Abstract
BACKGROUND
Previous studies have suggested that practicing functional tasks bilaterally instead of unilaterally may improve paretic limb performance after stroke.
OBJECTIVE
The purposes of this study were to determine whether the bilateral movement condition alters paretic limb performance of a functional task in people with post-stroke hemiparesis, and to identify specifically which parameters of performance may be affected.
METHODS
In this single-session study, we examined immediate effects of the bilateral vs. unilateral movement condition on performance of a reach-grasp-lift-release task at preferred-speed in 16 people with mild to moderate post-stroke hemiparesis and in 12 healthy controls. Performance was quantified using motion analysis variables, including durations of the reach and grasp phases, reach path straightness, maximum thumb-index finger aperture, efficiency of finger movement, peak grip force, and timing of release.
RESULTS
We found no evidence of immediate improvement in paretic-limb performance in the bilateral condition. In both groups, release timing occurred later when participants moved bilaterally instead of unilaterally, possibly representing a divided-attention effect. Other variables did not differ across conditions.
CONCLUSIONS
Our findings suggest little immediate impact of the bilateral condition on motor performance of a reach-grasp-lift-release task at preferred speed, in people with mild to moderate hemiparesis.
Keywords: motor control, hemiparesis, kinematic, reach, grasp, bilateral
INTRODUCTION
Advances in restorative neuroscience have revealed immense potential for use-dependent neural adaptation after stroke and have renewed the search for training methods that optimize recovery. For people with hemiparesis, although task-specific repetitive practice is clearly a key stimulus to promote motor learning, the choices of what to practice and how to practice remain challenging and are poorly guided by evidence. Current standards of care for post-stroke rehabilitation include sensorimotor training,1 but optimal methods to implement such training remain debatable, and the effects of altering specific characteristics of task performance are largely unknown.
Most therapeutic activities that constitute traditional post-stroke rehabilitation involve the use of the paretic arm by itself, or the use of both arms in a complementary fashion, either symmetrically or, more often, asymmetrically.2 Constraint-induced movement therapy emphasizes unilateral task performance by the paretic limb and intentionally limits participation by the non-paretic limb, in order to minimize learned non-use.3, 4 In contrast, several bilateral training paradigms have been developed5–9, each emphasizing repetitive movement practice using both upper limbs simultaneously, and involving the non-paretic limb for the purpose of facilitating paretic-limb performance.
One form of bilateral training involves repetitive, whole-arm practice of functional tasks that include reaching, grasping, moving or manipulating, and releasing objects. Tasks are practiced in a simultaneous symmetrical or reciprocal manner, such that movements of the two upper limbs occur separately and mirror each other. This differs from typical bilateral upper limb use in daily life, which often involves asymmetry and distinct, yet complementary, functional contributions by the two sides.
Interest in bilateral functional task training began more than a decade ago, based on a case series of twelve people with post-stroke hemiparesis.6, 10 After several sessions of practicing multijoint upper extremity tasks with only the paretic upper extremity, participants began practicing the same tasks using both upper extremities simultaneously and symmetrically. Raters quantified motor impairment by visually analyzing video recordings of task performance and rating various movement characteristics, each on a five-point scale. The observational scale included assessments of “joint ranges at the point of reaching the target, straightness and smoothness of the trajectory, accuracy of targeting, synchrony of limb parts, quality of grasp and presence of extraneous movements”.6 After switching from unilateral to bilateral training, each participant showed improved movement quality by the end of the first session, as well as an increased rate of improvement over multiple training sessions.
Since those initial findings were reported, three studies of bilateral functional task training have shown gains on the Fugl-Meyer Upper Extremity Test,11–13 and two have shown improved paretic-limb kinematics during the reaching phase of task performance.12, 13 Several other studies, however, have shown either no gains following bilateral functional task training,14, 15 or no differences in outcomes across unilateral vs. bilateral training groups.16–18 Results of two studies suggest that bilateral training may improve proximal joint motion more than unilateral training does, and therefore may be more beneficial for certain individuals.11, 19 Overall, outcome studies of bilateral functional task training have produced mixed results.
Within-session comparisons of unilateral versus bilateral task performance have identified reaching velocity as an aspect of movement quality that may be most likely to benefit from bilateral training. In three studies of reaching movements (without grasping), paretic arm peak velocity was greater in the bilateral movement condition, in people with mild or moderate post-stroke hemiparesis.20–22 Cunningham et al.23 reported that three of six people with chronic mild hemiparesis showed fewer discontinuous reach trajectories in bilateral movement trials compared to those performed unilaterally. Messier et al.,24 however, showed no improvement in shoulder or elbow joint excursion during bilateral reaching, and the bilateral condition was instead associated with increased compensatory trunk flexion. A more complete characterization of differences between bilateral and unilateral task performance is needed to determine whether effects are limited to the reaching phase, or if grasping performance may also benefit from the bilateral condition.
The purposes of this study were to determine whether the bilateral movement condition itself, in the absence of training, alters paretic limb performance of a functional task in people with post-stroke hemiparesis. Further, we sought to identify specifically which parameters of performance may be affected. We hypothesized that upper extremity movements performed bilaterally would be associated with faster and more direct reaching, more efficient finger movement, increased separation of the thumb and index finger, faster grasp formation and release, and grip force closer to that of healthy controls, compared to movements performed unilaterally. Using three dimensional motion analysis methods, we assessed timing, movement, and grip force during a reach-grasp-lift-release task that was chosen to represent movements commonly performed in daily life, in rehabilitation, and in outcome studies of bilateral functional task training. Two grip types were included, and a group of healthy adults provided control data for comparison.
