Abstract
Spot urinary albumin to creatinine ratio (ACR) measurement has been suggested as a surrogate to 24-hr urine collection for the assessment of microalbuminuria, and cystatin C (cysC) is known as an advantageous marker for renal function. The aim of this study was to evaluate the clinical values of spot urinary ACR and serum cysC for the assessment of diabetic nephropathy instead of 24-hr urine microalbumin in children and adolescents with diabetes. A total of 113 children and adolescents (age 12-19 yr, M:F = 47:66) with type 1 or 2 diabetes were enrolled. We evaluated the validity of spot urine ACR and serum cysC, and then compared them to 24-hr urine microalbumin and creatinine clearance. Spot urine ACR was correlated with 24-hr urine albumin excretion (R2 = 0.828, P = 0.001) and creatinine clearance (R2 = 0.249, P = 0.017). The ROC curve analysis of serum cysC demonstrated higher diagnostic accuracy than that of serum creatinine (AUC 0.732 vs 0.615). Both the measurements of spot urine ACR and serum cysC might better predict the presence of diabetic nephropathy than 24-hr urine microalbumin in childhood diabetic patients.
Keywords: Diabetic Nephropathies, Albumin to Creatinine Ratio, Cystatin C, Childhood Diabetes
INTRODUCTION
Diabetic nephropathy is a significant complication in diabetic patients, and it is becoming the most common cause of end stage renal disease (1). Childhood diabetic patients live long enough for nephropathy to develop because they develop diabetes early, usually before reaching adolescence. Therefore, preventing diabetic nephropathy or delaying the disease progression by way of early detection is very important (2, 3).
The determination of microalbuminuria has been suggested as an early predictor of diabetic nephropathy. The gold standard for measuring urine albumin excretion is still 24-hr urine collection. However, the standard clearance technique necessitates timed urine collection, which is not only time-consuming but also subject to error. Particularly, children with diabetes tend to collect 24-hr urine inaccurately because they are young and immature. Furthermore, 24-hr urinary albumin excretion rates can be altered by various conditions, such as intra-individual variability and day-to-day variation during the evolution of albuminuria (4).
Recently, the albumin to creatinine ratio (ACR), measured from a random urine sample, was suggested to be an effective surrogate to 24-hr urine collection for detecting microalbuminuria (5-7). ACR is convenient to perform, and is less affected by variation in urine concentration because it is a ratio between two measured substances.
Also, serum creatinine has been widely used as a marker of GFR, but it is not sensitive enough to detect decreased renal function. Therefore, various plasma low molecular weight proteins have been suggested as valuable markers of decreased renal function in place of serum creatinine (8). Among these markers, previous studies demonstrated that serum cystatin C (cysC) might be a superior marker for the evaluation of renal function than serum creatinine (9). However, the effectiveness of cysC for estimating GFR has not been sufficiently demonstrated in children with diabetes.
Therefore, the aim of this study was to investigate whether spot urine ACR and serum cysC are accurate and effective in assessing renal function instead of 24-hr urine microalbumin in children and adolescents with diabetes.
MATERIALS AND METHODS
A total of 113 children and adolescents (age 12-19 yr) with type 1 or 2 diabetes at Childhood Diabetes Clinic of Severance Children's Hospital were included in this study from January 2008 to August 2010. Ninety-eight (41 males and 57 females) patients were type 1 and 15 (6 males and 9 females) were type 2. Age, duration of disease, mean glycated hemoglobin (HbA1c), 24-hr urine microalbumin, ACR in spot urine, serum Cr, serum cysC, high sensitivity C-reactive protein (CRP), which rises in response to inflammation, were checked in all patients. Creatinine clearance was calculated using 24-hr urine collection. Estimated GFR (eGFR) was calculated with the Schwartz formula for patients under the age of 18 yr: eGFR (mL/min/1.73 m2) = k * Height (cm)/serum Cr (mg/dL) (k = 0.55 in children to 13 yr of age, k = 0.70 in adolescent males [the constant remains 0.55 for females]) (10) and Modification of Diet in Renal Disease (MDRD) formula for those of ages of 18 to 19 : eGFR = 186 * (Serum Cr) - 1.154 * (Age) - 0.203 * (0.742 if female) (11).
