Abstract
Background
We prospectively performed cytologic assessment and image analysis (IA) on matched nipple aspirate fluid (NAF) and mammary ductoscopy (MD) specimens to determine 1) the accuracy of these methods in cancer detection, and 2) whether the two collection methods provide complementary information.
Methods
NAF and MD specimens were collected from 84 breasts from 75 women (nine bilateral samples) who underwent breast surgery. Cytologic evaluation was performed on all samples. Image analysis was performed on slides with sufficient epithelial cells.
Results
Cytologic evaluation proved more accurate in patients without pathologic spontaneous nipple discharge (PND) than those with PND, mainly because of the potential false positive diagnosis in the latter. While the sensitivity of NAF and MD cytology was low (10% and 14%, respectively), both were 100% specific in cancer detection in the non-PND cohort. Combining NAF and MD cytology information improved sensitivity (24%) without sacrificing specificity. Similar to cytology, image analysis was more accurate in patients without PND having high specificity (100% for aneuploid IA), but relatively low sensitivity (36%). Combining NAF and MD cytology with aneuploid IA improved the sensitivity (45%) while maintaining high specificity (100%). The best predictive model was positive NAF cytology and/or MD cytology combined with IA aneuploidy, which resulted in 55% sensitivity and 100% specificity in breast cancer detection.
Conclusions
Cytologic evaluation and IA of NAF and MD specimens are complementary. The presence of atypical cells arising from an intraductal papilloma in ductoscopic specimens is a potential source of false positive diagnosis in patients with nipple discharge.
Keywords: nipple aspirate fluid, mammary ductoscopy, image analysis, cytology, breast cancer detection
INTRODUCTION
The currently accepted screening tools of mammography and physical examination miss up to 40% of early breast cancers. Moreover, when a mass is felt or mammographic abnormality identified, the subject must undergo an invasive diagnostic procedure, with its inherent risks and cosmetic implications. Over a million surgical or needle breast biopsies are performed annually in the United States to diagnose 240,000 new breast cancers [1]. Although 66-85% of the abnormalities are benign, an invasive procedure is necessary for cytologic or histologic evaluation of the lesions [2]. It is highly desirable to find markers that can identify women with early stage cancer or precancerous lesions without resorting to painful and expensive invasive procedures.
Cytologic evaluation of nipple fluid in women without pathologic spontaneous nipple discharge (PND), generally defined as one breast single duct spontaneous nipple discharge, was first performed by Papanicolaou [3]. Aspiration of the breast nipple is a safe, noninvasive method to collect breast epithelial cells, which are the source of the majority of breast cancers. We have extensive experience with collecting NAF to screen women for breast cancer risk [4-7]. While NAF is an excellent source for secreted proteins, the relatively low cellularity makes it less ideal for cytologic evaluation. Studies are in progress which combine promising secreted proteins in NAF with cytology in NAF and MD samples.
Another intraductal approach, ductal lavage (DL), allows the collection of ductal epithelial cells by placing a short catheter in one or more ducts through the nipple. The weakness of DL is that the collection is blind and the cell population represents samples of multiple areas of one duct or multiple ducts with or without an abnormality. Mammary ductoscopy (MD) on the other hand employs an endoscopic device that is placed in the duct and enables the operator to visualize an intraductal lesion and collect targeted samples by direct brushing or irrigating the area of visible pathology. The specimens are generally cellular and enriched in epithelial cells. MD is usually preceded by nipple aspiration, which identifies ducts containing a large volume of nipple aspirate fluid (NAF) and are therefore more likely to harbor proliferative disease, and also more likely to yield to successful endoscopy since the lumen is distended by the fluid. This technique has been used for over 18 years. [8,9] Most studies utilizing MD have evaluated women with PND [8,10,11]. One report [12] suggested that MD may also be useful in determining the optimal margins of resection for patients with in situ and invasive breast cancer. MD currently offers a safe alternative to ductography in guiding subsequent breast surgery in the treatment of PND.
Cytologic evaluation of mammary duct fluids is an accepted method of cancer risk assessment. However, most reports suggest that it has a low sensitivity for cancer detection [13]. However, low sensitivity does not eliminate the importance of NAF and MD cytology because unlike physical examination or radiologic imaging, a positive cytologic diagnosis can be used reliably to institute therapy. It would be beneficial to employ additional markers to improve the sensitivity of cytomorphology.
The assessment of abnormal DNA content by image analysis (IA) has proven to be a useful ancillary method in conjunction with morphology. We have found that aneuploidy in NAF [7] and in MD specimens [14] has good correlation with malignant intraductal cytology. The strengths and challenges involved with intraductal evaluation of the breast have been reviewed by other investigators. [15-17].
