivery of high radiation doses to the tumor while sparing the normal liver. Previous studies have shown that the radiation-induced liver injury associated with 3D-CRT is tolerable and the tumor response rates are 50$ to 70% in locally advanced HCC, indicating a dose-response relationship (10, 11).
Numerous clinical studies of TACE plus RT for patients with unresectable HCC have been performed in recent years (3-5, 12). Although the methods used to combine TACE and RT were heterogeneous, combination of these modalities appeared to have a synergistic effect according to several studies and meta-analyses (13). TACE and RT have been combined in three different ways (14). RT has been used as a "salvage" treatment for tumors unresponsive to repeated TACE (5, 12). However, in these cases, it is hard to explain why the combination of TACE and RT had a synergistic effect. The second approach is the use of RT to treat PVTT only (4, 15, 16). The rationale for this approach is that TACE is less effective in patients with PVTT and RT may make TACE more effective if PVTT can be eradicated by RT. The last approach, which we adapted in our study, is to deliver RT as a "consolidation" planned procedure to target residual hepatic tumors after TACE (3, 17, 18). The rationale for this approach is that RT targets cancer cells at the tumor periphery that may remain viable due to blood supply from the collateral circulation or recanalization of the embolized artery. Furthermore, tumor shrinkage after TACE allows the use of a smaller irradiation field, which permits higher tumor doses and improves normal liver tolerance (11). Additionally, any TACE anticancer drugs may have a radio-sensitizing effect. Adriamycin injected at the time of TACE has been shown to augment the anti-tumor efficacy of radiation (19). This drug is maintained at a relatively high concentration in the tumor for up to 27 days. Therefore, RT effect might be maximized if performed within a 27-day interval after TACE.
Based on these ideas, outcomes may vary according to the interval between TACE and RT. However, most published reports have used a wide range of treatment intervals ranging from 1 to 8 weeks, and even longer (3, 5, 12, 15, 18). Furthermore, most of these previous studies were retrospectively designed.
A short interval could theoretically maximize the radiation sensitizing effect of adriamycin, and minimize the repopulation of residual tumor cells after TACE. However, a certain interval is required to ensure the recovery of hepatic function, stabilization of lipiodol uptake, and the disappearance of the systemic effects of adriamycin.
Based on the previous TACE plus RT studies, we hypothesized that a two-week interval between TACE and RT would be tolerable (20). Under this background, we conducted this trial using a two-week interval between TACE and RT (START) in patients with unresectable HCC. START interval was planned according to liver function after TACE (2-3 weeks, or exclusion), and it was related significantly with the presence of main PVTT and marginally with Child-Pugh class. It might suggest that livers of the patient who had main PVTT and/or Child-Pugh class B are damaged more by TACE than the others. Therefore, when planning RT in those patients, radiation oncologist should consider extending of recovery interval after TACE.
Although the subjects of this study had poor prognostic factors such as PVTT, a large tumor size, and repeated TACE refractoriness, START yielded comparable tumor responses and survival curves to those reported for previous TACE plus RT studies. With the exception of an unexpected death due to hepatic failure after START, the other toxicities were manageable. Based on our results, a two-week interval appears to be a good option in those patients. Despite the tumor response and local control, however, the frequency of intrahepatic and/or distant metastases was high. In our trial, these developed in 54.1% and 44.6% of patients, respectively, and were the major failure pattern. Therefore, strategies that combine systemic treatments might be required to reduce intrahepatic and distant metastases after START.
Our study had several limitations. First, data were collected from a single institution, indicating that the clinical outcomes we reported need to be validated. Second, this was not a direct survival comparison study according to the treatment interval. Therefore, we only could provide the information about the possibility of the safety and comparable results, and we could not state that a 2-week interval showed the best outcomes. More studies are needed to address these problems and provide appropriate answers.
In conclusion, START showed comparable results in HCC patients who had poor prognostic factors with previous TACE plus RT series. Furthermore, treatment-related toxicity was manageable in all cases but one. Despite the good local control, however, the frequency of intrahepatic and/or distant metastases after treatment was high. A two-week interval between TACE and RT shows relatively safe and efficient. Further studies are warranted to confirm the safety and efficacy of START, to determine the ideal interval between TACE and RT.