METHODS
Participants
Sixteen people with hemiparesis due to stroke were recruited from the St. Louis metropolitan area via the Brain Recovery Core Database and the Cognitive Rehabilitation Research Group Stroke Registry at Washington University, and from local support groups for people with stroke. Potential participants were included if they 1) had a diagnosis of ischemic or hemorrhagic stroke by a stroke neurologist, 2) had persistent hemiparesis, as evidenced by upper extremity Medical Research Council muscle test scores 25 that were at least one muscle grade lower on the paretic side compared to the non-paretic side, 3) were able to reach, grasp and lift a vertical cylinder (3.4 cm diameter, 420 grams) using palmar and 3-finger grip types, as necessary to complete the study procedures, and 4) had the ability to follow 2-step commands. We excluded people who 1) had severe aphasia as indicated by a score of 2 or 3 on the Best Language item of the National Institutes of Health Stroke Scale (NIHSS) 26, 2) had severe hemispatial neglect, as indicated by a score of 2 on the Extinction and Inattention item on the NIHSS, 3) had musculoskeletal or other medical conditions besides stroke that limited either upper extremity, or 4) were unable to give informed consent.
Twelve healthy adults also participated, primarily to provide reference data needed to interpret results in the hemiparetic group. Volunteers who had no known neurological disease and no disability or injury affecting their upper extremity on either side were recruited from the Volunteer for Health Research Participant Registry at Washington University. To evenly represent both genders across the age range, we recruited one male and one female within each decade between 30 and 89 years of age. This study was approved by the Washington University Human Research Protection Office, and all participants gave their informed consent prior to beginning the study.
Clinical Assessments
Clinical tests were used to describe the participants with post-stroke hemiparesis (Table 1). We assessed upper extremity function using the Action Research Arm Test (ARAT) on the affected side27–29 and the Activities of Daily Living and Hand Function domains of the Stroke Impact Scale, version 3.0.30, 31 Maximum grip strength was measured on each side using a Jamar grip dynamometer in its second position.32, 33 Maximum pinch strength was measured on each side with a Jamar hydraulic pinch gauge positioned between the thumb and the lateral side of the index finger’s middle phalanx.34, 35 Sensation on the palmar surface of the distal index finger was evaluated using Semmes-Weinstein monofilaments.36 Spasticity of the elbow flexors was assessed on the affected side using the Modified Ashworth Scale.37 When possible, medical records were reviewed to identify lesion locations.
Table 1.
Characteristics of participants with hemiparesis
| Age (years) | 58 ± 11 (38–88) |
|---|---|
| Gender | 9 Male, 7 Female |
| Tested side | 10 Dominant 6 Non-dominant 8 Right 8 Left |
| Type of stroke | 14 Ischemic 2 Hemorrhagic |
| Years since stroke | 1.2 ± 2.7 (0.04–9.2) |
| Grip strength (paretic side in kg) | 21.8 ± 8.9 (10.0–36.0) |
| Grip strength (paretic side as % of non-paretic side) | 67 ± 27% (34–113) |
| Pinch strength (paretic side in kg) | 5.2 ± 2.3 (0–8) |
| Pinch strength on paretic side (paretic side as % of non-paretic side) | 65 ± 27% (0–114) |
| Sensation a | 2.83 n = 7 3.61 n = 5 4.31 n = 2 6.65 n = 2 |
| Spasticity b | 0 n = 8 1 n = 7 2 n = 1 |
| Action Research Arm Test on paretic side c | 41 ± 9 (24–53) |
| Stroke Impact Scale d Activities in a Typical Day Subscale | 62 ± 15 (43–88) |
| Stroke Impact Scale d Hand Function Subscale | 49 ± 22 (0–85) |
Mean ± 1 standard deviation (range)
Size of the smallest Semmes Weinstein monofilament sensed in 3 of 5 trials on the anterior distal index finger on the paretic side
Modified Ashworth Scale score for the elbow flexors on the paretic side
Range of possible scores is 0 to 57, 57 = normal
Range of possible scores is 0 to 100, 100 = normal
Task and Movement Conditions
We investigated immediate effects of the bilateral movement condition on performance, by comparing across unilateral versus bilateral conditions within a single session. Thus, no effects of training were evaluated. Upper extremity movement and grip force were measured during reach-grasp-lift-release movements in bilateral and unilateral conditions using palmar and 3-finger grip types. In order to represent the bilateral functional task training paradigm, the task included all movement phases (reach, grasp, lift and release) and was performed simultaneously and symmetrically, at the participant’s self-selected, comfortable speed. Palmar and 3-finger grip types were chosen because they have been well characterized as two discrete patterns of prehension with different levels of accuracy and precision, because neural control of the two grip types may differ, and because they represent a range of actions observed in daily life.38–41 We tested the paretic upper extremity of participants with hemiparesis, and one randomly selected side for control participants.
Apparatus
The target object that was grasped by the tested side (Figure 1a) consisted of a custom-fabricated vertical cylinder (3.4 cm diameter, 11.3 cm height) attached to a rectangular base (13.5 cm by 6 cm) that was designed to hold a Tekscan I-scan electronic interface (Tekscan, Inc. South Boston, MA). The cylindrical portion of the object was covered with a Tekscan pressure sensor (11.2 × 11.2 cm, 1936 sensels, spatial resolution 15.5 sensels/cm2). Combined weight of the object and electronics was 420 grams. An identical object without the pressure sensor was grasped by the non-tested side during bilateral movement trials.
