Abstract
Cystic fibrosis (CF) is a life-shortening disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene 1. Although bacterial lung infection and the resulting inflammation cause most of the morbidity and mortality, how loss of CFTR first disrupts airway host defense has remained uncertain 2–6. We asked what abnormalities impair eradication when a bacterium lands on the pristine surface of a newborn CF airway? To investigate these defects, we interrogated the viability of individual bacteria immobilized on solid grids and placed on the airway surface. As a model we studied CF pigs, which spontaneously develop hallmark features of CF lung disease 7,8. At birth, their lungs lack infection and inflammation, but have a reduced ability to eradicate bacteria 8. Here we show that in newborn wild-type pigs, the thin layer of airway surface liquid (ASL) rapidly killed bacteria in vivo, when removed from the lung, and in primary epithelial cultures. Lack of CFTR reduced bacterial killing. We found that ASL pH was more acidic in CF, and reducing pH inhibited the antimicrobial activity of ASL. Reducing ASL pH diminished bacterial killing in wild-type pigs, and increasing ASL pH rescued killing in CF pigs. These results directly link the initial host defense defect to loss of CFTR, an anion channel that facilitates HCO3− transport 9–13. Without CFTR, airway epithelial HCO3− secretion is defective, ASL pH falls and inhibits antimicrobial function, and thereby impairs killing of bacteria that enter the newborn lung. These findings suggest that increasing ASL pH might prevent the initial infection in patients with CF and that assaying bacterial killing could report on the benefit of therapeutic interventions.
Proposed origins of CF lung disease include reduced mucociliary clearance due to decreased ASL volume or altered mucus, reduced bacterial killing by ASL antimicrobials, defective bacterial elimination by phagocytes, abnormal inflammatory responses, reduced or increased bacterial binding by airway epithelia, and other defects 2–6. One or more of these defects could be responsible. Two factors have made it difficult to distinguish between hypotheses and identify initiating insults. First, as with many diseases, clinical manifestations may not reflect original defects, but it is problematic to study CF at its onset in newborn human infants. Second, mice with disrupted CFTR genes do not develop typical CF 14.
To circumvent these obstacles, we generated CFTR−/− pigs (CF pigs) 7. Within months of birth, CF pigs spontaneously develop airway inflammation, infection, tissue remodeling, mucus accumulation, and airway obstruction 7,8, hallmark features of CF lungs. Although at birth they exhibit none of these features, they already manifest a host defense defect against bacteria. Thus, newborn CF pigs provide an unprecedented opportunity to investigate mechanisms impairing host defense and initiating disease because they allow CF:non-CF comparisons without secondary confounds.
In previous work, we instilled Staphylococcus aureus into airways and four hours later found more bacteria in CF than non-CF pigs 8. However, that study revealed little about responsible mechanisms; we do not know whether bacteria were removed or killed within the lung, whether bacteria grew following instillation, whether phagocytic cells eliminated bacteria, whether bacteria bound to surfaces, or whether deposition and sampling were identical in all animals.
To investigate initial host defense defects, we developed a simple assay that tested viability of individual bacteria. We chemically linked biotin to S. aureus, bound streptavidin to gold grids, and combined them to attach S. aureus to grids (Fig. 1a,b). We chose S. aureus because we frequently isolate it from porcine CF lungs, and it is the most common organism isolated from young children with CF 8,15. A fluorescent live/dead stain revealed the state of bacteria. Exposing grids to ethanol killed most S. aureus (Fig. 1c). Importantly, placing grids on the porcine tracheal surface in vivo also killed bacteria.
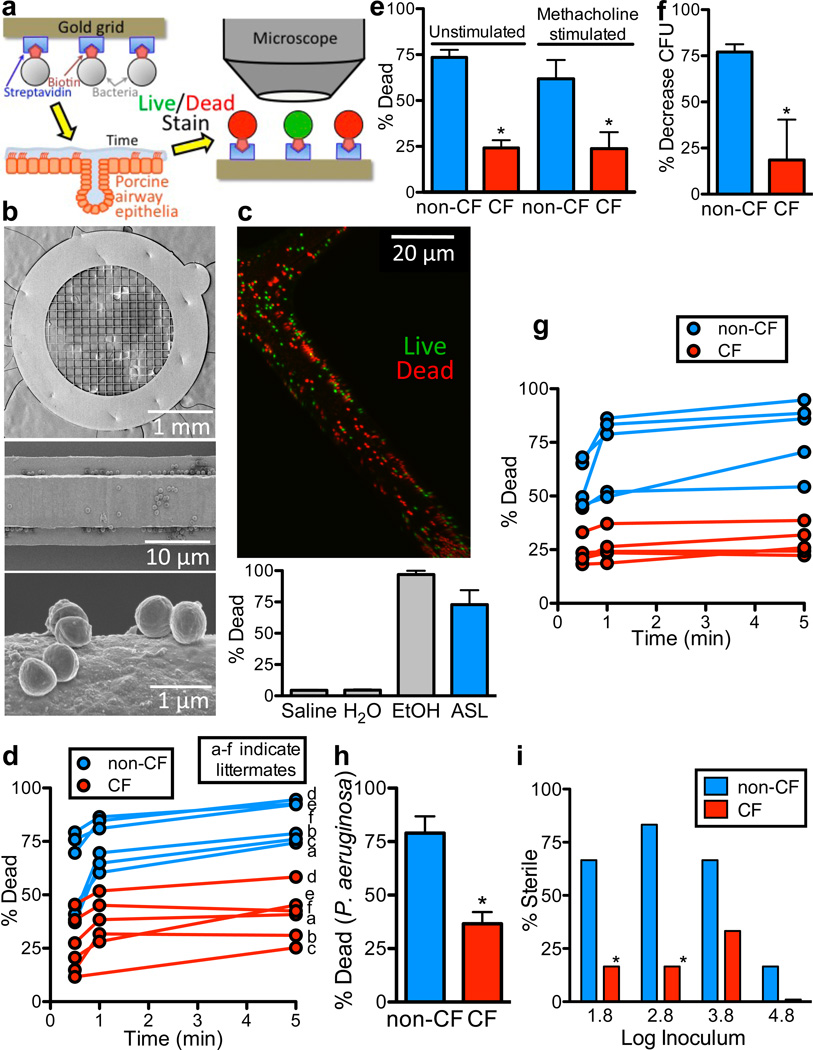
Figure 1. Bacterial killing is impaired in CF ASL.
a. Schematic showing biotin/streptavidin linking S. aureus to gold grids that were placed on the airway surface. After removal, bacteria were exposed to fluorescent live/dead stain (SYTO 9/propidium iodide), imaged, and counted.
