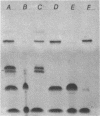
Abstract
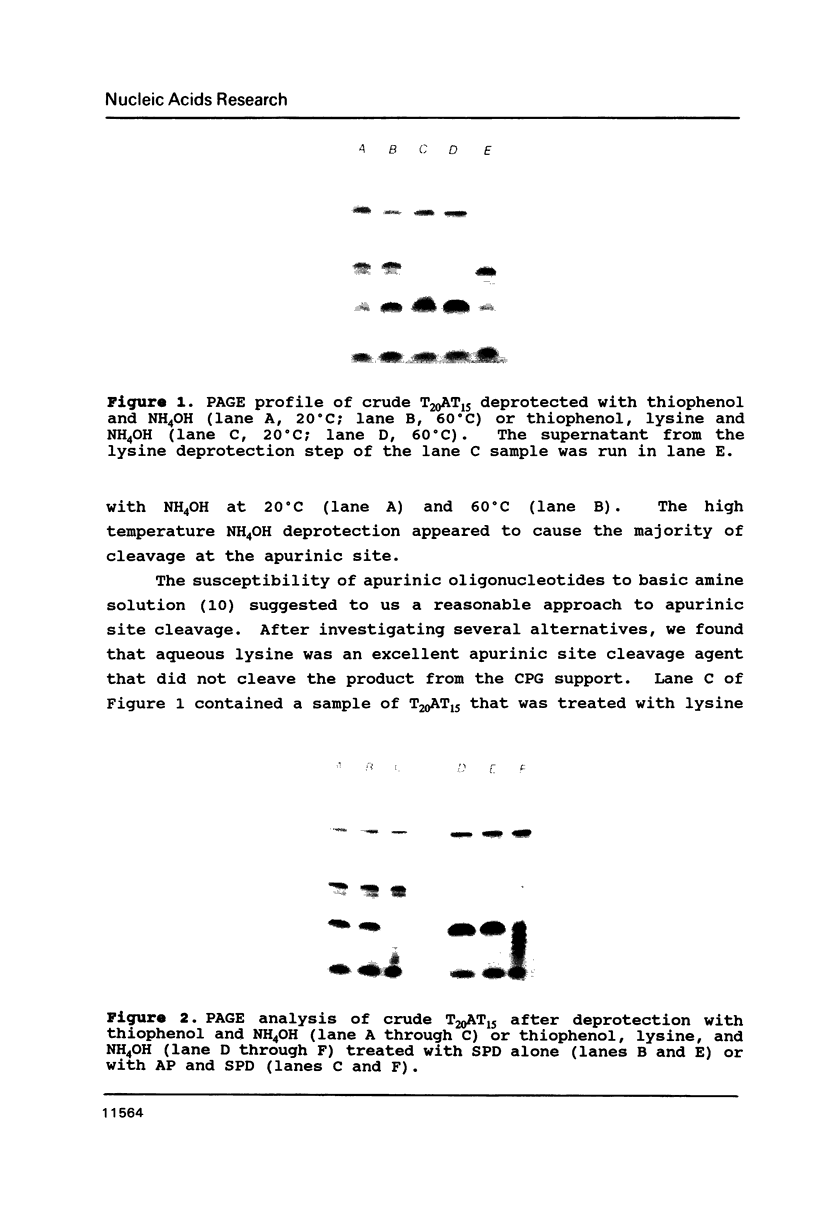
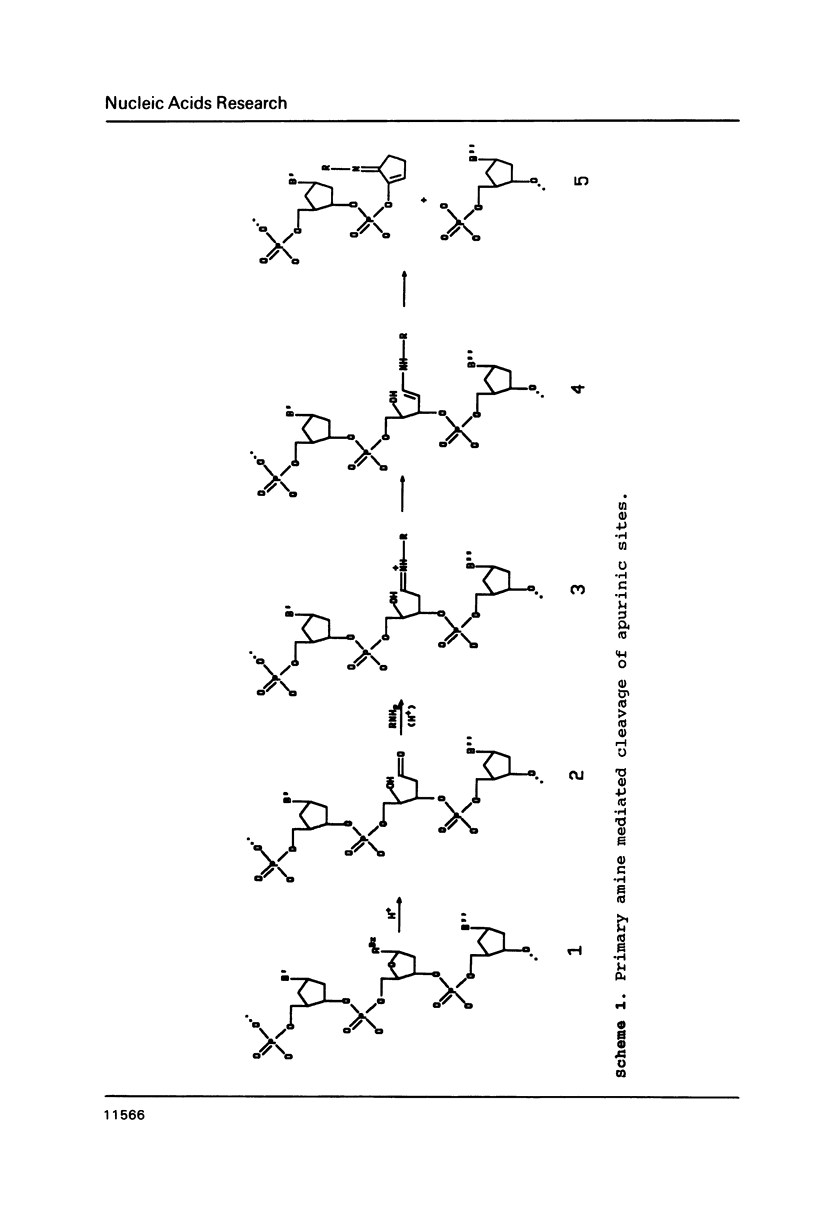
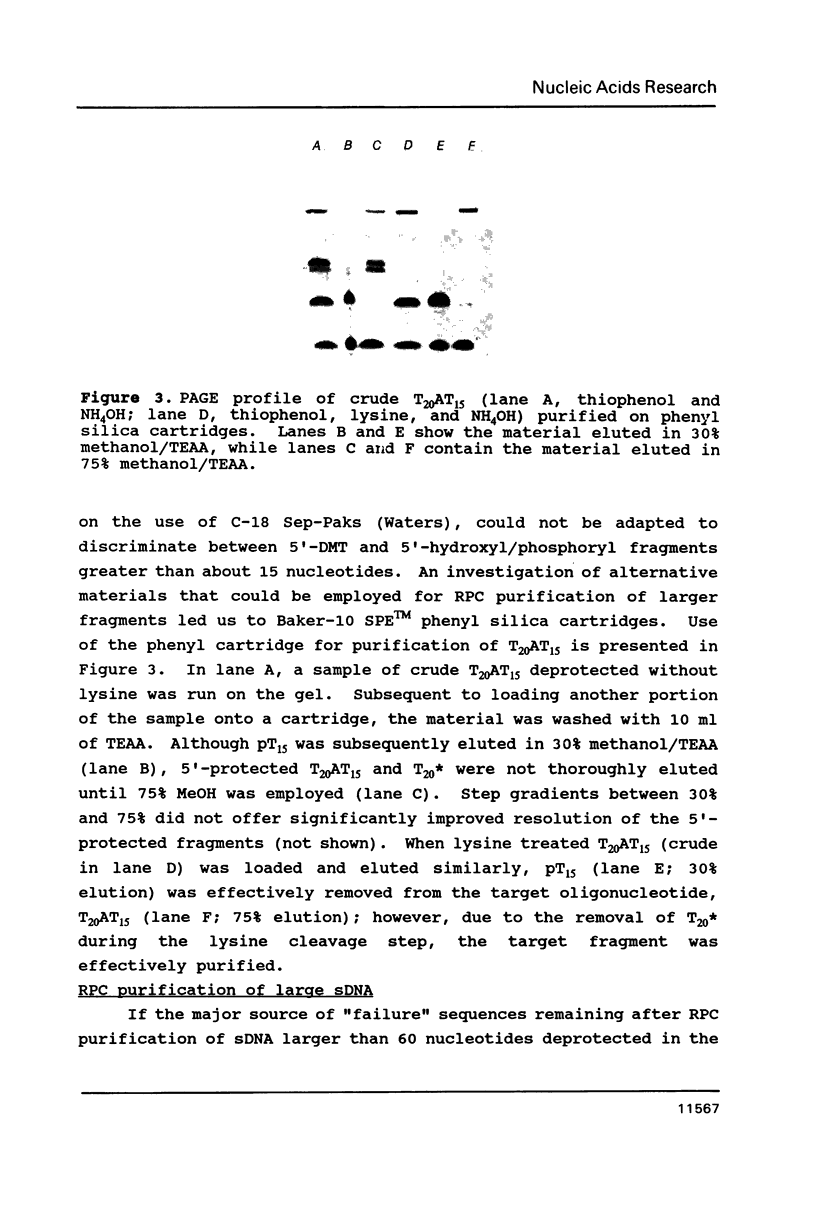
Rapid purification methods for synthetic oligonucleotides involving reverse-phase cartridges (RPC) and enzymatic hydrolysis have been introduced. These methods are based on a discrimination between the desired target fragment protected with a 5'-DMT group and incompletely elongated products possessing a 5'-hydroxyl function. For target products over 60 nucleotides, the rapid methods are of little use as reported to date. We have found that the problem is due to the presence of truncated 5'-DMT fragments generated from apurinic sites within the target product during NH4OH deprotection. These side products are co-purified with the target fragment when the rapid purification procedures are employed. If a step is included during deprotection to cleave the apurinic sites prior to removal of the crude product from the solid support (1 M lysine-HCl, pH 9 for 90 min at 60 degrees C), fragments up to 118 bases can be purified by RPC to near homogeneity.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Kingston I. B. Isolation of a genomic clone for bovine pancreatic trypsin inhibitor by using a unique-sequence synthetic DNA probe. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6838–6842. doi: 10.1073/pnas.80.22.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs M. M., Livingston D. C. Reaction of apurinic acid with aldehyde reagents. Biochim Biophys Acta. 1969 Jan 21;174(1):161–173. doi: 10.1016/0005-2787(69)90239-1. [DOI] [PubMed] [Google Scholar]