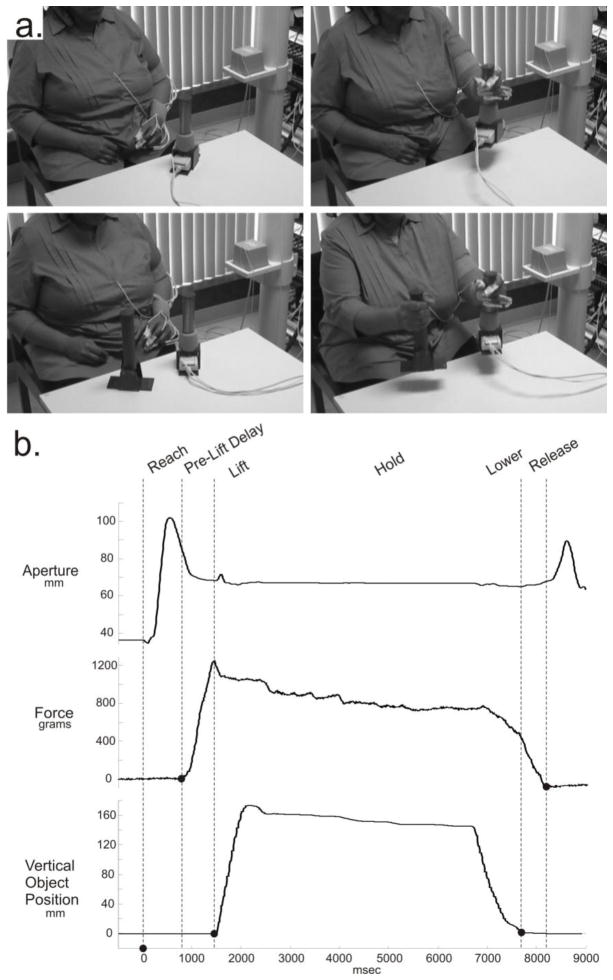
Fig. 1.
Assessment of motor performance. a) Illustration of the experimental set-up and a participant performing the reach-grasp-lift-release task with a palmar grip in unilateral (upper row), and bilateral (lower row) conditions. b) Example data from one trial. Vertical dashed lines demonstrate division of the task into movement phases, as described in the ‘Analysis’ section.
Measurement of grip force is a novel use of pressure sensor technology. This method was chosen instead of a more typical strain gauge system because it does not require that participants place their hand or fingers on specific locations, and instead allows for more natural grasping performance. A disadvantage of the pressure sensor system is that it only measures grip forces (normal forces) and is unable to measure load forces (tangential or shear forces). For use in this study, the advantage of capturing natural movements outweighed the disadvantage of limiting our force analysis to grip (i.e. normal) forces.
Experimental Procedures
Participants were seated in a chair with back support for all data collection (Figure 1). To prepare for motion capture using an electromagnetic tracking system (The MotionMonitor, Innovative Sports Training, Chicago, IL), nine sensors were attached to the trunk and upper extremity, as follows: 1) trunk: midline below the sternal notch, 2) upper arm: proximal to the lateral epicondyle, bisecting the upper arm mass, 3) forearm: midpoint between the radial and ulnar styloids on the dorsum of the forearm, 4) hand: midpoint of the third metacarpal on the dorsum of the hand, and 5 through 9) thumb and fingers: on the nail of each digit. One additional sensor was attached to the object, at the base of the cylindrical portion.
A table was placed with its closest edge across the participant’s mid-thighs and the height was adjusted to be as low as possible without contacting the thighs, in order to allow clearance of the table edge while reaching. The object was placed on the table at a standardized distance from the participant (90% of the length of the arm from shoulder to wrist). In the frontal plane, the object was aligned with the mid-clavicle. For bilateral trials, these criteria also determined placement of the non-instrumented object placed on the opposite side.
Four trial types were collected in random order, each characterized by the unilateral or bilateral movement condition and by the type of grip (i.e. palmar unilateral, palmar bilateral, 3-finger unilateral, and 3-finger bilateral). Prior to each trial, the participant was instructed to rest both hands in their lap with thumb and fingers together, wait for the word ‘go’, then grasp and lift the object(s), hold the object(s) above the table for about 5 seconds until the examiner said ‘done’, then put the object(s) down and return to the starting position. Further verbal instructions and demonstrations were also provided regarding grip type and the bilateral or unilateral movement condition. Before bilateral trials, participants were asked to perform the task with both arms at the same time. In order to capture natural performance, however, no instructions were provided regarding speed, or where vision and/or attention should be focused. Throughout each trial, three-dimensional movements of the tested upper extremity and the target object were recorded at 50 Hz, and pressure on the target object was recorded at 100 Hz. Three trials of each type were recorded consecutively, with approximately 10 seconds of rest between trials.
Analysis
Pressure data were converted to grams of force, using Tekscan software to multiply recorded pressure by the sensor’s spatial area. After low-pass filtering of kinematic data at 6 Hz using a second-order Butterworth filter, sensor position data were extracted using MotionMonitor software (Innovative Sports Training, Chicago, IL). Subsequent analysis was then completed using custom software written in MATLAB (The MathWorks, Inc., Natick, MA).
Durations of movement phases were determined based on hand velocity, force on the object, and object position, as follows (Figure 1B). The reach phase began when velocity of the hand sensor first exceeded 5 mm/s, and ended when force on the object first exceeded 5 grams. Pre-lift delay began at the end of the reach, and ended when the vertical position of the object increased by 3 mm from its initial value. Release timing was calculated as the difference between the time when the object returned to within 3 mm of its initial vertical position, and the time when force on the object returned to within 5 grams of its baseline value. In some cases, force returned to baseline prior to the object reaching a final stable position. In these cases, the release timing variable was negative, indicating release of the object before it was placed securely on the table. In other cases, the object reached a stable position before force returned to baseline, yielding a positive value for the release timing variable. Note that finger aperture was not used to indicate release because, as seen in the aperture trace at the top of Figure 1B, the majority of finger opening occurred after force was removed from the object.
