Abstract
The balance between tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor type 1 (PAI-1) regulates fibrinolysis. PAI-1 expression increases in atherosclerotic arteries and vascular smooth muscle cells (VSMCs) are one of major constituents of atheroma. We investigated the impact of lysophosphatidylcholine (lysoPC), an active component of oxidized low-density lipoprotein, on the plasminogen activator system of the rat VSMCs. The lysoPC stimulated the protein and gene expressions of PAI-1 but did not affect the protein expression of t-PA. Fibrin overlay zymography revealed that lysoPC increased the activity of PAI-1 in the conditioned media, while concurrently decreasing that of free t-PA. Vitamin E inhibited the lysoPC-induced PAI-1 expression. Further, lysoPC increased the intracellular reactive oxygen species (ROS) formation. Caffeic acid phenethyl ester, an inhibitor of NF-κB, blocked this lysoPC effect. Indeed, lysoPC induced the NF-κB-mediated transcriptional activity as measured by luciferase reporter assay. In addition, genistein, an inhibitor of protein-tyrosine kinase (PTK), diminished the lysoPC effect, while 7,12-dimethylbenz[a]anthracene, a stimulator of PTK, stimulated PAI-1 production. In conclusion, lysoPC does not affect t-PA expression but induces PAI-1 expression in the VSMC by mediating NF-κB and the genistein-sensitive PTK signaling pathways via oxidative stress. Importantly, lysoPC stimulates the enzyme activity of PAI-1 and suppresses that of t-PA.
Keywords: Lysophosphatidylcholines; NF-kappa B; Oxidative Stress; Plasminogen Activator Inhibitor 1; Protein-Tyrosine Kinase; Muscle, Smooth, Vascular
INTRODUCTION
Coronary heart disease (CHD) is the leading cause of death in developed countries. Although the pathophysiology of CHD is not fully understood, thrombosis plays a pivotal role in the development and progression of atherosclerosis. The coagulation and fibrinolytic systems are two separate, but interlinked enzyme cascades that regulate the production and breakdown of fibrin. Fibrinolysis occurs by the conversion of plasminogen to plasmin by tissue-type plasminogen activator (t-PA), which is also balanced by a specific inhibitor, plasminogen activator inhibitor type 1 (PAI-1). An increased blood level of PAI-1 antigen is associated with an increased risk of CHD (1), and PAI-1 gene expression is increased in atherosclerotic arteries (2). Moreover, elevated circulating levels might be attributed to localized synthesis and release of PAI-1 at atherosclerotic lesions (3).
Oxidative stress, another key component in the development of atherosclerosis, results in the oxidative modification of low-density lipoprotein (LDL). Oxidized LDL impacts not only the monocyte/macrophage and vascular endothelial cell (VEC), but also the vascular smooth muscle cell (VSMC) in various adverse ways (4). Lysophosphatidylcholine (lysoPC), a major phospholipid component, mediates many actions of oxidized LDL. These actions include not only the chemotaxis of the monocyte (5) and the proliferation of the macrophage (6), but also the induction of cell adhesion molecules in the VEC (7). Further actions of the oxidized LDL include modulation of the plasminogen activator (PA) system in the VEC (8) and proliferation of the VSMC (9, 10).
PAI-1 expression, detected primarily in VECs of healthy arteries (2), is also found in early atherosclerotic lesions of the intima of the VSMC. The fibrous cap PAI-1 messenger ribonucleic acid (mRNA) expression in the VSMC is increased in advanced atheromatous plaques compared with that in the adjacent media or that in the normal arterial tissue (11).
The purpose of the present study was to investigate the impact of lysoPC on the PA system in cultured VSMC. In addition, we explored the mechanisms underlying the lysoPC action.
MATERIALS AND METHODS
Materials
Sprague-Dawley rats were purchased from Charles River Japan (Hino, Japan). Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 medium (DMEM/F-12) without phenol red, fetal bovine serum, trypsin-ethylenediaminetetraacetic acid, and penicillin-streptomycin were purchased from GIBCO BRL (Grand Island, NY, USA). LysoPC, genistein, calphostin C, KT-5720, 7,12-dimethylbenz[a]anthracene (DMBA), and all other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Human plasminogen and bovine fibrinogen were obtained from Enzyme Research Laboratories Inc. (Upland, Swamsea, UK). Human urokinase, bovine thrombin, caffeic acid phenylethyl ester (CAPE) and vitamin E were purchased from Calbiochem (Darmstadt, Germany). Monoclonal antibody for α-smooth muscle actin was purchased from DAKO (Glostrup, Denmark), anti-β-actin antibody from Sigma Chemical Co., and 2',7'-dichlorofluorescin diacetate (DCF-DA) was obtained from Molecular Probes (Eugene, OR, USA). DCF-DA and CAPE were dissolved in dimethyl sulfoxide. Vitamin E was dissolved in ethanol, and all the other chemicals were dissolved in water.