The goal of this prospective study was to perform cytologic and image analysis (IA) assessment on matched NAF and MD specimens to determine 1) which intraductal material provided more predictive information, and 2) whether the two methods would provide complementary information.
METHODS
Overview
The protocol used in this study was approved by the Institutional Review Board. After informed consent was obtained, specimens were prospectively collected by one surgeon (ERS) in the operating theater as part of a planned surgical procedure to remove a lesion that was palpable, suspicious for cancer by imaging criteria, or a breast with pathologic nipple discharge. All specimens were processed using a predetermined protocol, and all Papanicolaou (Pap) stained slides were evaluated by one cytopathologist (HE). All Feulgen stained slides were interpreted by one pathologist (AK-S). Both pathologists have extensive experience evaluating cell preparations of breast epithelial cells [5,7,13,18]. All women underwent nipple aspiration prior to MD. Cytologic review was performed on all NAF and MD specimens. Image analysis was performed on all NAF and MD specimens with adequate cellularity, based on criteria outlined below under cytology interpretation. IA data are presented only for cases in which the analysis could be performed on both NAF and MD specimens.
Specimen procurement, preparation and interpretation
Nipple aspiration was routinely attempted to identify the duct(s) which provide fluid. The procedure used has been previously described [19]. NAF samples were collected into capillary tubes. One half of the fluid was stored at −80 °C for molecular studies, while the other half was placed into Shandon cytology fixative (ThermoShandon, Pittsburgh, PA) for cytologic evaluation and image analysis. MD was then performed on the duct(s) (1 or 2) which provided NAF.
The duct(s) that provided NAF were gently dilated sufficiently to introduce a 0.9 mm Acueity (Palo Alto, CA) endoscope. Once a duct was entered, the distance traveled within the duct varied based on the duct’s internal diameter (if it narrowed quickly, we advanced for a shorter distance). If a lesion was identified, the duct wall in the area of the lesion was gently abraded with the tip of the scope. If no lesion was identified, a random area of the duct(s) was abraded. The duct was then irrigated with normal saline. The specimens from a given breast were combined if a lesion was not seen or kept separate if a lesion was identified. The fluids were centrifuged to a pellet, resuspended in Hank’s balanced salt solution, and then divided into three parts, two to prepare cell lysates and the other for whole cell studies. The analysis of the cell lysates is ongoing. This report focuses on whole cell studies.
Cytology
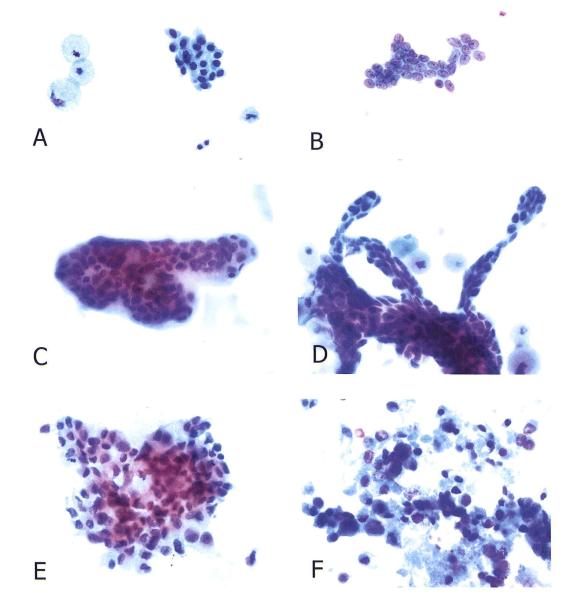
NAF and MD samples were centrifuged, the supernatant removed, and the cells fixed in Shandon Cytology Fixative (ThermoShandon). Each sample was cytocentrifuged onto ten glass slides for cytologic analysis and IA. The Papanicolaou-stained smears were examined in blinded fashion. The pathologist examined NAF and MD specimens at different times and without the knowledge of the results of the other sample or the surgical biopsy. Each NAF and MD specimen was classified into one of the following five categories: Inadequate (less than 10 epithelial cells), benign (encompassing normal and hyperplastic epithelial cells), mild atypia (nuclear enlargement with mild chromatin and nuclear membrane abnormalities), marked atypia/suspicious for carcinoma (presence of nuclear enlargement, variation is size, hyperchromasia, pronounced irregularity of nuclear membrane, with or without prominent nucleoli, that either quantitatively or qualitatively fall short of unequivocal malignant criteria), and malignant (unequivocal cytologic criteria of malignancy, frequently associated with loss of cohesiveness and necrosis) [7,20]. (Figure 1)
Figure 1.