Other variables of interest included reach path ratio, peak aperture, aperture path ratio, and peak grip force. Reach path ratio was defined as the length of the actual path of the forearm sensor (just proximal to wrist joint) during the reach phase, divided by the length of a straight line path. A reach path ratio close to one indicates a straight, direct reach, and a value greater than one indicates greater curvature of the reach path. Peak aperture was the maximum three-dimensional distance between sensors on the thumbnail and the index fingernail during the reach phase. Aperture path ratio quantified the smoothness/efficiency of thumb and index finger movement during the reach phase, and was calculated as follows (modified from Lang et al.42, 43):
An aperture path ratio equal to one indicates smooth and direct separation of the thumb and index finger to the maximum aperture value, followed by smooth and direct closing onto the object. Higher values indicate abnormal, inefficient opening and closing of the thumb and index fingers, typically seen when participants make multiple attempts to open their hand and then close it on the object. Peak grip force was defined as the maximum force applied to the object. Reliability of kinematic reaching variables has been shown to be adequate in healthy individuals and people with post-stroke hemiparesis.44, 45 In a recent evaluation of a reach-to-grasp task resembling the task used in the current study, Patterson et al.46 reported excellent reliability for reach duration, reach path ratio, and peak aperture (r > 0.75) in a group of people with hemiparesis after stroke.
Variables were calculated separately for each trial. Each participant’s performance in each movement condition was represented by the mean of three trials. Kolmogorov-Smirnov tests were used to determine whether data were normally distributed. Since all data met the normality assumption (p > 0.05), parametric statistics were used. For each variable, we determined effects of movement condition (unilateral vs. bilateral), grip type (3-finger vs. palmar), and group (control vs. hemiparetic) using mixed-effects analysis of variance, where movement condition and grip type were within-subjects factors, and group was a between-subjects factor. Statistica software was used for normality testing and analysis of variance (Version 6.1 Statsoft Inc., Tulsa, OK). The criterion for significance was set at p < 0.05 and the software accounted for unequal numbers in each group.
Statistical power was analyzed for the unilateral vs. bilateral comparisons in the hemiparetic group. Observed effect sizes were calculated using Hedges’ g, which is equal to the mean difference between conditions divided by the pooled unbiased standard deviation. For each variable, the sample size that would have been needed to achieve statistical significance was estimated using G*Power3 software,47 a paired t test design, observed effect sizes, and assumptions that power = 0.80 and 2-tailed alpha = 0.05. Influences of participant characteristics on unilateral vs. bilateral differences were evaluated graphically for each motion analysis variable in the hemiparetic group. Statistical analyses of such effects were precluded by the small sample size.
RESULTS
Characteristics of the 16 participants with hemiparesis are provided in Table 1. Time since stroke ranged from two weeks to nine years, and was less than four months in all except three participants. Severity of sensorimotor impairment and functional limitation ranged from mild to moderate, as shown by the strength measures and scores on the ARAT and SIS assessments. Lesion locations also varied, including three cortical, seven subcortical, four that were both cortical and subcortical, and two unknown.
Twelve healthy adults participated, including six males and six females between 32 and 81 years of age (mean 53.0, SD 15.8 years). Ten were right handed and two were left handed, by self report. Random selection of the side to be tested resulted in seven rights and five lefts. The dominant side was tested in seven participants (six right-handed, one left-handed). Group differences were not significant with respect to age (t-test, p = 0.30), gender (Mann-Whitney U, p = 0.77), hand dominance (Mann-Whitney U, p = 0.62), or dominant/non-dominant status of the tested side (Mann-Whitney U, p = 0.85).
Unilateral vs. bilateral effects
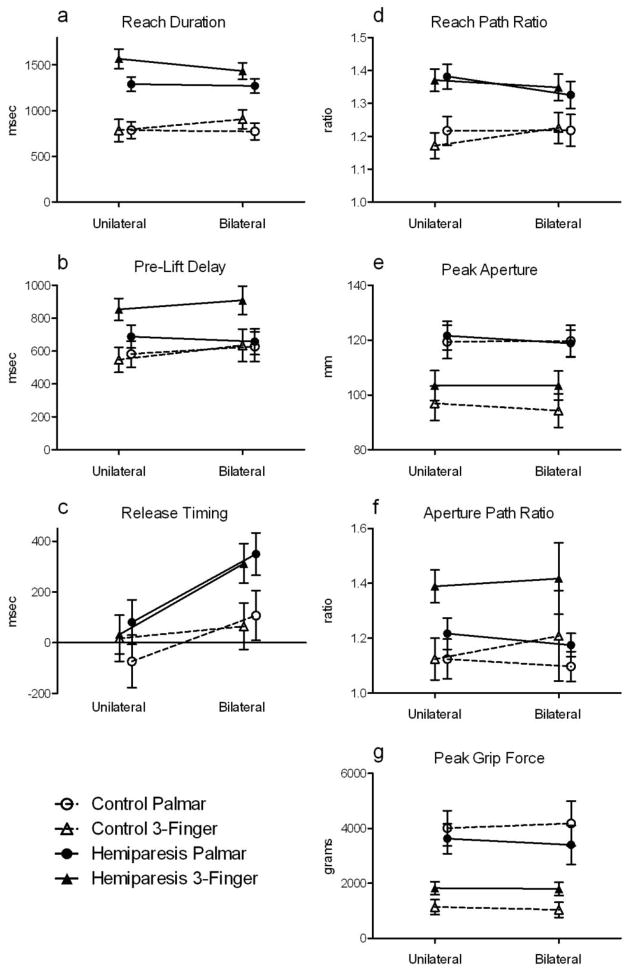
Results for each of the 7 variables are presented in Table 2. The primary comparison of interest for this study (unilateral vs. bilateral performance), was tested via the main effect of condition and the condition by group interaction effect. Contrary to our hypothesis, none of the variables showed improved motor performance in the bilateral condition, in either group. This finding is illustrated graphically in Figure 2A–G. One variable, release timing, was significantly different in the unilateral vs. bilateral conditions (Figure 2C). Participants in both groups released the object later, with respect to when the object contacted the table, when moving bilaterally instead of unilaterally (main effect of movement condition, F(1,26) = 13.1, p < 0.05, g = 0.74). For another variable, reach duration, a significant condition by group interaction was found (F(1,26) = 5.7, p < 0.05). Post-hoc testing, however, failed to find a difference between the unilateral and bilateral conditions for either group (p = 0.17 for the hemiparetic group, p = 0.57 for the control group). No main effects of condition or condition by group interaction effects were found for the other variables.