b. Scanning electron photomicrographs of bacteria-coated grid (top), grid bar (middle), and individual bacteria (bottom).
c. Image of bacteria-coated grid (green=live, red=dead) after placement for 5min on tracheal surface of 1-month-old, wild-type pig. Bottom shows percentage of bacteria that were dead after immersion in saline, water, 70% ethanol, or placement on tracheal surface. n=3 each. Here and elsewhere, bars are SEM.
d. S. aureus-coated grids were placed on tracheal surface of newborn CF and non-CF pigs for indicated times. Data are percentage dead bacteria. Each set of 3 time-points is from a single animal. Letters (a–f) indicate littermates; there is no 1min time point for CF pig in litter c due to experimental error. For each pig, 2–3 grids were used at each time point, 5–16 fields were counted per grid, each field contained ~100–1000 bacteria (Fig. S6), and data from each field were averaged. Operators were blinded to genotype. CF was different from non-CF at all time points, P<0.01.
e. S. aureus-coated grids were placed for 30sec on tracheal surface before and ~5min after methacholine stimulated secretion. n=6/genotype. *P<0.02. For each genotype, data with and without methacholine do not differ significantly.
f. ASL was removed from methacholine-stimulated pigs, bacteria (1×106 CFU/ml) were incubated in 1µl ASL for 10min. in a micro CFU assay, and bacteria were counted by dilution plating. n=6/genotype. *P<0.05.
g. S. aureus-coated grids were placed on surface of primary epithelial cultures for indicated times, and percentage dead bacteria was determined. Each set of data points represents mean from epithelia from a different animal. CF was different from non-CF at all time points, P<0.001.
h. P. aeruginosa-coated grids were placed on surface of cultured epithelia for 5min. n=5 cultures from different pigs per genotype. *P<0.001.
i. S. aureus in 100nl H2O were applied to apical surface of cultured epithelia. Apical surface was washed 24hr later and bacteria counted by dilution plating. Data are % of epithelia that showed no bacterial growth. n=18/genotype. *P<0.005, Fisher's exact test.
In 6–15 hr-old pigs, we made a small tracheal incision and placed bacteria-coated grids on the airway surface. Even 30-sec applications on non-CF airways killed S. aureus (Fig. 1d). Applying grids to littermate CF pigs killed approximately half as many bacteria. We administered methacholine to stimulate secretion of submucosal glands, which produce substantial amounts of antimicrobials 16,17, and to allow us to collect ASL for other studies. After methacholine, CF:non-CF differences persisted (Fig. 1e). We predicted that antimicrobial activity would also be detected if we removed methacholine-stimulated ASL and studied it with conventional colony-forming unit (CFU) assays. Indeed, bacterial killing was reduced in CF secretions (Fig. 1f).
We also applied S. aureus-coated grids to primary cultures of porcine airway epithelia and found reduced killing in CF (Fig. 1g). Previous data suggest that the host-defense defect involves many different bacteria 8,15. Therefore, we tested Pseudomonas aeruginosa-coated grids and found defective killing by CF epithelia (Fig. 1h). We also added S. aureus directly to cultured epithelia. Most non-CF epithelia eliminated low inocula of bacteria, but bacteria grew on most CF epithelia (Fig. 1i). At the highest inocula, S. aureus infected both CF and non-CF epithelia.
These data indicate that ASL rapidly kills bacteria, and CF impairs killing. The defect was partial, as CF ASL retained some activity. The assays allow several conclusions. a) Defective bacterial killing was not due to dysfunctional mucociliary clearance or abnormal killing by phagocytes; neither would explain results with grids in vivo or studies of cultured epithelia. b) We cannot attribute CF:non-CF differences to altered bacterial-epithelial binding because we saw the difference with bacteria attached to grids and with ASL studied ex vivo. c) Our earlier finding that newborn CF airways lack inflammation 8 and the killing defect in cultured epithelia indicate that abnormal inflammation was not responsible. d) Our bacteria-coated grid method also excludes differences in bacterial delivery, sampling, or growth. Therefore, we reasoned that defective killing arose either from reduced amounts of ASL antimicrobial factors or inhibition of their function.
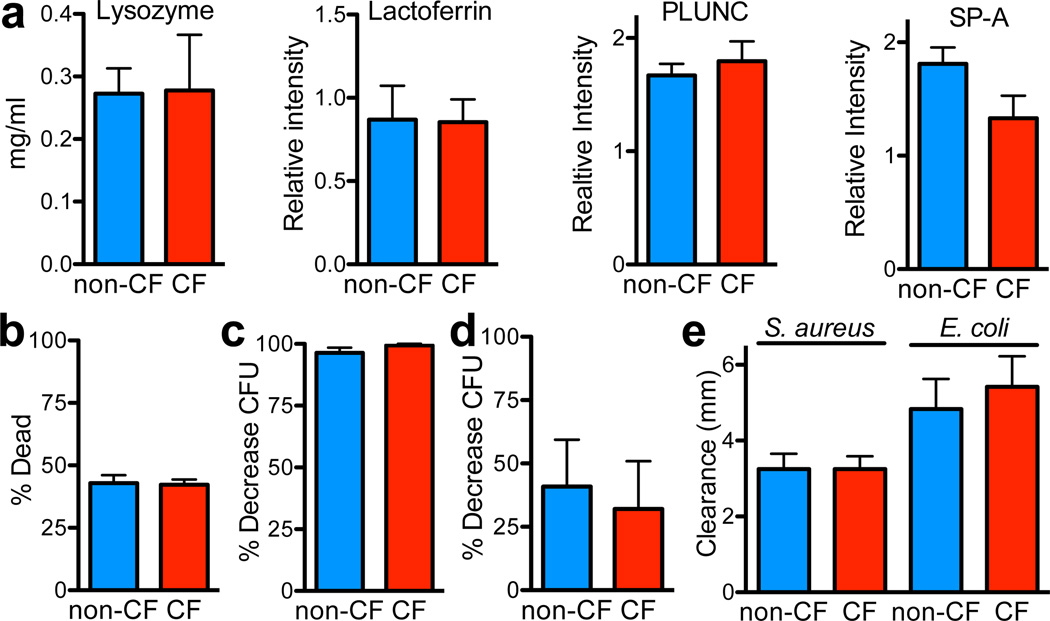
We investigated antimicrobials by measuring mRNA, protein, and aggregate activity under optimal conditions. The abundance of transcripts for secreted antimicrobial proteins (Table S1, Fig. S1) and proteins with known host defense functions revealed no consistent differences between genotypes (Table S2). In methacholine-stimulated ASL, concentrations of the two most abundant antimicrobials, lysozyme and lactoferrin, as well as PLUNC and SP-A did not differ by genotype (Fig. 2a). To assay aggregate ASL antimicrobial function, we did four experiments in which we maximized activity by reducing ionic strength close to zero 16,18,19. First, we added isotonic, salt-free buffer to apical surfaces of cultured airway epithelia. Under these controlled conditions, both genotypes showed equivalent killing of bacteria on grids (Fig. 2b). Second, ASL removed from cultured CF and non-CF epithelia with water killed bacteria to the same extent (Fig. 2c, S2). Third, ASL removed from pigs and diluted 1:100 with water showed genotype-independent killing (Fig. 2d). Fourth, radial diffusion assays with 10 mM Na phosphate in 1% agarose revealed areas of clearance for S. aureus and E. coli that were similar for both genotypes (Fig. 2e). These data indicate that non-CF and CF ASL had similar amounts of antimicrobials. Thus, they suggested that CF:non-CF bacterial killing disparities derived from other differences in ASL composition.
