SUMMARY
Streptococcus gordonii is a common oral commensal bacterial species in tooth biofilm (dental plaque) and specifically binds to salivary amylase through the surface exposed amylase-binding protein A (AbpA). When S. gordonii cells are pretreated with amylase, amylase bound to AbpA facilitates growth with starch as a primary nutrition source. The goal of this study was to explore possible regulatory effects of starch, starch metabolites and amylase on the expression of S. gordonii AbpA. An amylase ligand-binding assay was used to assess the expression of AbpA in culture supernatants and on bacterial cells from S. gordonii grown in defined medium supplemented with 1% starch, 0.5 mg ml−1 amylase, with starch and amylase together, or with various linear malto-oligosaccharides. Transcription of abpA was determined by reverse transcription quantitative polymerase chain reaction. AbpA was not detectable in culture supernatants containing either starch alone or amylase alone. In contrast, the amount of AbpA was notably increased when starch and amylase were both present in the medium. The expression of abpA was significantly increased (P < 0.05) following 40 min of incubation in defined medium supplemented with starch and amylase. Similar results were obtained in the presence of maltose and other short-chain malto-oligosacchrides. These results suggest that the products of starch hydrolysis produced from the action of salivary α-amylase, particularly maltose and maltotriose, regulate AbpA expression in S. gordonii.
Keywords: amylase-binding protein A, dental plaque, oral cavity, saliva
INTRODUCTION
Amylase-binding streptococci are numerous in the supra-gingival plaque of host species that express functional salivary α-amylase (Scannapieco et al., 1992, 1993, 1994). A representative amylase-binding species is Streptococcus gordonii, a commensal bacterium typically associated with oral health (Kolenbrander, 2000). Streptococcus gordonii binds salivary amylase to its surface with high efficiency and specificity via the surface expressed 20-kDa amylase-binding protein A (AbpA) (Scannapieco et al., 1992). AbpA is attached to the nascent cell wall at sites of cell division, and later, as the cell matures, is shed into the environment (Scannapieco et al., 1992). Though AbpA is the major protein responsible for amylase binding to bacterial cell surface, it is transiently associated with the cell wall and abundantly present in the culture supernatants (Roger et al., 1998). Mutation of abpA in S. gordonii eliminated amylase binding to bacterial surface, decreased biofilm formation and reduced adhesion to saliva-coated and amylase-coated hydroxylapatite in vitro (Rogers et al., 2001).
Salivary α-amylase is an abundant enzyme in saliva and is best known for its ability to degrade starch by hydrolysing 1,4-glycosidic linkages, with subsequent formation of maltose and various linear malto-oligosaccharides. Previous studies found that in vitro bound salivary amylase remains active and can be used by S. gordonii for carbohydrate metabolism in the presence of starch (Scannapieco et al., 1990; Douglas et al., 1992). In the absence of host amylase in the milieu, S. gordonii does not readily use starch and grows very poorly with starch as the sole nutrition source (Douglas et al., 1992; Rogers et al., 2001). Hence, the ability of S. gordonii to bind salivary amylase may be important for growth of this and other amylase-binding streptococcal species.
Previous studies have shown that carbohydrates in the milieu could influence the expression of AbpA, particularly by carbon catabolite repression (Rogers & Scannapieco, 2001). Indeed, AbpA was undetectable in cultures of bacteria in defined medium (DM) supplemented with 1% glucose, and greatly reduced in cultures of bacteria in brain–heart infusion medium containing 1% glucose (Rogers & Scannapieco, 2001). A catabolite responsive element was identified in the promoter region of abpA that probably regulates AbpA through the action of regulatory protein RegG, a CcpA-like catabolite control protein homologue (Rogers & Scannapieco, 2001). Mutation of regG eliminated the repressive effect of glucose on AbpA expression. These findings suggested that AbpA is likely to be a component of the bacterial carbohydrate utilization machinery, and is under the control of regulatory mechanisms governing carbohydrate metabolism.
Considering the fact that starch, as the substrate for bound amylase, is hydrolysed into smaller carbohydrates by the action of amylase, we hypothesized that starch could play a role in the regulation of expression of AbpA. In the presence of a potential food source (starch), it would be advantageous for the bacterium to exploit host amylase by increasing the expression of amylase-binding protein. Alternatively, we could not rule out the possibility of amylase itself exerting a regulatory effect on AbpA expression in the presence of starch. Hence, the goal of this study was to explore the regulatory effects of starch, starch metabolites and amylase on the expression of S. gordonii AbpA.
METHODS
Bacterial strains and culture conditions
Streptococcus gordonii CH1 Challis (GenBank, accession number s_gordonii_challis_n CP000725) was cultivated from frozen stocks on Todd–Hewitt (Difco, Detroit, MI) agar and incubated overnight at 37°C in a candle jar. For routine experiments, bacteria were cultured in the DM of Jenkinson, as modified by Loo (Jenkinson, 1986; Loo et al., 2000).
Isolation of human salivary α-amylase
Human parotid saliva from several healthy donors was duct-collected, as previously described (Miller, 1960; Bradway et al., 1989). This saliva was extensively dialysed against distilled water, lyophilized, resuspended in chromatography buffer (Scannapieco et al., 1989), and subjected to Bio-Gel P60 gel-filtration chromatography (Bradway et al., 1989; Scannapieco et al., 1989). The peaks corresponding to amylase were analysed for purity by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blot; selected peaks were pooled, dialysed, lyophilized and stored at −20°C. Isolated amylase appeared as a single band on SDS–PAGE stained with Coomassie Brilliant Blue as previously described (Scannapieco et al., 1989). Amylase peaks were also proven to be free from sIgA (Nikitkova et al., 2012). For the experiments in this study amylase was re-suspended in distilled water and used to supplement DM for bacterial growth experiments.
