Abstract
Drosophila melanogaster is widely used to study immune system function in insects. However, little work has been done in D. melanogaster on the effect of temperature on the immune system. Here we describe experiments that demonstrate that cooler temperatures enhance survival after infection and alter expression of immune-related genes in flies. This effect appears to be due not only to the fact that colder temperatures slow down bacterial growth, but also to the beneficial effects of cooler temperature on immune function. We explore the possibility that heat shock proteins, and in particular, Hsp83, may improve immune function at cool temperatures. We have long known that temperature can alter immune responses against microbial pathogens in insects. The approach described here allows us to determine whether this effect is due primarily to temperature-specific effects on the host or on its pathogen. These results suggest that both may be important.
Keywords: Temperature, Immunity, Infection, Hsp83, Drosophila melanogaster
1. Introduction
A half century of research has taught us much about the ways in which environmental factors influence immune function in insects (Boorstein and Ewald, 1987; Kluger et al., 1975; Steinhaus, 1960). In recent years, many researchers have focused on so-called `behavioral fever', where insects alter their temperature through thermoregulatory behavior, and thereby increase their ability to fight off pathogens. Much of this work has been done on the locust–fungus model system. Elliot, Thomas, and Blanford, among others, discovered that when locusts were infected with a pathogenic fungus, placing them at warmer temperatures prolonged their survival. In fact, when given the choice, the infected locusts relocate themselves to warmer areas of their environment (Blanford et al., 1998; Elliot et al., 2002; Thomas and Blanford, 2003). Similar affects of temperature on fungal infections have also been seen in grasshoppers (Inglis et al., 1996, 1997) and houseflies (Watson et al., 1993). Though much of the work on insects, immunity, and temperature points to warmer temperatures being beneficial, there is some evidence that warmer temperatures are not always helpful. In the cockroach–acanthocephalan system, researchers found that warmer temperatures did not affect cockroach survival and in fact reduced fecundity in infected hosts (Guinnee and Moore, 2004). Similarly, when the parasitic conopid fly infects bumblebees, colder temperatures are associated with higher bumblebee survival (Muller and Schmid-Hempel, 1993).
Despite a substantial amount of work on the problem, we know little about how and why temperature affects the ability of insects to overcome infection. One possibility is that behavioral fever increases host survival by placing the infectious agent in a suboptimal environment (Blanford and Thomas, 2001; Kluger et al., 1975). But the situation may be more complex. The efficacy of an insect's immune system may depend in part on temperature-dependent physiological changes in the host (Blanford et al., 1998; Elliot et al., 2002; Thomas and Blanford, 2003). There are myriad changes in metabolism, the nervous and endocrine systems, behavior, and the like, that occur in insects as their temperature increases (Neven, 2000). At a molecular level, temperature changes can lead to changes in the expression of various heat shock proteins (hsps; Feder and Hofmann, 1999; Lindquist and Craig, 1988). Studies of hemocyte counts and hemolymph proteins in thermoregulating locusts have found that warmer temperatures increase immunocompetence early in infection (Ouedraogo et al., 2002, 2003). It is not clear, however, why colder rather than warmer temperatures are beneficial in some interactions, nor do we understand the underlying mechanisms by which temperature might shape immunity.
Fortunately, we can begin to understand whether or how temperature affects the immune system thanks to recent advances in our understanding of the molecular basis of immunity in insects. Over the last decade, researchers working with Drosophila have described the many genes and gene interactions that lead from the initial encounter by an insect host with a particular pathogen, to the specific cellular and molecular mechanisms used to ward off the pathogen (see Hoffmann, 2003; Hultmark, 2003; Uvell and Engstrom, 2007 for review). There are two primary genetic pathways that provide humoral immunity through the production of antimicrobial peptides. The Imd pathway defends against gram-negative bacteria, while the Toll pathway defends against gram-positive bacteria and fungi (De Gregorio et al., 2001; Hedengren et al., 1999; Leclerc and Reichhart, 2004; Lemaitre et al., 1995). In addition, insects are also capable of mounting a cellular immune response, by which they can phagocytose a variety of pathogens (Rolff and Siva-Jothy, 2004; Wilson et al., 2001).
At the same time that researchers have uncovered the molecular basis of immunity in flies, others have focused on the molecular basis of their thermal ecology. We have long known about the effects of temperature on survival and reproduction in insects (Crill et al., 1996; Faurby et al., 2005; Huey and Berrigan, 2001; Mockett and Sohal, 2006). Other work with Drosophila has looked at temperature fluctuations in the wild, and how flies can survive extreme temperatures. At both hot and cold extremes, flies produce hsps, which provide a variety of protective mechanisms (Feder and Hofmann, 1999; Lindquist and Craig, 1988; Qin et al., 2005). Hsps are also released in response to other stresses, including infection (Guedes et al., 2005). In humans, Hsp60 assists in the activation of macrophages, part of the innate immune system (Kol et al., 1999, 2000). However, the direct or indirect role that hsps play in immunity in insects has not been well studied.
In light of the breadth of knowledge on both the molecular biology of immune function and of thermal ecology in Drosophila melanogaster, flies offer an ideal system with which to test hypotheses about the effects of temperature on the ability of insects to survive infection. While most work on behavioral fever has been confined to the Orthoptera, there is some information on the effects of temperature on immunity in flies. For example, flies infected with a sterilizing nematode can recover their fertility when moved to higher temperatures. Unlike Orthopteran species, however, these infected flies do not appear to choose higher temperatures (Ballabeni et al., 1995). One study on D. melanogaster showed that temperature affects resistance of flies selected to overcome attack by a parasitic wasp. However, the effect was not consistent. Most selection lines showed an increased ability to encapsulate the wasp eggs at warmer temperatures, but some selection lines were less able to survive (encapsulate) in warmer environments (Fellowes et al., 1999).
Here we describe experiments that examine the effects of temperature on host survival in a D. melanogaster–bacteria model system. The initial experiment is used to resolve how temperature affects the ability of flies to survive bacterial infection. We then carry out a series of further experiments to determine whether the effects of temperature on survival are due to changes in bacterial growth or host immunocompetence. We first measure the effects of temperature on bacterial growth within the host. To examine the effect temperature has on host immune function, we take an indirect approach and examine levels of gene expression in immune-related genes after an immune challenge at different temperatures.
In the wild, flies experience a range of temperatures within and between days. In a final set of studies, we examine the potential impact of these rapid changes on immune function by switching flies, either before or after infection, between thermal regimes. Studies on the biology of aging in flies have used switch experiments to great effect. It has long been known that rearing fruit flies on a calorically restricted diet can extend lifespan (Chippindale et al., 1993). By switching flies from restricted to standard diets, researchers learned that the life-extending effects of diet restriction (DR) are due to the immediate effects of DR on mortality, rather than long-term effects of reduced rates of damage accumulation under DR (Good and Tatar, 2001; Mair et al., 2004). Here we use temperature switching to determine if the initial temperature at which a non-infected fly is held can confer costs or benefits once the fly is infected and placed at a standard temperature. We also determine whether early temperature experience in infected flies alters long-term dynamics of the host–bacteria interaction, once the host is placed at a standard temperature.
Taken together, these experiments help us to better understand not only the effects of temperature on immune function, but also who is in control of these effects—the host or its pathogen.
