Abstract
Mitotic centromere associated kinesin (MCAK) is a kinesin related protein with the ability to stimulate microtubule depolymerization. It is found at spindle poles, where it may be involved in poleward microtubule flux, and at kinetochores and centromeres where it plays a role in correcting chromosome alignment errors. Its microtubule depolymerase activity and recruitment to centromeres is regulated by phosphorylation, but little is known about how MCAK is maintained at appropriate levels. We previously reported that MCAK accumulates during the cell cycle and is then degraded during mitosis. Using proteomic analysis, we have now identified a new phosphorylation site on MCAK that is responsible for its degradation. Mutation of the site to prevent phosphorylation prolonged the stability of the protein beyond the metaphase to anaphase transition and into the subsequent cell cycle whereas a phosphomimetic mutation accelerated degradation. Unexpectedly, the mutation that prevented phosphorylation also inhibited the removal of MCAK from centromeres causing it to remain attached throughout the cell cycle. Even low expression of phosphorylation-resistant MCAK delayed mitosis and interfered with cell division. Mitotic defects were also observed by overexpressing a GFP-tagged version of wild-type MCAK that similarly escaped degradation and accumulated to toxic levels, but didn't remain associated with kinetochores during interphase. The results demonstrate that degradation is an important mechanism for controlling the activity of MCAK.
Keywords: mitosis, kinesin, kif2c, GFP-MCAK, phosphorylation, cell cycle
Introduction
Kinesin related proteins comprise a large superfamily of ATP driven microtubule motor proteins characterized by highly homologous microtubule binding motor domains but a diverse array of structures and functions [Miki et al., 2005]. Many members of this family are designed to bind cargo and translocate it through the cell along microtubule tracks, but others play more specific roles in mitotic spindle assembly and chromosome segregation [Civelekoglu-Scholey and Scholey, 2010]. Members of one particularly interesting subclass of kinesin related proteins have an internal motor domain and have therefore been called Kin I kinesins, but a more recent classification system assigns these proteins to the kinesin-13 family [Lawrence et al., 2004]. One of the first members of the kinesin-13 family to be identified was mitotic centromere associated kinesin (MCAK) or Kif2c. Kin 13 proteins such as MCAK have been shown to bind microtubules in vitro, diffuse to the microtubule ends, and stimulate disassembly [Desai et al., 1999; Helenius et al., 2006]. This activity is believed to be important for the ability of MCAK and MCAK-like proteins to correct inappropriate microtubule attachments at kinetochores, thereby ensuring the faithful segregation of chromosomes during mitosis [Kline-Smith et al., 2004].
MCAK contains several phosphorylation sites that have been shown to be important for controlling the location and activity of the protein [Ems-McClung and Walczak, 2010]. In addition, recent evidence indicates that protein degradation plays an important role in regulating MCAK function. Mammalian MCAK and a homolog in Leishmania major have been shown to accumulate as the cell cycle progresses, reaching a maximum concentration in mitosis [Dubessay et al., 2006; Ganguly et al., 2008]. Once the mitotic checkpoint is satisfied by the alignment of chromosomes at the metaphase plate, the protein undergoes proteasomal degradation before being synthesized again during the next cell cycle [Ganguly et al., 2008]. It was previously reported that mitotic MCAK forms two bands on SDS gels and that the slower migrating band results from a mitosis-specific phosphorylation [Ganguly et al., 2008; Shimo et al., 2008]. We now report the identification of this phosphorylation site and demonstrate that it regulates MCAK stability and persistence at kinetochores.
Materials and Methods
Cell Lines and Antibodies
CHO cells [Cabral et al., 1980] were maintained in alpha modification of minimal essential medium (Mediatech Inc.) supplemented with 5% fetal bovine serum (Gemini Bio-Products). Mouse Flag-M2 and DM1α antibodies were purchased from Sigma-Aldrich, MCAK polyclonal antibody came from Cytoskeleton, and actin antibody C4 was from Millipore. All Alexa-conjugated secondary antibodies came from Invitrogen. Chemicals were from Sigma-Aldrich unless otherwise stated.
