Abstract
Functional diversification of taste cells is crucial for proper discrimination of taste qualities. We found homeodomain protein Skn-1a/Pou2f3 is expressed in sweet, umami, and bitter taste cells. The Skn-1a–deficient mice lacked electrophysiological and behavioral responses to sweet, umami, and bitter tastes, due to complete absence of sweet, umami, and bitter cells with concomitant expansion of sour cells. Skn-1a is critical for generating and balancing the diverse composition of taste cells.
Chemical cues contained in foods are received by taste receptor cells (TRCs) in the taste buds and evoke taste sensations consisting of five basic taste modalities: sweet, umami, bitter, sour, and salty. Individual TRCs are dedicated to one of these five modalities1,2, and genetic ablation of a selective functional subset of TRCs causes the loss of specific taste responses3. TRCs in the taste buds are derived from the local epithelium4 and undergo continuous turnover every one to two weeks5. Therefore, strictly controlled differentiation is critical for the maintenance of TRC diversity and the balance of TRC composition in taste buds.
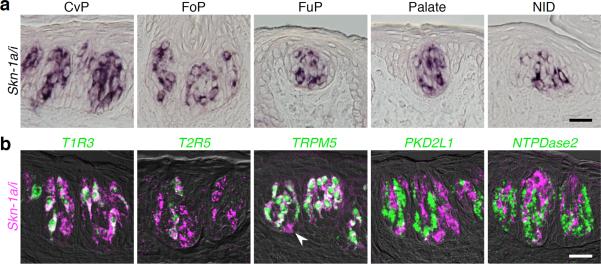
Transcription factors play important roles in the generation and fate determination of various cell types6–8. It has been hypothesized that some transcription factors may regulate the differentiation of TRCs, which are continuously self–renewing even in adult animals. We conducted a genome–wide comparative survey of gene expression profiles from isolated taste buds, circumvallate epithelium, and non–gustatory tongue epithelium, and found that Skn-1a/i, transcription factors originally identified in the epidermal keratinocytes9,10, is expressed in a subset of taste bud cells (Fig. 1a). Double fluorescence in situ hybridization analysis revealed that Skn-1a/i mRNA is expressed in T1R3–positive sweet and umami TRCs, T2R5–positive bitter TRCs, and a small population of oval cells in the basal region of the taste buds, but not in PKD2L1–positive sour TRCs and NTPDase2–positive cells with unknown functions (Fig. 1b). A detailed analysis using specific probes for Skn-1a and -1i (probes a-2 and i, respectively, in Supplementary Fig. 1a) showed expression of only Skn-1a mRNA (Supplementary Fig. 1b), and the 5-kb upstream region of the mouse skn-1a gene efficiently induced GFP expression in Skn-1a/i–positive TRCs in transgenic mice (skn1a–FinG) (Supplementary Fig. 1c). These results indicate that Skn-1a is the major transcript of the skn-1 locus in sweet, umami, and bitter TRCs, as well as in basal cells in the taste buds.
Figure 1.
Skn-1a is exclusively expressed in sweet, umami, and bitter taste receptor cells. (a) Expression of transcript(s) from the skn-1a/i locus in a subset of taste bud cells in every taste bud distributed within the oropharyngeal epithelium. (b) Double–labeling fluorescent in situ hybridization to reveal the subset of cells expressing Skn-1a/i mRNA. White arrowhead indicates basal cell expressing only Skn-1a/i mRNA. Scale bars indicate 20 μm.
