Abstract
Radiographic lesions suggesting old healed tuberculosis (TB) is considered a risk factor for the subsequent development of active TB. The aim of this study was to estimate the positive rates of tuberculin skin test (TST) and interferon-gamma release assay (IGRA) in persons with old healed TB. Participants with lesions suggesting old healed TB on chest images and controls without such lesions were prospectively enrolled between January 1, 2010, and January 31, 2011. TST and the QuantiFERON-TB Gold In-Tube test (QFT-GIT) were performed. In total, 193 participants with old healed TB and 126 controls were recruited. The rates of positive TST and QFT-GIT among patients with old healed TB were 54.6% and 77.7%, respectively. The rates of positive TST and QFT-GIT among patients without old healed TB were 38.9% and 61.9%. Sixteen percent of participants with old healed TB showed negative results by both TST and QFT-GIT. The positive rate of TST waned among participants with old healed TB who were older than 60 yr, whereas QFT-GIT positivity was unaffected by age. The positive rates of TST and IGRA among participants with radiographic lesions suggesting old healed TB was higher than without those lesions. In addition, IGRA may be more accurate than TST for the detection of latent TB infection, especially in populations of individuals older than 60 yr.
Keywords: Radiology and Other Imaging, Tuberculosis, Pulmonary
INTRODUCTION
Among infectious diseases, tuberculosis (TB) is one of the leading causes of mortality and morbidity worldwide. There were 9.4 million new cases and 1.3 million deaths among HIV-negative people in 2009 (1). To control TB, diagnosis and treatment of active TB is not enough because there are many cases of undetected latent TB infection (LTBI) that can develop into active disease over a period of time.
Among patients with LTBI, groups at high risk for the development of active TB include those with advanced HIV infection, those with lesions suggesting old healed TB on chest radiographs (CXR), those with chronic renal failure, those who have undergone anti-TNF-α treatment, those with poorly controlled diabetes, those with silicosis, those who are underweight (> 10% below normal), and those who have undergone gastrectomies (2). Among these, the presence of radiographic lesions consistent with old healed TB is one of the strongest risk factors for development of active TB (3). The 5-yr incidence of TB in tuberculin skin test (TST)-positive persons with old healed TB lesions on their CXR was 14.3 per 1,000 person-years. Among individuals with fibrotic lesions larger than 2 cm2, the incidence was 21.3 per 1,000 person-years (4). In another report, the presence of calcified nodular densities or fibrosis together with nodular densities in the mid and/or upper lung zones was associated with 8.8-fold higher odds for the subsequent development of active TB (5).
There is no reference standard for detection of LTBI, therefore TST had been the only test to evaluate the possibility of infection for a long time. Recently, a whole blood interferon-γ (IFN-γ) release assay (IGRA) which measures cellular immune responses to the tuberculosis-specific proteins ESAT-6 and CFP-10, has become commercially available and been regarded as superior modality than TST in detection of LTBI (6).
Although the presence of lesions suggesting old healed TB has been regarded as a hallmark of LTBI, there is little information on the positive rates of TST and IGRA in patients with radiographic lesions consistent with old healed TB. The objective of the present study was to estimate the positive rates of TST and IGRA in persons with radiographic lesions suggesting old healed TB.
MATERIALS AND METHODS
Participants
Participants were recruited prospectively between January 1, 2010, and January 31, 2011, at Seoul National University Hospital, a tertiary referral hospital in Seoul, Republic of Korea. A board-certified radiologist reviewed CXRs and, if available, chest computed tomography (CT) scans, of patients who were admitted or referred to the respiratory clinic during the study period. If patients satisfied the enrollment criteria, they were asked to participate in this study. Participants who had chest imaging abnormalities consistent with old healed TB were enrolled as a case group and those without such lesions were enrolled as a control group. Participants were excluded if they had taken immune-suppressive drugs during the previous 3 months, had positive HIV test results, had isolation of nontuberculous mycobacteria, were undergoing renal replacement therapy, or had uncontrolled diabetes. Participants in the control group were excluded if they had a past history of treatment for active TB, had pleural effusion, or had undergone resection of part of their lung. The possibility of active pulmonary TB was excluded in any patient who could expectorate sputum by three times of sputum that was negative for acid-fast staining and mycobacterial culture.