NAF and MD cytology. (A) Benign epithelial cells and foam cells in NAF. (B) Benign epithelial cells in MD sample. (C) A three-dimensional cluster of benign epithelial cells in NAF of a patient with papilloma. (D) A branching papillary cluster of cohesive, slightly atypical epithelial cells in the MD specimen from a patient with papilloma (same as C). A few foam cells are evident in the background. (E) Malignant cells in NAF of patient with ductal carcinoma. (F) Malignant cells in an MD sample from a patient with ductal carcinoma. Necrosis is present. (Papanicolaou stain, original magnification × 500)
Image analysis
A standardized quantitative DNA staining kit (Feulgen, Tripath, Burlington, NC) was used following the manufacturer’s instructions. In brief, after rehydration the slides were placed in 5N HCl, transferred to the staining solution (Schiff’s reagent), rinsed, dehydrated and mounted with synthetic resin. A Fairfield DNA ploidy system for image analysis (Fairfield Imaging Ltd., Nottingham, UK) was used. This system, employing a light microscope, a multicolor solid state camera and a digital computer has the ability to process cell images and calculate cell cycle and ploidy parameters [21]. The parameters calculated were DNA index (DI), S phase fraction (SPF), and the presence of hypertetraploid cells (cells with a DI > 2.0). DI values between 0.80-1.19 were defined as diploid, and values out of this range considered aneuploid. The reported DI for each specimen represents the mean value of the individually measured cells. All epithelial cells if less than 100 cells were present on a slide, or a minimum of 100 cells if more cells were present were analyzed. All specimens were evaluated in blinded fashion.
Statistical analysis
Contingency table analyses for two-way cross-classifications were performed using the χ2 test of independence and the Fisher’s exact test when feasible in order to determine whether various MD and NAF cytological and pathological characteristics and image analysis were related to each other in a statistically significant way. When appropriate, Mc Nemar’s test was used. The same NAF and MD cytology and image analysis variables were cross-classified with cancer in order to determine the differences in their sensitivity and specificity as a test for cancer. These same variables were then combined to determine whether the sensitivity of the test improved. Then various cytology variables were combined with image analysis variables and again cross-classified with cancer and analyzed. These steps were taken in order to determine whether NAF and MD characteristics provided complementary information and in order to find the test for breast cancer with the highest specificity. All statistical models were performed using SAS version 9.1 for Windows.
RESULTS
Overview
We successfully performed MD or nipple aspiration on 93 consecutive breasts. NAF and matched MD were collected from 87 of these breasts. Eliminating those in whom we collected both samples but, for one reason or another, did not undergo the planned surgical procedure, the sample size was 84. These 84 matched specimens from 75 women serve as the basis for our report (Table 1). The pathologic findings in the surgical specimens included 32 with ductal carcinoma in situ (DCIS) or invasive breast carcinoma (IBC), 3 precancerous conditions (lobular carcinoma in situ or atypical duct hyperplasia), 14 papillomas, 4 atypical papillomas, 22 duct hyperplasia, and 9 without pathologic changes (classified as normal). Three of the 38 women with PND had breast cancer, whereas 29/46 women without PND had breast cancer. Thus, among our cohort of women requiring diagnostic breast surgery, PND was negatively associated with cancer (p < .0001), and a sensitive predictor of the absence of breast cancer.
TABLE 1.
Demographics and clinical data (N= 75)
| Demographics | |
| Age (yrs) | |
| Mean (Range) | 51 (25-81) |
| Median | 49 |
| Clinical Data | |
| Menopausal Status* | |
| Premenopausal | 35 |
| Postmenopausal | 37 |
| Ever used hormone replacement therapy (HRT)* | 16 |
| Ever pregnant | 64 |
Information unavailable for 3 women for menopausal status and 1 woman for use of HRT
Cytologic findings
Of 32 breasts that contained cancer, cytology was malignant in 4 MD, and none of the NAF samples (Table 2). Using Mc Nemar’s test this difference was found to be significant (p=0.05). In none of MD samples and 3 of NAF specimens cytology was suspicious. In our statistical analysis we considered a suspicious or malignant cytologic diagnosis as positive cytology for NAF and MD samples. With this definition, the sensitivity of cytology was 13% for MD and 9% for NAF (Table 3). There were six false positive MD cytology (5 suspicious, one malignant) and 1 false positive NAF cytology (called suspicious, Figure 2) samples, resulting in a specificity of 88% for MD and 98% for NAF. All false positive diagnoses were made on the breasts with PND, and with one exception in those harboring papillomas. Therefore, the specificity of positive cytology was 100% in both NAF and MD samples from breasts without PND (Table 3). Combining NAF and MD results improved the sensitivity of cytology from 10% for NAF and 14% for MD cytology to 24%, maintaining 100% specificity in breasts without PND.