Figure 2. CF and non-CF ASL have similar antimicrobial concentrations and aggregate antimicrobial activity under optimal conditions.
a. Lysozyme concentration was measured with lysoplates; n=8/genotype. Quantitative western blots assayed lactoferrin, PLUNC, and SP-A; data are relative intensity of blots, n=12/genotype.
b. 60µl isotonic xylitol was added to apical surface of airway cultures (diluting ASL ~100:1). 3min later, two S. aureus-coated grids were placed on epithelial surface for 1min, then removed and counted. n=6 epithelia/genotype, each from different pigs.
c. ASL was removed from cultured epithelia by washing with 100µl H2O. ASL was incubated with S. aureus (3.3×103 CFU/ml) for 60min, and micro CFU assays were used to measure antimicrobial activity. n=12/genotype. See also Fig. S2.
d. Methacholine-stimulated ASL was diluted 1:100 in H2O, incubated with S. aureus (1×106 CFU/ml) for 60min, and CFU assays were used to measure antimicrobial activity. n=5/genotype.
e. Radial diffusion assays were used to measure antimicrobial activity of ASL collected from epithelial cultures. n=6/genotype.
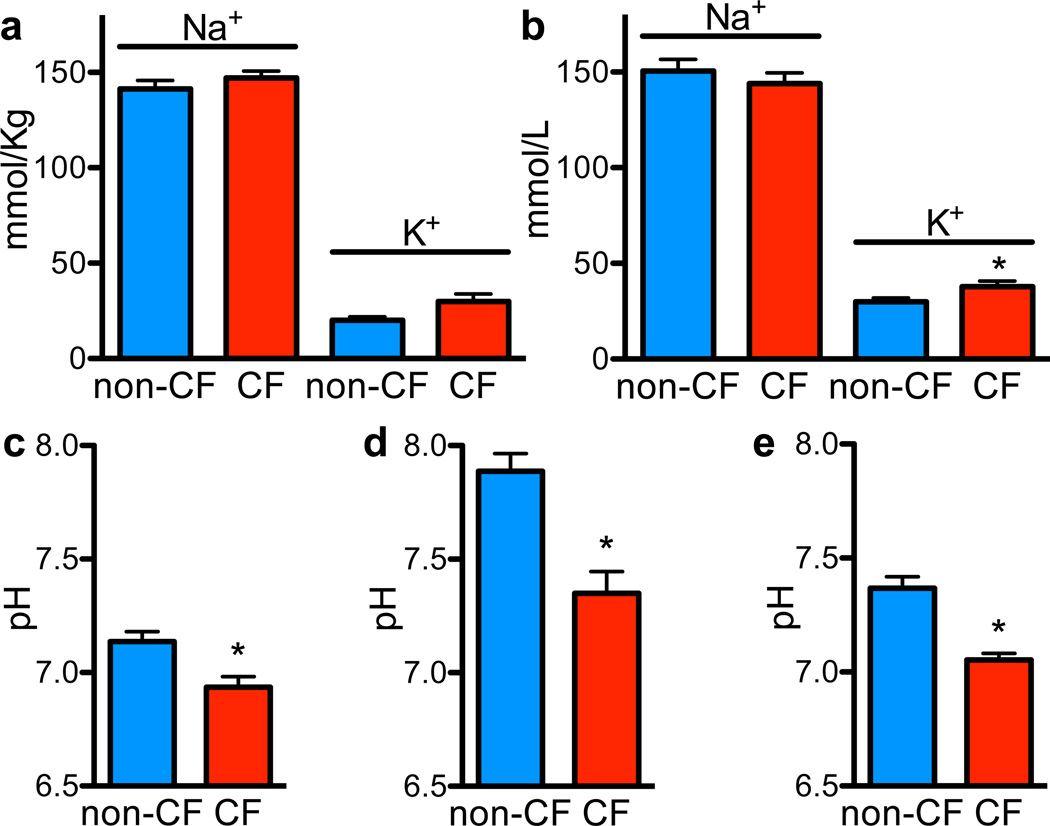
An increased ionic strength inhibits activity of many antimicrobials 16,18,19. Studies of human CF airway epithelia in culture or xenografts reported either higher 20,21 or the same 22,23 ASL NaCl concentrations as non-CF, but an in vivo study 24 reported similar concentrations. Therefore, we measured Na+ and K+ concentrations in ASL collected from newborn pigs and found that they did not differ by genotype (Fig. 3a). In addition, ASL collected after methacholine stimulation showed ion concentrations similar to those measured under basal conditions and only minor differences in K+ concentration between CF and non-CF (Fig. 3b). Thus, different ASL Na+ and K+ concentrations do not explain defective bacterial killing in CF.
Figure 3. ASL pH is more acidic in CF than non-CF.
a. ASL was collected under basal conditions from tracheal surface using Parafilm-coated paper; Na+ and K+ concentrations were measured as described in Methods (Table S3). n=8 non-CF and 6 CF.
b. ASL Na+ and K+ concentrations in methacholine-stimulated ASL. n=16 non-CF and 14 CF pigs. *P<0.05.
c. ASL pH measured in vivo using pH-sensitive planar optical probe placed on tracheal surface. n=6 non-CF and 7 CF; littermates were used with one extra CF. *P<0.05. Studies were done with animals in 5% CO2; therefore, ASL CO2 concentration was likely >5% due to CO2 production by the pigs.
d. Methacholine-stimulated ASL was removed, and pH was measured with a micro-optical pH probe. pH was measured 10min after removal in ambient CO2, which likely increased pH values compared to in vivo. n=10 pigs/genotype; littermates were used. *P<0.0005.
e. ASL pH measured in cultured airway epithelia using fluorescent pH indicator. N=5epithelia/genotype, each from a different pig. *P<0.01. Calculated HCO3− concentrations using measured pH, the 5% CO2 concentration, and Henderson-Hasselbalch equation were non-CF 28.1±4.2mM (n=8) and CF 13.1±2.4mM (n=8), p=0.007.