Serum creatinine was determined by an enzymatic method (Kodak Ektachem 700 XR-C system, Eastman Kodak, Rochester, NY, USA). Serum cysC was measured by automated particle-enhanced immunonephelometry using a BN100 nephelometer (Dade Behring, Marburg, Germany).
Data were analyzed by the SAS program (version 9.1; SAS Institute, Cary, NC, USA). We used the generalized linear regression model and chi-squared test to verify the demographic trends of continuous and categorical variables over the ordinal variable of time, respectively. Significance was determined as P < 0.05. The sensitivity and specificity of serum cysC, serum creatinine, and creatinine clearance for the detection of reduced GFR were assessed by the ROC curve.
Ethics statement
This study was approved by the Institutional Review Board of Yonsei University Severance Hospital (IRB number 4-2012-0001). Written informed consent was exempted from all subjects as well as their parents.
RESULTS
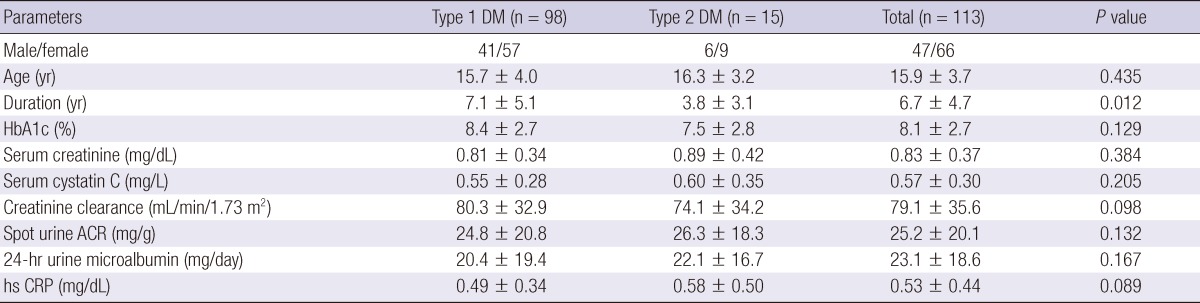
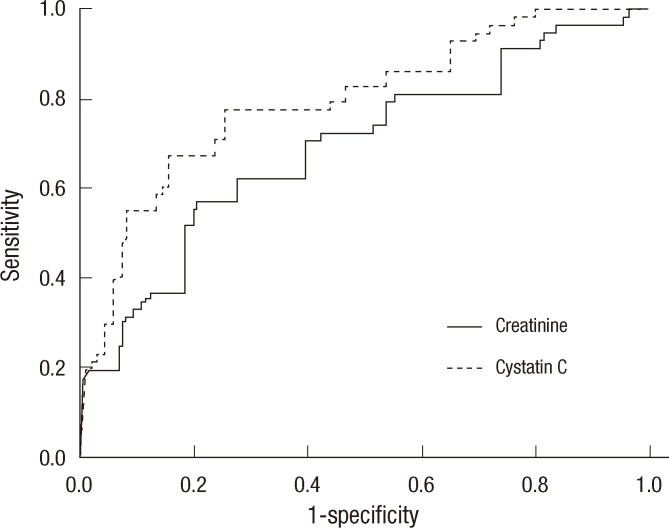
Baseline patient characteristics are shown in Table 1. There were no significant differences between the type 1 and 2 diabetes groups regarding mean HbA1c, serum creatinine, cysC and creatinine clearance. Ninety six patients (85%) had normoalbuminuria, 17 (15%) had microalbuminuria and no patient had macroalbuminuria. We compared the microalbuminuria group with the normoalbuminuria group (Table 2). Age, duration of disease, and mean HbA1c levels were significantly higher in the microalbuminuria group than in the normoalbuminuria group, suggesting that the incidence of diabetic nephropathy increases according to the progress of the disease and poor glycemic control. Serum creatinine level was not significantly different between the two groups, but serum cysC was significantly higher in the microalbuminuria group (P = 0.040). Creatinine clearance was significantly lower in the microalbuminuria group. High sensitivity CRP did not differ between the two groups. Spot urine ACR was positively correlated with 24-hr urine albumin excretion (R2 = 0.828 and P = 0.001) (Fig. 1) and negatively correlated with creatinine clearance (R2 = 0.249 and P = 0.017).