TABLE 2.
Cytologic Characteristics of NAF and Ductoscopic Specimens Based on Pathologic Diagnosis*
| Pathology | N | NAF/Ductoscopic Cytology (%) |
||||
|---|---|---|---|---|---|---|
| Inadequate Epith cells |
Normal/DH | Atypia | Suspicious for Cancer |
Malignant | ||
| Normal | 9 | |||||
| NAF | 4(44) | 5(56) | 0 | 0 | 0 | |
| Ductoscopy | 4(44) | 2(22) | 3(34) | 0 | 0 | |
| Hyperplasia | 22 | |||||
| NAF | 16(73) | 3(13.5) | 3(13.5) | 0 | 0 | |
| Ductoscopy | 8(36) | 8(36) | 5(23) | 1(5) | 0 | |
| Papilloma | 14 | |||||
| NAF | 4(29) | 7(50) | 3(21) | 0 | 0 | |
| Ductoscopy | 5(36) | 5(36) | 1(7) | 2(14) | 1(7) | |
| Papilloma with atypia | 4 | |||||
| NAF | 2(50) | 1(25) | 0 | 1(25) | 0 | |
| Ductoscopy | 0 | 0 | 2(50) | 2(50) | 0 | |
| Atypical hyperplasia | 1 | |||||
| NAF | 0 | 1(100) | 0 | 0 | 0 | |
| Ductoscopy | 1(100) | 0 | 0 | 0 | 0 | |
| Lobular carcinoma in situ | 2 | |||||
| NAF | 1(50) | 1(50) | 0 | 0 | 0 | |
| Ductoscopy | 1(50) | 1(50) | 0 | 0 | 0 | |
| Ductal carcinoma in situ | 12 | |||||
| NAF | 3(25) | 7(58) | 1(13.5) | 1(13.5) | 0 | |
| Ductoscopy | 2(17) | 7(58) | 2(17) | 0 | 1(8) | |
| Invasive breast cancer | 20 | |||||
| NAF | 8(40) | 8(40) | 2(10) | 2(10) | 0 | |
| Ductoscopy | 7(35) | 8(40) | 2(10) | 0 | 3(15) | |
| TOTAL | ||||||
| NAF | 84 | 38(45.2) | 33(39.2) | 9(10.7) | 4(4.7) | 0 |
| Ductoscopy | 84 | 28(33.3) | 31(36.9) | 15(17.8) | 5(5.9) | 5(5.9) |
NAF: nipple aspirate fluid; DH: ductal hyperplasia; epith: epithelial.
TABLE 3.
Sensitivity (Se) and Specificity (Sp) of Nipple Aspirate Fluid (NAF) and Mammary Ductoscopy (MD) Cytology1
| Cytology2 | All cases(N=84) |
PND3 (N=38) |
No PND (N=46) |
|||
|---|---|---|---|---|---|---|
| Se | Sp | Se | Sp | Se | Sp | |
| Positive NAF | 9 | 98 | 0 | 97 | 10 | 100 |
| Positive MD | 13 | 88 | 0 | 83 | 14 | 100 |
| Positive NAF and/or MD |
22 | 88 | 0 | 83 | 24 | 100 |
Three of the 38 women with PND had breast cancer, whereas 29/46 women without PND had breast cancer.
Positive cytology= suspicious or malignant diagnosis. In three cases, NAF cytology was positive but the MD was not positive.
PND: pathologic spontaneous nipple discharge
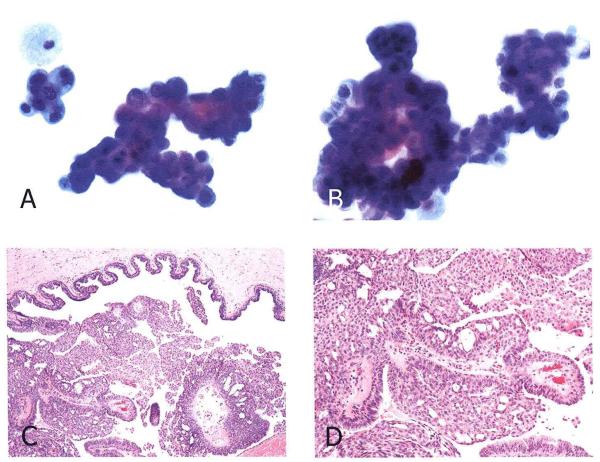
Figure 2.