Earlier studies indicated that pH can affect antimicrobial activity 19,25. Human and porcine airway epithelia exhibit CFTR-dependent HCO3− secretion 11,12, and CF reduces ASL pH 26,27. To assess ASL pH in vivo, we placed a planar pH-sensitive probe on the tracheal surface. pH was lower in CF than non-CF ASL (Fig. 3c). Methacholine-stimulated ASL removed from CF pigs and measured with an optical pH probe was more acidic than non-CF (Fig. 3d); pH was measured after removal in ambient CO2, likely contributing to the higher absolute pH values. We also measured ASL pH in primary airway epithelial cultures using a fluorescent pH indicator and found reduced pH in CF (Fig. 3e). Although absolute pH values varied in different preparations, in all three, CF ASL was more acidic.
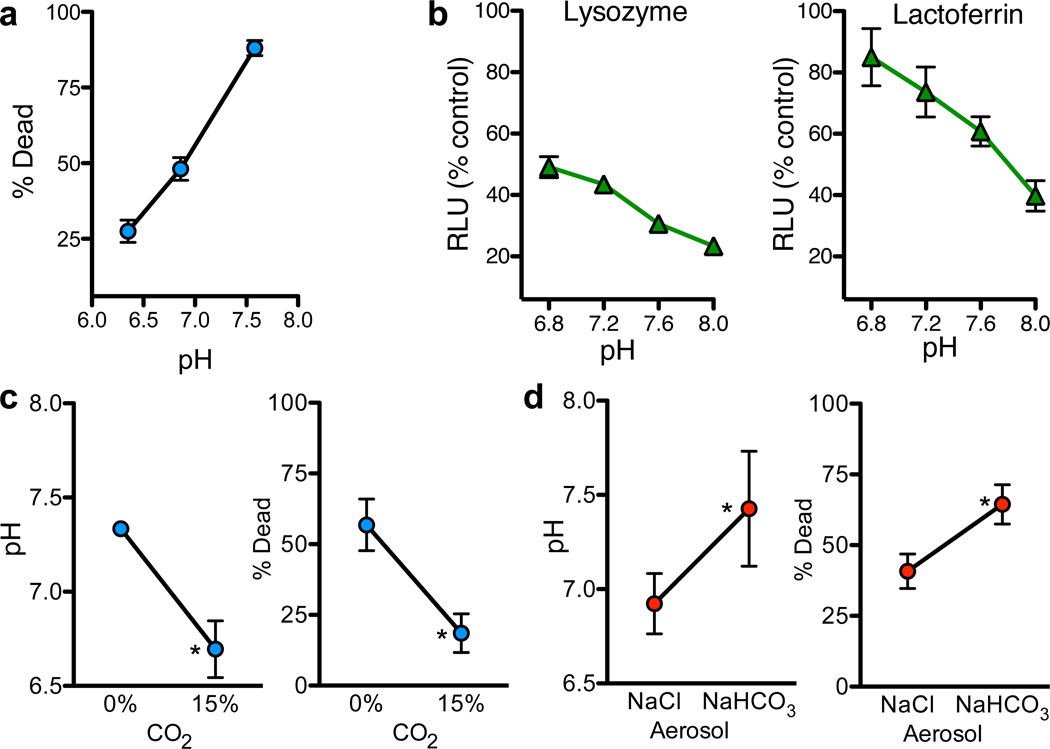
We tested whether pH affects ASL antimicrobial activity by removing ASL from newborn non-CF pigs, adjusting pH, and applying S. aureus-coated grids. Killing was pH-dependent, increasing as pH increased (Fig. 4a, S3). We also tested lysozyme and lactoferrin; increasing pH increased S. aureus and E. coli killing (Fig. 4b,S4,S5).
Figure 4. Increasing ASL pH enhances antimicrobial activity.
a. Methacholine-stimulated ASL was removed from non-CF pigs, pH was adjusted with HCl, and antimicrobial activity was measured with S. aureus-coated grids. n=6. See Fig. S3, Table S3.
b. Effect of pH on antimicrobial activity of 1.25mg/ml lysozyme and 4mg/ml lactoferrin in S. aureus luminescence assay. Data are relative luminescence compared to control at 30min. for lysozyme and 60min. for lactoferrin (Fig. S5). Similar results were obtained with E. coli (Fig. S4).
c. Tracheas of non-CF pigs were exposed to 0% or 15% CO2 in vivo. pH was measured with pH-sensitive planar electrode. n=6/genotype. Bacterial killing was measured with S. aureus-coated grids placed on surface for 30sec. n=4/genotype. *P<0.05.
d. NaHCO3 or NaCl (50µl, 100mM) was aerosolized onto airway surface of CF pigs. pH (n=6/genotype) and bacterial killing (n=5/genotype) were measured as in 4c. *P<0.05.
If pH is responsible for differences in bacterial killing, we predicted that reducing ASL pH would inhibit bacterial killing in wild-type pigs, and raising pH would enhance killing in CF pigs. In non-CF pigs, elevating airway CO2 reduced ASL pH and inhibited bacterial killing (Fig. 4c). In CF pigs, we aerosolized NaHCO3 into the trachea. Compared to NaCl, NaHCO3 increased ASL pH and enhanced killing (Fig. 4d).
Our results directly link CFTR mutations to defective bacterial eradication. CFTR is an anion channel that conducts HCO3− and works in concert with Cl−/HCO3− exchange and H+ secretion to regulate ASL pH 2,9,10,13; its loss prevents airway epithelia from secreting HCO3− 11,12. The resulting decreased ASL pH inhibits antimicrobial function, thereby impairing killing of bacteria that enter the lung. Our findings with bacteria-coated grids in vivo, ASL removed from pigs, and primary epithelial cultures all point to this defect.
What about other defects that might commence CF lung disease? First, progression from the pristine lung of a newborn to the chronically infected, inflamed, remodeled, and obstructed lung of a person with advanced CF entails many steps. Our findings suggest that reduced antibacterial activity may initiate this downward spiral. However, we cannot exclude additional abnormalities at the onset of disease. These might include abnormal mucociliary clearance, bacterial binding, inflammation, or phagocytic function. As disease progresses, the relative importance of various defects may also change. As one example, reduced ASL pH might alter mucus secretion 2 and thereby impair mucociliary clearance either at the genesis of disease or as mucus secretion increases with inflammation and remodeling. Second, ASL contains a complex mixture of peptides, proteins and lipids with individual 16,28,29 and synergistic antimicrobial effects 29,30. Our data do not assess relative importance or abundance of each factor or CF:non-CF differences.