Table 2.
Effects of unilateral vs. bilateral movement condition, group, and grip type
| Group | Unilateral Palmar | Bilateral Palmar | Unilateral 3-Finger | Bilateral 3-Finger | |
|---|---|---|---|---|---|
| Reach Duration (msec) b c d | Hemiparesis | 1291 ± 80 | 1272 ± 79 | 1570 ± 106 | 1435 ± 89 |
| Control | 787 ± 92 | 774 ± 91 | 785 ± 122 | 906 ± 103 | |
| Pre-Lift Delay (msec) e | Hemiparesis | 688 ± 69 | 657 ± 78 | 853 ± 66 | 908 ± 86 |
| Control | 582 ± 79 | 627 ± 90 | 547 ± 76 | 634 ± 99 | |
| Release timing (msec) a | Hemiparesis | 81 ± 87 | 349 ± 83 | 32 ± 77 | 312 ± 78 |
| Control | −73 ± 103 | 107 ± 98 | 17 ± 91 | 65 ± 92 | |
| Reach Path Ratio c | Hemiparesis | 1.38 ± 0.04 | 1.33 ± 0.04 | 1.37 ± 0.03 | 1.35 ± 0.04 |
| Control | 1.22 ± 0.04 | 1.22 ± 0.05 | 1.17 ± 0.04 | 1.23 ± 0.05 | |
| Peak Aperture (mm) d | Hemiparesis | 122 ± 5 | 119 ± 5 | 103 ± 5 | 103 ± 5 |
| Control | 119 ± 6 | 120 ± 6 | 97 ± 6 | 94 ± 6 | |
| Aperture Path Ratio | Hemiparesis | 1.22 ± 0.06 | 1.17 ± 0.04 | 1.39 ± 0.06 | 1.42 ± 0.13 |
| Control | 1.12 ± 0.07 | 1.10 ± 0.05 | 1.12 ± 0.08 | 1.21 ± 0.16 | |
| Peak Grip Force (grams) d | Hemiparesis | 3625 ± 549 | 3400 ± 707 | 1824 ± 234 | 1798 ± 237 |
| Control | 4007 ± 634 | 4175 ± 816 | 1140 ± 270 | 1036 ± 274 |
Mean ± 1 standard error
Main effect of unilateral vs. bilateral condition, p < 0.05
Condition × group interaction effect, p < 0.05
Main effect of group, p < 0.05
Main effect of grip, p < 0.05
Grip × group interaction effect, p < 0.05
Fig. 2.
Comparisons of reach-grasp-lift-release task performance when moving unilaterally versus bilaterally. Values are means ± 1 standard error. The only variable that differed across the unilateral versus bilateral conditions was release timing (panel c). Participants in both groups released the object later when moving bilaterally instead of unilaterally. Refer to results section for effects of group and grip type.
Observed effect sizes for unilateral vs. bilateral comparisons in the hemiparetic group are presented in Table 3. The only large effects (g ≥ 0.5) were for release timing, which occurred later during bilateral compared to unilateral trials, in both grip types. Effect size was medium (0.3 ≤ g ≤ 0.5) for reach path ratio in palmar grip trials, which tended to be lower in the bilateral condition although the mean difference did not reach significance. The remaining effect sizes were small (0.1 ≤ g ≤ 0.3) or minimal (g < 0.1). For all variables except release timing, a substantially larger sample would be required in order for the observed effects to reach statistical significance (Table 3). Graphs of lesion location, time since stroke, and paretic limb ARAT score versus the unilateral/bilateral difference in each variable showed no effects of participant characteristics.
Table 3.
Post-hoc power analysis for comparisons of unilateral vs. bilateral conditions in participants with hemiparesis
| Palmar | 3-Finger | |||
|---|---|---|---|---|
| Observed Effect Size a | Estimated N b | Observed Effect Size a | Estimated N b | |
| Reach Duration | 0.05 | >1000 | 0.28 | 101 |
| Pre-Lift Delay | 0.12 | 569 | 0.15 | 348 |
| Release timing | 0.80 | 15 | 0.88 | 13 |
| Reach Path Ratio | 0.33 | 73 | 0.13 | 456 |
| Peak Aperture | 0.12 | 521 | 0.00 | na c |
| Aperture Path Ratio | 0.21 | 183 | 0.06 | >1000 |
| Peak Grip Force | 0.12 | 569 | 0.02 | >1000 |
Hedges’ g
Estimated sample size required for observed effect to reach statistical significance at p = 0.05
not applicable, since effect size was 0
Effects of group and grip type
Significant effects of group and grip type are indicated in Table 2. As expected, reach duration and reach path ratio were greater in the hemiparetic group compared to the controls, across unilateral/bilateral conditions and grip types (main effect of group on reach duration F(1,26) = 23.3, p < 0.05, g = 1.36; main effect of group on reach path ratio F(1,26) = 9.0, p < 0.05, g = 1.00). In the hemiparetic group only, pre-lift delay was greater for the 3-finger grip compared to the palmar grip, across conditions (group by grip type interaction F(1,26) = 6.8, p < 0.05, post-hoc p < 0.05 for 3-finger vs. palmar in hemiparetic group only, g = 0.91). Reach duration was greater for the 3-finger grip compared to the palmar grip, across conditions and groups (main effect of grip type F(1,26) = 8.9, p < 0.05, g = 0.60). Peak aperture and peak grip force were greater for the palmar grip compared to 3-finger grip, across conditions and groups (main effect of grip type on peak aperture F(1,26) = 56.1, p < 0.05, g = 1.38; main effect of grip type on peak grip force F(1,26) = 29.6, p < 0.05, g = 0.97).