- Gao X., Gaffney B. L., Senior M., Riddle R. R., Jones R. A. Methylation of thymine residues during oligonucleotide synthesis. Nucleic Acids Res. 1985 Jan 25;13(2):573–584. doi: 10.1093/nar/13.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K. M., Jones S. S., Hackett N. R., Khorana H. G. Specific amino acid substitutions in bacterioopsin: Replacement of a restriction fragment in the structural gene by synthetic DNA fragments containing altered codons. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2285–2289. doi: 10.1073/pnas.81.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson K. J., Chung B. C., Urdea M. S., Miller W. L. Study of cholesterol side-chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes. Endocrinology. 1986 Apr;118(4):1296–1305. doi: 10.1210/endo-118-4-1296. [DOI] [PubMed] [Google Scholar]
- McBride L. J., McCollum C., Davidson S., Efcavitch J. W., Andrus A., Lombardi S. J. A new, reliable cartridge for the rapid purification of synthetic DNA. Biotechniques. 1988 Apr;6(4):362–367. [PubMed] [Google Scholar]
- Philippsen P., Thiebe R., Wintermeyer W., Zachau H. G. Splitting of phenylalanine specific tRNA into half molecules by chemical means. Biochem Biophys Res Commun. 1968 Dec 30;33(6):922–928. doi: 10.1016/0006-291x(68)90400-2. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Urdea M. S. Use of unpurified synthetic deoxynucleotide primers for rapid dideoxynucleotide chain termination sequencing. DNA. 1984 Aug;3(4):339–343. doi: 10.1089/dna.1.1984.3.339. [DOI] [PubMed] [Google Scholar]