Cell Synchronization
CHO cells were synchronized by incubating them overnight in medium containing 5 mM thymidine, reversing the S-phase block for 4 h, adding 35 ng/ml nocodazole for approximately 3 h, and shaking off mitotic cells as previously described [Ganguly et al., 2008]. Cell cycle blockage was confirmed using a flow cytometer (Guava,Technologies, Hayward, CA) and also by staining a small aliquot of the cells with 2 μg/ml HOECHST 33342 and observing the chromosome organization by fluorescence microscopy. More than 90% of the mitotic shake-off cells were typically found to be in prometaphase.
Immunoprecipitation and Mass Spectrometry
A stable CHO cell line expressing 8X the endogenous level of FLAG-MCAK was synchronized in prometaphase, lysed in microtubule buffer (20 mM Tris-HCl, pH 6.8, 0.5% Nonidet P-40, 1 mM MgCl2, 2 mM EGTA and 140 mM NaCl), and centrifuged at 12,000 × g for 15 min at 4 °C to remove cellular debris. The supernatant was then incubated for 14 h at 4 °C with anti-FLAG antibodies (M2, Sigma) that were immobilized on Protein G agarose beads, the immunoprecipitate was dissolved in SDS sample buffer, and the proteins were resolved on a 7.5% polyacrylamide gel. Coomassie blue staining revealed two closely spaced major bands having a mobility consistent with MCAK. Each band was separately excised and sent for phosphopeptide mapping by mass spectrometry (Center for Functional Genomics, University at Albany, Rensselaer, NY).
The gel pieces were washed, reduced, alkylated, and digested with trypsin. Peptides extracted from the gel were enriched for phosphopeptides using a TiO2 column and then concentrated and dissolved in 5% formic acid for LC-MS/MS using a CapLC and Q-Tof2 (both from Waters Co., Milford, MA). PKL files containing mass and intensity values were generated using Masslynx 3.5 software (Waters). MASCOT 2.2 (Matrix Science, London, UK) was used to compare the data to the sequence of MCAK.
Site Directed Mutagenesis
Human MCAK cDNA (GenBank Accession No. BC014924) was obtained from the American Type Culture Collection and cloned into the tetracycline-regulated mammalian expression vector, pTOPneo [Ganguly et al., 2008; Gonzalez-Garay et al., 1999]. The MCAK cDNA also contained a FLAG epitope tag at the 5'-end for convenient detection of the protein. Site-directed mutagenesis was carried out with the QuickChange mutagenesis kit (Invitrogen) and all mutations were confirmed by sequencing.
Isolation of Stably Transfected Cell Lines
CHO tTA6.6a cells expressing a tetracycline-regulated transactivator [Gonzalez-Garay et al., 1999] were seeded into 35-mm tissue culture dishes and transfected with WT or mutant pTOP/FLAG-MCAK using Lipofectamine (Invitrogen). Following transfection, the cells were grown overnight in the presence of 1 μg/ml tetracycline, then trypsinized and replated in 100-mm dishes containing growth medium, 1 μg/ml tetracycline, and 2 mg/ml G418. After 10 days the drug resistant cells were trypsinized and stored as a total G418-resistant population. From the stable G418 population about 100 cells were plated on 60 mm dishes in tetracycline and G418 and incubated 7-10 days until they formed visible colonies. The colonies were isolated and screened by Western blot analysis for FLAG-MCAK production. Cell lines with low production (< 4 times endogenous MCAK) were chosen for further studies.
Immunofluorescence
Cells grown on glass coverslips were pre-extracted for 1 min at 4°C with microtubule buffer containing 4 μg/ml paclitaxel and then fixed in methanol at –20°C for 20 min. The fixed cells were stained with a 1:50 dilution of mouse FLAG-specific antibody M2 followed by goat antimouse IgG conjugated to Alexa 488 at a 1:100 dilution. The secondary antibody solution also contained 1 μg/ml DAPI to stain chromosomes.