AbpA expression in overnight cultures with various growth conditions
Bacteria were streaked on Todd–Hewitt (Difco, Detroit, MI) agar from frozen stocks and incubated overnight at 37°C in a candle jar. Several colonies were transferred to 10 ml liquid medium and incubated for 16–18 h (overnight) statically at 37°C in a candle jar until the optical density of the culture at 600 nm (OD600) reached 0.6–0.8. The overnight conditions tested were as follows: DM supplemented with 0.5 mg ml−1 purified human salivary amylase, which approximates the normal concentration of amylase in saliva (Jacobsen et al., 1972); DM supplemented with 1% (weight/volume; W/V) soluble starch (Fisher Scientific, Fair Lawn, NJ); DM supplemented with 1% (W/V) soluble starch and 0.5 mg m−1 purified human salivary amylase. Brain–heart infusion broth (Bacto™, BD, Sparks, MD) was tested as a positive control, because S. gordonii shows high expression of AbpA in this medium (Rogers & Scannapieco, 2001). Additionally, 0.5 mg ml−1 salivary amylase was added to the bacterial culture grown overnight in DM 1% soluble starch, and incubated for 15 min (SA15 in Fig. 1). Bacterial cultures were centrifuged at 6000 g for 5 min; the supernatants were collected and used for precipitation of AbpA and amylase ligand-binding assay.
Figure 1.
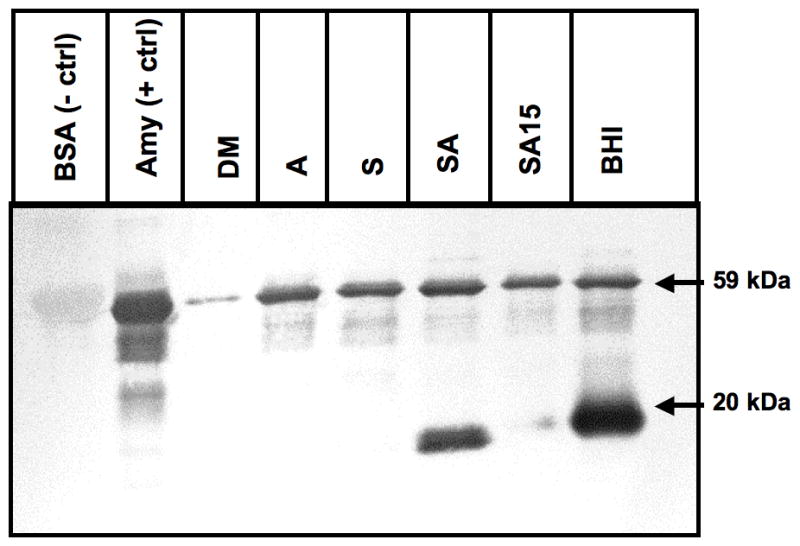
Expression of AbpA protein in culture supernatants of various growth conditions. Amylase ligand-binding assay of overnight culture supernatants precipitated with amylase. Bovine serum albumin as a negative control (BSA –ctrl), and salivary amylase as a positive control (Amy +ctrl). Growth conditions: defined media (DM); DM with 0.5 mg ml−1 amylase alone (A); DM supplemented with 1% starch (S); DM 1% starch + 0.5 mg ml−1 amylase (SA); DM with 1% starch grown overnight and 0.5 mg ml−1 amylase added and incubated for 15 min (SA15); brain–heart infusion (BHI). All supernatants were adjusted to have equal total protein content. AbpA protein (20 kDa band). Salivary amylase (59 kDa) used for precipitation is present in all lanes except negative control.
Precipitation of AbpA from culture supernatants
To precipitate proteins, culture supernatants were filtered through a 0.2-μm filter, adjusted to 1 mg/ml total protein and incubated for 1 h at room temperature with 50 μg ml −1 purified salivary amylase in a 1-ml total volume (Li et al., 2002; Chaudhuri et al., 2007). The amylase-precipitated proteins were collected by centrifugation at 10,000 g at room temperature for 10 min, resuspended in loading buffer (0.06 M Tris–HCl, pH 6.8, 10% glycerol, 2% SDS, 0.05% 2-β-mercaptoethanol and 0.00125 mg bromophenol blue), and boiled for 3 min before loading into 12% SDS–PAGE gels. Purified salivary amylase (1 μg) was loaded as a positive control and bovine serum albumin (1 μg) was loaded as a negative control. SDS–PAGE gels were then transblotted and used in the amylase ligand-binding assay.
Amylase ligand-binding assay
The proteins were electro-transferred from SDS–PAGE gels to Immobilon-P membranes (Millipore, Bedford, MA) and blocked overnight with 5% skim milk in Tris-buffered saline with 0.1 % Tween (TBST). After each change of reagent, the membrane was washed three times for 10 min in TSBT. Following blocking, the membrane was incubated with 10 mg ml−1 amylase in TBST, washed, exposed to rabbit anti-amylase antibody (Calbiochem - Merck, Rockland, MA) for 30 min, washed and incubated with goat anti-rabbit IgG (Bio-Rad, Hercules, CA) for 30 min. The membrane was then developed using Sigma FAST BCIP/NBT (Sigma, St Louis, MO).
RNA isolation and reverse transcription
Streptococcus gordonii was cultured in 120 ml DM with 0.2% glucose to the mid-log phase (0.5–0.6 OD600). The culture was then divided into 12 aliquots and centrifuged at 6000 g for 5 min at 19°C to pellet bacterial cells. The supernatant was removed and the bacteria were resuspended in fresh DM (2 ml) supplemented with one of the following: 1% (W/V) D-glucose (Fisher Scientific), 1% soluble starch (Fisher Scientific), 1% starch + 0.5 mg ml−1 purified salivary amylase, 1% maltose, 1% maltotriose, 1% maltotetraose, 1% maltopentaose 1% maltohexaose, 1% maltoheptaose, 1% maltoheptaose + 0.5 mg ml−1 amylase, 0.5 mg ml−1 amylase, or DM alone, and incubated in the candle jar at 37°C for 40 min. The malto-oligosaccharides were obtained from Sigma. After the bacterial suspensions were centrifuged at 6000 g for 5 min at 19°C, the supernatant was removed and stored at − 20°C until analysed for proteins, and the bacterial pellets were used to isolate RNA.