After providing written consent, each participant was asked about his/her past medical history including TB, smoking status, and co-morbidities. Additionally, the presence of BCG vaccination scar was screened and their medical records were reviewed.
Study protocol
Interpretation of CXR and Chest CT
The presence of old healed TB was defined based on criteria proposed by Linh et al. (5) and the ATS/CDC guidelines (7). Lesions compatible with old healed TB were categorized as probable or possible cases. Probable cases were defined as; calcified nodular densities in mid and/or upper lung zones; fibrosis and nodular densities in mid and/or upper lung zones. Possible cases included lesions such as; calcified nodular densities in lower zones; fibrosis or nodular densities in mid and/or upper lung zones; single calcified nodule; significant basal pleural thickening (≥ 10 mm); bronchiectasis in upper lobe.
IGRA
The QuantiFERON-TB Gold In-Tube test (QFT-GIT) was performed according to the manufacturer's instructions (8).
TST
After collection of blood samples for the QFT-GIT, the TST was performed on the volar side of the forearm according to the Mantoux method using a 2-TU dose of purified protein derivative RT23 (Statens Serum Institut, Copenhagen, Denmark), and any induration was measured in millimeters after 48-72 hr using the ballpoint method (9). We defined a positive test as an induration of ≥ 10 mm (10).
Statistical analysis
Statistical test of comparison was made using Pearson's chi-squared test or Fisher's exact test for categorical variables and Student's t-test for continuous variables. Statistical significance was defined by a 2-tailed P value of ≤ 0.05. Agreement between the TST and QFT-GIT results was assessed using κ coefficients (11). All statistical analyses were conducted using the PASW software (Ver. 17.0; SPSS Inc., Chicago, IL, USA).
Ethicis statement
The informed consent was obtained from every participants. The study protocol was approved by the ethics review committee of the Seoul National University Hospital (IRB No. H-0909-054-295).
RESULTS
Demographics of the participants
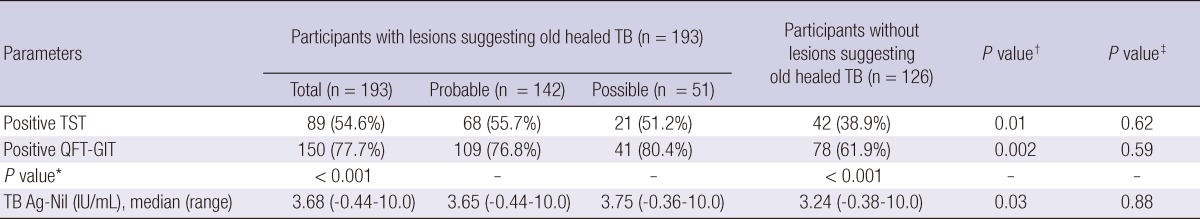
In total, 193 participants with old healed TB on their CXR and 126 without evidence of old healed TB were recruited. The median age of the case group was 64 (range, 36-88) yr and that of the control group was 60 (range, 18-81) yr (P = 0.01). There were 120 (62.2%) males in the case group and 67 (53.2%) in the control group. No participant in either group reported contact with active TB patients in recent 6 months. There was no significant difference between the two groups in the prevalence of comorbidities, including diabetes, chronic liver disease, chronic renal failure, and malignancies (Table 1). Available CT scans were reviewed among 84.0% of participants.
Table 1.