2. Materials and methods
In the following section we describe four specific sets of experiments. The initial experiment is designed to determine directly the effect of temperature on the ability of flies to survive an intra-thoracic injection of bacteria. To determine the extent to which these results are due to temperature-dependent interactions either in the host or the pathogen, we then carry out three additional experiments. First, we measure the effect of host temperature on bacterial growth, using measures of bacterial growth in solid or liquid culture. Second, we measure the effect of temperature on expression levels of several host genes associated with immune function. And third, to determine when the temperature causes the change in survival rate as well as how temperature affects gene expression in the absence of infection, we carry out a set of switch experiments in which animals are housed at one temperature initially, and then switched to a different temperature at a later time.
2.1. Fly stocks
D. melanogaster flies were collected in August 2003 from the University of Georgia horticulture farm in Watkins-ville, GA. Thirty-six isofemale, inbred lines were used to reconstitute a large outbred population in May of 2004. Flies were kept in a large population cage with overlapping generations at room temperature, approximately 22.5 °C, until September of 2005. The flies were then maintained in 15 vials, with non-overlapping generations until the completion of the experiment, June 2007. Each generation a subset of emerging adults, approximately 120 female–male pairs, were collected, randomized, and used to start the next generation (of 15 vials). All flies were raised at 25 1C with a 12-day generation time and a 12:12 h L/D cycle. Flies were cultured in vials containing approximately 3 ml of a standard cornmeal–molasses–agar medium, supplemented with a pinch of live yeast in each vial to facilitate egg production. Eggs were trimmed to approximately 150 in each vial in order to ensure that larvae were not overcrowded. This provided approximately 2250 eggs to start the next generation. For the experiments, females were collect as virgins from the culture vials and then were aged for 3 days. This controlled for both age and mating status, which have both been shown to affect immune function (Fedorka et al., 2007; Fedorka and Zuk, 2005; Lawniczak and Begun, 2004; McGraw et al., 2004; Zerofsky et al., 2005).
2.2. Bacterial strains
Flies were infected with two strains of bacteria that have been found in natural fly populations (Corby-Harris et al., 2007; Lazzaro et al., 2006), the gram-negative bacteria, Pseudomonas aeruginosa and the gram-positive bacteria, Lactococcus lactis. Both stocks were obtained from Dr. Brian Lazzaro at Cornell University. The L. lactis strain was isolated from a natural fly population (Lazzaro et al., 2006); the P. aeruginosa strain was derived from a laboratory culture (strain PA01). Bacteria were kept at 4 °C in liquid LB broth (Luria–Bertani, a standard medium for culturing bacteria) and then raised at 37 °C, their optimal growing temperature (Bhatti et al., 1976; Molina-Hoppner et al., 2003), for 24 h prior to each experiment. A spectrophotometer was used to standardize the concentration of bacteria at 600 nm, and the bacterial solution was then diluted to an optical density of 0.01Å. A 0.1 mm stainless steel pin mounted on the end of a plastic pipette tip was dipped into the bacterial solution, such that the needle contained between 10 and 15 bacteria (based on colony counts; data not shown). For each infection, the pin was dipped in 70% ethanol, wiped dry, dipped in the bacteria solution, and inserted into the thorax of the fly (modified from Lazzaro et al., 2004).
2.3. Temperature and survival
To determine how temperature affects overall survival, females were infected with P. aeruginosa, L. lactis, or sterile broth (sham) as a control. After infection, females were placed in one of three treatments, 17, 25, or 29 °C. These temperatures represent a range the flies may encounter in nature (Feder et al., 2000), and are mild enough that flies can survive for extended periods of time. After infection, mortality was recorded every day for 4 days, by which time flies either succumbed to the infection or recovered at each of the temperatures. For each bacteria×temperature treatment, we carried out five replicates using a total of 250–300 flies for each bacterial infection at each temperature (or 55 flies for the sham-infected flies).
Given that not all flies succumb to the infection, we compared survival in two ways. First, to compare the percentage of flies that died at different temperatures following infection, we conducted an ANOVA on arcsin square root transformed data. To test for the effect of infection on mortality rates among those flies that died, we used a Cox Proportional Hazards (PH) model (Parmar and Machin, 1995; Therneau and Grambsch, 2000), where temperature and replicate were the independent variables, and number dead at each time point was the dependent variable. In the PH model, between 15% and 18% of the flies survived the infection and were treated as censored data at the end of the 4-day period. All statistics were carried out using JMP 5.1 (JMP 5.1, 2007). Standard errors shown on graphs represent error between replicates.
2.4. Colony counts
To determine how temperature affects bacterial growth, females were infected with P. aeruginosa, L. lactis, or a sterile broth and placed at 17, 25, or 29 °C. Flies were then removed at 1.5, 3, 8, or 13 h post-infection, anesthetized using CO2, and ground up using a sterile pestle in individual 1.5 ml eppendorf tubes containing 25 μl of Drosophila Ringer's Solution (Ashburner, 1989). The mix was then plated out on Pseudomonas or Lactococcus selective agar plates (plates made in lab from powder concentrates, MP Biomedicals, LLC#1008817; Fisher Scientific #0XCM0817B, respectively). Plates were incubated at 37 °C for 24 h in order to determine how many CFUs were present in the fly when they were ground up. Colonies were counted by hand, and if there were too many colonies to count on a plate, that plate was scored as `lawn'. We conducted the whole experiment four times for each strain of bacteria. For P. aeruginosa infections there were a total of 70 plates per temperature at 1.5 h post-infection, and 40 plates per temperature at 3, 8, and 13 h post-infection. For L. lactis infections, there were 70 plates per temperature at 1.5 and 3 h, and 40 plates per temperature at 8 and 13 h. Flies injected with sterile broth were ground up at 13 h to confirm that the only bacteria present on the plates were those with which we infected the flies (N = 16 flies). Colony-count data are highly non-normal, and those plates with a solid lawn of bacteria could not be counted. Accordingly, within each temperature-time treatment, colony count data, including `lawn' plates, were transformed to ordinal ranks, and the non-parametric Kruskal–Wallis test was used to test for differences among treatments. Lawn plates were given a value of 2200, which was a value higher than our highest count. Separate analyses were carried out for Pseudomonas and Lactococcus infections. A Nemenyi test (a non-parametric version of the Tukey test) was used to conduct post-hoc pair-wise comparisons between the temperature×time treatments on the ranked data. In addition, growth curves of the bacteria were conducted at 17, 25, and 29 °C in order to examine changes in growth of the bacteria in vitro at the three different temperatures.
We also measured bacterial growth at 17, 25, 29, and 37 °C in vitro to confirm the results in the colony count experiment. We measured the optical density at 600 nm of the bacteria at 5, 10, 20, 30, 44 and 52 h after being diluted to 0.01Å from log phase growth. This allowed us to determine if the bacterial growth seen in the fly is similar to that seen in vitro for a given temperature.
2.5. Real time RT-PCR
Two gene-expression studies were carried out. In the first, we used Real time RT-PCR to study expression levels in six immunity-related genes, including three in the Imd pathway (Pgrp-LC, Relish (Rel), and Diptericin (Dpt)), for the gram-negative infections, and three in the Toll pathway (Spatzle (Spz), Cactus (Cact), and Metchnikowin (Mtk)), for the gram-positive infections. These genes represent the recognition, signaling, and antimicrobial peptides of their respective immune pathways. A second experiment (see Switch Experiments) measured Spz, Mtk, and a heat shock protein, Hsp83, in uninfected flies. Primer sequences for all genes are provided in Table 1.