For RNA isolation, the bacterial pellet from each test condition described above was resuspended in salivary buffer (5 ml) followed by the addition of hot acid phenol as previously described (Vickerman et al., 2007). The RNA was then treated with TurboDNase (Applied Biosystems/Ambion, Austin, TX) according to manufacturer’s protocol and purified with the RNeasy minikit columns (Qiagen, Valencia, CA) using the manufacturer’s cleanup protocol. Total RNA was quantified using the Nanodrop 2000 spectrophotometer and RNA integrity was determined by agarose gel electrophoresis. Total RNA was used immediately for cDNA synthesis. The S. gordonii total RNA was reverse transcribed to cDNA using a modified protocol from the Pathogen Functional Genomics Resource Center (PFGRC) at the J. Craig Venter Institute http://pfgrc.jcvi.org/index.php/microarray.html. Briefly, 4 μg total RNA was reverse transcribed using Superscript II or III (Invitrogen, Carlsbad, CA) reverse transcriptase in a deoxynucleotide mixture. After alkaline hydrolysis to remove the RNA template, cDNA was purified with QIAquick PCR columns (Qiagen) and used in the quantitative reverse transcription polymerase chain reaction (RT-qPCR) assay.
RT-qPCR
The concentration of cDNA was determined by Nanodrop 2000 spectrophotometer, and each sample was normalized to 2 ng μl−1 for RT-qPCR. Gene-specific primers were designed using Primer BLAST (National Center for Biotechnology Information, www.ncbi.nlm.nih.gov), and produced by Invitrogen. Amplification of the gyrase A (gyrA) gene was used as an endogenous control, and the non-supplemented DM condition was used as the calibrator. For gyrA: forward primer GyrA-F 5′-GCGGATTGTTGTAACCGAGT-3′, reverse primer GyrA-R 5′-ACGGACACCCTCACGATTAG-3′ was used in the reaction. For the abpA: forward primer AbpA-F 5′-GCTGCAGCACCAGTTTTCTCTGCT-3′, reverse primer AbpA-R 5′-GCGCCGTCGTTGTTACGAGC-3′. The efficiency of the assay, tested using serial dilutions of cDNA template, was between 90 and 100%. Dissociation curve analysis was performed to verify amplification of a single product. Each reaction mixture (total volume, 25 μl) contained 1 μl template, 10 μl diethyl pyrocarbonate-treated water, 12 μl Power SYBR green PCR master mixture(Applied Biosystems, Foster City, CA) and 2 μl primer mix (100 nM). Relative RT-qPCR was performed on the ABI 7500 thermal cycler (Applied Biosystems), using uniform cycling conditions (95°C for 10 min and then 40 cycles of 30 s at 95°C and 1 min at 56°C) for Power SYBR Green (Applied Biosystems). Each reaction was run in triplicate, and non-template controls were run for each primer set. The RT-qPCR was performed on samples from the five independent experiments conducted on distinct days.
Statistical analysis
The log2 relative quantity values of the RT-qPCR data were analysed using Student’s t-test to compare expression of abpA in cells grown in one of the supplemented conditions with the expression of the abpA in cells of non-supplemented DM (n > 3). Significant values showed difference in abpA expression in tested growth condition compared with control condition at P < 0.05.
RESULTS
To investigate the role of starch and amylase in the regulation of expression of AbpA, we assessed the amounts of AbpA protein in the supernatants of overnight cultures of S. gordonii grown in the presence of starch alone, amylase alone, or starch and amylase together. All supernatants were adjusted with DM to contain the similar amount of the total protein. The DM has a low concentration of glucose (0.2%) and so was used to minimize catabolite repression of AbpA expression (Rogers & Scannapieco, 2001) and to avoid interference with complex carbohydrates that might be present in complex media. AbpA was abundantly present in overnight culture in the presence of both starch and bound amylase (Fig. 1). When bacteria were grown in DM with 1% starch, the appearance of AbpA was only observed after addition of amylase and 15 min of incubation, which suggests that time is required for the bacteria to express AbpA after exposure of the cell to starch and amylase or the products of their interaction. AbpA was not detectable in overnight cultures with starch alone, or with amylase alone. Together, these results suggest that expression of AbpA was influenced by the presence of both starch and amylase in the milieu, and that it takes time to express the protein in the supernatant after exposure.
Considering the fact that starch is digested by salivary amylase to form abundant malto-oligosaccharides, with maltose as the final end product (Ramasubbu et al., 2004), it was not clear if the observed up-regulation of AbpA was the result of the presence of the amylase–starch complex or of the effect of malto-oligosaccharides derived from starch digestion by amylase. Therefore, we further investigated the influence of linear starch-derived malto-oligosaccharides on the expression of abpA. Bacteria were grown to mid-log phase, where maximal AbpA protein expression was observed previously (Scannapieco et al., 1992; Gwynn & Douglas, 1994). At mid-log phase the bacteria were exposed to different substrates for 40 min before isolation of total RNA from bacterial cells, and collection of culture supernatant for protein analysis. The expression of abpA was elevated sixfold (P < 0.05) in the presence of starch and amylase together (Fig. 2), but not in the presence of starch or amylase alone. Expression of abpA was also increased in the presence of short-chain malto-oligosaccharides and maltose. Up to sixfold (P < 0.05) greater expression of abpA was observed in cells incubated with maltotriose, and greater than twofold increased expression was found in the presence of other malto-oligosaccharides (Fig. 2). Hence, the presence of the products of starch degradation in the milieu influences the expression of the abpA gene by S. gordonii.
Figure 2.