Several potential fates await bacteria that land on the airway surface. They might be killed. They might remain metabolically active, but unable to reproduce. They might replicate. And/or they might be removed. The balance between these options and their timing determines whether or not infection ensues. Our grid assay tested one of these alternatives independent of the others, and the data indicate that ASL killed many, but not all bacteria. The speed of killing was remarkable, although killing might have continued after grids were withdrawn from ASL, rinsed and placed in indicator solution. Quick action is consistent with rapid bacterial permeabilization by antimicrobial proteins 19. Our other assays showing reduced numbers of growing bacteria (CFUs) after being placed on epithelial cultures or in ASL reflect consequences of immediate killing plus potential effects of ASL on replication. Results from our earlier delivery of bacteria into porcine airways reflect all these host defenses plus bacterial removal and phagocytosis 8. Although multiple processes protect lungs when bacteria enter, ASL antimicrobials may be key to rapidly reducing numbers of viable organisms and thereby decreasing the probability that they will replicate, escape other mechanisms, and colonize the lung.
Aerosolizing HCO3− onto CF airways in vivo increased bacterial killing. Our mechanistic findings and this result suggest that correcting ASL pH might prevent the initial CF infection. That might be accomplished by delivering HCO3− into airways, altering pH regulation by airway epithelia, enhancing activity of ASL antimicrobials, delivering pH-insensitive antimicrobials, or targeting mutant CFTR. These results also suggest that adapting the bacteria-coated grid method to assay bacterial killing in patients could be useful for assessing potential therapies. We also speculate that increasing ASL pH might prevent or treat airway infections in other diseases.
METHODS SUMMARY
The Supplementary Information provides full descriptions of experimental methods and procedures.
METHODS
CFTR−/− and CFTR+/+ pigs
We previously reported generation of CFTR−/− pigs 7,31. Animals were produced by mating CFTR+/− male and female pigs. Newborn littermates were obtained from Exemplar Genetics. Animals were studied 8–15 hours after birth. Euthanasia was with IV Euthasol (Virbac). The University of Iowa Animal Care and Use Committee approved all animal studies.
Preparation of differentiated primary cultures of airway epithelia
Epithelial cells were isolated from the trachea and bronchi by enzymatic digestion, seeded onto collagen-coated, semi-permeable membranes (0.6 cm2 Millicell-PCF; Millipore, Bedford, MA), and grown at the air-liquid interface as previously described 12,32. Culture medium, a 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium (DMEM/F12), was supplemented with 2% Ultroser G (PALL France SAS; Saint Germain-en-Laye, France). Differentiated epithelia were used at least 14 days after seeding.
Bacteria-coated grids
Gold grids (TEM grids, 200 mesh, Ted Pella Inc.) were immersed in 1 mM 11-mercaptoundecanoic acid (HS(CH2)10COOH, MUA, Aldrich) solution for 30 min at room temperature and then exposed to 1-ethyl-3-(3-diethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS) (molar ratio 1:2) for 30 min to activate the carboxyl groups of MUA. Grids were then placed in a phosphate buffer solution (PBS), pH 7.4, containing 10 µg/ml streptavidin (Sigma) for 30 min and then rinsed with PBS. Streptavidin-coated grids were immersed in 1 M glycine for 30 min to quench the reaction and then rinsed with PBS. We used S. aureus isolate 43SA (isolated from a CF pig with pulmonary infection 8), and P. aeruginosa PAO1. Molecular typing and antibiotic susceptibility profiling of S. aureus isolate 43SA was performed using standard methods. We determined that the 43SA strain is methicillin-sensitive and belongs to sequence type 398 (ST398), which is very common in pigs and often transferred to farmers 33. Bacteria were cultured to log phase growth and 1×108 bacteria were mixed with 0.1 mg/ml N-hydroxysulfosuccinimide (sulfo-NHS)-biotin (Thermo Scientific) for 1 h at room temperature. Streptavidin-coated grids were then incubated with biotinylated bacteria for 30 min and rinsed in PBS.
Bacteria-coated grids were used in three preparations. They were placed on the airway surface in vivo in pigs. They were tested in ASL removed from pigs after methacholine stimulation. They were also placed on the apical surface of primary cultures of differentiated airway epithelia. In each case, after removal from ASL, they were immediately (2–3 sec) rinsed with PBS and then immersed in PBS containing the fluorescent indicators SYTO 9 and propidium iodide (Live/Dead BacLight™ Bacterial Viability assay, Invitrogen). Propidium iodide staining and this assay indicate the percentage of dead cells (Invitrogen, Molecular Probes product information and Fig. S7). After 15 min, the grids were rinsed with PBS and placed on slides for imaging with a laser scanning confocal microscope (Olympus FV1000). For each experiment, we tested bacteria-coated grids that were immersed in saline rather than exposed to ASL; the percentage of dead bacteria was 2–6%. In Fig. 1d and 1g, the shortest exposure to ASL was 30 sec. When bacteria-coated grids were placed on the airway surface of wild-type pigs in vivo for a shorter time (5 sec), 15±2% (n=4) of the bacteria were dead. Of note, a study using high-speed atomic force microscopy found that antimicrobial proteins disrupted the bacterial surface at variable rates ranging from ~10 sec to minutes 34. During development of the bacteria-coated grid assay, we assessed other methods of labeling bacteria including use of bacteria expressing GFP and bacteria labeled with streptavidin-Alexa Fluor 647, other substrates including fibers and planar films, and other methods of attaching bacteria including antibody and protein A. The methods we adopted proved to be the most reliable for detecting live and dead bacteria.
For each experimental condition, time point, and/or animal, 2 or 3 grids were examined. For each grid, all live and dead bacteria were counted in 5–16 individual microscopic (60X) fields; each microscopic field contained ~100–1000 bacteria. The percentages of dead bacteria in each field were then averaged to determine the percentage of dead bacteria for the experimental data point. Operators were blinded to genotype. In some experiments, two different operators obtained similar results.
The total number of bacteria (live plus dead) attached to grids varied from experiment to experiment. For an individual grid, the number of bacteria per microscopic field also varied from field to field with an average coefficient of variation of 0.56 for grids applied to ASL in vivo and in vitro. Figure S6 shows the average number of bacteria per microscopic field for the data in Fig. 1d and 1g. The total number of bacteria (live plus dead) in randomly selected microscopic fields did not differ between grids exposed to CF or non-CF ASL or in conditions where many or a few bacteria were killed. In addition, the total number of bacteria per microscopic field (live plus dead, mean±SD) did not differ for bacteria exposed for 30 sec to water (232±94 total bacteria per field, 4.6% dead), saline (230±103 total bacteria per field, 4.3% dead) or 70% alcohol (228±108 total bacteria per field, 97% dead).