Table 1.
Primer sets for the real time rt-PCR genes used in this study
| Gene | Sense | Antisense |
|---|---|---|
| Pgrp-LC | AAGATCCGGCGCAAACC | CCTTGCGTCCGACAGTGTT |
| Rel | CGGCCATACTCCCTTGGAA | GGACCACCCGGTCATTTG |
| Dipt | AAATTACGATGACAGGCGGTCT | TCGAAGGTTCTCTCGACAGTTTA |
| Cact | CAGGCAACTGTCATGGGATTG | GCTTTGGTGATCCTCGCTATTT |
| Spz | TGATGACGCCCATGTGGAT | GTGGCAAAGAAGGCGAACA |
| Mtk | GTGCTGGCAGAGCCTCATC | GCGACGGCCTCGTATCG |
| Hsp83 | GCACCAAGATCGTGCTGTACA | CAAGGAGGACCAGACCG |
| Act5c | CCGAGCGCGGTTACTCTTT | CTCCTTGATGTCACGGACGAT |
To examine gene expression at the three temperatures after infection, Real time RT-PCR was conducted on flies inoculated either with lipopolysaccharides (LPS) derived from P. aeruginosa (1 mg per 50 μl sterile broth), or with L. lactis (1000 μl) grown to saturation, autoclaved for 15 min, spun down and the pellet re-suspended with 50 μl sterile broth. Septic injury was used to inoculate the flies, as in the previous infection experiments. Sham inoculations were also conducted with sterile broth and used as a calibrator for gene expression. Dead L. lactis or LPS were used in the inoculations in order to eliminate any confounding effects of bacterial growth. This insured that any temperature-dependent changes in gene expression reflected physiological changes in the host, and not temperature-related changes in pathogen levels.
Flies were inoculated, placed at 17, 25, or 29 °C for either 3 or 8 h, and then groups of five flies were anesthetized under CO2, and frozen at −80 °C in 300 μl of Trizol. RNA was extracted from the flies using a standard protocol (Khodarev et al., 2002), treated with DNase (Promega, RQ1 RNase-free DNase), and 2.5 μg of RNA was converted to cDNA (Roche, Transcriptor First Strand cDNA Synthesis Kit). A 1:50 dilution of the cDNA was mixed with 12.5 μl Sybr Green (Applied Biosystems), 3.5 μl H2O, and 2 μl of primer for each gene of interest. Controls using just the DNase treated RNA were carried out to confirm there was no left over DNA. For the gene expression studies, three replicates of five flies each were analyzed for each temperature×infection×time point.
Real time RT-PCR measures gene expression levels (mRNA) by converting mRNA to cDNA, and then amplifying cDNA with PCR. The original amount of cDNA will determine the time it takes for the amplified DNA to reach a specific `cycle threshold' (ct). In order to compare expression levels between genes, the ct has to first be normalized to control for variation in the quantity of cDNA added to each reaction. The observed ct is subtracted from that of an internal control, Actin 5c, to give us a Δct. We confirmed that Actin5c expression did not differ due to the effect of temperature (ANOVA, F2 = 2.6515, P = 0.0911). To compare genes between treatments, we further calibrated the Δct against the sham-inoculated control. This calibrated value, ΔΔct, is then transformed (2−ΔΔct) in order to obtain the Relative Quantification (RQ), which is typically used to compare gene expression. RQ values were highly non-normal, however. Accordingly, for all statistical tests we used a natural log transformation, which greatly improved normality. To determine if there was a difference in levels of gene expression between LPS (or dead L. lactis) inoculated flies and sham-inoculated flies, we tested RQ values using a one-group, two-tailed t-test, asking if the average value of ln(RQ) was significantly different from 0. A Tukey–Kramer HSD test was used to determine if temperatures differed within genes. By examining gene expression at three different temperatures, we were able to determine how temperature affects the immune system in flies.
This experiment includes a large number of individual tests for changes in gene expression in infected vs. sham flies (two bacterial species×three host genes measured per bacterial infection×two time points×three temperatures, for a total of 36 tests). With this large number of tests, we increase the risk of type-I error (falsely rejecting a true null hypothesis). To correct for this effect of multiple comparisons, we used the Benjamini–Hochberg correction (Benjamini and Hochberg, 1995). This test is less conservative than the more commonly used Bonferroni correction (see Biometry, Sokal and Rohlf, 2000), which we consider overly conservative. To test for significance using the Benjamini–Hochberg correction, we first rank each P-value from largest to smallest. The corrected P-value is considered statistically significant if P(i)≤αm/i, where α is the standard significance level, i is the ordinal rank of the ith P-value, and m is the number of samples being tested.
2.6. Temperature switches
In order to understand how and when temperature affects survival rate we conducted several experiments in which temperature was changed before or after infection.
2.6.1. Three-hour switch treatment
We were interested in determining how host survival after infection would be affected by a brief period at a low, moderate or high temperature, followed by a more protracted incubation period at moderate temperature. For this experiment, we infected flies with P. aeruginosa, L. lactis, or sterile broth, and placed them immediately after infection at 17, 25, or 29 °C for 3 h, and then returned them all to 25 °C. Flies kept at 25 °C for the whole experiment were labeled as controls (sham flies were inoculated with a sterile broth). In previous experiments, peak mortality occurred between 24 and 33 h post-infection at 25 °C. Accordingly, we recorded mortality every hour from 24 to 33 h post-infection. Differences in mortality rates between control and treatment flies were compared using the Proportional Hazards model. Three replicates were conducted, with a total of N = 150 at each temperature (before moving to 25 °C) for each bacteria, and N = 30 for the sham infections at each temperature.
2.6.2. Preheat treatment
To examine the effects of temperature on immunity in the absence of infection, at 2 days after eclosion and prior to infection, flies were placed at 17, 25, or 29 °C for 24 h. After 24 h at this initial temperature, the flies were infected with P. aeruginosa, L. lactis, or a sterile broth and all the flies were placed at 25 °C. Mortality was recorded every hour from 24 to 33 h post-infection and the effect of temperature on age-specific mortality was tested using a Proportional Hazards model. Three replicates were conducted, with a total of N = 150 for each temperature for each bacteria, and N = 30 for the sham infections at each temperature.
2.6.3. Gene expression in preheated flies
For the preheat experiment, in addition to measuring survival, we also used real time RT-PCR to measure gene expression in non-infected flies kept for 24 h at 17, 25, or 29 °C. These flies were not infected and gene expression levels at 17 or 29 °C were measured relative to those in flies kept at 25 °C. Three genes were examined, including two immune genes from the Toll pathway (Spz, Mtk) and Hsp83, a hsp that is induced both during cold stress (Qin et al., 2005) and in larvae infected with both gram-positive and gram-negative bacteria (Guedes et al., 2005). Only Toll genes were examined (no Imd genes) due to limited resources, and due to the response that we see in gram-positive infected flies after the preheat treatment. Three replicates of five flies at each temperature were analyzed. To compare expression levels at 17 or 29 °C relative to 25 °C, we used a two-tailed t-test on ln-transformed values of RQ.