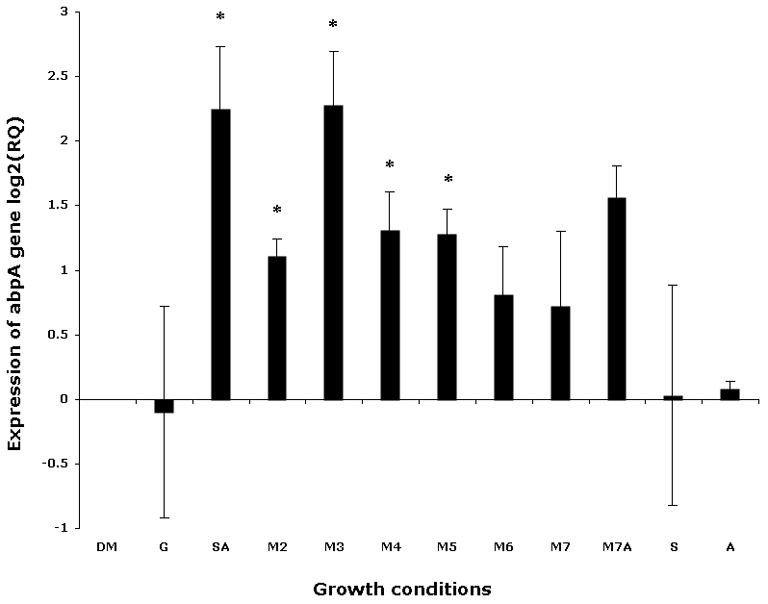
Expression of abpA gene in Streptococcus gordonii by quantitative reverse transcription–polymerase chain reaction. Bacteria were cultured to mid-log phase and pelleted. To the pellet one of the following was added and the mixture was incubated for 40 min in a candle jar at 37°C: fresh defined medium (DM); DM supplemented with 1% glucose (G); 1% starch and 0.5 mg ml−1 amylase (SA); 1% maltose (M2); 1% maltotriose (M3); 1% maltotetraose (M4); 1% maltopentaose (M5); 1% maltohexaose (M6); 1% maltoheptaose (M7); 1% maltoheptaose with 0.5 mg ml−1 amylase (M7A); 1% starch (S); 0.5 mg ml−1 amylase (A). The gyrA gene was used as endogenous control. Incubation in minimal fresh DM was used as the calibrator; log2 of relative quantity (RQ) is presented.
We also analysed the amount of AbpA protein in the supernatants after 40 min exposure of bacterial cells to various substrates by using amylase precipitation and the amylase ligand-binding assay. As expected, more AbpA protein was detected in the supernatants in the presence of starch and amylase together compared with cells incubated with un-supplemented DM or DM supplemented with glucose (Fig. 3). AbpA protein was minimal in the supernatants of cells incubated with starch or amylase alone. However, AbpA protein levels were elevated in the supernatants of cells incubated with short-chain malto-oligosaccharides (Fig. 3).
Figure 3.
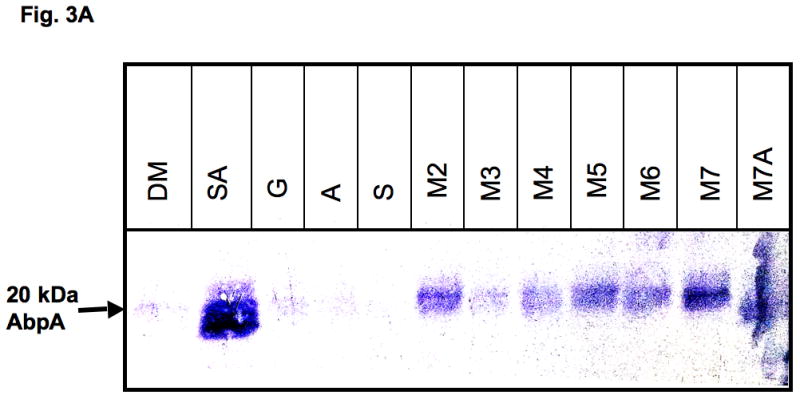
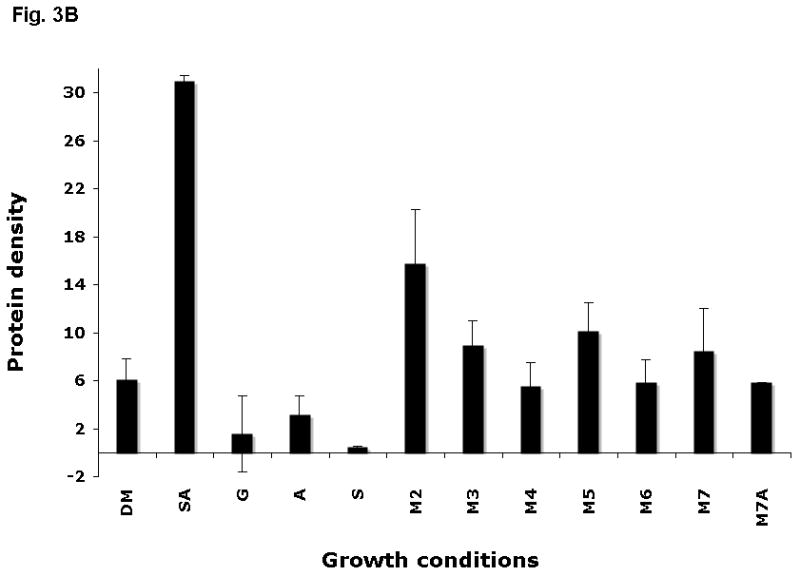
Expression of AbpA protein in culture supernatants grown with different malto-oligosaccharides. (A) Bacterial cells derived from the same culture were incubated for 40 min in DM supplemented with one of the following: 1% starch and 0.5 mg ml−1 amylase (SA); 1% glucose (G); 0.5 mg ml−1 amylase (A); 1% starch (S); 1% maltose (M2); 1% maltotriose (M3); 1% maltotetraose (M4); 1% maltopentaose (M5); 1% maltohexaose (M6); 1% maltoheptaose (M7); maltoheptaose with 0.5 mg ml−1 amylase (M7A). Total protein precipitated from 1 ml supernatant was loaded onto a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel, electro-transferred to polyvinylidene difluoride (PVDF) membrane, and the amylase ligand-binding assay was performed. (B) Densitometry analysis of AbpA protein on the same PVDF membranes described above.