This study protocol was reviewed and approved by the institutional animal care and use committee (IACUC) of Sungkyunkwan University School of Medicine (Permission No. H-A9-003), which is an accredited facility by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) and abides by the Institute of Laboratory Animal Resources (ILAR) guide.
Cell culture
The thoracic aortas from 3-month-old Sprague-Dawley rats (160-180 g) were removed, and the VSMCs were isolated using an enzyme digestion method as previously described (12). The VSMCs were grown in DMEM/F-12 (50:50) without phenol red containing antibiotics and 10% fetal bovine serum. The cells positively stained for α-smooth muscle actin. Cells in an almost confluent state were made quiescent by incubation for at least 48 hrs in a defined serum-free medium containing insulin (0.5 µM), transferrin (5 mg/mL), and ascorbate (0.2 mM).
Western blot analysis
Cells were lysed in radioimmunoprecipitation buffer (50 mM Tris-HCl [pH, 7.5], 200 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]) containing 1 mM phenylmethylsulfonyl fluoride for 30 min on ice. Thirty micrograms of cellular proteins were separated by SDS-polyacrylamide gel electrophoresis and electrotransferred to a polyvinyldifluoride transfer membrane (Schleicher & Schuell, Keene, NH, USA). They were then blocked and incubated with rabbit polyclonal anti-PAI-1 antibody (American Diagnostica Inc., Stamford, CT, USA). After washing, blots were incubated with anti-rabbit/anti-mouse horseradish peroxidase conjugated secondary antibody (Amersham Biosciences, Buckinghamshire, UK), then washed and incubated with enhanced chemiluminescence reagents (Amersham Biosciences) to detect the protein levels. β-actin was used as a loading control.
Enzyme-linked immunosorbent assay (ELISA)
The t-PA was quantified using an ELISA (Molecular Innovations, Inc. Novi, MI, USA). The color reaction was performed with a 3,3',5,5'-tetramethylbenzidine substrate and measured at 450 µm by a SmartSpec 3000 spectrophotometer (Bio-Rad, Hercules, CA, USA).
RNA preparation and real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay
The total RNA was extracted immediately from cells using a mirVana™ miRNA Isolation Kit (Ambion Inc., Austin, TX, USA) according to the manufacturer's instructions. The total RNA concentrations were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE, USA). The total RNA (1 µg) was reverse transcribed using the random hexamer primers in a high-capacity complementary deoxyribonucleic acid Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Real-time RT-PCR was performed using Taqman® Gene Expression Assays (Applied Biosystems) with the ABI 7900 Sequence Detection System (Applied Biosystems). All reactions were performed in triplicate using 10 µL samples containing 80 ng complementary deoxyribonucleic acid. The reaction protocol involved heating for 2 min at 50℃ and 10 min at 95℃, followed by 40 cycles of amplification (15 sec at 95℃ and 1 min at 60℃). Analysis was performed using ABI PRISM 7000 Sequence Detection software (Applied Biosystems). The difference in the cycle threshold (ΔCt) for PAI-1 gene expression from each sample was calculated by normalization with glyceraldehyde 3-phosphate dehydrogenase. Primers for rat PAI-1 were 5'-CAATGGAAGACCCCCTTCTTAGAG-3' (forward) and 5'-CATGGGCACGGAGATGGT-3' (reverse). Primers for rat glyceraldehyde 3-phosphate dehydrogenase were 5'-GTATCGGACGCCTGGTTACC-3' (forward) and 5'-TTGATGGCAACAATGTCCACTTTG-3' (reverse).
Fibrin overlay zymography and reverse fibrin overlay zymography
The conditioned media (CM) was subjected to gel electrophoresis using a 9% polyacrylamide gel containing 0.1% SDS. After completion of electrophoresis, the polyacrylamide gel was washed twice in 2.5% Triton X-100 for 1 hr to remove the SDS. In order for the fibrin overlay assay to detect PA activity, the polyacrylamide gel was treated using a particular method. After a brief rinse in distilled water, the polyacrylamide gel was placed on an opaque fibrin indicator gel containing 1% low gelling temperature agarose (FMC BioProducts, Rockland, ME, USA), human plasminogen (12.5 µg/mL), bovine thrombin (0.5 U/mL), and bovine fibrinogen (2 mg/mL). The polyacrylamide/fibrin indicator gel combination was then incubated in a humidified chamber at 37℃. The PA activity was detected by the appearance of clear bands in the opaque indicator gel. Samples of CM were normalized to cell protein content.