3. Results
3.1. Temperature and survival
The proportion of flies that died did not differ among the temperature treatments for either bacteria (P. aeruginosa: ANOVA, F2 = 0.3122, P = 0.7376; L. lactis: F2 = 0.5199, P = 0.6191). However, we found a significant difference in the rate at which flies died among the 17, 25, and 29 °C treatments for both P. aeruginosa and L. lactis (P. aeruginosa: , P<0.0001; L. lactis: , P<0.0001). Median time to death for P. aeruginosa flies was 3, 2, and 1 days post-infection for 17, 25, and 29 °C, respectively (Fig. 1a). Median time to death for L. lactis flies was 4, 2, and 1 days for 17, 25, and 29 °C, respectively (Fig. 1b).
Fig. 1.
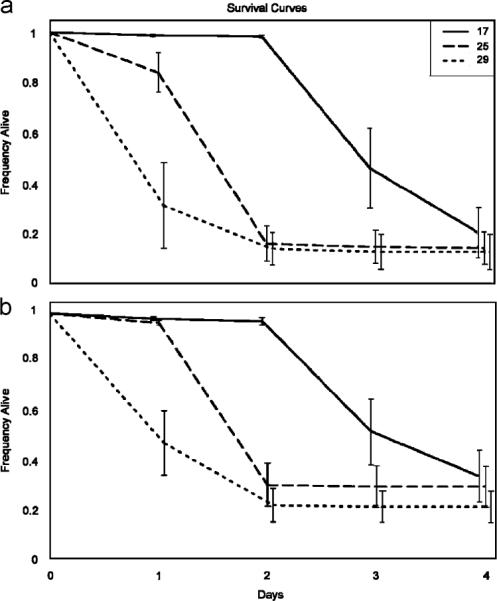
Survival curves (±1 S.E.) for females infected with P. aeruginosa (a) or L. lactis (b) at 17, 25, and 29 °C. In both cases, there is a significant difference between the temperature treatments (Proportional Hazards model, a: , P<0.0001; b: , P<0.0001). Median time to death (days post-infection) for P. aeruginosa: 17 °C = 3 days, 25 °C = 2 days, 29 °C = 1 day; L. lactis: 17 °C = 4 days, 25 °C = 2 days, and 29 °C = 1 day.
Overall survival rates were relatively low (15.9% of the flies survived infection with P. aeruginosa and 18.5% survived infection with L. lactis). Accordingly, we repeated the experiment with a lower dose (LD50, on average). Again, while rates of mortality increased with temperature, the proportion that survived the infection did not vary with temperature (data not shown). No sham-injected flies died during the mortality assay period for any of the experiments.
3.2. Colony counts
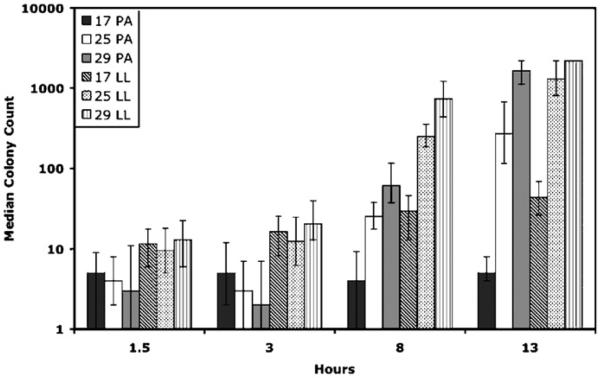
At 8 and 13 h post-infection, bacterial concentration in flies, as measured by colony counts, was consistently greater at higher temperatures (Table 2). No differences were observed at 1.5 h post-infection, and in one case (P. aeruginosa, 3 h post-infection), flies held at 17 °C produced greater colony counts than flies held at 29 °C (Fig. 2). No bacteria were observed on the agar plates for the sham-inoculated flies. Growth of the bacteria in vitro showed a similar pattern to that of the in vivo growth at 8 and 13 h post-infection, growth rate of bacteria increased with temperature (Fig. 3).
Table 2.
Colony count comparisons for P. aeruginosa (PA) and L. lactis (LL) at different time points after infectiona
| Comparison | Hour | Bacteria | χ2-statistics | d.f. | P-value | Nemenyi |
|---|---|---|---|---|---|---|
| 17–25–29 | 1.5 | PA | 1.312 | 2 | 0.519 | ns |
| 17–25–29 | 3 | PA | 6.249 | 2 | 0.044 | 17>29* |
| 17–25–29 | 8 | PA | 62.66 | 2 | <0.0001 | 17<25*** |
| 17<29*** | ||||||
| 25<29* | ||||||
| 17–25–29 | 13 | PA | 82.32 | 2 | <0.0001 | 17<25*** |
| 17<29*** | ||||||
| 25<29*** | ||||||
| 17–25–29 | 1.5 | LL | 1.753 | 2 | 0.416 | ns |
| 17–25–29 | 3 | LL | 29.08 | 2 | 0.011 | 25<29** |
| 17–25–29 | 8 | LL | 77.86 | 2 | <0.0001 | 17<25*** |
| 17<29*** | ||||||
| 25<29** | ||||||
| 17–25–29 | 13 | LL | 75.77 | 2 | <0.0001 | 17<25*** |
| 17<29*** | ||||||
| 25<29* |
Pair-wise comparisons using the Nemenyi test were conducted on the ranked data to determine if there were significant differences between the treatments.
P = 0.05.
P = 0.01.
P = 0.001.
Fig. 2.
Median colony counts for P. aeruginosa (solid bars) and L. lactis (hashed bars) infections, shown with first and third quartiles on a log scale. Early on in infection (1.5 and 3 h) there is not a clear pattern with regards to the effect of temperature on bacterial growth. However, at 8 and 13 h after infection, flies held at warmer temperatures had higher colony counts than those at cooler temperatures for both bacteria. Lawn plates were given a value of 2200. In the 29 °C treatment at 13 h, for L. lactis the majority of plates were lawns, and the median and quartiles were also lawns, so there are no quartile bars for that data point.
Fig. 3.
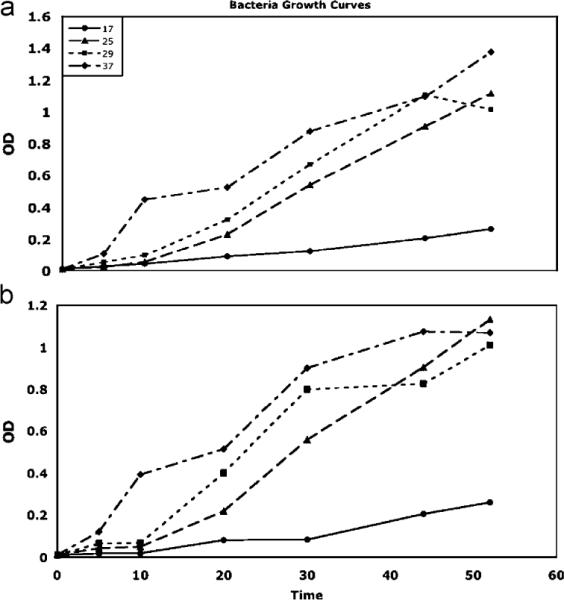
Bacterial growth of P. aeruginosa (a) and L. lactis (b) in vitro at 17, 25, 29, and 37 °C. We find that warmer temperatures lead to faster bacterial growth (higher optical density).