Footnotes
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education, Science, and Technology (2010-0011107).
References
- 1.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 2.Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer. 1994;73:2259–2267. doi: 10.1002/1097-0142(19940501)73:9<2259::aid-cncr2820730905>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Oh D, Lim do H, Park HC, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC, Lim HK, Lee WJ, et al. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol. 2010;33:370–375. doi: 10.1097/COC.0b013e3181b0c298. [DOI] [PubMed] [Google Scholar]
- 4.Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 5.Seong J, Park HC, Han KH, Lee DY, Lee JT, Chon CY, Moon YM, Suh CO. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000;47:1331–1335. doi: 10.1016/s0360-3016(00)00519-8. [DOI] [PubMed] [Google Scholar]
- 6.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 7.Hidajat N, Wust P, Felix R, Schröder R. Radiation dose of the radiologist and the assistant in transarterial hepatic chemoembolization: comparison with the dose limits. Rofo. 2006;178:185–190. doi: 10.1055/s-2005-858789. [DOI] [PubMed] [Google Scholar]
- 8.Dawson LA, Guha C. Hepatocellular carcinoma: radiation therapy. Cancer J. 2008;14:111–116. doi: 10.1097/PPO.0b013e31816a0e80. [DOI] [PubMed] [Google Scholar]
- 9.Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal cein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2011;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150–155. doi: 10.1016/s0360-3016(02)02864-x. [DOI] [PubMed] [Google Scholar]
- 11.Seong J. Challenge and hope in radiotherapy of hepatocellular carcinoma. Yonsei Med J. 2009;50:601–612. doi: 10.3349/ymj.2009.50.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005;25:1189–1196. doi: 10.1111/j.1478-3231.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 13.Meng MB, Cui YL, Lu Y, She B, Chen Y, Guan YS, Zhang RM. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and metaanalysis. Radiother Oncol. 2009;92:184–194. doi: 10.1016/j.radonc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Ma S, Jiao B, Liu X, Yi H, Kong D, Gao L, Zhao G, Yang Y, Liu X. Approach to radiation therapy in hepatocellular carcinoma. Cancer Treat Rev. 2010;36:157–163. doi: 10.1016/j.ctrv.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Park MK, Gwak GY, Lim do H, Choo SW, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. The efficacy of combined transarterial chemoembolization and 3-dimensional conformal radiotherapy for hepatocellular carcinoma with main portal vein thrombosis. Hepatogastroenterology. 2010;57:801–806. [PubMed] [Google Scholar]
- 16.Yu JI, Park HC, Lim do H, Park W, Yoo BC, Paik SW, Koh KC, Lee JH. Prognostic index for portal vein tumor thrombosis in patients with hepatocellular carcinoma treated with radiation therapy. J Korean Med Sci. 2011;26:1014–1022. doi: 10.3346/jkms.2011.26.8.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi SB, Kim KS, Park YN, Choi JS, Lee WJ, Seong J, Han KH, Lee JT. The efficacy of hepatic resection after neoadjuvant transarterial chemoembolization (TACE) and radiation therapy in hepatocellular carcinoma greater than 5 cm in size. J Korean Med Sci. 2009;24:242–247. doi: 10.3346/jkms.2009.24.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu LT, Zhou ZH, Lin JH, Chen Z, Wang K, Wang P, Zhu XY, Shen YH, Meng ZQ, Liu LM. Clinical study of transarterial chemoembolization combined with 3-dimensional conformal radiotherapy for hepatocellular carcinoma. Eur J Surg Oncol. 2011;37:245–251. doi: 10.1016/j.ejso.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989;170:783–786. doi: 10.1148/radiology.170.3.2536946. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Yu J, Wang L, Li C, Zhou T, Zhai L, Xing L. Study of local three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for patients with stage III hepatocellular carcinoma. Am J Clin Oncol. 2003;26:e92–e99. doi: 10.1097/01.COC.0000077936.97997.AB. [DOI] [PubMed] [Google Scholar]