Demographic and clinical characteristics of participants
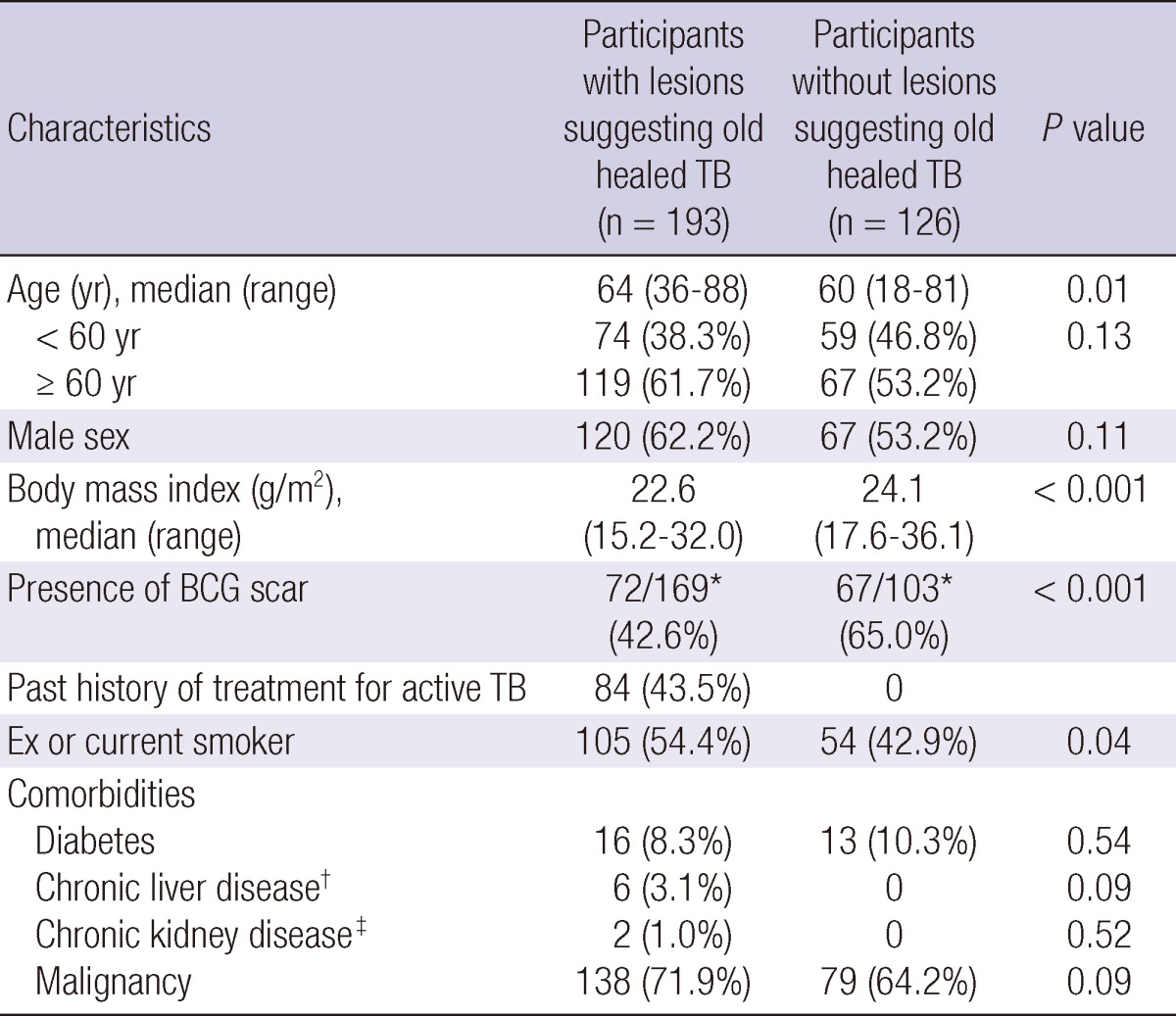
*Number of participants with available information on presence of BCG scar; †Chronic viral hepatitis and liver cirrhosis with Child-Pugh Class A; ‡Estimated GFR (MDRD formula) < 50 mL/min/1.73 m2.
Results of TST and QFT-GIT among participants
TST results were missing in 48 participants (30 in the case group and 18 in the control group), whereas QFT-GIT results were available for all participants. No indeterminate QFT-GIT result was reported.
The TST positive response rate was higher in the case group than in the controls (54.6% vs 38.9%; P = 0.01). The QFT-GIT positive response rate was also higher in the case group than in the controls (77.7% vs 61.9%; P = 0.002). When compared to TST, positive rates of the QFT-GIT are higher in both groups (P < 0.001). No difference in the TST and QFT-GIT positive rates was identified between participants with probable and possible old healed TB (P = 0.62 and 0.59, respectively) (Table 2).
Table 2.
Results of tuberculin skin test and QuantiFERON-TB Gold In-Tube assay
*Comparison between results of TST and QFT-GIT; †Comparison between old healed TB group vs control group; ‡Comparison between probable old healed group vs possible old healed group.