3.3. Real time RT-PCR
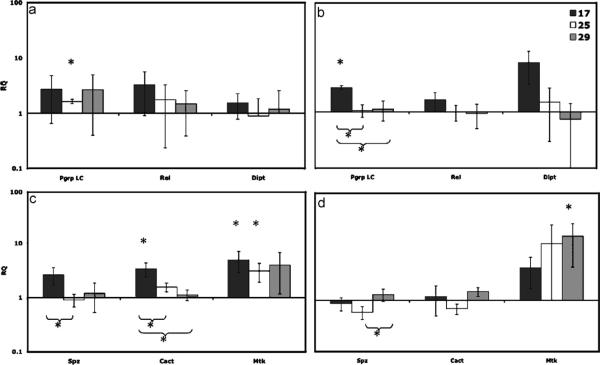
After the Benjamini–Hochberg correction, in flies at 17 °C, we observed an up-regulation of Cact and Mtk at 3 h (in dead L. lactis inoculations), and Pgrp-LC at 8 h (in LPS inoculations) compared to sham flies (Fig. 4b and c). At 25 °C at 3 h Mtk and Pgrp-LC were up-regulated compared to sham. Finally at 29 °C, only Mtk at 8 h was up-regulated (Fig. 4d). When comparing between temperatures within one gene, at 17 °C gene expression was significantly higher than 25 and 29 °C in Pgrp-LC and Cact, and higher than 25 °C in Spz. We also found expression at 29 was higher than 25 °C in Spz (Table 3).
Fig. 4.
Measures of gene expression from real time RT-PCR analysis of flies infected with LPS at (a) 3 h and (b) 8 h; and with flies infected with killed L. lactis at (c) 3 h and (d) 8 h. Relative quantification (RQ) is on the y-axis (with 95% confidence intervals) and the immune genes are on the x-axis. An asterisk indicates a significant difference (P<0.05) in gene expression between the infected and sham flies. An asterisk with a bracket indicates a significant difference in gene expression between the two temperatures. We see an up-regulation of Cact, Mtk, and Pgrp-LC genes at 17 °C an up-regulation of Pgrp-LC and Mtk at 25 °C and only an up-regulation of Mtk at 29 °C.
Table 3.
Real time rt-PCR comparisons within a temperature between infected and sham inoculated fliesa
| Gene | Hour | Temperature | t-statistics | P-value | Tukey-HSD |
|---|---|---|---|---|---|
| Pgrp-LC | 3 | 17 | 1.46 | ns | ns |
| 25 | 10.81 | 0.0085 | |||
| 29 | 0.99 | ns | |||
| Rel | 3 | 17 | 3.05 | 0.093 | ns |
| 25 | 0.83 | ns | |||
| 29 | 0.48 | ns | |||
| Dipt | 3 | 17 | 1.32 | ns | ns |
| 25 | −0.75 | ns | |||
| 29 | −0.29 | ns | |||
| Pgrp-LC | 8 | 17 | 18.75 | 0.0339 | 17>25* |
| 25 | 0.80 | ns | 17>29* | ||
| 29 | 0.30 | ns | |||
| Rel | 8 | 17 | 2.11 | ns | ns |
| 25 | −0.20 | ns | |||
| 29 | −0.54 | ns | |||
| Dipt | 8 | 17 | 5.06 | ns | 17>29† |
| 25 | 0.54 | ns | |||
| 29 | −0.97 | ns | |||
| Spz | 3 | 17 | 4.65 | 0.043 | 17>25* |
| 25 | −0.82 | ns | |||
| 29 | 0.32 | ns | |||
| Cact | 3 | 17 | 7.39 | 0.018 | 17>25* |
| 25 | 4.73 | 0.042 | 17>25** | ||
| 29 | 0.93 | ns | |||
| Mtk | 3 | 17 | 6.43 | 0.023 | ns |
| 25 | 5.73 | 0.029 | |||
| 29 | 2.98 | ns | |||
| Spz | 8 | 17 | −1.00 | ns | 25>29* |
| 25 | −3.91 | 0.060 | |||
| 29 | 1.73 | ns | |||
| Cact | 8 | 17 | 0.20 | ns | ns |
| 25 | −3.22 | 0.085 | |||
| 29 | 3.57 | 0.071 | |||
| Mtk | 8 | 17 | 4.68 | 0.043 | ns |
| 25 | 3.17 | 0.089 | |||
| 29 | 5.41 | 0.033 |
Results are based on two-tailed t-tests. P-values that are significant after the Benjamini–Hochberg correction are shown in bold. A Tukey–Kramer HSD test was conducted for the temperature comparisons within each gene.
P = 0.1.
P = 0.05.
P = 0.01.
3.4. Temperature switches
3.4.1. Three hour switch treatment
In the 3-h switch experiment, there was a significant difference among the temperature treatments (P. aeruginosa infections, , P<0.0001, L. lactis, , P<0.0001; Fig. 5). Flies that were held at 17 °C for 3 h after infection had higher survival rates than flies held at 29 °C for 3 h (median time to death post-infection for P. aeruginosa: 17 °C = 30 h, 25 °C = 28 h, and 29 °C = 27 h; L. lactis 17 °C 30 h, 25 °C = 28 h, and 29 °C = 26 h. Final mortality was checked the next day, and no differences in overall mortality were seen between the three temperature treatments (data not shown).
Fig. 5.
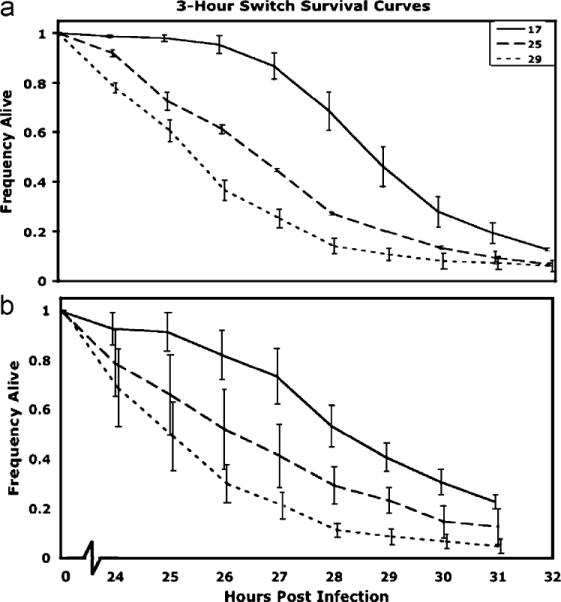
Survival curves (±1 S.E.) for the 3-h switch experiment for flies infected with (a) P. aeruginosa or (b) L. lactis at 17, 25, and 29 °C. After just 3 h at the respective temperatures, we see a significant difference in subsequent survival rate (Proportional Hazards model: P. aeruginosa, , P<0.0001; L. lactis, , P<0.0001). Median time to death for P. aeruginosa: 17 °C = 30 h, 25 °C = 28 h, 29 °C = 26 h; L. lactis: 17 °C = 30 h, 25 °C = 28 h, and 29 °C = 27 h.