DISCUSSION
Streptococcus gordonii is not able to use starch as a nutritional source unless extracellular amylase is present (Scannapieco et al., 1990; Douglas, 1990; Rogers et al., 2001). Salivary amylase binds to a specific receptor, AbpA, on the surface of S. gordonii, and retains its activity to degrade starch to provide a source of nutrition for the bacteria (Douglas, 1990; Douglas et al., 1992). The results of this study indicate that expression of the abpA gene is induced by both amylase and starch in the growth medium, whereas the presence of either component alone did not significantly alter expression of abpA. In the oral cavity, where amylase is always present, S. gordonii probably expresses only a basal level of AbpA. The presence of dietary starch together with available amylase increases AbpA expression enabling it to capture the enzyme, so that the available starch can be hydrolysed into a usable form. Amylase quickly hydrolyses starch at 1,4-glycosidic linkages to yield malto-oligosaccharides. Hence, it appears advantageous for the bacterium to be able to maximally capture the exogenous enzyme when starch is present by increasing the expression of the surface-associated AbpA. This would maximize the binding of the host enzyme to maximally increase the amount of usable malto-oligosaccharides in close proximity to the bacterium.
We observed that short-chain malto-oligosaccharides and maltose induced expression of abpA in a manner similar to starch and amylase together. The greatest expression was noted in the presence of maltotriose, the linear oligosaccharide starch metabolite consisting of three glucose moieties. These results suggest that products of starch degradation play a regulatory role in abpA gene expression.
Our previous work found that abpA is regulated by a catabolite repression mechanism through the effect of a catabolite control protein A (CcpA) homologue, the transcriptional regulator known in S. gordonii as RegG (Rogers & Scannapieco, 2001). A catabolite-responsive element (cre) was identified in the promoter region of the abpA and mutation of regG eliminated the catabolite repression effect of glucose on the expression of abpA (Rogers & Scannapieco, 2001). This mechanism of catabolite repression is probably similar to the ATP-dependent protein–protein interaction previously described in other gram-positive bacteria (Saier et al., 1996), where one of the elements of the glucose transport phosphotransferase system, the HPr protein, is phosphorylated on Ser-46 during glucose transport through phosphotransferase, and together with the phosphorylated glucose metabolite binds to a CcpA homologue, RegG (Rogers & Scannapieco, 2001). The latter changes conformation to acquire a high affinity toward cre (Saier et al., 1996), which in our case was positioned in the promoter region of abpA (Rogers & Scannapieco, 2001). The binding of RegG to cre is likely to prevent transcription of abpA (Fig. 4A). The present results support the previous findings, as we did not observe increased expression of abpA in the presence of glucose in the milieu. The present work provides further evidence for upregulation of abpA through maltose/maltodextrin induction. We observed that the levels of AbpA were elevated in cells exposed to 1% maltose and malto-oligosaccharides as determined by transcript levels and protein levels. These results differ from our previous observation where growth in FMC medium (Terleckyj et al., 1975) supplemented with maltose did not upregulate AbpA expression (Rogers & Scannapieco, 2001). This discrepancy, however, is probably the result of differences in experimental design between the two studies. In the previous studies, the bacteria were grown in the maltose-supplemented medium to mid-log phase for 10 h. In this case, the concentration of maltose diminished with time as the bacteria metabolized maltose to glucose. Hence, the expression of AbpA was probably repressed. In the present experiments, however, a minimal (0.2%) concentration of glucose in the DM was used to minimize catabolite repressive effects, and the time of bacterial exposure to 1% maltose was limited to 40 min. Hence, in the present experiments, the relative concentration of maltose was obviously high enough to induce AbpA expression (Fig. 3).
Figure 4.
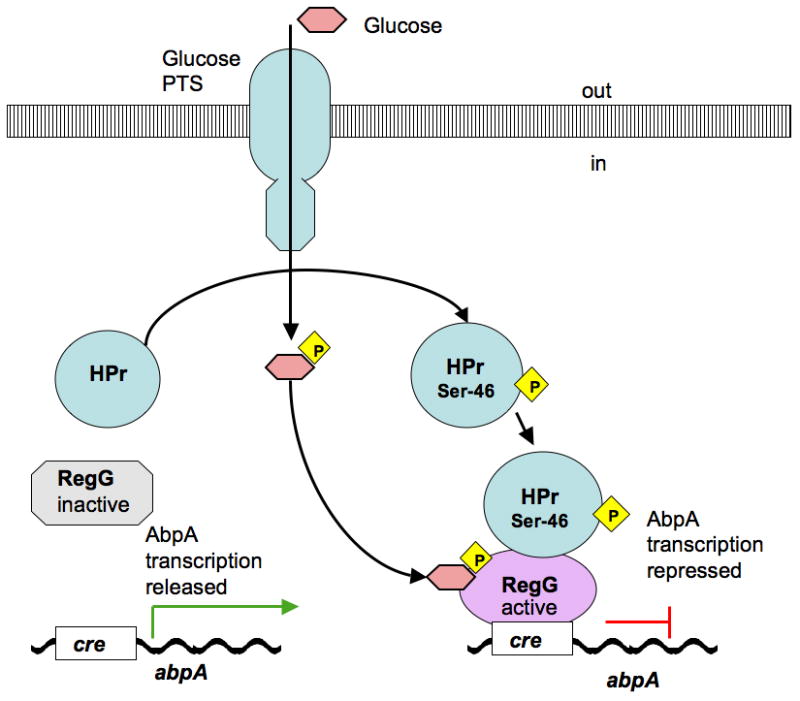
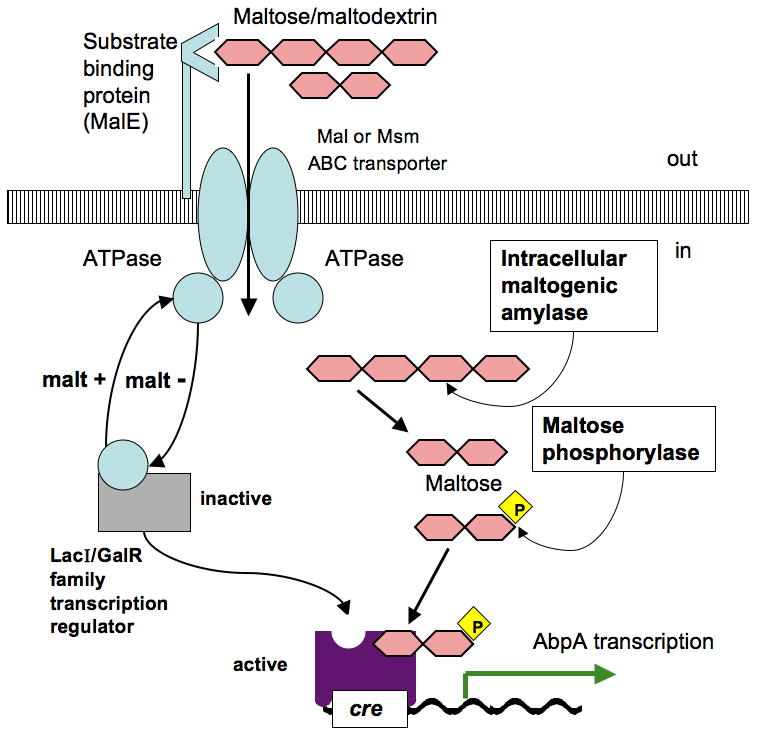