Comparison of TST and QFT-GIT results between different age groups
To examine the impact of age on TST and QFT-GIT results, participants were classified into two groups: < 60 and ≥ 60 yr of age. The TST positive rate was lower among ≥ 60-yr-old participants with radiographic lesions suggesting old healed TB than among the younger participants (43.3% vs 71.2%; P < 0.01). However, no difference in the QFT-GIT positive rate was observed between the two age groups in participants with radiographic lesions suggesting old healed TB (78.2% vs 77.0%; P = 0.86) (Table 3).
Table 3.
Results of tuberculin skin test and QuantiFERON-TB Gold In-Tube assay among different age groups
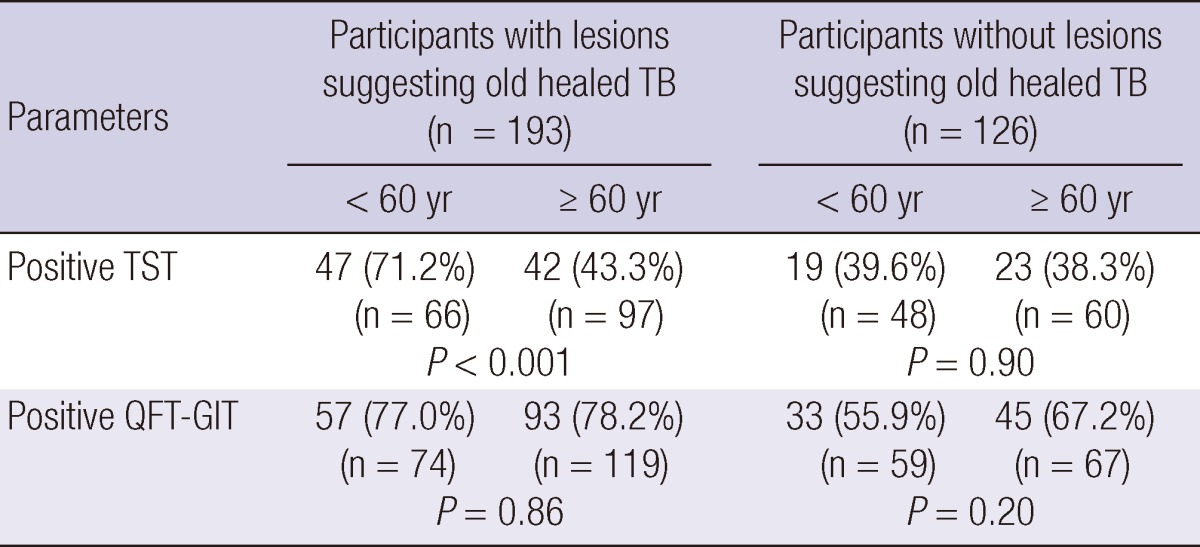
P values are from comparisons of positive rates between participants < 60 and ≥ 60 yr.
Comparison of TST and QFT-GIT results between participants with and without a prior history of TB
The TST and QFT-GIT results were not affected by past history of TB treatment. The TST positive response rate in case group was 52.2% in participants without history of previous TB and was 57.7% in those with history of TB (P = 0.48). In addition, the QFT-GIT positive response rate in case group was 76.1% in participants without history of previous TB and 79.8% in those with history of TB (P = 0.55).
Concordance of TST and QFT-GIT results
In total, 48.5% of participants with radiographic lesions suggesting old healed TB had positive results for both TST and QFT-GIT, 29.4% showed negative TST and positive IGRA results. In addition, 6.1% showed positive TST and negative QFT-GIT results, and 16.0% showed negative results by both tests (Table 4).
Table 4.
Classification of participants with old healed TB based on tuberculin skin test and QuantiFERON-TB Gold In-Tube assay
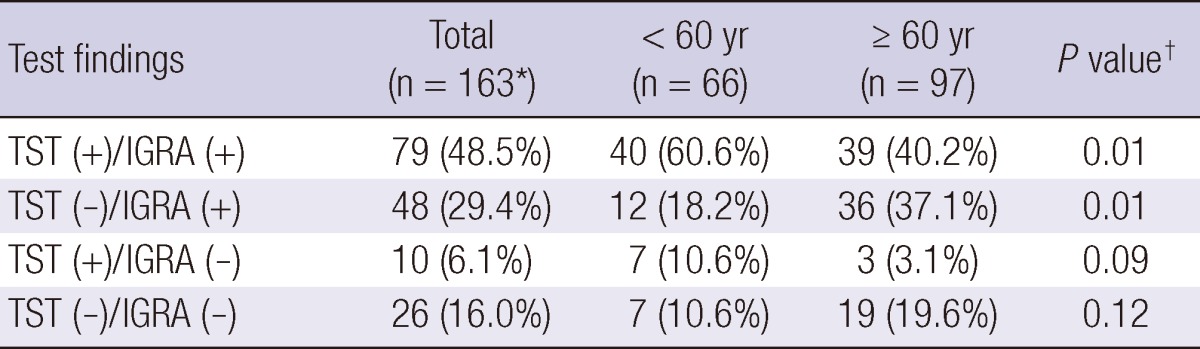
*Results of TST were missing in 30 participants with old healed TB; †Comparison between the number included in specific category and the others.