3.4.2. Preheat treatment
In flies that were exposed to 17, 25 or 29 °C for 24 h prior to infection and then infected with P. aeruginosa, mortality rates were not affected by pre-infection treatment (, P = 0.75; Fig. 6a). Among flies infected with L. lactis, there was a significant difference among pre-infection heat treatment (, P = 0.0019; Fig. 6b). Median time to death was 27 h for all treatments, but a Proportional Hazards test between 17 and 29 °C shows 29 °C flies have a higher mortality rate than 17 °C flies (, P = 0.0021). In both sets of infections, the proportion of flies that survived the infection did not differ among treatments (data not shown).
Fig. 6.
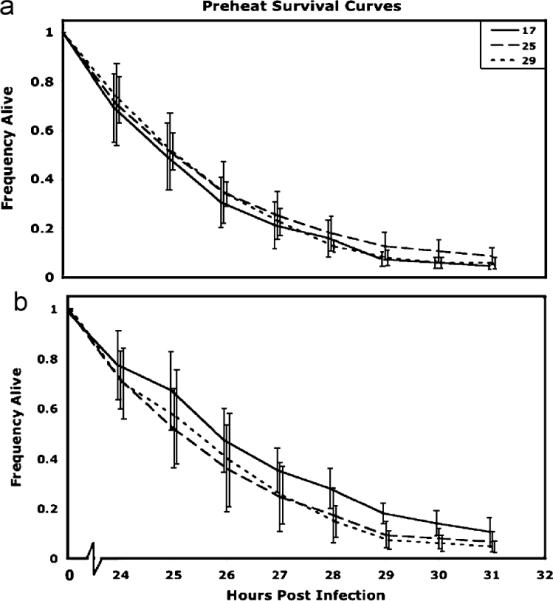
Survival curves (±1 S.E.) for flies maintained at 17, 25, or 29 °C before infection with (a) P. aeruginosa or (b) L. lactis. (a) There is no effect of pre-infection temperature regime on mortality when flies are infected with the gram-negative bacteria (Proportional Hazards model , P = 0.756). (b) However, in flies infected with the gram-positive bacteria, there is a significant difference in mortality rate (Proportional Hazards model, , P = 0.0019), with 29 °C flies having a greater mortality rate than 17 °C flies (Proportional Hazards model , P = 0.0021).
3.4.3. Gene expression in preheated flies
Flies showed an increase in expression of Spz, Mtk and Hsp83 at both 17 and 29 °C relative to 25 °C. However, while the magnitude of the effects was often quite large (Fig. 7), only two of the six comparisons were close to significance (Hsp8317 vs. 25: t2 = 4.135, P = 0.036; Mtk17 vs. 25: t2 = 2.77, P = 0.109).
Fig. 7.
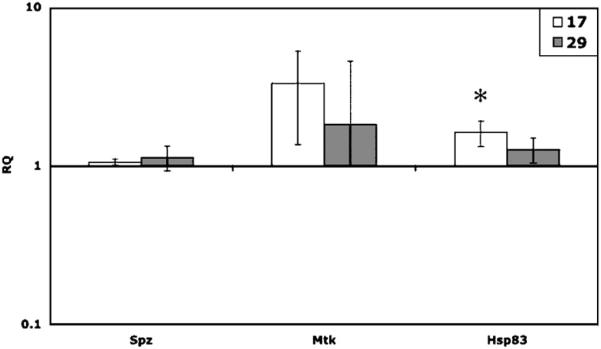
Mean gene expression (RQ; with 95% confidence intervals) for flies kept at 17 or 29 °C for 24 h, with no infection, relative to gene expression in 25 °C flies. Hsp83 is significantly higher in flies held at 17 °C than in flies held at 25 °C (t2 = 5.13, P 0.036). Though not statistically significant, Mtk also shows a trend of increased expression at 17 °C (t2 = 2.77, P = 0.109).
4. Discussion
To fully understand how temperature affects immune function in insects, we teased apart the effects of temperature on host physiology from the effects of temperature on the pathogen. Our results showed that flies infected with bacteria survive for longer when held at lower temperatures. This effect appeared to be due to the influence of temperature on both bacterial growth and the physiological responses in the host.
Infected flies held at lower temperatures survived longer, but the benefit of cooler temperature was just an hour or two. Could this brief period of survival increase fitness in infected flies? While fecundity is reduced in many insects after infection (Cole et al., 2003; Elsawaf et al., 1994; Zerofsky et al., 2005), females still continue to produce eggs. In fact, some bacteria, such as Wolbachia, can even lead to increased fecundity in flies (Fry et al., 2004). While we did not examine fecundity in this experiment, flies held at cooler temperatures might lay a few more eggs while they are still alive. This effect could be further enhanced if, as our Switch experiment suggests, a period at cooler temperatures were to enhance immunocompetence, and then a period at warmer temperatures increased egg production (Partridge et al., 1995). Future studies should measure the specific fitness consequences of the interaction between temperature and infection.
We examined gene expression at various temperatures in the hope of more completely understanding how temperature affects immunity in insects. In our gene expression assays, we discovered that infected flies held at cooler temperatures tended to show a greater up-regulation of immune genes than those held at warmer temperatures. This result was contrary to what we expected, since rates of other physiological systems such as metabolism and respiration generally increase at warmer temperatures (Gilchrist and Huey, 2001; Imasheva et al., 1998; Neven, 2000). One explanation for our data could be that in our gram-negative infected flies (LPS), genes at 29 °C were up-regulated more quickly, the infection was cleared, and the genes returned to basal expression levels by 8 h (Fig. 4a and b). However, this explanation is not consistent with our data from flies injected with heat-killed gram-positive bacteria (L. lactis). In these flies, we saw greater expression at 17 than at 29 °C at 3 h (Fig. 4c) and there was still an up-regulation of Mtk at 29 °C at 8 h (i.e. the immune system was still fighting off the infection; Fig. 4d). Previous research has shown that up-regulation of genes in response to infection may last for 48 h or more, at least when flies are infected with live bacteria and held at 25 °C (De Gregorio et al., 2001). Although we cannot be certain that genes in the gram-negative pathway do not return to basal levels by 8 h, our data do suggest that in the gram-positive pathway, immune genes in flies held at 17 °C show greater levels of expression after infection than those in flies held at 29 °C. It remains to be seen whether these temperature-dependent effects on gene expression are also found in proteins involved in the immune system.
We hoped that the 3-h switch experiment would help us to determine when the effect of temperature on survival occurred, and whether the effect of temperature on survival was transient or permanent. Switch experiments have been used previously to better understand how a particular treatment affects mortality rate. For example, Good and Tatar as well as Mair et al. carried out switch experiments to determine how DR extends life span in flies. They moved flies from a reduced diet to a full diet and examined age-specific mortality rates. These switch experiments enabled the authors to distinguish between two hypotheses. If DR increases life expectancy by decreasing the immediate risk of death, then one would expect to see a transient effect, with lower mortality rates occurring only when animals were exposed to the DR regime. However, if DR worked by slowing the accumulation of irreversible damage, then any time spent on DR should decrease mortality rates, even after the organism has been switched to a standard diet. In both studies, when the flies were switched from one diet to the other, the mortality rate also switched. Based on these experiments, one can conclude that DR extends survival by decreasing the immediate risk of death (Good and Tatar, 2001; Mair et al., 2004).