DISCUSSION
In this single-session study, we found no evidence of an immediate improvement in paretic-limb performance of a reach-grasp-lift-release task when participants with mild-to-moderate hemiparesis moved bilaterally instead of unilaterally. The only significant difference across the unilateral vs. bilateral conditions was for release timing, which occurred later in the bilateral condition. Post-hoc power analysis showed that the observed effects for most variables were small and would not have reached statistical significance unless the sample size was much larger. Large, rapid improvements in paretic limb motor performance, observed visually in the initial case studies of bilateral functional task training,6, 10 were not confirmed in this study using three-dimensional motion analysis methods.
Several possible explanations merit exploration. First, the reach-grasp-lift-release task that was chosen for this study may not have adequately engaged the neural mechanisms involved in interlimb coupling. For example, temporal and spatial symmetry are likely to be enhanced during fast, cyclical bilateral movements that require cooperation of the two limbs.48 Consistent with study purposes, we chose the reach-grasp-lift-release task in order to represent all phases of movements commonly performed in daily life and rehabilitation. Further, we chose to have participants perform the task at their preferred speed and in a parallel fashion (both sides simultaneously but separately), in order to match the methods employed in outcome studies of bilateral functional task training.6, 11–14, 17 The lack of significant differences in our study suggest that, while the bilateral movement condition can influence reaching movements performed under specific contexts, it may have little impact on the functional reach-grasp-lift-release movement studied here.
A second possibility is that benefits of the bilateral movement condition may be limited to reaching performance, with little impact distally. In our study, reach paths tended to be straighter in the bilateral condition in the hemiparetic group, although the difference did not reach significance. We did not see an increase in reach velocity in the bilateral condition, which others have shown,20–22 however that may have been due to differences in speed-related instructions. In each of the previous studies, reaching movements were performed as fast as possible, and in our study participants moved at their natural preferred speed. We extended the investigation of bilateral effects to measures of grasping performance (e.g. pre-lift delay, aperture path ratio, peak aperture, peak grip force), and found no benefit of the bilateral condition on those variables. Our results, combined with previous findings, suggest that the bilateral condition may primarily affect the reaching phase of task performance, and perhaps only in certain movement contexts (e.g. during fast, cyclical motions).
A third possibility is that bilateral training effects emerge over the course of many repetitions within a session and thus are not detectable unless bilateral practice trials precede assessment. Certain forms of experience-dependent neural adaptation (e.g. alterations in cortical excitability, strengthening of pre-existing latent synaptic connections) can occur on a relatively short time scale, but require repeated movement practice.49 Since our study was designed to investigate immediate effects of the bilateral condition itself, and did not explore training effects, we are unable to determine whether multiple repetitions of bilateral reach-grasp-lift-release would have improved paretic limb performance in the bilateral condition by the end of one or more practice sessions. The single-session design of this study limits its implications for rehabilitation. Nevertheless, the substantial single-session improvements in paretic limb movement quality that were observed visually in the initial case studies of bilateral training6, 10 were not confirmed in this study using highly objective methods. Our findings add support to the results of recent clinical trials, which showed equivalent paretic-limb improvements regardless of whether functional task training was performed bilaterally or unilaterally. 16, 17
Across both groups and both grip types, timing of the release relative to object contact with the table occurred later when the task was performed bilaterally. This may represent an effect of divided attention, as participants may have alternated their gaze between the two objects as both were returned to the table and released at the same time. Similar unilateral/bilateral differences in the two groups suggest a normal contextual effect on movement, rather than a stroke-related impairment. Nevertheless, slow, difficult grip release is a common problem experienced by many people with stroke, and this finding suggests that the bilateral movement condition may further impede performance.
Expected effects of grip type were also observed in this study. In both groups, movements using palmar grip were characterized by shorter reach durations, increased peak apertures, and increased peak grip force, as compared to movements using 3-finger grip. These differences are consistent with previous reports and with the fundamental differences between grip types used for power vs. precision (ie. palmar vs. 3-finger).38, 40, 41, 50–52 The only grip effect that differed across groups was for pre-lift delay. In the hemiparetic group only, pre-lift delay was longer during 3-finger grips compared to palmar grips. This may reflect difficulty with dexterity and finger individuation, which are common impairments post-stroke.
CONCLUSION
In this single-session study, we found no evidence that the bilateral movement condition improves paretic limb performance of a reach-grasp-lift-release task at preferred speed. Thus, we cannot infer which characteristics of motor performance may be most likely to change after bilateral training of similar tasks. Combined with previous reports showing improved kinematics when fast reaching movements are performed bilaterally instead of unilaterally, our findings suggest context-specific changes in the bilateral movement condition. Movements that engage the neural mechanisms involved in bilateral coupling (i.e. fast, cyclical proximal limb motions) may be most likely to benefit, while slower, discrete tasks and distal motions may be unaffected. These observations further suggest that bilateral training paradigms may differ in important ways and that evidence related to each should be weighed separately.