Bacteria-coated grids were also prepared for scanning electron microscopy using standard procedures. Briefly, the grids were fixed in 2.5% gluteraldehyde in 0.1M cacodylate buffer followed by post fixation in 1% osmium tetroxide. The grids were then dehydrated in a graded series of ethanol, transitioned to hexamethyldisilizane, air dried overnight and mounted on aluminum stubs. Following sputter coating with gold/palladium, the samples were imaged in a Hitachi S-4800 scanning electron microscope (Pleasanton, CA).
Micro-CFU and radial diffusion assays
We used S. aureus strain 43SA and strain E. coli pCGLS-1. Micro-CFU assays were performed as previously described 35. When ASL was collected in vivo, each micro-CFU assay contained an initial inoculum of approximately 1×106 CFU/ml. When ASL was collected from cultured epithelia, each micro-CFU assay had an initial inoculum of approximately 3.3×103 CFU/ml. Radial diffusion assays with 10 mM sodium phosphate, pH 7.4 in 1% agarose were performed with a final O.D. of bacteria of 0.02 as previously described 36.
Collection of ASL for protein and antimicrobial studies
ASL was collected from pigs anesthetized with ketamine (20 mg/kg, IM) and xylazine (2 mg/kg, IM), and maintained with propofol (2 mg/kg, IV). The neck was dissected to expose the trachea. Tracheal secretion was stimulated by administering methacholine (2.5 mg/kg, IV). After approximately 5 min, tracheal secretions were collected by making a small incision in the anterior tracheal wall and inserting a sterile polyester tipped applicator (Puritan Medical Products Co.; Guilford, ME, USA) to collect ASL. The probe was then inserted into a microcentrifuge tube and secretions were recovered by centrifugation. This procedure produced approximately 10–20 µL of ASL fluid from each animal. For assays of ASL proteins, samples were immediately placed on ice and frozen at −80°C until use. For assays of antimicrobial activity, samples were used immediately.
ASL was also collected from primary cultures by rinsing the apical surface with 100 µL H2O. Based on our earlier work 20, we estimate that collection produced an approximate 1:80 to 1:125 dilution of ASL.
Measurement of amounts of ASL antimicrobial proteins
To immunoblot for lactoferrin, PLUNC, and SP-A, 10 µl of a 1:10 dilution of the ASL was separated on 4–15% Tris-HCl gels and transferred to PVDF membranes, followed by blocking in TBS-Tween containing 2% BSA. Membranes were incubated with a primary antibody (rabbit anti-human lactoferrin, cat#RLAC-80A, Immunology Consultants; monoclonal anti-human PLUNC, cat.# MAB1897, R&D Systems; or polyclonal antisera against porcine SP-A 37). Membranes were washed 4 times using TBS-Tween, then incubated with secondary antibody conjugated to horseradish peroxidase (Thermo Fisher Scientific; Rockford, IL, USA) at a 1:20,000 dilution for 1 hr. After 5 more washes in TBS-Tween, protein bands were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). Membranes were then exposed to film and densitometry performed. The same ASL samples were used for each western blot and stripped between westerns with Restore Western Blot Stripping Buffer (Thermo Scientific).
Lysozyme was measured using lysoplates as previously described 36. Undiluted methacholine-stimulated secretions (5 µl) were used and compared to a standard of human lysozyme (L1667, Sigma, St. Louis, MO).
Collection and determination of ASL Na+ and K+ concentrations
Pigs were anesthetized with ketamine (20 mg/kg, IM) and xylazine (2 mg/kg, IM), followed by propofol (2 mg/kg, IV). The trachea was surgically exposed and accessed anteriorly using heat cautery. We then made a small anterior incision through the tracheal rings using heat cautery to prevent bleeding. To ensure that air was completely humidified the animal was studied in a humidified chamber (100% relative humidity, 25–30°C).
We collected ASL using a procedure designed to minimize the generation of excessive capillary forces during sampling. We fused thin lens paper (VWR Scientific Products, West Chester, PA) with Parafilm M (Pechiney Plastic Packaging, Menasha, WI) in an oven (205°C) for 70–90 sec. This procedure further reduced the volume of liquid that the paper would absorb and minimized evaporation from the surface not touching ASL. We prepared 0.5×2 cm strips, washed them 3 times in double distilled water, and dried them overnight at 40°C.
The Parafilm-fused paper strips were weighed and then gently placed in contact with the luminal surface of the posterior trachea for 15 sec. Immediately after removal from the trachea, Parafilm-fused paper strips were placed (paper side up) on a precision balance (Mettler-Toledo XP26DR; Columbus, OH). Mass measurements were recorded by a synchronized computer 10 times per sec for 200 sec (BalanceLink; Mettler-Toledo; Columbus, OH) while evaporation occurred. The amount of ASL collected was determined by plotting mass vs. time, fitting a one-phase exponential decay to the data (GraphPad Prism 5; GraphPad Software, Inc.), and extrapolating to mass at time 0, i.e., the time at which the strip was removed from the airway surface. We then dried the strips overnight at 40°C and measured the dry mass. 3.0 ± 0.5 µl of ASL (n=14) was collected per cm2.
To dissolve the dried ASL contents, the strips were placed in 1 ml flame photometer internal standard solution (Instrumentation Laboratory; Bedford, MA) overnight. We then measured Na+ and K+ content with an IL943 Flame Photometer and compared output to calibration curves of solutions containing known mole contents (Instrumentation Laboratory; Bedford, MA). ASL Na+ and K+ concentrations were calculated by dividing the mole content of cation by the mass of solvent. The mass of ASL solvent was determined as the difference between the initial ASL sample mass and the dry sample mass.
We also measured Na+ and K+ concentrations after methacholine administration (2.5 mg/kg, IV) to stimulate submucosal gland and goblet cell secretion 38. To collect the readily visible secretions, we lightly applied a sterile polystyrene applicator (Puritan Medical Products Co.) to the tracheal surface. The applicator tip was then suspended and sealed in a microcentrifuge tube, and liquid was isolated by centrifugation through a layer of water-saturated oil. We diluted secretions 1:10 in double distilled H2O, added 10 µl to 1 ml of flame photometer Internal Standard, measured Na+ and K+ content by flame photometry, and calculated the ion concentrations.
Measurement of ASL pH
To assess pH in vivo, we used non-invasive dual lifetime referencing to interrogate a 3 × 3 mm planar optode (pH sensitive foil, PreSens GmbH, Regensburg, Germany) 39 applied directly to the tracheal surface. The device used to transmit and receive the excitation and emission light was a single channel pH meter (pH-1 mini; PreSens GmbH). The tip of the fiberoptic pH meter was kept at the same distance from the tracheal surface in all samples and confirmed by recording the amplitude registered by the device. Calibration before each set of measurements was done by placing the planar optode on the surface of a flat filter soaked in standard pH buffers. To minimize alterations in CO2 during placement of the probe, the experiments were done in an environment of 5% CO2; hence the CO2 concentration in ASL was likely >5% due to CO2 production by the pigs. Thus, the pH values are likely lower than normally occur.