Theoretical model of regulation of abpA gene through catabolite repression by glucose (A) and substrate induction by maltose (B). The proposed model for abpA is adapted from regulatory pathways of carbohydrate utilization machinery described in various microorganisms (Saier et al., 1996; Boos & Shuman, 1998; Rogers & Scannapieco, 2001; Shelburne et al., 2006, 2008a, 2008b; Gorke & Stulke, 2008; Webb et al., 2008). (A) HPr protein becomes phosphorylated on Ser-46 during glucose transport through glucose phosphotransferase, and together with the phosphorylated glucose metabolite binds to RegG; the latter changes conformation and acquires high affinity for cre, which prevents transcription of abpA. (B) LacI/GalR transcriptional regulator is inactive while bonded to ATPase protein, a component of a Mal or Msm ABC transporter; during maltose/maltodextrin transport ATPase releases a LacI/GalR transcriptional regulator; latter binds internalized phosphorylated maltose to its phosphosugar site and becomes active; active LacI/GalR transcriptional regulator acquires high affinity for cre and initiates transcription of abpA.
Maltose/maltodextrin gene regulation has been previously described in various microorganisms (Nieto et al., 1997; Boos & Shuman, 1998; Boos & Bohm, 2000; Shelburne et al., 2006). In Listeria monocytogenes, the maltodextrin ABC transporter system is induced by maltose/maltodextrin and repressed by glucose, which seems to be similar to what is observed for regulation of abpA in S. gordonii. Interestingly, L. monocytogenes does not have extracellular amylase and depends on neighboring organisms that are able to hydrolyse starch to provide this function (Gopal et al., 2010). Previous findings suggest that maltose/maltodextrin induces the expression of the maltose ABC transporting system where maltose has the major role of inducer in L. monocytogenes (Gopal et al., 2010). Intracellular amylase generates maltose from maltodextrins in the cytoplasm, which become phosphorylated by the action of maltose phosphorylase. Phosphorylated maltose binds to and activates a LacI/GalR family transcriptional regulator, which in turn acts as a transcriptional activator (Gopal et al., 2010).
A similar mechanism of maltose-induced transcriptional activation is present in Lactococcus lactis where MalR, the LacI/GalR family regulator, activates expression of the maltose operon (Andersson & Radstrom, 2002).
Maltodextrin use is linked to virulence factor production in selected species of streptococci (Shelburne et al., 2008a, 2008b). In Streptococcus mutans, two maltose permease ABC transporter systems for maltose/maltodextrin uptake have been described (Webb et al., 2007, 2008). One system is encoded by malE, malF, malG and malK in the mal operon and the other system, the multiple sugar metabolism (Msm) transporter, is encoded by the msm operon. Interestingly, S. mutans UA159 isogenic double mutant strain (KCL82), although lacking these two functional operons was still able to ferment maltose as a primary carbon source (Webb et al., 2007). The principal maltose uptake system in this S. mutans strain was a phosphoenolpyruvate-dependent phosphotransferase system ptsG, which has sequence similarity with Enterococcus faecalis MalT (Webb et al., 2007, 2008).
To be able to better understand the observed regulation of abpA through the maltose/maltodextrin transport system, we searched the S. gordonii genome for oligosaccharide transporter gene candidates. Using BLAST search and the KEGG database we identified several genes as potential oligosaccharide transporters with sequence similarity with Msm and Mal-like transporters of S. mutans. Potential Mal-like ABC transporters in S. gordonii are represented by annotated genes malD SGO_0102, malC SGO_0103, maltose/maltodextrin-binding protein precursor SGO_0104, and ATP-binding protein SGO_0352. A potential transcriptional regulator for this operon is the upstream positioned transcriptional repressor SGO_0100, which is a LacI/GalR family protein with a sugar-binding domain and a DNA-binding domain. Considering the fact that the promoter region of abpA possesses a cre element known to be a binding site of LacI/GalR regulators (Francke et al., 2008; Rogers & Scannapieco, 2001), the proposed maltose/maltodextrin transporting system could possibly play a role in activation of abpA expression. Fig. 4B shows a hypothetical model adapted for abpA from previously described maltose/maltodextrin regulation pathways of other microorganisms (Saier et al., 1996; Boos & Bohm, 2000; Gorke & Stulke, 2008; Shelburne et al., 2006, 2008a, 2008b; Webb et al., 2008). However, it is not certain if the transcriptional repressor SGO_0100 would play the role of activator of a mal operon as in the case of the L. monocytogenes LacI/GalR regulator (Gopal et al., 2010), or is it a repressor in S. gordonii. It is also not yet clear if this annotated maltose transport system is in fact a maltose/maltodextrin transporter, and if it is, the role it plays in AbpA induction.