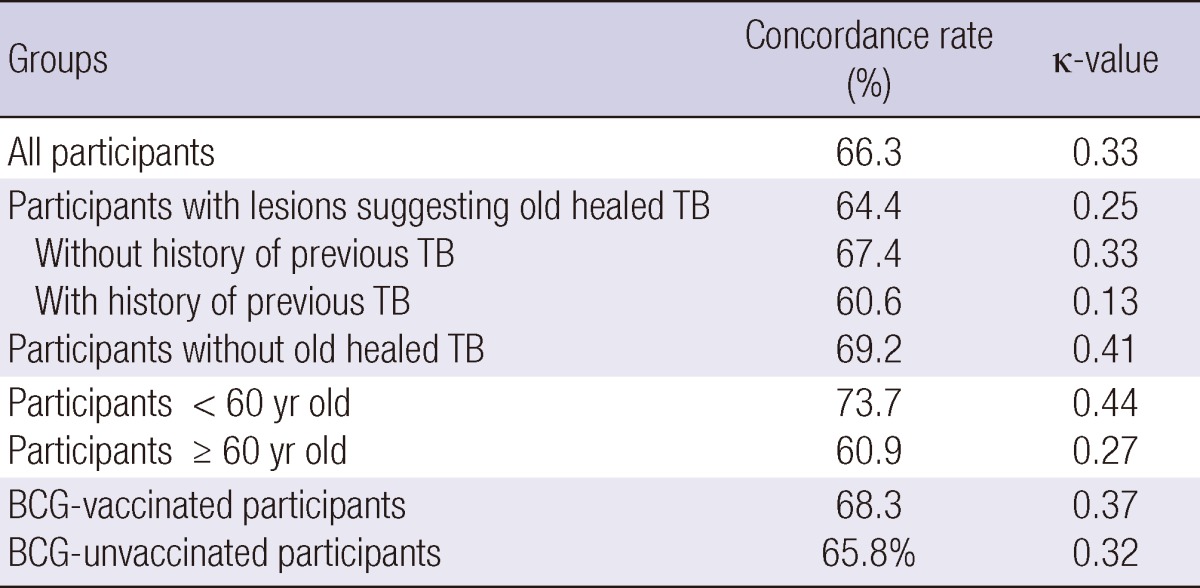
The overall agreement between the TST and QFT-GIT in participants was poor (κ = 0.33). The TST and QFT-GIT concordance rate was poor in participants with radiographic lesions suggesting old healed TB (κ = 0.25), but was better in the control group (κ = 0.41). Agreement between the two tests was better among participants < 60 yr of age (κ = 0.44) than among older participants (κ = 0.27). However, agreement was not different between participants with BCG scars (κ = 0.37) and without BCG scar (κ = 0.32) (Table 5).
Table 5.
Concordance rates of tuberculin skin test and QuantiFERON-TB Gold In-Tube assay
DISCUSSION
Although old healed TB on CXR has been regarded as one of the strongest risk factors for development of active TB, as well as presence of LTBI (12-14), the actual positive rates of TST and IGRA among patients with these lesions is unclear. This prospective study showed that the positive rates of TST and QFT-GIT were 54.6% and 77.7%, respectively, higher than in participants without radiographic lesions suggesting old healed TB (38.9% by TST and 61.9% by QFT-GIT).