The PAI activity was detected using a reverse overlay assay in which the polyacrylamide gel was overlaid on a fibrin indicator gel similar to the ones described above, except with the addition of human urokinase (1.5 U/mL). The polyacrylamide/reverse fibrin indicator gel combination was then placed in a humidified chamber at 37℃. In this assay, all of the fibrin in the reverse indicator gel is lysed, with the exception of the areas containing PAI. Opaque bands on the indicator gel delineate the presence of PAI.
Analysis of intracellular formation of reactive oxygen species
Intracellular free radical production was determined using DCF-DA, a cell-permeable oxidation-sensitive probe. Highly fluorescent DCF is formed by the reaction with membrane-impermeable intracellular reactive oxygen species (ROS). The VSMCs were cultured on the chamber slide, and the quiescent cells were treated with lysoPC in combination with 50 µM DCF-DA for 1 hr. After being washed twice with phosphate-buffered saline, cells were observed with a Nikon Diaphot 300 inverted fluorescence microscope coupled to an argon laser with a 488 ηm excitation band. For quantitative analysis of the ROS formation, flow cytometry analysis was used. The VSMCs were cultured on 60 mm dishes, and quiescent cells were incubated with 10 µM DCF-DA and lysoPC. After being washed with phosphate-buffered saline, cells were trypsinized and resuspended in phosphate-buffered saline containing 1 mM trypsin-ethylenediaminetetraacetic acid. The cells were immediately analyzed by flow cytometry (10,000 cells/sample) using a 488 ηm excitation beam. The median fluorescence intensity was quantitatively determined by CellQuest software (Becton-Dickinson, San Jose, CA, USA) analysis of the recorded histograms.
In vitro transient transfection and reporter assay
To study the NF-κB activation, the VSMCs were transfected with a reporter plasmid containing the luciferase reporter gene linked to five repeats of the NF-κB binding sites. The VSMCs (1 × 105 cells/well) were plated in 24-well plates and grown to about 70% confluence. Cells were then transiently cotransfected with 1 µg of NF-κB-luciferase reporter plasmid and 1 µg of β-galactosidase plasmid using Lipofectamine plus (Invitrogen, Carlsbad, CA, USA). At 6 hr post-transfection, cells were starved for 48 hr before stimulation with lysoPC. Transfected cells were exposed to 10 µM lysoPC for the indicated time periods. Luciferase activity was measured using a luciferase assay kit (Promega, Madison, WI, USA) with signal detection for 5 sec in a luminometer (Panomics Inc., Fremont, CA, USA). A β-galactosidase enzyme assay (Promega) was used to determine the β-galactosidase activity at 420 ηm with a SmartSpec 3000 spectrophotometer. The results are expressed relative to the NF-κB activity compared with controls after normalizing for β-galactosidase activity and protein concentration.
Data analysis and statistics
Data are expressed as mean ± SEM, and N refers to the number of experiments performed. The Kruskal-Wallis test and Wilcoxon rank sum test were applied for statistical analysis. A two-tailed value of P < 0.05 was considered statistically significant.
RESULTS
LysoPC induced the expressions of PAI-1 protein and mRNA in the VSMC
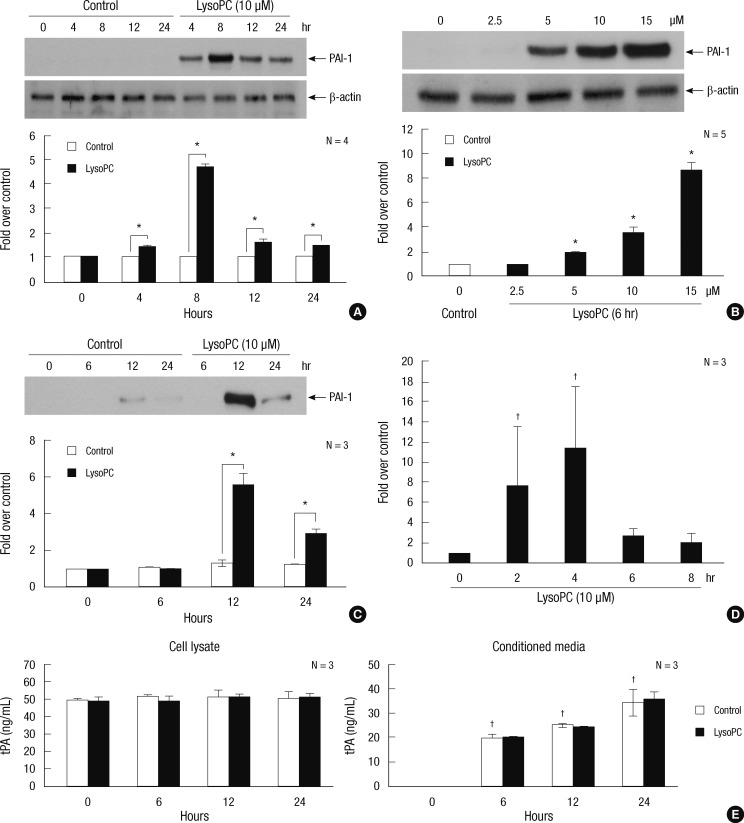
Western blot analysis demonstrated PAI-1 induction by lysoPC in the VSMC. There was a 4-hr increase in expression of PAI-1 in a time-course experiment with 10 µM lysoPC, and a peak was reached at 8 hr (Fig. 1A). The dose-response experiment showed that the 6-hr lysoPC treatment increased the PAI-1 expression in a dose-dependent manner (Fig. 1B). Significant increments were observed at 5 µM and higher concentrations. After 10 µM lysoPC treatment, the PAI-1 secretion into the CM increased over time, with a peak at 12 hr (Fig. 1C).