False positive cytology in a case of intraductal papilloma with atypia. (A) Two clusters of epithelial cells exhibiting nuclear enlargement, hyperchromasia and distinct nucleoli. A mitotic figure is evident in the center of left upper cluster. This NAF specimen was called suspicious for malignancy. (B) MD cytology from the same patient which was independently called suspicious for carcinoma. A large, branching papillary cluster of epithelial cells exhibits marked nuclear enlargement and hyperchromasia. (Papanicolaou stain, original magnification × 500) (C, D) histologic section of the excisional biopsy exhibiting an intraductal papilloma with areas of atypical hyperplasia (H & E stain, original magnifications C, ×50; D, ×100)
Image analysis (IA) findings
Thirty-nine matched NAF and MD samples with IA information were available (Table 4). In general, IA indices (aneuploidy, presence of hypertetraploid cells, DNA index, and SPF) were similar in NAF and MD specimens, with the sensitivity ranging from 31% to 46%, and specificity from 73% to 96% (Table 5). Similar to cytology, false positive results occurred mostly in the PND cohort, particularly in NAF specimens. A specificity of 100% was achieved by NAF IA when PND cases were excluded. Combining NAF and MD image analysis results improved the sensitivity with no decrease in specificity in non-PND cases.
TABLE 4.
Image Analysis of Nipple Aspirate Fluid (NAF) and Mammary Ductoscopy (MD) Samples Based on Pathologic Diagnosis1
| Pathology | Image Analysis |
|||
|---|---|---|---|---|
| Total | Aneuploid | Hypertetraploid | SPF > 20 | |
| Normal | 4 | |||
| NAF | 0 | 0 | 0 | |
| MD | 0 | 1 | 1 | |
| Hyperplasia | 9 | |||
| NAF | 2 | 0 | 2 | |
| MD | 1 | 0 | 2 | |
| Papilloma | 10 | |||
| NAF | 1 | 1 | 1 | |
| MD | 1 | 0 | 2 | |
| Papilloma with atypia | 2 | |||
| NAF | 0 | 0 | 0 | |
| MD | 2 | 2 | 2 | |
| Atypical hyperplasia | 0 | |||
| NAF | 0 | 0 | 0 | |
| MD | 0 | 0 | 0 | |
| Lobular carcinoma in situ | 1 | |||
| NAF | 0 | 0 | 0 | |
| MD | 0 | 0 | 0 | |
| Ductal carcinoma in situ | 3 | |||
| NAF | 0 | 1 | 1 | |
| MD | 1 | 1 | 1 | |
| Invasive breast cancer | 10 | |||
| NAF | 4 | 3 | 5 | |
| MD | 4 | 4 | 3 | |
| Total | ||||
| NAF | 39 | 7 | 5 | 9 |
| MD | 39 | 9 | 8 | 11 |
Only samples with epithelial cells were imaged for morphometric analysis. Image analysis was not routinely performed on NAF specimens until midway into the protocol.
Diploid defined as DNA Index (DI) = 0.8-1.2, aneuploid as any value above or below diploid. The scores are based on overall DI. Hypertetraploidy is defined as any case that has one or more cells with a DI > 2.0.
TABLE 5.
Percent Sensitivity (Se) and Specificity (Sp) of Image Analysis of Nipple Aspirate Fluid (NAF) and Mammary Ductoscopy (MD) Samples
| All cases (N=39) |
PND1 (N=20) |
No PND (N=19) |
||||
|---|---|---|---|---|---|---|
| Se | Sp | Se | Sp | Se | Sp | |
| NAF | ||||||
| - Aneuploid | 31 | 88 | 0 | 83 | 36 | 100 |
| - Hypertetraploid2 | 31 | 96 | 0 | 94 | 36 | 100 |
| - SPF3 > 20 | 46 | 88 | 50 | 83 | 45 | 100 |
| MD | ||||||
| - Aneuploid | 38 | 85 | 50 | 78 | 36 | 100 |
| - Hypertetraploid | 38 | 88 | 0 | 89 | 45 | 88 |
| - SPF > 20 | 31 | 73 | 50 | 67 | 27 | 88 |
| NAF and/or MD | ||||||
| - Aneuploid | 46 | 85 | 50 | 72 | 45 | 100 |
| - Hypertetraploid | 46 | 85 | 0 | 83 | 55 | 88 |
| - SPF > 20 | 46 | 69 | 50 | 61 | 45 | 100 |
PND: pathologic spontaneous nipple discharge
Diploid defined as DNA Index (DI) = 0.8-1.2, aneuploid as any value above or below diploid. The scores are based on overall DI. Hypertetraploidy is defined as any case that has one or more cells with a DI > 2.0.