We cannot rule out the possibility that abpA regulation could be achieved through one of the Msm transporters (Webb et al., 2008). One Msm transporter candidate in S. gordonii is annotated as: msmE SGO_1305, msmF SGO_1304, and msmG SGO_1303 with transcriptional regulator msmR SGO_1306; another Msm-like ABC transporter SGO_0120, SGO_0121, SGO_0122 with each gene having up to 54% amino acid identity with Msm proteins of S. mutans and closely positioned on the chromosome phosphosugar-binding transcriptional regulator SGO_0127; and yet another sugar transporter SGO_1765, having 41% protein identity with the Msm permease of S. mutans. These hypothetical transporting systems are good candidates for a proposed model of positive regulation of abpA (Fig. 4B). However, though the function of these genes is annotated as oligosaccharide-transporting systems based on their similarity to the known proteins and conserved domains, their function in S. gordonii has not yet been established experimentally and requires further studies.
We also searched for the homologue of MalT in the S. gordonii identified phosphotransferase system IIBC component SGO_0505 with 70% protein identity to S. mutans MalT (ptsG), which has been suggested to play a major role in maltose transport in S. mutans (Webb et al., 2007). It is possible, but not likely, that MalT is involved in abpA induction, because in S. mutans MalT is responsible solely for the maltose transport (Webb et al., 2007). In our study, however, we observed upregulation of abpA not only in the presence of maltose, but also in the presence of other malto-oligosaccharides. Future studies will investigate and determine experimentally the role of SGO_0100, SGO_0127, SGO_1306 and other potentially promising LacI/GalR transcriptional activators on abpA gene expression.
Because AbpA is regulated in part through catabolite repression and maltose/maltodextrin induction, it probably plays a role in carbohydrate utilization systems. It is expected that one of the maltose/maltodextrin transporting systems in S. gordonii plays a regulatory role for AbpA in the presence of products of dietary starch degradation by salivary amylase.
Now that we have established that AbpA is positively regulated by maltose/maltodextrin, which are abundant substrates in the oral cavity during food consumption, several questions remain. First, it is necessary to determine which of the hypothetical maltose/maltodextrin transporting systems and transcription regulators play a role in AbpA induction. Second, the consequence of upregulation of AbpA for S. gordonii colonization remains to be determined. Though in vitro studies have shown that AbpA plays a role in biofilm formation on saliva-coated hydroxylapatite (Loo et al., 2000; Rogers et al., 2001), in vivo studies have argued against such a role (Tanzer et al., 2003). Our recent studies suggest that the binding of salivary amylase to AbpA on S. gordonii results in differential gene expression with a concomitant increase in bacterial growth and fitness (Nikitkova et al., 2012). Third, it is of interest to determine if the observed phenomenon of S. gordonii AbpA regulation affects host oral health in a positive or negative way. The intake of foods with higher starch content versus glucose content may increase the expression of AbpA, and alter the course of S. gordonii colonization. This in turn may influence the subsequent colonization of the oral cavity by potential pathogenic bacteria. The mechanisms of AbpA regulation and the role of AbpA in the metabolic processes of S. gordonii require further study to better understand S. gordonii colonization and interaction with the host environment.
Acknowledgments
This work was supported by the National Institute of Dental and Craniofacial Research and National Institutes of Health grants DE09838 and DE007034.
References
- Andersson U, Radstrom P. Physiological function of the maltose operon regulator, MalR, in Lactococcus lactis. BMC Microbiol. 2002;2:28. doi: 10.1186/1471-2180-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W, Bohm A. Learning new tricks from an old dog: MalT of the Escherichia coli maltose system is part of a complex regulatory network. Trends Genet. 2000;16:404–409. doi: 10.1016/s0168-9525(00)02086-2. [DOI] [PubMed] [Google Scholar]
- Boos W, Shuman H. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol Mol Biol Rev. 1998;62:204–229. doi: 10.1128/mmbr.62.1.204-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradway SD, Bergey EJ, Jones PC, Levine MJ. Oral mucosal pellicle. Adsorption and transpeptidation of salivary components to buccal epithelial cells. Biochem J. 1989;261:887–896. doi: 10.1042/bj2610887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri B, Rojek J, Vickerman MM, Tanzer JM, Scannapieco FA. Interaction of salivary alpha-amylase and amylase-binding-protein A (AbpA) of Streptococcus gordonii with glucosyltransferase of S. gordonii and Streptococcus mutans. BMC Microbiol. 2007;7:60. doi: 10.1186/1471-2180-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas CW. Characterization of the alpha-amylase receptor of Streptococcus gordonii NCTC 7868. J Dent Res. 1990;69:1746–1752. doi: 10.1177/00220345900690110701. [DOI] [PubMed] [Google Scholar]
- Douglas CW, Heath J, Gwynn JP. Enzymic activity of salivary amylase when bound to the surface of oral streptococci. FEMS Microbiol Lett. 1992;71:193–197. doi: 10.1016/0378-1097(92)90511-l. [DOI] [PubMed] [Google Scholar]