Fig. 1.
The effects of lysoPC on PAI-1 expression in VSMCs. (A), (B) and (C) Induction of PAI-1 protein as detected by Western blot analysis. Representative blots are shown in the upper section of each panel. The intensity of the bands was densitometrically determined and normalized to that of corresponding β-actin bands. Graphic data are depicted in the lower part of the panel. (A) Time-course effects of 10 µM lysoPC in the cell lysate. (B) Dose-response effects of lysoPC at 6 hr of treatment in the cell lysate. (C) Time-course effects of 10 µM lysoPC in the conditioned media normalized to the cell protein content. (D) Time-course effects of 10 µM lysoPC on gene expression of PAI-1 as evaluated by real-time RT-PCR. (E) Time-course effects of 10 µM lysoPC on tPA in the cell lysate and conditioned media as assessed by ELISA. *P < 0.05 vs vehicle-treated control; †P < 0.05 vs baseline.
The effect of lysoPC was also investigated at the level of gene expression. The time-course experiment using 10 µM lysoPC showed that PAI-1 mRNA induction was evident at 2 hr and reached peak expression after 4 hr as assessed by RT-PCR (Fig. 1D).
Constitutive production of t-PA in the VSMC remained unchanged and the secretion into the CM increased with time, as analyzed by ELISA. Neither the production nor the secretion was altered by lysoPC (Fig. 1E).
LysoPC stimulated PAI-1 activity and suppressed t-PA activity in the CM
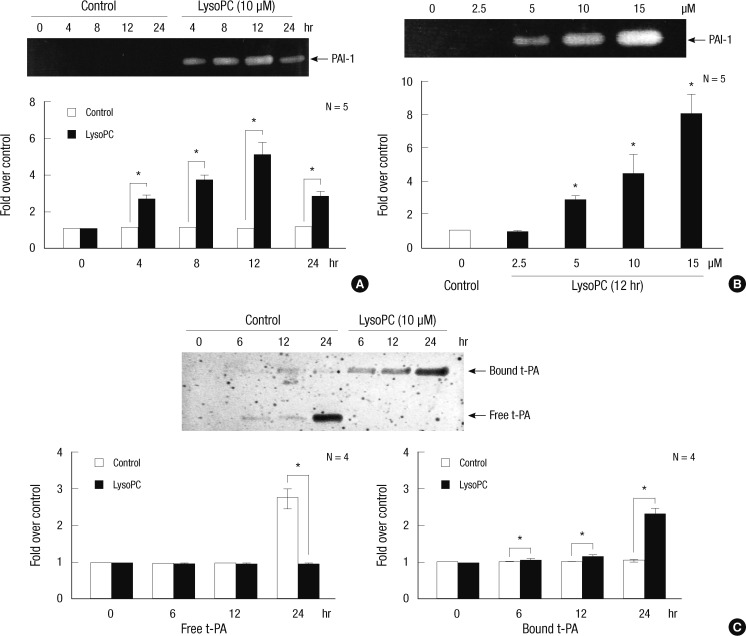
Zymography was used to evaluate the changes in the activity of the PA system in the CM following lysoPC treatment. PAI-1 activity was assessed by reverse fibrin overlay zymography. As shown in Fig. 2A, the time-course experiment showed that 10 µM lysoPC stimulated PAI-1 activity. A significant increase was evident at 4 hr, and maximal activity was seen at 12 hr. Treating the VSMC with 2.5 to 15 µM lysoPC for 12 hr also increased the PAI-1 activity in a dose-dependent manner (Fig. 2B).
Fig. 2.
The effects of lysoPC on the activities of plasminogen activator and its inhibitors in the CM harvested from cultured VSMCs. Representative zymographs are shown in the upper section of each panel. The intensity of the bands was densitometrically determined, and graphic data are depicted in the lower part of the panel. (A) and (B) Time- and dose-dependent activity of PAI-1 after lysoPC treatment as assessed by reverse fibrin overlay zymography. (C) Time-course effects of 10 µM lysoPC on activity of t-PA as assessed by fibrin overlay zymography. *P < 0.05 vs vehicle-treated control.