Table 1.
Baseline characteristics of patients with type 1 or 2 diabetes
HbA1c, Glycated hemoglobin; ACR, Albumin to creatinine ratio; hs CRP, High sensitivity C-reactive protein.
Table 2.
Comparison between the normoalbuminuria and microalbuminuria groups
NormoA, Normoalbuminuria; MicroA, Microalbuminuria; HbA1c, Glycated hemoglobin; ACR, Albumin to creatinine ratio; hs CRP, high sensitivity C-reactive protein.
Fig. 1.
Correlation between 24-hr microalbumin (mg/24 hr) and spot urine albumin to creatinine ratio (ACR) (mg/g). The lower cutoff for microalbuminuria; 30 (mg/24 hr) in 24-hr urine microalbumin represents 36 (mg/g) in spot urine ACR.
We analyzed serum creatinine, cysC and spot urine ACR between the patients with creatine clearance < 60 mL/min/1.73 m2 (group I) and those with creatine clearance > 60 mL/min/1.73 m2 (group II) (Table 3). Serum creatinine did not differ between the two groups, but spot urine ACR and serum cysC were significantly greater in group I.
Table 3.
Comparison of serum parameters between the creatinine clearance < 60 and creatinine clearance > 60 groups
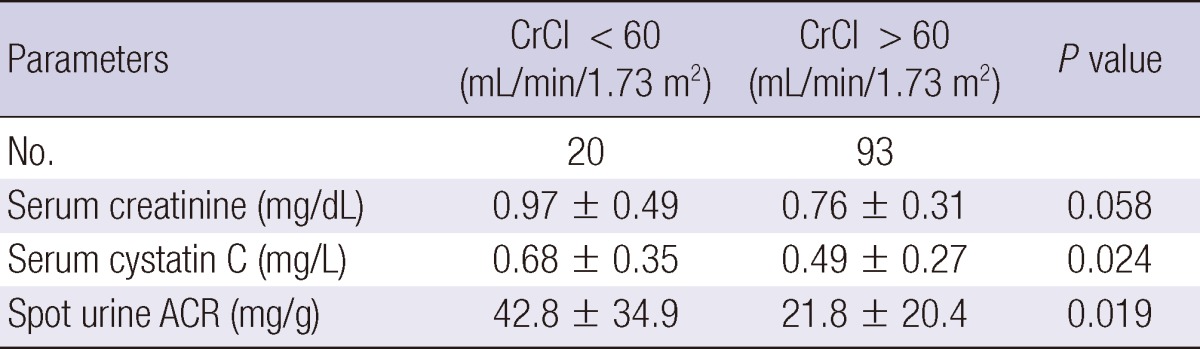
CrCl, Creatinine clearance; ACR, Albumin to creatinine ratio.
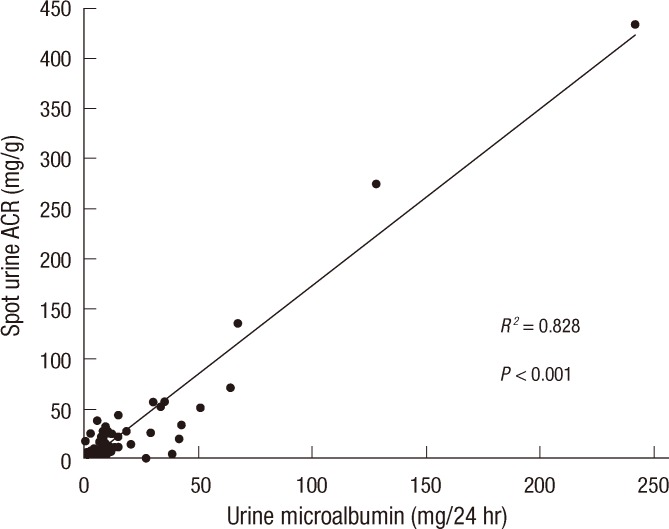
The sensitivity and specificity of serum cysC and creatinine compared with creatinine clearance were estimated via ROC curves (Fig. 2). The area under the curves (AUC) with the cut-off value of 60 mL/min/1.73 m2 was 0.732 for serum cysC and 0.615 for serum creatinine. The AUC was significantly higher for serum cysC (P = 0.028), but not for serum creatinine (P = 0.069). The sensitivity and specificity of cysC were 87.3% and 66.2%, respectively, with the upper reference limit as the cut-off. However, serum creatinine showed 74.3% and 58.3%, respectively. The ROC curve analysis of serum cysC demonstrated higher diagnostic accuracy than that of serum creatinine.