To assess pH ex vivo, secretion was stimulated with methacholine, and 5 min later, ASL was removed using the same methods as described for measurement of ion concentrations. Ten min after removal from the pig, ASL pH was measured using a needle-type Fiber Optic pH Meter (World Precisions Instruments Inc., #502123, Sarasota, FL). The pH meter was calibrated before each set of measurements. After removal and during measurement, the CO2 over the sample was ambient, i.e., approximately zero, which likely accounts for the higher pH values in the ex vivo ASL.
To assess pH in primary cultures of airway epithelia, we used the fluorescent ratiometric pH indicator SNARF conjugated to dextran (Molecular Probes Inc., D-3304). SNARF was prepared as a suspension in perfluorocarbon and 200 µl was added to the apical surface. Two hr later, epithelia were studied in a humidified, 5% CO2 atmosphere at 37°C on the stage of an inverted laser scanning microscope (Zeiss 510 Meta NLO). SNARF was excited at 488 nm, fluorescence intensity was measured at 580 nm and 640 nm, and pH was calculated as described 40.
Methods for changing CO2 and for aerosolizing HCO3−
To decrease airway pH in non-CF pigs, CO2 concentration was controlled in a humidified chamber (100% measured relative humidity, 25–30 °C) using 0 or 15% CO2 and 21% O2 in N2. To increase airway pH in CF pigs, 50 µl of either 100 mM NaHCO3 or 100 mM NaCl (as a control) were aerosolized (FMJ-250 and 1A-1C, Penn-Century, Wyndmoor, PA) at 20 cm over the exposed trachea; that approach achieved an average deposition of ~1 µL/cm2 of fluid.
Luminescence antibacterial assay
To assess the effect of pH on the antibacterial activity of lysozyme and lactoferrin, we used a luminescence assay of bacterial viability that has been described previously 30. The bacteria used were S. aureus Xen29 (Caliper LifeSciences Bioware™, Hopkinton, MA) and E. coli DH5α (GIBCO BRL, Life Technologies, Grand Island, NY) expressing a luminescence plasmid pCGLS1. E. coli were grown in Luria-Bertani medium containing ampicillin (100 µg/ml, to maintain the plasmid) at 30°C with shaking. For S. aureus, kanamycin 10 µg/ml was used instead of ampicillin. Log phase bacteria were centrifuged and resuspended in 1% Tryptic Soy Broth medium with 10 mM HEPES titrated to pH values from 6.8–8.0. E. coli (5 × 106 CFU) or S. aureus (5 × 105 CFU) were incubated with the indicated concentrations of lysozyme or lactoferrin in 96-well plates (Optiplate-96, Perkin Elmer, Waltham, MA) in a total volume of 120 µl. Controls were the same conditions in the same plates, but without lysozyme or lactoferrin. After incubation at 30°C for the indicated times, luminescence was measured with a luminometer (Spectra Max L, Molecular Devices, Sunnyvale, CA) and was reported as relative light units (RLU). A previous study 41 determined that reductions in luminescence have an excellent correlation with a decrease in CFU.
Microarray analysis
Trachea and bronchus tissue samples were dissected from newborn piglets within 12 hours of birth. Samples were cut into ~5 mm3 pieces and stored in RNAlater RNA Stabilization Reagent (Ambion; Austin, TX) using the manufacturer’s recommended protocols. Primary cultures of differentiated CF and non-CF tracheal epithelia were prepared as described above. Total RNA was isolated with TRIzol Reagent (Invitrogen; Carlsbad, CA). Only RNA samples attaining a minimum of 7.0 RNA Integrity Number on the Agilent 2100 Bioanalyzer (Agilent Technologies; Palo Alto, CA) were processed. 5 µg of total RNA was used to generate biotinylated cRNA using the Affymetrix Gene Chip one-cycle target labeling kit (Affymetrix, Inc.; Santa Clara, CA) according to the manufacturer’s recommended protocols, and then hybridized to the Affymetrix Porcine GeneChip (23,937 probe sets that interrogate approximately 23,256 transcripts from 20,201 S. scrofa genes). Each sample hybridization (trachea, bronchus, and cultured tracheal epithelia) was performed on a separate day, with all genotypes represented in each run. The arrays were washed, stained, and scanned using the Affymetrix Model 450 Fluidics Station and Affymetrix Model 3000 scanner, and data collected using the GeneChip Operating Software (GCOS), v.1.4, using the manufacturer’s recommended protocols. Partek Genomics Suite Software (Partek Inc., St. Louis, MO)(one-way ANOVA analysis) was used to analyze the data.
Quantitative RT-PCR
Primers to specifically amplify porcine GAPDH, lactoferrin, lysozyme, S100A9, and PBD-2 were designed and validated using standard procedures. Total RNA was harvested using TRIzol (manufacturer’s recommended protocol) from the trachea and bronchus of 6 CFTR+/+ and 6 CFTR−/− pigs and from primary cultures of tracheal epithelia from 8 CFTR+/+ and 8 CFTR−/− pigs. Reverse transcription with 1 µg total RNA was performed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). Ten nanograms of cDNA and 50 picomoles of both forward and reverse primers were used per reaction for quantitative PCR. POWER SYBR Green PCR master mix (Applied Biosystems) was used for quantification. GAPDH cDNA levels were used to normalize expression of lactoferrin, lysozyme, S100A9, and PBD-2. Fold change was generated using average ΔΔCt values for each genotype. Error bars were generated using standard error of ΔΔCt values of each sample. The primers we used were:
-
GAPDH
Forward: GACTTCGAGCAGGAGATGG
Reverse: GCACGGTGTTGGTGTTGGCGTAGAGG
-
Lactoferrin
Forward: AGCCATCGCTACCTGAAACATGC
Reverse: ATCATGAAGGCACAGGCTTCCAG
-
Lysozyme
Forward: TGCAAAGAGGGTTGTCAGAG
Reverse: AAGAGACAAGGTGAGCTGAAG
-
S100A9
Forward: TCAGCCAGAGCCCTATAAATGCTG
Reverse: TCTTCCTGCACTCTGTCCAAGC
-
PBD-2
Forward: GGATTGAAGGGACCTGTTACAG
Reverse: GCAAATACTTCACTTGGCCTG
Statistical analysis
Data are presented as means ± standard errors of the mean (SEM). Unless otherwise indicated, statistical analysis used an unpaired t test. Differences were considered statistically significant at P < 0.05.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jennifer Bartlett, Xitiz Chamling, Jeng-Haur Chen, Lakshmi Durairaj, Nick Gansemer, Emma Hornick, Pamela Hughes, Paula Ludwig, Theresa Mayhew, Kelli Mohn, Lynda Ostedgaard, Michael Rector, Leah Reznikov, Lindsey Schneider, Annie Shelton, Tim Starner, Ping Tan, Alex Tucker, Ameya Walimbe, and Timothy Yahr for assistance and/or discussion. This work was supported by the NIH (HL51670, HL091842, HL102288) and the Cystic Fibrosis Foundation. DAS was supported by AI076671 and Gilead Sciences Research Scholars Program in Cystic Fibrosis. HPH was supported by Program Grant (RGP001612009-C) of the Human Frontier Science Program. MJW is an HHMI Investigator. MJW was co-founder of Exemplar Genetics, a company licensing materials and technology related to this work.