Using fibrin overlay zymography, Fig. 2C shows that 10 µM of lysoPC suppressed the free t-PA activity. The time-course experiment demonstrated that the dramatic decrease in free t-PA activity paralleled a substantial increase in higher molecular weight complexes. The latter were most likely to be inactive t-PA bound to PAI-1.
Up-regulation of PAI-1 expression was ROS-dependent and required NF-κB activation in the lysoPC-treated VSMC
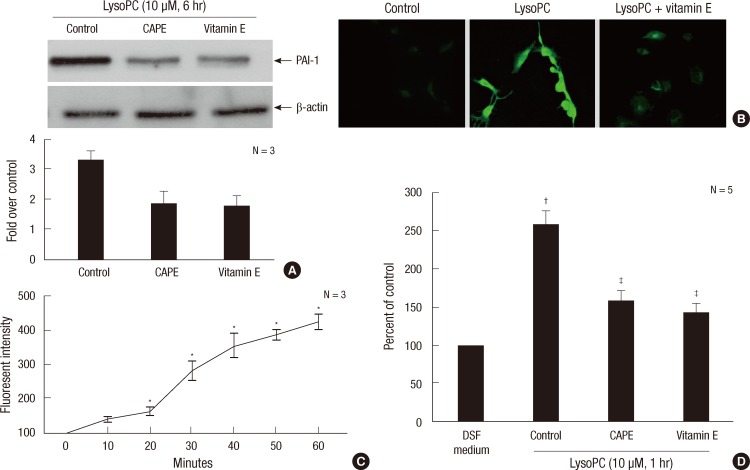
Our group (13) reported that lysoPC induces oxidative stress in the VSMC. NF-κB, a transcription factor and important regulator of gene expression, responds to many stimuli, including proinflammatory cytokines and growth factors (14, 15). We studied the relevance of the ROS and NF-κB activation in the effect of lysoPC on PAI-1 expression. We used vitamin E, a potent antioxidant or CAPE, as the inhibitor of NF-κB. Fig. 3A shows that both vitamin E and CAPE significantly suppressed lysoPC-induced PAI-1 expression.
Fig. 3.
The involvement of oxidative stress and nuclear transcription factor NF-κB activation in the induction of PAI-1 in VSMCs stimulated with 10 µM lysoPC. (A) Effects of vitamin E (50 µM), a potent antioxidant, or CAPE (20 nM), an inhibitor of NF-κB, on PAI-1 expression induced by lysoPC for 6 hr as assessed by Western blot analysis. Representative blots are shown in the upper section of each panel. The intensity of the bands was densitometrically determined and normalized to that of corresponding β-actin bands. Graphic data are depicted in the lower part of the panel. (B) Intracellular ROS production detected by DCF fluorescence under confocal microscopy (× 100). (C) Kinetics of intracellular ROS production as assessed by flow cytometry analysis using DCF-DA. (D) Effects of lysoPC for 1 hr on NF-κB-mediated transcriptional activity as measured by luciferase reporter assay. *P < 0.05 vs baseline; †P < 0.05 vs DSF medium; ‡P < 0.05 vs vehicle-treated control.
Under confocal laser microscopy, the lysoPC induction of intracellular ROS production was observed (Fig. 3B). Flow cytometry revealed the kinetics of ROS formation by lysoPC (Fig. 3C).
Impacts of lysoPC on NF-κB-mediated transcriptional activity were examined using a luciferase reporter assay. As shown in Fig. 3D, lysoPC increased the luciferase activity, indicating that lysoPC induced the NF-κB-dependent transcriptional activity. Vitamin E, however, decreased the luciferase activity. CAPE was used as a negative control.