SPF: S phase fraction
Combining cytology and IA information
We combined cytologic and IA findings which resulted in improved sensitivity of NAF, MD or NAF + MD in non-PND samples, with minimal or no decrease in specificity (Table 6). The best model was a positive NAF cytology and/or MD cytology combined with IA aneuploidy, which resulted in 55% sensitivity and 100% specificity in detection of cancer in non-PND samples. In PND samples, combining cytologic and IA results did not consistently improve cancer prediction.
TABLE 6.
Percent Sensitivity and Specificity of Positive Cytology and Image Analysis Findings in Nipple Aspirate Fluid (NAF) and Mammary Ductoscopy (MD) Samples in Breast without PND (N=19)1
| Cytology and IA results | Sensitivity | Specificity |
|---|---|---|
| Positive NAF cytology and/or | ||
| - Aneuploid | 45 | 100 |
| - Hypertetraploid3 | 36 | 100 |
| - SPF4 > 20 | 55 | 100 |
| Positive MD cytology and/or | ||
| - Aneuploid | 45 | 100 |
| - Hypertetraploid | 55 | 88 |
| - SPF > 20 | 36 | 88 |
| Positive NAF cytology and/or | ||
| Positive MD cytology and/or | ||
| - Aneuploid | 55 | 100 |
| - Hypertetraploid | 64 | 88 |
| - SPF > 20 | 55 | 88 |
Positive cytology= suspicious or malignant diagnosis.
PND: pathologic spontaneous nipple discharge
Diploid defined as DNA Index (DI) = 0.8-1.2, aneuploid as any value above or below diploid. The scores are based on overall DI. Hypertetraploidy is defined as any case that has one or more cells with a DI > 2.0.
SPF: S phase fraction
DISCUSSION
Treatment of breast cancer requires definitive morphologic diagnosis of malignancy by invasive needle aspiration or surgical biopsies. A malignant neoplasm is found in only approximately 20% of the biopsies that are performed on the basis of a palpable lesion, an abnormal mammogram or nipple discharge. [2] Noninvasive and minimally invasive procedures are presently under review to replace invasive diagnostic procedures. These procedures include nipple aspiration, ductal lavage, and ductoscopy. Each of these techniques has its advantages and limitations. NAF is a completely noninvasive and inexpensive method requiring a simple device [18]. The fluid is rich in secreted proteins, but scant in cellularity, hence insensitive for the detection of cancer and precancerous lesions and unsuitable for evaluation of intracellular proteins. DL is expensive and time-consuming. It provides more cellular specimens, but the samples are random rather than representing specific abnormal area(s) within a duct. MD is also expensive and time-consuming, but has the advantage of providing a large number of cells from specific abnormal area(s) within a duct under direct visualization. Therefore, at least in theory, MD should afford higher sensitivity of cytologic studies and adequate samples for assessment of intracellular proteins. For these reasons we hypothesized that the cytology and image analysis in MD and NAF might be complementary. This was confirmed in our study, as in three women with breast cancer NAF cytology was positive but the MD sample was not (Table 3). This is not surprising, since NAF is often derived from several ducts, and the duct which was scoped may not have been the one containing the neoplastic cells. It is of note that although we combined the “suspicious” and “malignant” diagnoses as positive cytology, there were more malignant diagnoses in MD specimens and more suspicious diagnoses in NAF specimens. This difference is most likely because of the higher cellularity of the MD specimens enabling the pathologist to render a more definitive interpretation.
In our previous studies we have shown that cytologic and image analysis evaluations are highly specific but not very sensitive in cancer prediction [4,6,14]. Another goal of our study was to see whether combining cytology and IA data from NAF and MD samples obtained from the same patient could improve the sensitivity without sacrificing the specificity. We also wanted to investigate whether the accuracy of these tests was different in patients with and without PND. As in previous studies, cytologic assessment in both NAF and MD samples demonstrated high specificity but low sensitivity. The specificity was lower in samples from breasts with PND than in those without PND. Indeed, there were no false positive diagnoses in the non-PND group in either NAF or MD samples (100% specificity). This is consistent with earlier reports [22]. The only false-positive NAF (called suspicious) and all 6 false-positive MD samples (5 suspicious, 1 malignant) were in patients with PND. The histopathology in all except one case was intraductal papilloma and ductal hyperplasia in the remaining case (suspicious MD). The most common cause of PND in our patients was papilloma, and 17 of 18 papilloma cases were in subjects with PND. Only 3 of the 38 patients with PND had breast cancer as compared to 29 of the 46 women without PND. The atypical cells shed from intraductal papillomas, particularly in MD samples in which the cells are forcibly removed, can mimic cancer. This is an important message of our study that cytopathologists should exercise caution in interpretation of atypical cells in ductoscopic samples from patients with PND. Furthermore, in our study the sensitivity of both NAF and MD were lower in PND patients than those without PND, suggesting that these methods might have a limited role in the evaluation of patients with PND. However considering the fact that only 3 of our PND patients proved to have cancer, this finding should not be generalized until larger series have been reported.