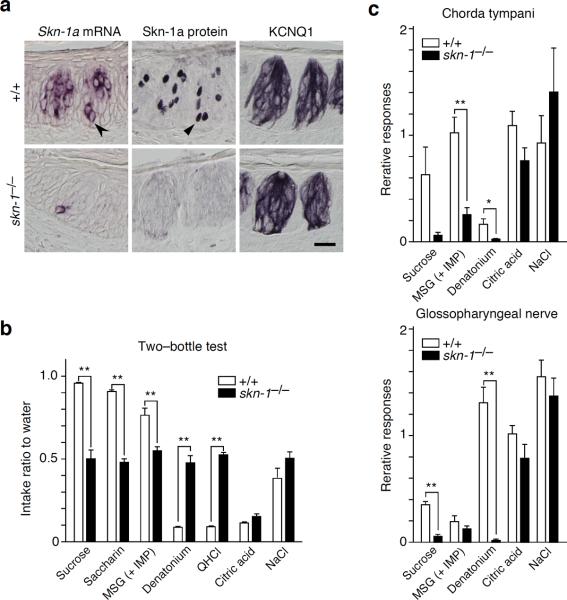
To elucidate the function of Skn-1a in the gustatory system, we generated gene–targeted mice with a null mutation of skn-1a/i (Supplementary Fig. 2). Skn-1a protein was completely absent in the taste buds of homozygous skn-1a/i null mutants, while a small population of basal cells expressed truncated, non–functional Skn-1a mRNA (Fig. 2a). Skn-1a–deficient mice were viable and fertile and displayed no overt abnormalities in the gross morphology of the taste buds, as assessed by the expression of KCNQ1, a marker for nearly all taste bud cells11 (Fig. 2a). However, the Skn-1a–deficient mice showed no preference for sweet and umami tastes and no avoidance of bitter taste, whereas their aversive response to sour taste appeared normal (Fig. 2b and Supplementary Fig. 3a). Electrophysiological recordings of peripheral nerves containing gustatory fibers displayed a significant decrease in their responses to sweet, umami, and bitter tastes, while, consistent with the behavioral phenotypes, normal responses to sour and salty tastes were maintained (Fig. 2c and Supplementary Fig. 3b). These findings indicate that Skn-1a protein is required for the normal development and/or function of the gustatory system, particularly for sweet, umami, and bitter taste reception.
Figure 2.
Skn-1a is essential for the reception and perception of sweet, umami, and bitter tastes. (a) Skn-1a mRNA and protein in skn-1−/− mice. The morphological phenotype of the taste bud was investigated by immunohistochemistry for KCNQ1. Black arrowhead and triangle indicate basal cell expressing Skn-1a/i mRNA and protein, respectively. Scale bar indicates 20 μm. (b) The two–bottle test for seven taste solutions of five taste modalities. Taste preferences of wild–type (n = 8) and skn-1−/− (n = 12) mice were measured relative to water. Preference to sweet and umami tastes and avoidance of bitter taste were disappeared in skn-1−/− mice. (c) Neural responses of chorda tympani (top panel) and the glossopharyngeal nerve (bottom panel) to sweet, umami, bitter, sour, and salty tastants by whole–nerve recording. Integrated neural responses of wild–type (n = 5–6) and skn-1−/− (n = 5) mice to taste solution were standardized to the magnitude of the response to ammonium chloride. Responses to sweet, umami, and bitter tastes were dramatically reduced in skn-1−/− mice. Significance was assessed by the t–test: *P < 0.05; **P < 0.01. Data are means ± s.e.m.
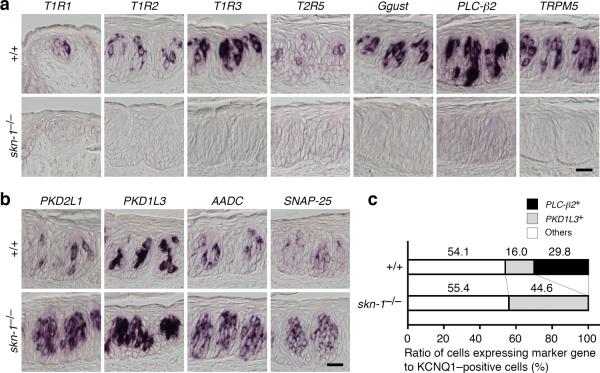
In Skn-1a–deficient mice, no mRNA for either the receptors or the signal transducing molecules mediating sweet, umami, and/or bitter taste stimuli were expressed (Fig. 3a). In striking contrast, a significant increase was observed in the mRNA levels of genes expressed in sour TRCs including PKD2L1, PKD1L3, AADC, and SNAP-25 (Fig. 3b, Supplementary Fig. 4). It should be noted that the amount of increase in PKD1L3 mRNA–positive cells in Skn-1a–deficient mice was equivalent to that of the decrease in PLC-β2 mRNA–positive cells (Fig. 3c and Supplementary Fig. 5). These data suggest that the population of sour TRCs was expanded in the taste buds of Skn-1a–deficient mice at the expense of sweet, umami, and bitter TRCs. In the taste buds of transgenic mice (skn1a–FinG) harboring GFP under the control of skn-1a promoter on the skn-1−/− background, GFP protein was not detectable in any cells, while GFP mRNA was detected in only a minority of the basal cells (Supplementary Fig. 6), as observed for Skn-1a mRNA and protein in the Skn-1a–deficient mice (Fig. 2a).