Protein-tyrosine kinase was involved in the signaling pathway mediating the lysoPC-induced response
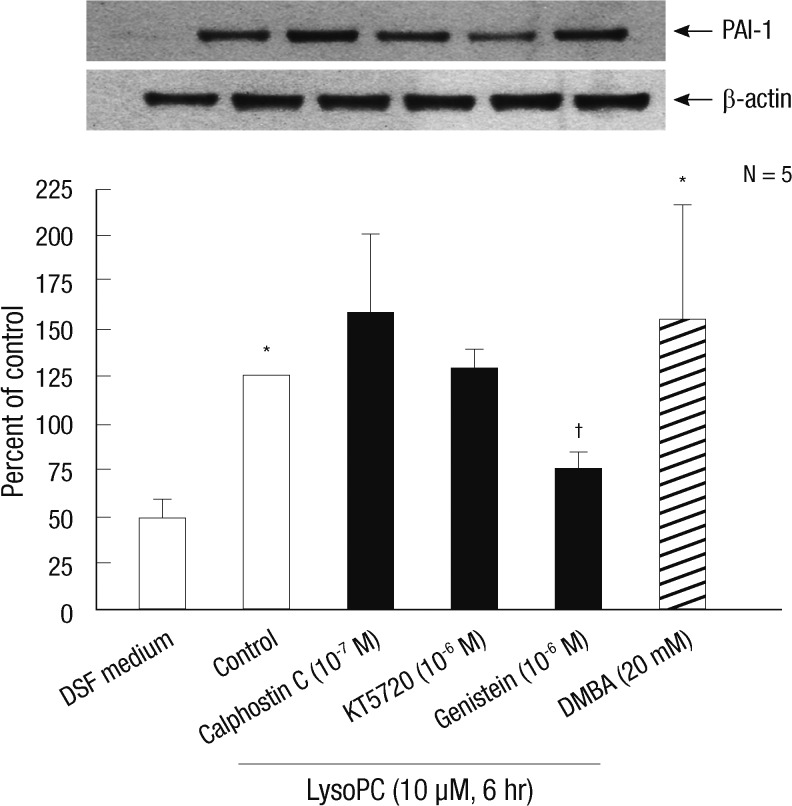
Western blot analysis evaluated the signaling mechanisms involved in the lysoPC action on PAI-1. We examined the effects of KT-5720, an inhibitor of protein kinase A, and calphostin C, an inhibitor of protein kinase C, as shown in Fig. 4. Neither agent significantly altered PAI-1 expression. However, genistein, an inhibitor of protein-tyrosine kinase (PTK), diminished the lysoPC effect. Further, DMBA, a stimulator of PTK, stimulated PAI-1 production in the VSMC.
Fig. 4.
The effects of inhibitors of major cell signaling pathways on the expression of PAI-1 stimulated with lysoPC in vascular smooth muscle cells. Representative blots are shown in the upper panel. The intensity of the bands was densitometrically determined and normalized to that of corresponding β-actin bands. Graphic data are depicted in the lower panel. Western blot analysis of whole cell extracts from vascular smooth muscle cells treated for 6 hr with 10 µM lysoPC in the presence of calphostin C (10-7 M, an inhibitor of protein kinase C), KT-5720 (10-6 M, an inhibitor of protein kinase A) or genistein (10-6 M, an inhibitor of PTK). Effect of DMBA (20 mM, a stimulator of PTK) on PAI-1 expression is also shown. *P < 0.05 vs DSF medium; †P < 0.05 vs vehicle-treated control.
DISCUSSION
The local alterations in fibrinolytic activity may be more responsible for intravascular thrombosis in CHD than is systemic impairment (16). The t-PA, the principal mediator of fibrinolysis in vivo (17), diffuses from the vascular cells and converts plasminogen, adsorbed to the fibrin clot, into plasmin. Plasmin then degrades the fibrin polymer into smaller fragments that are cleared by the monocyte-macrophage scavenger system. PAI-1 binds to t-PA in circulation and inhibits plasminogen activation (18). The level of PAI-1 mRNA in human arteries has been correlated with the degree of atherosclerosis (2). Importantly, atheromatous plaque is mainly composed of VSMC. Therefore, direct modulation of the PA system by oxidized LDL in VSMC might have relatively greater clinical relevance compared with that in VEC (8).
This study demonstrated that lysoPC stimulated PAI-1 synthesis in the VSMC in vitro. This finding is consistent with a previous study (19) which reported that both oxidized LDL and lysoPC induced PAI-1 in the human aortic VSMC. In addition, the present study demonstrated that lysoPC augmented the enzyme activity of PAI-1. In contrast to the thrombin responses (20), early release of PAI-1 was not observed. However, the secretion and enzyme activity in the CM displayed a similar time-course to PAI-1 protein synthesis in the VSMC.
We also examined in detail the impact of lysoPC on t-PA. Thrombin (21) or angiotensin II (22) induced simultaneous PAI-1 and t-PA expressions in the VSMC. A 24 hr treatment of lysoPC, however, did not alter the production or secretion of t-PA. Of note, lysoPC decreased the free t-PA activity in the CM but simultaneously increased the activity of bound t-PA. This situation might reflect increased PAI-1 secretion over time. Accordingly, lysoPC might pose a more negative direct impact on local fibrinolysis with regard to the pathogenesis of CHD.