Footnotes
CONTRIBUTIONS
Designed studies: AAP, XXT, MJH, MHAA, SR, DAS, PBM, MJW, JZ. Performed experiments: AAP, XXT, MJH, MHAA, SR, TOM, PHK, CLW, HPH, MvE, BB, ARH, DAS. Wrote the manuscript: AAP, XXT, MJH, MHAA, SR, DAS, PBM, MJW, JZ.
REFERENCES
- 1.Welsh MJ, Ramsey BW, Accurso F, Cutting GR. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver CR, et al., editors. McGraw-Hill; 2001. pp. 5121–5189. [Google Scholar]
- 2.Quinton PM. Role of epithelial HCO3− transport in mucin secretion: lessons from cystic fibrosis. Am. J. Physiol. Cell Physiol. 2010;299:C1222–C1233. doi: 10.1152/ajpcell.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 4.Wine JJ. The genesis of cystic fibrosis lung disease. J. Clin. Invest. 1999;103:309–312. doi: 10.1172/JCI6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guggino WB. Cystic fibrosis and the salt controversy. Cell. 1999;96:607–610. doi: 10.1016/s0092-8674(00)80570-x. [DOI] [PubMed] [Google Scholar]
- 6.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am. J. Physiol. Cell Physiol. 2006;291:C218–C230. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 7.Rogers CS, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoltz DA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J. Membr. Biol. 2006;211:139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J. Clin. Invest. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J-H, et al. Loss of anion transport without increased sodium absorption characterize newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garnett JP, et al. Novel Role for Pendrin in Orchestrating Bicarbonate Secretion in Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)-expressing Airway Serous Cells. J. Biol. Chem. 2011;286:41069–41082. doi: 10.1074/jbc.M111.266734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol. Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 15.Foundation CF. Cystic Fibrosis Foundation Patient Registry: Annual Data Report 2010. Bethesda, MD: 2010. [Google Scholar]
- 16.Ganz T. Antimicrobial polypeptides. J. Leukoc. Biol. 2004;75:34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- 17.Wine JJ, Joo NS. Submucosal glands and airway defense. Proc. Am. Thorac. Soc. 2004;1:47–53. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- 18.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 19.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 20.Zabner J, Smith JJ, Karp PH, Widdicombe JH, Welsh MJ. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol. Cell. 1998;2:397–403. doi: 10.1016/s1097-2765(00)80284-1. [DOI] [PubMed] [Google Scholar]
- 21.Goldman MJ, et al. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 22.Matsui H, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 23.Tarran R, et al. The CF salt controversy: in vivo observations and therapeutic approaches. Mol. Cell. 2001;8:149–158. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 24.Knowles MR, et al. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J. Clin. Invest. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selsted ME, Szklarek D, Lehrer RI. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect. Immun. 1984;45:150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am. J. Physiol. Cell. Physiol. 2006;290:C741–C749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 27.Coakley RD, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. U. S. A. 2003;100:16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laube DM, Yim S, Ryan LK, Kisich KO, Diamond G. Antimicrobial peptides in the airway. Curr. Top. Microbiol. Immunol. 2006;306:153–182. doi: 10.1007/3-540-29916-5_6. [DOI] [PubMed] [Google Scholar]
- 29.Do TQ, et al. Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. J. Immunol. 2008;181:4177–4187. doi: 10.4049/jimmunol.181.6.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am. J. Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
METHODS REFERENCES
- 31.Rogers CS, et al. Production of CFTR null and ΔF508 heterozygous pigs by AAV-mediated gene targeting and somatic cell nuclear transfer. J. Clin. Invest. 2008;118:1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karp PH, et al. Wise C. Epithelial Cell Culture Protocols. Vol. 188. Humana Press, Inc.; 2002. pp. 115–137. [Google Scholar]
- 33.Smith TC, et al. Methicillin-resistant Staphylococcus aureus MRSA strain ST398 is present in midwestern U.S. swine and swine workers. PLoS ONE. 2009;4:e4258. doi: 10.1371/journal.pone.0004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fantner GE, Barbero RJ, Gray DS, Belcher AM. Kinetics of antimicrobial peptide activity measured on individual bacterial cells using high-speed atomic force microscopy. Nat. Nanotechnol. 2010;5:280–285. doi: 10.1038/nnano.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole AM, Wu M, Kim YH, Ganz T. Microanalysis of antimicrobial properties of human fluids. J. Microbiol. Methods. 2000;41:135–143. doi: 10.1016/s0167-7012(00)00140-8. [DOI] [PubMed] [Google Scholar]
- 36.Lehrer RI, Rosenman M, Harwig SSL, Jackson R, Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods. 1991;137:167–173. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 37.van Eijk M, et al. Porcine pulmonary collections show distinct interactions with influenza A viruses: role of the N-linked oligosaccharides in the carbohydrate recognition domain. J. Immunol. 2003;171:1431–1440. doi: 10.4049/jimmunol.171.3.1431. [DOI] [PubMed] [Google Scholar]
- 38.Singer M, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat. Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 39.Blossfeld S, Gansert D. A novel non-invasive optical method for quantitative visualization of pH dynamics in the rhizosphere of plants. Plant Cell Environ. 2007;30:176–186. doi: 10.1111/j.1365-3040.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 40.Shuba YM, Dietrich CJ, Oermann E, Cleemann L, Morad M. Local extracellular acidification caused by Ca2+-dependent exocytosis in PC12 cells. Cell Calcium. 2008;44:220–229. doi: 10.1016/j.ceca.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Travis SM, et al. Activity of abundant antimicrobials of the human airway. Am. J. Respir. Cell Mol. Biol. 1999;20:872–879. doi: 10.1165/ajrcmb.20.5.3572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.