Figure 3.
Loss of Skn-1a alters the composition of taste receptor cells. (a) Expression of genes involved in sweet, umami, and bitter taste reception and perception in wild–type (top panel) and in the skn-1−/− (bottom panel) mice. The robust expression of T1R1, T1R2, T1R3, T2R5, Ggust, PLC-β2, and TRPM5 observed in wild-type mice was completely absent in skn-1−/− mice. (b) Expression of genes closely associated with sour TRCs in wild–type (top panel) and skn-1−/− (bottom panel) mice. The robust expression of PKD2L1, PKD1L3, aromaticl-amino acid decarboxylase (AADC), and SNAP-25 detected in wild–type mice was further increased in skn-1−/− mice. (c) Composition of TRCs in wild–type and skn-1−/− mice. Populations of sweet, umami, and bitter TRCs and sour TRCs as PLC-β2 and PKD1L3 mRNA–expressing cells, respectively, were counted relative to KCNQ1–expressing cells. The remaining PLC-β2 and PKD1L3 mRNA–expressing cells were categorized as others. Scale bars indicate 20 μm.
Taken together, our findings provide compelling evidence that Skn-1a is a crucial transcription factor for generating sweet, umami, and bitter TRCs. Because Skn-1a is expressed in mature TRCs as well as in the immature taste bud cells, it is likely that Skn-1a plays plural roles not only in the specification, but also in the differentiation and maintenance of sweet, umami, and bitter TRCs. We also unraveled the genetic relationships among the three cell lineages giving rise to sweet/umami/bitter TRCs, sour TRCs, and the remaining taste bud cells, including the subpopulation of salty TRCs2. The loss of sweet, umami, and bitter TRCs and the expansion of sour TRCs caused by Skn-1a–deficiency suggest the binary differentiation competence of progenitor cells into sweet/umami/bitter and sour TRCs.
Supplementary Material
Acknowledgements
We thank Y. Sato for assistance with the BAC library screening, staffs of the Research Resource Centre, RIKEN, for assistance with generating chimera and transgenic mice, and T. Kondo for useful comments on the manuscript.
Footnotes
Author Contribution I.M. and K.A. designed the research. I.M., M.O., and Y.Y. generated the transgenic and gene-targeting mice with M.O. I.M. and M.O. performed histological experiments. M.N. performed electrophysiological and behavioral experiments. I.M. and M.O. analyzed the data. I.M., Y.Y. and K.A. wrote the paper.
The authors declare no competing financial interests.
References
- 1.Chandrashekar J, Hoon MA, Ryba NJP, Zuker CS. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 2.Chandrashekar J, et al. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang AL, et al. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone LM, Finger TE, Tam PPL, Tan S-S. Proc. Natl. Acad. Sci. USA. 1995;92:1916–1920. doi: 10.1073/pnas.92.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beidler LM, Smallman RL. J. Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan AK, Rosenfeld MG. Genes Dev. 1997;11:1207–1225. doi: 10.1101/gad.11.10.1207. [DOI] [PubMed] [Google Scholar]
- 7.Jessell TM. Nat. Rev. Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 8.Guillemot F. Development. 2007;134:3771–3780. doi: 10.1242/dev.006379. [DOI] [PubMed] [Google Scholar]
- 9.Andersen B, et al. Science. 1993;260:78–82. doi: 10.1126/science.7682011. [DOI] [PubMed] [Google Scholar]
- 10.Andersen B, et al. Genes Dev. 1997;11:1873–1884. doi: 10.1101/gad.11.14.1873. [DOI] [PubMed] [Google Scholar]
- 11.Ohmoto M, Matsumoto I, Misaka T, Abe K. Chem. Senses. 2006;31:739–746. doi: 10.1093/chemse/bjl016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.