In this study, 10 µM lysoPC enhanced the intracellular production of ROS within one hour. This lysoPC-stimulated PAI-1 expression was abolished by vitamin E. These findings support a critical role of oxidative stress in the pathogenesis of atherosclerosis (23). Since ROS exert their effects via activation of specific intracellular signaling pathways, they profoundly influence both normal physiology and the course of vascular disease in the VSMC (24). Although the ROS-mediated signal transduction is very complex and remains elusive, the ROS may not only generate classic second-messengers (calcium and lipid mediators), but may also act as second-messengers (25). The present study revealed that the activation of NF-κB was involved in PAI-1 expression in the lysoPC-stimulated VSMC. NF-κB is one of the transcription factors that may be controlled by the redox status of the cell (26). This study further examined the relationship between ROS generation and NF-κB activation. Vitamin E inhibited luciferase activity, which suggests that the increase in the intracellular ROS level after lysoPC treatment is required for the NF-κB-dependent transcriptional activity. Kinases are also frequently involved as mediators of redox-sensitive signal transduction. We demonstrated that genistein-sensitive PTK was a target for ROS action in the VSMC. Moreover, the stimulation of PTK led to induction of PAI-1. Further studies are required to define the specific mechanisms underlying the activation of NF-κB and genistein-sensitive PTK by ROS.
Newly synthesized PAI-1 is also deposited into the extracellular matrix of the vessel. By limiting extracellular proteolysis in the developing atherosclerotic lesions, PAI-1 may play a significant role in the organization and incorporation of mural thrombi within the plaque (2). Further research focusing on this aspect of PAI-1 action is warranted. Although VSMCs from the rat aorta have been thought to be a classic in vitro model, cells derived from a human coronary artery might be more appropriate for use in further studies.
In conclusion, we have demonstrated that lysoPC, a major phospholipid component of LDL, does not affect t-PA expression but induces PAI-1 expression by mediating NF-κB and genistein-sensitive PTK signaling pathways via oxidative stress in the VSMC. Importantly, lysoPC stimulates the enzyme activity of PAI-1 and suppresses that of t-PA.
Footnotes
This work was supported in part by a Samsung Biomedical Research Institute grant (#SBRI C-95-036 and #SBRI C-A3-220-1), the Sungkyunkwan University Foundation for Corporate Collaboration (S-2010-0228-000), and the Technology Development Program for Agriculture and Forestry, Ministry of Agriculture, Forestry and Fisheries, Republic of Korea (109127-03-3-SB010).
References
- 1.Ilić M, Majkić-Singh N, Lalić K. Plasminogen activator inhibitor in patients with acute myocardial infarction and re-infarction in syndrome X. Clin Lab. 2002;48:125–128. [PubMed] [Google Scholar]
- 2.Schneiderman J, Sawdey MS, Keeton MR, Bordin GM, Bernstein EF, Dilley RB, Loskutoff DJ. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci U S A. 1992;89:6998–7002. doi: 10.1073/pnas.89.15.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schäfer K, Muller K, Hecke A, Mounier E, Goebel J, Loskutoff DJ, Konstantinides S. Enhanced thrombosis in atherosclerosis-prone mice is associated with increased arterial expression of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2003;23:2097–2103. doi: 10.1161/01.ATV.0000097766.36623.DF. [DOI] [PubMed] [Google Scholar]
- 4.Cherubini A, Vigna GB, Zuliani G, Ruggiero C, Senin U, Fellin R. Role of antioxidants in atherosclerosis: epidemiological and clinical update. Curr Pharm Des. 2005;11:2017–2032. doi: 10.2174/1381612054065783. [DOI] [PubMed] [Google Scholar]
- 5.Rong JX, Berman JW, Taubman MB, Fisher EA. Lysophosphatidylcholine stimulates monocyte chemoattractant protein-1 gene expression in rat aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2002;22:1617–1623. doi: 10.1161/01.atv.0000035408.93749.71. [DOI] [PubMed] [Google Scholar]
- 6.Sakai M, Miyazaki A, Hakamata H, Sasaki T, Yui S, Yamazaki M, Shichiri M, Horiuchi S. Lysophosphatidylcholine plays an essential role in the mitogenic effect of oxidized low density lipoprotein on murine macrophages. J Biol Chem. 1994;269:31430–31435. [PubMed] [Google Scholar]