In light of these experiments, we were interested in determining whether the effect of temperature was due to an immediate and transient effect, or whether exposure prior to or early after infection would have long-term consequences on the ability to resist infection. In our switch experiment, we did not observe a change in the mortality rate of flies when they were switched between temperatures. In fact, and in marked contrast with the previous `switch' studies on DR, we saw that after only 3 h at a given temperature, the mortality rate of the flies appeared to be set permanently (Fig. 5). This result may have been due to the lower temperature either slowing bacterial growth and effectively giving the immune system a head start, or causing some physiological change in flies that allowed them to fight off the pathogen more efficiently. Future studies on bacterial growth rates at different temperatures as well as more gene expression studies will help resolve this difference.
Finally, we found that low temperature led to an up-regulation of Hsp83 as well as a trend of Mtk up-regulation. Given that cold stress induces Hsp83 (Qin et al., 2005), our data suggest that flies may be undergoing cold stress at 17 °C. Other work has also shown that Hsp83 is present in infected D. melanogaster (Guedes et al., 2005). The relationship between cold stress, immunity, and Hsp83 is no doubt a complicated one. We also saw a slight up-regulation of Mtk, an antimicrobial peptide normally activated by infection, at 17 °C, even in the absence of infection. It is possible that the cold stress activated some component of the immune system in our flies. Further work is needed to determine whether the heat-shock response system interacts with known immune pathways in insects, as has already been suggested by work with human cell lines (Kol et al., 1999, 2000).
5. Conclusion
We examined several aspects of temperature, immune function, and gene expression in D. melanogaster. In contrast with previous studies showing that warmer temperature helps insects fight fungal infections, we found that in flies infected with bacteria, colder temperatures increase the length of survival. Our data suggest that this effect is due not only to the fact that cooler temperatures slow the growth of the pathogen, but also that cooler temperatures enhance the ability of flies to fight off bacterial infections. Just how these effects influence fitness remains to be determined. As we tease apart the role of temperature on immune function in insects, we may also gain a better understanding of a broad range of ecological problems, including biological control of pests, determinants of species distributions, and host-parasite interactions.
Acknowledgments
Members of the Promislow lab and Dave Hall provided helpful comments on a previous version of this manuscript. Roger Deal and the Meagher lab gave invaluable help with the Real time RT-PCR experiments. Funding for this work was provided by a Sigma Xi Grant in Aid of Research to JEL, the ARCS Fellowship Foundation award to JEL, and a Senior Scholar award from the Ellison Medical Foundation to DELP. During the course of this work, JEL was supported in part by the University of Georgia Genetics NIH Training Grant, and by the Alton Fellowship in Genetics.
References
- Ashburner M. Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory Press; USA: 1989. [Google Scholar]
- Ballabeni P, Benway H, Jaenike J. Lack of behavioral fever in nematode-parasitized Drosophila. Journal of Parasitology. 1995;81:670–674. [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- Bhatti AR, Devoe IW, Ingram JM. Cell division in Pseudomonas aeruginosa—participation of alkaline-phosphatase. Journal of Bacteriology. 1976;126:400–409. doi: 10.1128/jb.126.1.400-409.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford S, Thomas MB. Adult survival, maturation, and reproduction of the desert locust Schistocerca gregaria infected with the fungus Metarhizium anisopliae var acridum. Journal of Invertebrate Pathology. 2001;78:1–8. doi: 10.1006/jipa.2001.5031. [DOI] [PubMed] [Google Scholar]
- Blanford S, Thomas MB, Langewald J. Behavioural fever in the Senegalese grasshopper, Oedaleus senegalensis, and its implications for biological control using pathogens. Ecological Entomology. 1998;23:9–14. [Google Scholar]
- Boorstein SM, Ewald PW. Costs and benefits of behavioral fever in Melanoplus-Sanguinipes infected by Nosema-Acridophagus. Physiological Zoology. 1987;60:586–595. [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution: nutrition and the cost of reproduction. Journal of Evolutionary Biology. 1993;6:171–193. [Google Scholar]
- Cole TJ, Eggleston P, Hurd H. Juvenile hormone titre and egg production in Tenebrio molitor infected by Hymenolepis diminuta: effect of male and/or female infection, male age and mating. Journal of Insect Physiology. 2003;49:583–590. doi: 10.1016/s0022-1910(03)00030-1. [DOI] [PubMed] [Google Scholar]
- Corby-Harris V, Pontaroli A, Shimkets L, Bennetzen J, Havel K, Promislow D. The geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Applied and Environmental Microbiology. 2007:3470–3479. doi: 10.1128/AEM.02120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WD, Huey RB, Gilchrist GW. Within- and between-generation effects of temperature on the morphology and physiology of Drosophila melanogaster. Evolution. 1996;50:1205–1218. doi: 10.1111/j.1558-5646.1996.tb02361.x. [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12590–12595. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot SL, Blanford S, Thomas MB. Host-pathogen interactions in a varying environment: temperature, behavioural fever and fitness. Proceedings of the Royal Society of London Series B-Biological Sciences. 2002;269:1599–1607. doi: 10.1098/rspb.2002.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsawaf BM, Elsattar SA, Shehata MG, Lane RP, Morsy TA. Reduced longevity and fecundity in Leishmania-infected sand flies. American Journal of Tropical Medicine and Hygiene. 1994;51:767–770. doi: 10.4269/ajtmh.1994.51.767. [DOI] [PubMed] [Google Scholar]
- Faurby S, Kjaersgaard A, Pertoldi C, Loeschcke V. The effect of maternal and grandmaternal age in benign and high temperature environments. Experimental Gerontology. 2005;40:988–996. doi: 10.1016/j.exger.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annual Review of Physiology. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Feder ME, Roberts SP, Bordelon AC. Molecular thermal telemetry of free-ranging adult. Oecologia. 2000;123:460–465. doi: 10.1007/s004420000334. [DOI] [PubMed] [Google Scholar]
- Fedorka KM, Zuk M. Sexual conflict and female immune suppression in the cricket, Allonemobious socius. Journal of Evolutionary Biology. 2005;18:1515–1522. doi: 10.1111/j.1420-9101.2005.00942.x. [DOI] [PubMed] [Google Scholar]
- Fedorka KM, Linder JE, Winterhalter W, Promislow D. Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proceedings of the Royal Society of London Series B-Biological Sciences. 2007;274:1211–1217. doi: 10.1098/rspb.2006.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellowes MDE, Kraaijeveld AR, Godfray HCJ. Cross-resistance following artificial selection for increased defense against parasitoids in Drosophila melanogaster. Evolution. 1999;53:966–972. doi: 10.1111/j.1558-5646.1999.tb05391.x. [DOI] [PubMed] [Google Scholar]
- Fry AJ, Palmer MR, Rand DM. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity. 2004;93:379–389. doi: 10.1038/sj.hdy.6800514. [DOI] [PubMed] [Google Scholar]
- Gilchrist GW, Huey RB. Parental and developmental temperature effects on the thermal dependence of fitness in Drosophila melanogaster. Evolution. 2001;55:209–214. doi: 10.1111/j.0014-3820.2001.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Good TP, Tatar M. Age-specific mortality and reproduction respond to adult dietary restriction in Drosophila melanogaster. Journal of Insect Physiology. 2001;47:1467–1473. doi: 10.1016/s0022-1910(01)00138-x. [DOI] [PubMed] [Google Scholar]
- Guedes SD, Vitorino R, Domingues R, Tomer K, Correia AJF, Amado F, Domingues P. Proteomics of immune-challenged Drosophila melanogaster larvae hemolymph. Biochemical and Biophysical Research Communications. 2005;328:106–115. doi: 10.1016/j.bbrc.2004.12.135. [DOI] [PubMed] [Google Scholar]
- Guinnee MA, Moore J. The effect of parasitism on host fecundity is dependent on temperature in a cockroach-acanthocephalan system. Journal of Parasitology. 2004;90:673–677. doi: 10.1645/GE-98R1. [DOI] [PubMed] [Google Scholar]
- Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, Hultmark D. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Molecular Cell. 1999;4:827–837. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Huey RB, Berrigan D. Temperature, demography, and ectotherm fitness. American Naturalist. 2001;158:204–210. doi: 10.1086/321314. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Drosophila immunity: paths and patterns. Current Opinion in Immunology. 2003;15:12–19. doi: 10.1016/s0952-7915(02)00005-5. [DOI] [PubMed] [Google Scholar]
- Imasheva AG, Loeschcke V, Zhivotovsky LA, Lazebny OE. Stress temperatures and quantitative variation in Drosophila melanogaster. Heredity. 1998;81:246–253. doi: 10.1046/j.1365-2540.1998.00384.x. [DOI] [PubMed] [Google Scholar]
- Inglis GD, Johnson DL, Goettel MS. Effects of temperature and thermoregulation on mycosis by Beauveria bassiana in grass-hoppers. Biological Control. 1996;7:131–139. [Google Scholar]
- Inglis GD, Johnson DL, Goettel MS. Effects of temperature and sunlight on mycosis (Beauveria bassiana) (Hyphomycetes: Sympodulosporae) of grasshoppers under field conditions. Environmental Entomology. 1997;26:400–409. [Google Scholar]
- JMP 5.1, S. I. I. 2007. SAS Campus Drive, Bldg S, Cary, NC 27513.