Acknowledgments
This work was supported in part by NIH R01HD055964 (CEL), NIH T32HD007434 (SLD), and the Foundation for Physical Therapy (SLD). The authors acknowledge Michael Strube, PhD for statistical advice, and Sydney Schaefer, PhD and Dustin Hardwick, DPT, PhD for assistance with data collection.
References
- 1.Teasell RW, Foley NC, Bhogal SK, Speechley MR. An evidence-based review of stroke rehabilitation. Top Stroke Rehabil. 2003;10:29–58. doi: 10.1310/8YNA-1YHK-YMHB-XTE1. [DOI] [PubMed] [Google Scholar]
- 2.McCombe Waller S, Whitall J. Bilateral arm training: Why and who benefits? NeuroRehabilitation. 2008;23:29–41. [PMC free article] [PubMed] [Google Scholar]
- 3.Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: Implications for rehabilitation. Eura Medicophys. 2006;42:241–256. [PubMed] [Google Scholar]
- 4.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The excite randomized clinical trial. Jama. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 5.Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: Electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33:1589–1594. doi: 10.1161/01.str.0000016926.77114.a6. [DOI] [PubMed] [Google Scholar]
- 6.Mudie MH, Matyas TA. Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disabil Rehabil. 2000;22:23–37. doi: 10.1080/096382800297097. [DOI] [PubMed] [Google Scholar]
- 7.Stinear JW, Byblow WD. Rhythmic bilateral movement training modulates corticomotor excitability and enhances upper limb motricity poststroke: A pilot study. J Clin Neurophysiol. 2004;21:124–131. doi: 10.1097/00004691-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 9.Whitall J, Waller SM, Sorkin JD, Forrester LW, Macko RF, Hanley DF, Goldberg AP, Luft A. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: A single-blinded randomized controlled trial. Neurorehabil Neural Repair. 2011;25:118–129. doi: 10.1177/1545968310380685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mudie MH, Matyas TA. Upper extremity retraining following stroke: Effects of bilateral practice. J Neuro Rehab. 1996;10:167–184. [Google Scholar]
- 11.Lin KC, Chang YF, Wu CY, Chen YA. Effects of constraint-induced therapy versus bilateral arm training on motor performance, daily functions, and quality of life in stroke survivors. Neurorehabil Neural Repair. 2009;23:441–448. doi: 10.1177/1545968308328719. [DOI] [PubMed] [Google Scholar]
- 12.Lin KC, Chen YA, Chen CL, Wu CY, Chang YF. The effects of bilateral arm training on motor control and functional performance in chronic stroke: A randomized controlled study. Neurorehabil Neural Repair. 2010;24:42–51. doi: 10.1177/1545968309345268. [DOI] [PubMed] [Google Scholar]
- 13.Summers JJ, Kagerer FA, Garry MI, Hiraga CY, Loftus A, Cauraugh JH. Bilateral and unilateral movement training on upper limb function in chronic stroke patients: A tms study. J Neurol Sci. 2007;252:76–82. doi: 10.1016/j.jns.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Lewis GN, Byblow WD. Neurophysiological and behavioural adaptations to a bilateral training intervention in individuals following stroke. Clin Rehabil. 2004;18:48–59. doi: 10.1191/0269215504cr701oa. [DOI] [PubMed] [Google Scholar]
- 15.Tijs E, Matyas TA. Bilateral training does not facilitate performance of copying tasks in poststroke hemiplegia. Neurorehabil Neural Repair. 2006;20:473–483. doi: 10.1177/1545968306287900. [DOI] [PubMed] [Google Scholar]
- 16.Desrosiers J, Bourbonnais D, Corriveau H, Gosselin S, Bravo G. Effectiveness of unilateral and symmetrical bilateral task training for arm during the subacute phase after stroke: A randomized controlled trial. Clin Rehabil. 2005;19:581–593. doi: 10.1191/0269215505cr896oa. [DOI] [PubMed] [Google Scholar]
- 17.Morris JH, van Wijck F, Joice S, Ogston SA, Cole I, MacWalter RS. A comparison of bilateral and unilateral upper-limb task training in early poststroke rehabilitation: A randomized controlled trial. Arch Phys Med Rehabil. 2008;89:1237–1245. doi: 10.1016/j.apmr.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 18.Platz T, Bock S, Prass K. Reduced skilfulness of arm motor behaviour among motor stroke patients with good clinical recovery: Does it indicate reduced automaticity? Can it be improved by unilateral or bilateral training? A kinematic motion analysis study. Neuropsychologia. 2001;39:687–698. doi: 10.1016/s0028-3932(01)00005-7. [DOI] [PubMed] [Google Scholar]
- 19.Stoykov ME, Lewis GN, Corcos DM. Comparison of bilateral and unilateral training for upper extremity hemiparesis in stroke. Neurorehabil Neural Repair. 2009 doi: 10.1177/1545968309338190. [DOI] [PubMed] [Google Scholar]
- 20.Harris-Love ML, McCombe Waller S, Whitall J. Exploiting interlimb coupling to improve paretic arm reaching performance in people with chronic stroke. Arch Phys Med Rehabil. 2005;86:2131–2137. doi: 10.1016/j.apmr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 21.McCombe Waller S, Harris-Love M, Liu W, Whitall J. Temporal coordination of the arms during bilateral simultaneous and sequential movements in patients with chronic hemiparesis. Exp Brain Res. 2006;168:450–454. doi: 10.1007/s00221-005-0235-3. [DOI] [PubMed] [Google Scholar]