- 7.Zou Y, Kim CH, Chung JH, Kim JY, Chung SW, Kim MK, Im DS, Lee J, Yu BP, Chung HY. Upregulation of endothelial adhesion molecules by lysophosphatidylcholine. Involvement of G protein-coupled receptor GPR4. FEBS J. 2007;274:2573–2584. doi: 10.1111/j.1742-4658.2007.05792.x. [DOI] [PubMed] [Google Scholar]
- 8.Kugiyama K, Sakamoto T, Misumi I, Sugiyama S, Ohgushi M, Ogawa H, Horiguchi M, Yasue H. Transferable lipids in oxidized low-density lipoprotein stimulate plasminogen activator inhibitor-1 and inhibit tissue-type plasminogen activator release from endothelial cells. Circ Res. 1993;73:335–343. doi: 10.1161/01.res.73.2.335. [DOI] [PubMed] [Google Scholar]
- 9.Chai YC, Binion DG, Macklis R, Chisolm GM., 3rd Smooth muscle cell proliferation induced by oxidized LDL-borne lysophosphatidylcholine. Evidence for FGF-2 release from cells not extracellular matrix. Vascul Pharmacol. 2002;38:229–237. doi: 10.1016/s1537-1891(02)00173-8. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Kim IW, Lee HY, Chae IH, Kim MA, Kim HS, Kim CH, Sohn DW, Oh BH, Lee MM, et al. The effect of naringin on the lysophosphatidylcholine-induced proliferation of vascular smooth muscle cells. Korean Circ J. 2002;32:61–70. [Google Scholar]
- 11.Lupu F, Bergonzelli GE, Heim DA, Cousin E, Genton CY, Bachmann F, Kruithof EK. Localization and production of plasminogen activator inhibitor-1 in human healthy and atherosclerotic arteries. Arterioscler Thromb. 1993;13:1090–1100. doi: 10.1161/01.atv.13.7.1090. [DOI] [PubMed] [Google Scholar]
- 12.Owens GK, Loeb A, Gordon D, Thompsin MM. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells. J Cell Biol. 1986;102:343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon BK, Oh WJ, Kessel B, Roh CR, Choi DS, Lee JH, Kim DK. 17β-estradiol inhibits proliferation of cultured vascular smooth muscle cells induced by lysophosphatidylcholine via a nongenomic antioxidant mechanism. Menopause. 2001;8:58–64. doi: 10.1097/00042192-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt C, Peng B, Li Z, Sclabas GM, Fujioka S, Niu J, Schmidt-Supprian M, Evans DB, Abbruzzese JL, Chiao PJ. Mechanisms of proinflammatory cytokine-induced biphasic NF-kappaB activation. Mol Cell. 2003;12:1287–1300. doi: 10.1016/s1097-2765(03)00390-3. [DOI] [PubMed] [Google Scholar]
- 15.Grilli M, Chiu JJ, Lenardo MJ. NF-kB and Rel: Participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 16.Collen D, Juhan-Vague I. Fibrinolysis and atherosclerosis. Semin Thromb Hemost. 1988;14:180–183. doi: 10.1055/s-2007-1002773. [DOI] [PubMed] [Google Scholar]
- 17.Nassar T, Akkawi S, Shina A, Haj-Yehia A, Bdeir K, Tarshis M, Heyman SN, Higazi AA. In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood. 2004;103:897–902. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- 18.Kruithof EK, Tran-Thang C, Bachmann F. The fast-acting inhibitor of tissue-type plasminogen activator in plasma is also the primary plasma inhibitor of urokinase. Thromb Haemost. 1986;55:65–69. [PubMed] [Google Scholar]
- 19.Dichtl W, Stiko A, Eriksson P, Goncalves I, Calara F, Banfi C, Ares MP, Hamsten A, Nilsson J. Oxidized LDL and lysophosphatidylcholine stimulate plasminogen activator inhibitor-1 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:3025–3032. doi: 10.1161/01.atv.19.12.3025. [DOI] [PubMed] [Google Scholar]
- 20.Cockell KA, Ren S, Sun J, Angel A, Shen GX. Effect of thrombin on release of plasminogen activator inhibitor-1 from cultured primate arterial smooth muscle cells. Thromb Res. 1995;77:119–131. doi: 10.1016/0049-3848(95)91618-u. [DOI] [PubMed] [Google Scholar]
- 21.Wojta J, Gallicchio M, Zoellner H, Hufnagl P, Last K, Filonzi EL, Binder BR, Hamilton JA, McGrath K. Thrombin stimulates expression of tissue-type plasminogen activator and plasminogen activator inhibitor type 1 in cultured human vascular smooth muscle cells. Thromb Haemost. 1993;70:469–474. [PubMed] [Google Scholar]
- 22.van Leeuwen RT, Kol A, Andreotti F, Kluft C, Maseri A, Sperti G. Angiotensin II increases plasminogen activator inhibitor type 1 and tissue-type plasminogen activator messenger RNA in cultured rat aortic smooth muscle cells. Circulation. 1994;90:362–368. doi: 10.1161/01.cir.90.1.362. [DOI] [PubMed] [Google Scholar]
- 23.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 24.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 25.Kondo T, Hirose M, Kageyama K. Roles of oxidative stress and redox regulation in atherosclerosis. J Atheroscler Thromb. 2009;16:532–538. doi: 10.5551/jat.1255. [DOI] [PubMed] [Google Scholar]
- 26.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]