- Khodarev NN, Yu J, Nodzenski E, Murley JS, Kataoka Y, Brown CK, Grdina DJ, Weichselbaum RR. Method of RNA purification from endothelial cells for DNA array experiments. Biotechniques. 2002;32:316–320. doi: 10.2144/02322mt02. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188:166–168. [PubMed] [Google Scholar]
- Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60 s activate human vascular endothelium, smooth muscle cells, and macrophages. Journal of Clinical Investigation. 1999;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. Journal of Immunology. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- Lawniczak MKN, Begun DJ. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome. 2004;47:900–910. doi: 10.1139/g04-050. [DOI] [PubMed] [Google Scholar]
- Lazzaro BP, Sceurman BK, Clark AG. Genetic basis of natural variation in D. melanogaster antibacterial immunity. Science. 2004;303:1873–1876. doi: 10.1126/science.1092447. [DOI] [PubMed] [Google Scholar]
- Lazzaro BP, Sackton TB, Clark AG. Genetic variation in Drosophila melanogaster resistance to infection: a comparison across bacteria. Genetics. 2006;174:1539–1554. doi: 10.1534/genetics.105.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc V, Reichhart JM. The immune response of Drosophila melanogaster. Immunological Reviews. 2004;198:59–71. doi: 10.1111/j.0105-2896.2004.0130.x. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Kromermetzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart JM, Hoffmann JA. A recessive mutation, immune-deficiency (Imd), defines 2 distinct control pathways in the Drosophila host-defense. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annual Review of Genetics. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Dietary restriction in Drosophila—response. Science. 2004;303:1611–1612. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Current Biology. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Sohal RS. Temperature-dependent trade-offs between longevity and fertility in the Drosophila mutant, methuselah. Experimental Gerontology. 2006;41:566–573. doi: 10.1016/j.exger.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Molina-Hoppner A, Sato T, Kato C, Ganzle MG, Vogel RF. Effects of pressure on cell morphology and cell division of lactic acid bacteria. Extremophiles. 2003;7:511–516. doi: 10.1007/s00792-003-0349-0. [DOI] [PubMed] [Google Scholar]
- Muller CB, Schmid-Hempel P. Exploitation of cold temperature as defense against parasitoids in bumblebees. Nature. 1993;363:65–67. [Google Scholar]
- Neven LG. Physiological responses of insects to heat. Postharvest Biology and Technology. 2000;21:103–111. [Google Scholar]
- Ouedraogo RM, Kamp A, Goettel MS, Brodeur J, Bidochka MJ. Attenuation of fungal infection in thermoregulating Locusta migratoria is accompanied by changes in hemolymph proteins. Journal of Invertebrate Pathology. 2002;81:19–24. doi: 10.1016/s0022-2011(02)00117-9. [DOI] [PubMed] [Google Scholar]
- Ouedraogo RM, Cusson M, Goettel MS, Brodeur J. Inhibition of fungal growth in thermoregulating locusts, Locusta migratoria, infected by the fungus Metarhizium anisopliae var acridum. Journal of Invertebrate Pathology. 2003;82:103–109. doi: 10.1016/s0022-2011(02)00185-4. [DOI] [PubMed] [Google Scholar]
- Parmar MKB, Machin D. Survival Analysis: A Practical Approach. Wiley; Chichester: 1995. [Google Scholar]
- Partridge L, Barrie B, Barton NH, Fowler K, French V. Rapid laboratory evolution of adult life-history traits in Drosophila melanogaster in response to temperature. Evolution. 1995;49:538–544. doi: 10.1111/j.1558-5646.1995.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Qin W, Neal SJ, Robertson RM, Westwood JT, Walker VK. Cold hardening and transcriptional change in Drosophila melanogaster. Insect Molecular Biology. 2005;14:607–613. doi: 10.1111/j.1365-2583.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- Rolff J, Siva-Jothy MT. Selection on insect immunity in the wild. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:2157–2160. doi: 10.1098/rspb.2004.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R, Rohlf FJ. Biometry. W.H. Freeman and Company; USA: 2000. [Google Scholar]
- Steinhaus EA. Symposium-selected topics in microbial ecology: importance of environmental factors in the insect-microbe ecosystem. Bacteriological Reviews. 1960;24:365–373. doi: 10.1128/br.24.4.365-373.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer; New York: 2000. [Google Scholar]
- Thomas MB, Blanford S. Thermal biology in insect-parasite interactions. Trends in Ecology and Evolution. 2003;18:344–350. [Google Scholar]
- Uvell H, Engstrom Y. A multilayered defense against infection: combinatorial control of insect immune genes. Trends in Genetics. 2007;23:343–349. doi: 10.1016/j.tig.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Watson DW, Mullens BA, Petersen JJ. Behavioral fever response of Musca-domestica (Diptera, Muscidae) to infection by Entomophthora-Muscae (Zygomycetes, Entomophthorales) Journal of Invertebrate Pathology. 1993;61:10–16. [Google Scholar]
- Wilson K, Cotter SC, Reeson AF, Pell JK. Melanism and disease resistance in insects. Ecology Letters. 2001;4:637–649. [Google Scholar]
- Zerofsky M, Harel E, Silverman N, Tatar M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell. 2005;4:103–108. doi: 10.1111/j.1474-9728.2005.00147.x. [DOI] [PubMed] [Google Scholar]