- Schulhof J. C., Molko D., Teoule R. The final deprotection step in oligonucleotide synthesis is reduced to a mild and rapid ammonia treatment by using labile base-protecting groups. Nucleic Acids Res. 1987 Jan 26;15(2):397–416. doi: 10.1093/nar/15.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. R., Chambers R. W. Synthesis and properties of oligodeoxynucleotides with an AP site at a preselected position. Nucleic Acids Res. 1987 Sep 25;15(18):7451–7462. doi: 10.1093/nar/15.18.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM C., SHAPIRO H. S., LIPSHITZ R., CHARGAFF E. Distribution density of nucleotides within a desoxyribonucleic acid chain. J Biol Chem. 1953 Aug;203(2):673–688. [PubMed] [Google Scholar]
- Warner B. D., Warner M. E., Karns G. A., Ku L., Brown-Shimer S., Urdea M. S. Construction and evaluation of an instrument for the automated synthesis of oligodeoxyribonucleotides. DNA. 1984 Oct;3(5):401–411. doi: 10.1089/dna.1984.3.401. [DOI] [PubMed] [Google Scholar]
- Wosnick M. A., Barnett R. W., Vicentini A. M., Erfle H., Elliott R., Sumner-Smith M., Mantei N., Davies R. W. Rapid construction of large synthetic genes: total chemical synthesis of two different versions of the bovine prochymosin gene. Gene. 1987;60(1):115–127. doi: 10.1016/0378-1119(87)90219-8. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Dinehart W. J., Jones B. K. Modifications of guanine bases during oligonucleotide synthesis. Nucleic Acids Res. 1988 May 25;16(10):4539–4554. doi: 10.1093/nar/16.10.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]