In a previous study that evaluated the prevalence of LTBI among healthcare workers in India, no difference in positive rates of TST as well as QFT-GIT was identified between participants with radiographic lesions suggesting old healed TB and participants without those lesions (TST positivity, 81.9% vs 84.0%; QFT-GIT positivity, 82.8% vs 79.6%) (15). Given the higher prevalence of active TB among the general population in India (249 per 100,000 persons in 2009) (1) than in Korea (115 per 100,000 persons in 2009) (1), as well as the higher prevalence of LTBI among healthcare workers (16), the association between presence of radiographic lesions suggesting old healed TB and positive TST or QFT-GIT might have been masked by the high prevalence of LTBI among participants with normal CXR in that study.
Although calcified nodular densities or fibrosis together with nodular densities in the mid and/or upper lung zones have been regarded as typical patterns of old healed TB, the positive rates of TST or QFT-GIT did not differ according to the characteristics of radiographic lesions in our study. Participants with radiographic lesions suggesting old healed TB were classified into probable and possible groups. Participants with typical radiographic findings of old healed TB were included in the probable group, and those with relatively minor abnormalities might be associated with previous TB infection in the possible group. The TST and QFT-GIT positive rates among participants with probable TB sequelae and those among participants with possible TB sequelae did not differ. This indicates that the presence of radiographic lesions suggesting old healed TB itself rather than a specific CXR pattern suggesting the presence of old healed TB is important in predicting the positive TST or QFT-GIT.
Increasing age is a risk factor for LTBI in areas where TB is common because older persons have had more chances to contact active TB patients. Based on the observation that TST positivity increased with age (17), TST has been suggested to be more sensitive than IGRA in detection of remote infections. However, in the present study, the TST positive rate was lower among participants with radiographic lesions suggesting old healed TB. Conversely, IGRA positivity was not affected by older age. This discrepancy between TST and IGRA in the aged group could be explained by waning immunity against tuberculin with age. False-negative TST results have been reported in older populations (18, 19), and this may be associated with decreased delayed type hypersensitivity with increasing age. Because IGRA is based on different T-cell immunity, rather than a delayed type hypersensitivity reaction, it may not be affected by aging as much as TST (12, 20, 21).
In our study, the TST and QFT-GIT results were not affected by past history of TB treatment among participants with lesions suggesting old healed TB. This observation could be explained by the fact the 32%-46% of patients with active TB had negative conversion of IGRA with anti-TB treatment (22, 23). However, the clinical meaning of negative IGRA conversion with anti-TB treatment among TB patients is not clear yet.
Most studies have shown that agreement between TST and QFT-GIT is poor-to-intermediate (κ = 0.16-0.55) (17, 24). Likewise, the present study showed that the overall agreement between the TST and QFT-GIT in participants was poor (κ = 0.33). A previous study suggested that the BCG vaccination status was as a major reason for this discrepancy (25). However, in the present study, the agreement did not differ between participants with (κ = 0.37) and without BCG scars (κ = 0.32). Instead, age was associated with discordance between the two tests. Agreement of the two tests was better among participants < 60 yr of age (κ = 0.44) than among older participants (κ = 0.27). Poor agreement in the older group was caused primarily by an increased proportion of participants with TST-negative and QFT-GIT-positive results among them. This result is consistent with previous observations that TST-negative and QFT-positive discordance increased with age (26, 27) and indicates that the QFT-GIT test may be more sensitive than the TST in the elderly.
Although the presence of lesions suggesting old healed TB has been regarded as a hallmark of LTBI, both TST and QFT-GIT were negative in 16.0% of participants with those lesions. That is, no evidence of LTBI was identified among them. This could be explained in two ways. First, neither TST nor QFT-GIT may be as sensitive as expected in the detection of LTBI. Previous studies confirming excellent sensitivity of QFT-GIT used active TB patients as the gold standard for TB infection (28). However, IFN-γ releasing capacity to TB antigens might be weaker among patients with LTBI than among patients with active TB. If this is true, then QFT-GIT sensitivity in the diagnosis of LTBI would be lower than expected. Second, the interpretation of radiographic lesions suggesting old healed TB could be inaccurate in some cases. In fact, radiographic lesions suggestive of TB may also be present in conditions such as histoplasmosis, pneumoconiosis, hypersensitivity pneumonitis, sarcoidosis, vasculitis, and siderosis. In this context, the presence of radiographic lesions suggesting old healed TB might not be a good surrogate for the presence of LTBI, although it has been widely used (29-31).