- Francke C, Kerkhoven R, Wels M, Siezen RJ. A generic approach to identify Transcription Factor-specific operator motifs; Inferences for LacI-family mediated regulation in Lactobacillus plantarum WCFS1. BMC Genomics. 2008;9:145. doi: 10.1186/1471-2164-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S, Berg D, Hagen N, et al. Maltose and maltodextrin utilization by Listeria monocytogenes depend on an inducible ABC transporter which is repressed by glucose. PLoS One. 2010;5:e10349. doi: 10.1371/journal.pone.0010349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorke B, Stulke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- Gwynn JP, Douglas CW. Comparison of amylase-binding proteins in oral streptococci. FEMS Microbiol Lett. 1994;124:373–379. doi: 10.1111/j.1574-6968.1994.tb07311.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson HF. Cell-surface proteins of Streptococcus sanguis associated with cell hydrophobicity and coaggregation properties. J Gen Microbiol. 1986;132:1575–1589. doi: 10.1099/00221287-132-6-1575. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- Li L, Tanzer JM, Scannapieco FA. Identification and analysis of the amylase-binding protein B (AbpB) and gene (abpB) from Streptococcus gordonii. FEMS Microbiol Lett. 2002;212:151–157. doi: 10.1111/j.1574-6968.2002.tb11259.x. [DOI] [PubMed] [Google Scholar]
- Loo CY, Corliss DA, Ganeshkumar N. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J Bacteriol. 2000;182:1374–1382. doi: 10.1128/jb.182.5.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JL. Method of pure parotid saliva collection without cannulization. J Dent Res. 1960;39:1075. doi: 10.1177/00220345600390051601. [DOI] [PubMed] [Google Scholar]
- Nieto C, Espinosa M, Puyet A. The maltose/maltodextrin regulon of Streptococcus pneumoniae. Differential promoter regulation by the transcriptional repressor MalR. J Biol Chem. 1997;272:30860–30865. doi: 10.1074/jbc.272.49.30860. [DOI] [PubMed] [Google Scholar]
- Nikitkova AE, Haase EM, Vickerman MM, Gill SR, Scannapieco FA. Response of fatty acid synthesis genes to the binding of human salivary amylase by Streptococcus gordonii. Appl Environ Microbiol. 2012 doi: 10.1128/AEM.07071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu N, Ragunath C, Mishra PJ, Thomas LM, Gyemant G, Kandra L. Human salivary alpha-amylase Trp58 situated at subsite -2 is critical for enzyme activity. Eur J Biochem. 2004;271:2517–2529. doi: 10.1111/j.1432-1033.2004.04182.x. [DOI] [PubMed] [Google Scholar]
- Rogers JD, Palmer RJ, Jr, Kolenbrander PE, Scannapieco FA. Role of Streptococcus gordonii amylase-binding protein A in adhesion to hydroxyapatite, starch metabolism, and biofilm formation. Infect Immun. 2001;69:7046–7056. doi: 10.1128/IAI.69.11.7046-7056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JD, Scannapieco FA. RegG, a CcpA homolog, participates in regulation of amylase-binding protein A gene (abpA) expression in Streptococcus gordonii. J Bacteriol. 2001;183:3521–3525. doi: 10.1128/JB.183.11.3521-3525.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH, Jr, Chauvaux S, Cook GM, et al. Catabolite repression and inducer control in Gram-positive bacteria. Microbiology. 1996;142 (Pt 2):217–230. doi: 10.1099/13500872-142-2-217. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Bergey EJ, Reddy MS, Levine MJ. Characterization of salivary alpha-amylase binding to Streptococcus sanguis. Infect Immun. 1989;57:2853–2863. doi: 10.1128/iai.57.9.2853-2863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco FA, Bhandary K, Ramasubbu N, Levine MJ. Structural relationship between the enzymatic and streptococcal binding sites of human salivary alpha-amylase. Biochem Biophys Res Commun. 1990;173:1109–1115. doi: 10.1016/s0006-291x(05)80900-3. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Haraszthy GG, Cho MI, Levine MJ. Characterization of an amylase-binding component of Streptococcus gordonii G9B. Infect Immun. 1992;60:4726–4733. doi: 10.1128/iai.60.11.4726-4733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco FA, Solomon L, Wadenya RO. Emergence in human dental plaque and host distribution of amylase-binding streptococci. J Dent Res. 1994;73:1627–1635. doi: 10.1177/00220345940730100701. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Torres G, Levine MJ. Salivary alpha-amylase: role in dental plaque and caries formation. Crit Rev Oral Biol Med. 1993;4:301–307. doi: 10.1177/10454411930040030701. [DOI] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Keith D, Horstmann N, et al. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc Natl Acad Sci U S A. 2008a;105:1698–1703. doi: 10.1073/pnas.0711767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Sumby P, Sitkiewicz I, et al. Maltodextrin utilization plays a key role in the ability of group A Streptococcus to colonize the oropharynx. Infect Immun. 2006;74:4605–4614. doi: 10.1128/IAI.00477-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, Davenport MT, Keith DB, Musser JM. The role of complex carbohydrate catabolism in the pathogenesis of invasive streptococci. Trends Microbiol. 2008b;16:318–325. doi: 10.1016/j.tim.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer JM, Grant L, Thompson A, Li L, Rogers JD, Haase EM, Scannapieco FA. Amylase-binding proteins A (AbpA) and B (AbpB) differentially affect colonization of rats’ teeth by Streptococcus gordonii. Microbiology. 2003;149:2653–2660. doi: 10.1099/mic.0.26022-0. [DOI] [PubMed] [Google Scholar]
- Terleckyj B, Willett NP, Shockman GD. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975;11:649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman MM, Iobst S, Jesionowski AM, Gill SR. Genome-wide transcriptional changes in Streptococcus gordonii in response to competence signaling peptide. J Bacteriol. 2007;189:7799–7807. doi: 10.1128/JB.01023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AJ, Homer KA, Hosie AH. A phosphoenolpyruvate-dependent phosphotransferase system is the principal maltose transporter in Streptococcus mutans. J Bacteriol. 2007;189:3322–3327. doi: 10.1128/JB.01633-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AJ, Homer KA, Hosie AH. Two closely related ABC transporters in Streptococcus mutans are involved in disaccharide and/or oligosaccharide uptake. J Bacteriol. 2008;190:168–178. doi: 10.1128/JB.01509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