Fig. 2.
Serum cystatin C showed better diagnostic accuracy than serum creatinine at the cutoff value of below 60 mL/min/1.73 m2 of creatinine clearance.
DISCUSSION
The gold standard for the assessment of diabetic nephropathy in children is still the quantitative analysis of microalbumin through 24-hr urine collection. However, many factors such as exercise, hypertension, hyperglycemia can influence daily urinary albumin excretion (12). Day-to-day variation according to the volume of collected urine can hinder the diagnosis of diabetic nephropathy (4). Discovery of microalbuminuria through 24-hr urine collection can also be erroneous because of improper collection, which commonly happens with children and adolescents. Therefore, a test using timed urine collection has been suggested to be repeated three times to confirm microalbuminuria, but this is very hard to apply to children.
Recent studies have demonstrated good correlation between spot urine ACR and 24-hr urinary albumin excretion (6, 13). Spot urine ACR was thought to be more accurate because it eliminates the possibility of improper collection. The method using spot urine samples may ensure better compliance than timed urine test, especially in childhood patients. Furthermore, the spot urine ACR has the advantage of being less affected by urine volume because it is a ratio of two measured substances (14).
Nevertheless, few studies have reported the efficacy and effectiveness of spot urine ACR compared with creatinine clearance through 24-hr urine collection in childhood patients with diabetes. The present study showed that spot urine ACR was closely correlated with 24-hr urine albumin excretion and creatinine clearance in children and adolescents with diabetes. However, we can not definitively support that spot urine ACR should be used in place of 24-hr urine collected miacroalbumin due to the small study population. We do, however, feel that spot urine ACR would be useful as a screening tool when timed urine collection is diffcult to obtain in out-patient settings.
The use of endogenous markers to evaluate renal function is also important in the evaluation of diabetic nephropathy. Various low molecular proteins, such as cysC, β2-microglobulin, and collagen type IV, instead of serum creatinine have been suggested as useful endogenous markers for evaluating renal function (8). Among these, previous studies have demonstrated that cysC might be a more sensitive indicator of GFR than serum creatinine, but there have been few studies on cysC as an endogenous marker reflecting GFR in children with diabetic nephropathy (15-17).
In the present study, the level of serum cysC, but not serum creatinine, was significantly different between the normoalbuminuria group and the microalbuminuria group. Estimated GFR according to the Schwartz and MDRD II formulas did not demonstrate any difference between the two groups, although creatinine clearance was significantly lower in the microalbuminuria group. It is thought that serum creatinine is unable to reflect the early signs of decreased renal function in childhood diabetic patients, while serum cysC can. However, we cannot say that serum cysC might be surrogate marker of diabetic nephropathy, because diabetic patients have the increased possibility of decreased renal function due to causes other than diabetic nephropathy. Nevertheless, cysC based GFR is suggested to be a useful method for estimating renal function (18). Recently, Schwartz et al. proposed an updated Schwartz formula using cysC (19). We can estimate that cysC based GFR may be a better method than creatinine based GFR on the findings in this study.
Our studies have some limitations in that we did not perform the measurement of exogenous substances such as insulin, 51Cr-EDTA, 99mTc-diethylenetriaminepentaacetic acid, iohexol (20) or DTPA renogram, which has been suggested as the most accurate method to evaluate renal function. However, DTPA renogram or other accurate measurements based on the measurement of exogenous substances are hard to perform, especially in childhood diabetic patients. More studies with a large sample population are needed to confirm that spot urine ACR can replace 24-hr urine microalbumin in childhood diabetes.
There was a discrepancy between eGFR and creatinine clearance through 24-hr urine collection in this study. For this we considered two possibilities. First, the discrepancy may implicate that urine collection is inaccurate. Second, the MDRD formula within the normal range of creatinine might not reflect accurate renal function.