Similar to the cytology results, IA findings from both NAF and MD samples were highly specific if collected from a breast without PND, but less so in breasts with PND. Among non-PND samples, there were no false positive IA in NAF samples and only one false positive in MD samples. Combining the IA results in NAF and MD samples improved the sensitivity in the non-PND samples but not in PND cases. Combining IA and cytology findings provided the highest predictive ability, with an increase in sensitivity and without a decrease in specificity in the non-PND samples. The best model in our study (Table 6) was positive NAF cytology and/or positive MD cytology combined with aneuploid IA, which resulted in a sensitivity of 55% and specificity of 100% in the non-PND cohort.
Based on our findings, in women without PND requiring surgery for a palpable mass or imaging abnormality, NAF and MD cytology and IA findings are complementary, providing highly specific information on whether a breast contains cancer. In the presence of PND, cytology and IA alone or in combination have very low sensitivity. While NAF cytology alone in this group of patients is highly specific (97%), the specificity is reduced when the results are combined with MD cytology and/or IA. This is mainly due to the high incidence of intraductal papilloma in breasts with PND, which results in the shedding of atypical cells in ductoscopic specimens. Our study confirms previous observations that aneuploidy per se is not a reliable marker of malignancy, since aneuploid cells can be identified in benign breast lesions [23].
An additional limitation of cytologic evaluation and image analysis of NAF and MD is the lack of adequate epithelial cells in many samples. To improve the sensitivity rates, other ancillary tests such as the analysis of extracellular proteins and/or molecular studies should be considered in conjunction with cytology.
Confirmation of our results by other studies will be necessary before these methods are implemented in standard clinical practice. Currently, inadequate expertise and lack of equipment in most centers are major limiting factors in the utilization of cytology and image analysis in NAF and MD specimens. Nonetheless, the skills required to collect the samples are not difficult to learn, and centralized evaluation of specimens at reference laboratories could be implemented until adequate expertise is more widely available.
Acknowledgments
Supported in part by NIH grant CA-87391 and by Natural Sciences and Engineering Research Council of Canada grant RGPIN92037
Abbreviations
- ADH
atypical ductal hyperplasia
- DCIS
ductal carcinoma in situ
- DI
DNA index
- IA
image analysis
- IBC
invasive breast cancer
- LCIS
lobular carcinoma in situ
- NAF
nipple aspirate fluid
- PND
pathologic spontaneous nipple discharge
- SPF
S phase fraction
REFERENCES
- 1.Society AC ACS: Cancer Facts and Figures 2007. ACS Journal. 2007 [Google Scholar]
- 2.Fahy BN, Bold RJ, Schneider PD, Khatri V, Goodnight JE., Jr Cost-benefit analysis of biopsy methods for suspicious mammographic lesions. Arch Surg. 2001;136(9):990–4. doi: 10.1001/archsurg.136.9.990. discussion 994-5. [DOI] [PubMed] [Google Scholar]
- 3.Papanicolaou GN, Holmquist DG, Bader GM, Falk EA. Exfoliative cytology of the human mammary gland and its value in the diagnosis of cancer and other diseases of the breast. Cancer. 1958;11:377–409. doi: 10.1002/1097-0142(195803/04)11:2<377::aid-cncr2820110223>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Sauter ER, Ehya H, Klein-Szanto AJ, Wagner-Mann C, MacGibbon B. Fiberoptic ductoscopy findings in women with and without spontaneous nipple discharge. Cancer. 2005;103(5):914–21. doi: 10.1002/cncr.20865. [DOI] [PubMed] [Google Scholar]
- 5.Sauter ER, Ehya H, Mammen A, Klein G. Nipple aspirate cytology and pathologic parameters predict residual cancer and nodal involvement after excisional breast biopsy. Br J Cancer. 2001;85(12):1952–7. doi: 10.1054/bjoc.2001.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauter ER, Ehya H, Schlatter L, MacGibbon B. Ductoscopic cytology to detect breast cancer. Cancer J. 2004;10(1):33–41. doi: 10.1097/00130404-200401000-00008. discussion 15-6. [DOI] [PubMed] [Google Scholar]