The strengths and limitations of this study must be acknowledged. In previous studies, the presence of radiographic lesions consistent with old healed TB was determined based on CXR. However, chest CT could differentiate old fibrotic lesions more clearly (32) and has the potential to play a role as a predictor of future development of TB, possibly better than CXR (33). The fact that chest CT was available among 84.0% of participants makes the present results more reliable. A limitation of this study is that a considerable proportion of participants had underlying comorbidities such as diabetes, which can increase susceptibility to TB infection. However, as the proportions of participants with comorbidities were not different between the two groups, the possibility of the results being affected by the presence of comorbidities might not be significant. However, the burden of TB is still intermediate to high in Korea, thus the interpretation of the results might not be applicable in general.
The positive rates of TST and IGRA among participants with radiographic lesions suggesting old healed TB is higher than those without such lesions. IGRA may be more accurate than TST for the detection of LTBI, especially in populations of individuals older than 60 yr.
References
- 1.World Health Organization. Global tuberculosis control: WHO report 2010. Geneva: World Health Organization; 2010. [Google Scholar]
- 2.Horsburgh CR., Jr Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060–2067. doi: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- 3.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 4.International Union Against Tuberculosis; International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ. 1982;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- 5.Linh NN, Marks GB, Crawford AB. Radiographic predictors of subsequent reactivation of tuberculosis. Int J Tuberc Lung Dis. 2007;11:1136–1142. [PubMed] [Google Scholar]
- 6.Schluger NW, Burzynski J. Recent advances in testing for latent TB. Chest. 2010;138:1456–1463. doi: 10.1378/chest.10-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161:S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 8.Cellestis. QuantiFERON®-TB Gold In-Tube Package Insert. 2006. [Google Scholar]
- 9.Sokal JE. Editorial: Measurement of delayed skin-test responses. N Engl J Med. 1975;293:501–502. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49:1–51. [PubMed] [Google Scholar]
- 11.Fleiss JL, Levin B, Paik M. The measurement of interrater agreement. In: Shewart WA, editor. Statistical methods for rates and proportions. 3rd ed. Hoboken, NJ: John Wiley & Sons; 2004. pp. 598–626. [Google Scholar]
- 12.Harada N, Nakajima Y, Higuchi K, Sekiya Y, Rothel J, Mori T. Screening for tuberculosis infection using whole-blood interferon-gamma and Mantoux testing among Japanese healthcare workers. Infect Control Hosp Epidemiol. 2006;27:442–448. doi: 10.1086/504358. [DOI] [PubMed] [Google Scholar]
- 13.Matulis G, Jüni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008;67:84–90. doi: 10.1136/ard.2007.070789. [DOI] [PubMed] [Google Scholar]
- 14.Seyhan EC, Sökücü S, Altin S, Günlüoğlu G, Trablus S, Yilmaz D, Koksalan OK, Issever H. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection in hemodialysis patients. Transpl Infect Dis. 2010;12:98–105. doi: 10.1111/j.1399-3062.2009.00469.x. [DOI] [PubMed] [Google Scholar]
- 15.Joshi R, Patil S, Kalantri S, Schwartzman K, Menzies D, Pai M. Prevalence of abnormal radiological findings in health care workers with latent tuberculosis infection and correlations with T cell immune response. PLoS One. 2007;2:e805. doi: 10.1371/journal.pone.0000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62–70. doi: 10.1136/thx.2010.143180. [DOI] [PubMed] [Google Scholar]
- 17.Arend SM, Thijsen SF, Leyten EM, Bouwman JJ, Franken WP, Koster BF, Cobelens FG, van Houte AJ, Bossink AW. Comparison of two interferon-gamma assays and tuberculin skin test for tracing tuberculosis contacts. Am J Respir Crit Care Med. 2007;175:618–627. doi: 10.1164/rccm.200608-1099OC. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Stable EJ, Flaherty D, Schecter G, Slutkin G, Hopewell PC. Conversion and reversion of tuberculin reactions in nursing home residents. Am Rev Respir Dis. 1988;137:801–804. doi: 10.1164/ajrccm/137.4.801. [DOI] [PubMed] [Google Scholar]