Nevertheless, to the best of our knowledge, the present study is the first to simultaneously check both serum cysC and spot urine ACR in children and adolescents with diabetes, and our study demonstrated that spot urine ACR might be a more accurate, convenient, and effective indicator for the detection of microalbuminuria instead of 24-hr urine microalbumin in diabetic children. CysC based GFR may be a more accurate method than creatinine based GFR to evaluate renal function.
References
- 1.Rossing P. Diabetic nephropathy: worldwide epidemic and effects of current treatment on natural history. Curr Diab Rep. 2006;6:479–483. doi: 10.1007/s11892-006-0083-y. [DOI] [PubMed] [Google Scholar]
- 2.Bangstad HJ, Osterby R, Dahl-Jørgensen K, Berg KJ, Hartmann A, Nyberg G, Frahm Bjørn S, Hanssen KF. Early glomerulopathy is present in young, type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1993;36:523–529. doi: 10.1007/BF02743268. [DOI] [PubMed] [Google Scholar]
- 3.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 4.Phillipou G, Phillips PJ. Variability of urinary albumin excretion in patients with microalbuminuria. Diabetes Care. 1994;17:425–427. doi: 10.2337/diacare.17.5.425. [DOI] [PubMed] [Google Scholar]
- 5.Eshøj O, Feldt-Rasmussen B, Larsen ML, Mogensen EF. Comparison of overnight, morning and 24-hour urine collections in the assessment of diabetic microalbuminuria. Diabet Med. 1987;4:531–533. doi: 10.1111/j.1464-5491.1987.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Assadi FK. Quantitation of microalbuminuria using random urine samples. Pediatr Nephrol. 2002;17:107–110. doi: 10.1007/s00467-001-0762-5. [DOI] [PubMed] [Google Scholar]
- 8.Aksun SA, Ozmen D, Ozmen B, Parildar Z, Mutaf I, Turgan N, Habif S, Kumanlioğluc K, Bayindir O. Beta2-microglobulin and cystatin C in type 2 diabetes: assessment of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2004;112:195–200. doi: 10.1055/s-2004-817933. [DOI] [PubMed] [Google Scholar]
- 9.Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin C: a replacement for creatinine as a biochemical marker of GFR. Kidney Int Suppl. 1994;47:S20–S21. [PubMed] [Google Scholar]
- 10.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 11.Macisaac RJ, Tsalamandris C, Thomas MC, Premaratne E, Panagiotopoulos S, Smith TJ, Poon A, Jenkins MA, Ratnaike SI, Power DA, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49:1686–1689. doi: 10.1007/s00125-006-0275-7. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz A, Vaeth M, Mogensen CE. Systolic blood pressure relates to the rate of progression of albuminuria in NIDDM. Diabetologia. 1994;37:1251–1258. doi: 10.1007/BF00399799. [DOI] [PubMed] [Google Scholar]
- 13.Jensen JS, Clausen P, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Detecting microalbuminuria by urinary albumin/creatinine concentration ratio. Nephrol Dial Transplant. 1997;12:6–9. [PubMed] [Google Scholar]
- 14.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 15.Donahue RP, Stranges S, Rejman K, Rafalson LB, Dmochowski J, Trevisan M. Elevated cystatin C concentration and progression to pre-diabetes: the Western New York study. Diabetes Care. 2007;30:1724–1729. doi: 10.2337/dc07-0040. [DOI] [PubMed] [Google Scholar]
- 16.Coll E, Botey A, Alvarez L, Poch E, Quintó L, Saurina A, Vera M, Piera C, Darnell A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 17.Chae HW, Shin JI, Kim BS, Kim HS, Kim DH. Effectiveness of serum cystatin C and β2-microglobulin as early predictive markers of diabetic nephropathy. J Korean Soc Pediatr Endocrinol. 2008;13:177–183. [Google Scholar]
- 18.Tan GD, Lewis AV, James TJ, Altmann P, Taylor RP, Levy JC. Clinical usefulness of cystatin C for the estimation of glomerular filtration rate in type 1 diabetes: reproducibility and accuracy compared with standard measures and iohexol clearance. Diabetes Care. 2002;25:2004–2009. doi: 10.2337/diacare.25.11.2004. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia LH, Bing XG, An XT. Serum cystatin C assay for the detection of early renal impairment in diabetic patients. J Clin Lab Anal. 2004;18:31–35. doi: 10.1002/jcla.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]