- 7.Sauter ER, Ross E, Daly M, Klein-Szanto A, Engstrom PF, Sorling A, Malick J, Ehya H. Nipple aspirate fluid: a promising non-invasive method to identify cellular markers of breast cancer risk. Br J Cancer. 1997;76(4):494–501. doi: 10.1038/bjc.1997.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki A, Okazaki M, Asaishi K, Satoh H, Watanabe Y, Mikami T, Toda K, Okazaki Y, Nabeta K, Hirata K. Fiberoptic ductoscopy of the breast: a new diagnostic procedure for nipple discharge. Jpn J Clin Oncol. 1991;21(3):188–93. others. [PubMed] [Google Scholar]
- 9.Teboul M. A new concept in breast investigation: echo-histological acino-ductal analysis or analytic echography. Biomed Pharmacother. 1988;42(4):289–95. [PubMed] [Google Scholar]
- 10.Shen KW, Wu J, Lu JS, Han QX, Shen ZZ, Nguyen M, Shao ZM, Barsky SH. Fiberoptic ductoscopy for patients with nipple discharge. Cancer. 2000;89(7):1512–9. [PubMed] [Google Scholar]
- 11.Berna JD, Garcia-Medina V, Kuni CC. Ductoscopy: a new technique for ductal exploration. Eur J Radiol. 1991;12(2):127–9. doi: 10.1016/0720-048x(91)90112-9. [DOI] [PubMed] [Google Scholar]
- 12.Dooley WC. Endoscopic visualization of breast tumors. JAMA. 2000;284(12):1518. doi: 10.1001/jama.284.12.1518-b. [DOI] [PubMed] [Google Scholar]
- 13.Sauter ER, Ehya H, Babb J, Diamandis E, Daly M, Klein-Szanto A, Sigurdson E, Hoffman J, Malick J, Engstrom PF. Biological markers of risk in nipple aspirate fluid are associated with residual cancer and tumour size. Br J Cancer. 1999;81(7):1222–7. doi: 10.1038/sj.bjc.6690832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauter ER, Klein-Szanto A, Ehya H, MacGibbon B. Ductoscopic cytology and image analysis to detect breast carcinoma. Cancer. 2004;101(6):1283–92. doi: 10.1002/cncr.20524. [DOI] [PubMed] [Google Scholar]
- 15.Dua RS, Isacke CM, Gui GP. The intraductal approach to breast cancer biomarker discovery. J Clin Oncol. 2006;24(7):1209–16. doi: 10.1200/JCO.2005.04.1830. [DOI] [PubMed] [Google Scholar]
- 16.Escobar PF, Crowe JP, Matsunaga T, Mokbel K. The clinical applications of mammary ductoscopy. Am J Surg. 2006;191(2):211–5. doi: 10.1016/j.amjsurg.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Lang JE, Kuerer HM. Breast ductal secretions: clinical features, potential uses, and possible applications. Cancer Control. 2007;14(4):350–9. doi: 10.1177/107327480701400405. [DOI] [PubMed] [Google Scholar]
- 18.Sauter ER, Daly M, Linahan K, Ehya H, Engstrom PF, Bonney G, Ross EA, Yu H, Diamandis E. Prostate-specific antigen levels in nipple aspirate fluid correlate with breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(12):967–70. [PubMed] [Google Scholar]
- 19.Sauter ER, Daly M, Linahan K, Ehya H, Engstrom PF, Bonney G, Ross EA, Yu H, Diamandis E. Prostate-specific antigen levels in nipple aspirate fluid correlate with breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(12):967–70. [PubMed] [Google Scholar]
- 20.Sauter ER, Ehya H, Schlatter L, MacGibbon B. Ductoscopic Cytology to Detect Breast Cancer. The Cancer J. 2004;10:33–41. doi: 10.1097/00130404-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Sudbo J, Kildal W, Risberg B, Koppang HS, Danielsen HE, Reith A. DNA content as a prognostic marker in patients with oral leukoplakia. N Engl J Med. 2001;344(17):1270–8. doi: 10.1056/NEJM200104263441702. [DOI] [PubMed] [Google Scholar]
- 22.Dinkel HP, Gassel AM, Muller T, Lourens S, Rominger M, Tschammler A. Galactography and exfoliative cytology in women with abnormal nipple discharge. Obstet Gynecol. 2001;97(4):625–9. doi: 10.1016/s0029-7844(00)01229-1. [DOI] [PubMed] [Google Scholar]
- 23.Stomper PC, DeBloom JR, 2nd, Budnick RM, Stewart CC. Flow cytometric DNA analyses of benign breast lesions detected by screening mammography. Clin Cancer Res. 1998;4(6):1543–7. [PubMed] [Google Scholar]