- 19.Woodruff CE, Chapman PT. Tuberculin sensitivity in elderly patients. Am Rev Respir Dis. 1971;104:261–263. doi: 10.1164/arrd.1971.104.2.261. [DOI] [PubMed] [Google Scholar]
- 20.Schablon A, Harling M, Diel R, Nienhaus A. Risk of latent TB infection in individuals employed in the healthcare sector in Germany: a multicentre prevalence study. BMC Infect Dis. 2010;10:107. doi: 10.1186/1471-2334-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diel R, Loddenkemper R, Meywald-Walter K, Gottschalk R, Nienhaus A. Comparative performance of tuberculin skin test, QuantiFERON-TB-Gold In Tube assay, and T-Spot.TB test in contact investigations for tuberculosis. Chest. 2009;135:1010–1018. doi: 10.1378/chest.08-2048. [DOI] [PubMed] [Google Scholar]
- 22.Lee SW, Lee CT, Yim JJ. Serial interferon-gamma release assays during treatment of active tuberculosis in young adults. BMC Infect Dis. 2010;10:300. doi: 10.1186/1471-2334-10-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobashi Y, Sugiu T, Ohue Y, Mouri K, Obase Y, Miyashita N, Oka M. Long-term follow-up of the QuantiFERON TB-2G test for active tuberculosis disease. Intern Med. 2008;47:1957–1961. doi: 10.2169/internalmedicine.47.1313. [DOI] [PubMed] [Google Scholar]
- 24.Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, Lange C, Losi M, Markova R, Migliori GB, et al. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J. 2011;37:88–99. doi: 10.1183/09031936.00115110. [DOI] [PubMed] [Google Scholar]
- 25.Kobashi Y, Mouri K, Obase Y, Fukuda M, Miyashita N, Oka M. Clinical evaluation of QuantiFERON TB-2G test for immunocompromised patients. Eur Respir J. 2007;30:945–950. doi: 10.1183/09031936.00040007. [DOI] [PubMed] [Google Scholar]
- 26.Nienhaus A, Schablon A, Diel R. Interferon-gamma release assay for the diagnosis of latent TB infection: analysis of discordant results, when compared to the tuberculin skin test. PLoS One. 2008;3:e2665. doi: 10.1371/journal.pone.0002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyrhol-Riise AM, Gran G, Wentzel-Larsen T, Blomberg B, Haanshuus CG, Mørkve O. Diagnosis and follow-up of treatment of latent tuberculosis; the utility of the QuantiFERON-TB Gold In-tube assay in outpatients from a tuberculosis low-endemic country. BMC Infect Dis. 2010;10:57. doi: 10.1186/1471-2334-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest. 2010;137:952–968. doi: 10.1378/chest.09-2350. [DOI] [PubMed] [Google Scholar]
- 29.Malaviya AN, Kapoor S, Garg S, Rawat R, Shankar S, Nagpal S, Khanna D, Furst DE. Preventing tuberculosis flare in patients with inflammatory rheumatic diseases receiving tumor necrosis factor-alpha inhibitors in India: an audit report. J Rheumatol. 2009;36:1414–1420. doi: 10.3899/jrheum.081042. [DOI] [PubMed] [Google Scholar]
- 30.Triverio PA, Bridevaux PO, Roux-Lombard P, Niksic L, Rochat T, Martin PY, Saudan P, Janssens JP. Interferon-gamma release assays versus tuberculin skin testing for detection of latent tuberculosis in chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24:1952–1956. doi: 10.1093/ndt/gfn748. [DOI] [PubMed] [Google Scholar]
- 31.Laffitte E, Janssens JP, Roux-Lombard P, Thielen AM, Barde C, Marazza G, Panizzon RG, Saurat JH. Tuberculosis screening in patients with psoriasis before antitumour necrosis factor therapy: comparison of an interferon-gamma release assay vs. tuberculin skin test. Br J Dermatol. 2009;161:797–800. doi: 10.1111/j.1365-2133.2009.09331.x. [DOI] [PubMed] [Google Scholar]
- 32.Im JG, Itoh H, Shim YS, Lee JH, Ahn J, Han MC, Noma S. Pulmonary tuberculosis: CT findings--early active disease and sequential change with antituberculous therapy. Radiology. 1993;186:653–660. doi: 10.1148/radiology.186.3.8430169. [DOI] [PubMed] [Google Scholar]
- 33.Richeldi L, Losi M, D'Amico R, Luppi M, Ferrari A, Mussini C, Codeluppi M, Cocchi S, Prati F, Paci V, et al. Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest. 2009;136:198–204. doi: 10.1378/chest.08-2575. [DOI] [PubMed] [Google Scholar]