- 22.Rose DK, Winstein CJ. The co-ordination of bimanual rapid aiming movements following stroke. Clin Rehabil. 2005;19:452–462. doi: 10.1191/0269215505cr806oa. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham CL, Stoykov ME, Walter CB. Bilateral facilitation of motor control in chronic hemiplegia. Acta Psychol (Amst) 2002;110:321–337. doi: 10.1016/s0001-6918(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 24.Messier S, Bourbonnais D, Desrosiers J, Roy Y. Kinematic analysis of upper limbs and trunk movement during bilateral movement after stroke. Arch Phys Med Rehabil. 2006;87:1463–1470. doi: 10.1016/j.apmr.2006.07.273. [DOI] [PubMed] [Google Scholar]
- 25.Platz T, Winter T, Muller N, Pinkowski C, Eickhof C, Mauritz KH. Arm ability training for stroke and traumatic brain injury patients with mild arm paresis: A single-blind, randomized, controlled trial. Arch Phys Med Rehabil. 2001;82:961–968. doi: 10.1053/apmr.2001.23982. [DOI] [PubMed] [Google Scholar]
- 26.Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, Zivin J. Underlying structure of the national institutes of health stroke scale: Results of a factor analysis. Ninds tpa stroke trial investigators. Stroke. 1999;30:2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 27.Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: Properties of the action research arm test. Arch Phys Med Rehabil. 2006;87:1605–1610. doi: 10.1016/j.apmr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
- 30.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 31.Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the stroke impact scale. Stroke. 2002;33:1840–1844. doi: 10.1161/01.str.0000019289.15440.f2. [DOI] [PubMed] [Google Scholar]
- 32.Fess EE. Grip strength. In: Casanova JS, editor. Clinical assessment recommendations. Chicago: American Society of Hand Therapists; 1992. pp. 41–45. [Google Scholar]
- 33.Schmidt RT, Toews JV. Grip strength as measured by the jamar dynamometer. Arch Phys Med Rehabil. 1970;51:321–327. [PubMed] [Google Scholar]
- 34.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: Normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 35.Werle S, Goldhahn J, Drerup S, Simmen BR, Sprott H, Herren DB. Age- and gender-specific normative data of grip and pinch strength in a healthy adult swiss population. J Hand Surg Eur Vol. 2009;34:76–84. doi: 10.1177/1753193408096763. [DOI] [PubMed] [Google Scholar]
- 36.Bell-Krotoski J. Advances in sensibility evaluation. Hand Clin. 1991;7:527–546. [PubMed] [Google Scholar]
- 37.Bohannon RW, Smith MB. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 38.Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: An fmri study. J Neurophysiol. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- 39.Landsmeer JM. Power grip and precision handling. Ann Rheum Dis. 1962;21:164–170. doi: 10.1136/ard.21.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Napier JR. The prehensile movements of the human hand. J Bone Joint Surg Br. 1956;38-B:902–913. doi: 10.1302/0301-620X.38B4.902. [DOI] [PubMed] [Google Scholar]
- 41.Pouydebat E, Laurin M, Gorce P, Bels V. Evolution of grasping among anthropoids. J Evol Biol. 2008;21:1732–1743. doi: 10.1111/j.1420-9101.2008.01582.x. [DOI] [PubMed] [Google Scholar]
- 42.Lang CE, Wagner JM, Bastian AJ, Hu Q, Edwards DF, Sahrmann SA, Dromerick AW. Deficits in grasp versus reach during acute hemiparesis. Exp Brain Res. 2005;166:126–136. doi: 10.1007/s00221-005-2350-6. [DOI] [PubMed] [Google Scholar]
- 43.Lang CE, Wagner JM, Edwards DF, Sahrmann SA, Dromerick AW. Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabil Neural Repair. 2006;20:444–454. doi: 10.1177/1545968306289299. [DOI] [PubMed] [Google Scholar]
- 44.Caimmi M, Carda S, Giovanzana C, Maini ES, Sabatini AM, Smania N, Molteni F. Using kinematic analysis to evaluate constraint-induced movement therapy in chronic stroke patients. Neurorehabil Neural Repair. 2008;22:31–39. doi: 10.1177/1545968307302923. [DOI] [PubMed] [Google Scholar]
- 45.Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Phys Ther. 2008;88:652–663. doi: 10.2522/ptj.20070255. [DOI] [PubMed] [Google Scholar]
- 46.Patterson TS, Bishop MD, McGuirk TE, Sethi A, Richards LG. Reliability of upper extremity kinematics while performing different tasks in individuals with stroke. J Mot Behav. 2011;43:121–130. doi: 10.1080/00222895.2010.548422. [DOI] [PubMed] [Google Scholar]
- 47.Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 48.Kelso JA, Southard DL, Goodman D. On the coordination of two-handed movements. J Exp Psychol Hum Percept Perform. 1979;5:229–238. doi: 10.1037//0096-1523.5.2.229. [DOI] [PubMed] [Google Scholar]
- 49.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 50.Castiello U, Bennett KM, Stelmach GE. The bilateral reach to grasp movement. Behav Brain Res. 1993;56:43–57. doi: 10.1016/0166-4328(93)90021-h. [DOI] [PubMed] [Google Scholar]
- 51.Flanagan JR, Burstedt MK, Johansson RS. Control of fingertip forces in multidigit manipulation. J Neurophysiol. 1999;81:1706–1717. doi: 10.1152/jn.1999.81.4.1706. [DOI] [PubMed] [Google Scholar]
- 52.Johansson RS. Sensory control of dexterous manipulation in humans. In: Wing AM, Haggard P, Flanagan JR, editors. Hand and brain. New York: Academic; 1996. pp. 381–414. [Google Scholar]