Electrophoretic Techniques
Cells were lysed in 1% SDS, and the proteins were precipitated with 5 volumes of ice-cold acetone and then pelleted at 12,000 × g for 5 min. The pellet was solubilized in SDS sample buffer (0.0625 M Tris-HCl, pH 6.8, 2.5% SDS, 5% 2-mercaptoethanol, 10% glycerol), fractionated on 7.5% polyacrylamide SDS minigels, and transferred to a nitrocellulose membrane. The membranes were then blocked in 2% milk in PBS containing 0.05% Tween-20 (PBST) for 1 h, washed 3 times in PBST, and incubated in 1:2,000 dilutions of rabbit anti-MCAK or mouse anti-FLAG M2 antibody. A 1:30,000 dilution of actin antibody C4 or a 1:4,000 dilution of tubulin antibody DM1A was also added as a control for sample loading. Antibody incubations were carried out for 2 h at room temperature. Reactive bands were detected with 1:2,000 dilutions of Alexa 647-conjugated goat anti-mouse and anti-rabbit IgGs and visualized with a STORM 860 imager (Molecular Dynamics Inc.).
Results
Identification of Novel Phosphorylation Sites
SDS gel analysis has shown that MCAK from mitotic, but not interphase cells migrates as two distinct bands and that the upper (slower migrating) band reverts to normal mobility when the extracts are treated with alkaline phosphatase [Ganguly et al., 2008; Shimo et al., 2008]. It was further found that the upper band did not arise from phosphorylation by Aurora B, a kinase that is known to phosphorylate MCAK at multiple sites [Ganguly et al., 2008]. Finally, it was reported that the upper band was preferentially lost at metaphase, but it could be stabilized by treating the cells with the proteasomal inhibitor MG-132, thus implying that phosphorylation was a signal for MCAK degradation.
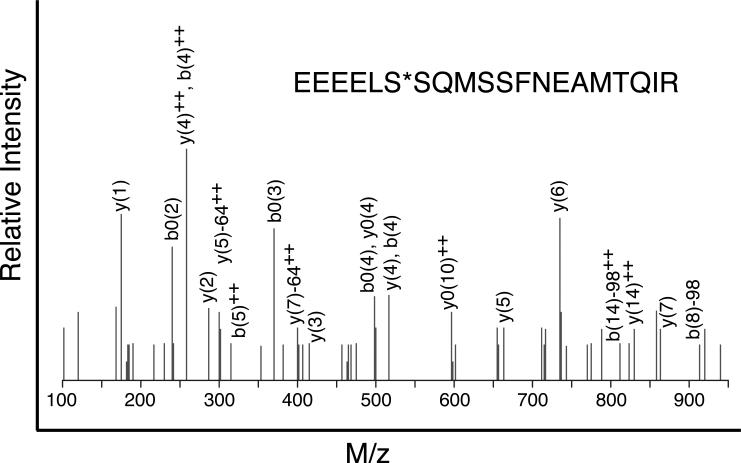
To identify the phosphorylation sites responsible for degradation, a mitotic cell lysate from CHO cells expressing FLAG-tagged human MCAK was prepared. A FLAG antibody immobilized on protein-G agarose beads was then added and proteins in the immunoprecipitate were resolved by SDS gel electrophoresis. Phosphate containing peptides from the upper and lower MCAK bands were enriched by TiO2 affinity chromatography and analyzed by mass spectrometry. This approach led to the identification of two phosphorylated amino acids, S166 and S628, that were present in the upper band, but not in the lower band. The MS/MS spectrum of the tryptic peptide containing S628 is shown in Fig. 1 and its location relative to the domain structure of MCAK is shown in Fig. S1.
Fig. 1. MS/MS fragmentation of the peptide containing S628.
The tryptic peptide containing S628 (indicated by an asterisk) was fragmented and analyzed by mass spectrometry. The fragmentation data indicated phosphorylation of S628.
Mutation of S628 and S629 Alters the Electrophoretic Mobility of MCAK
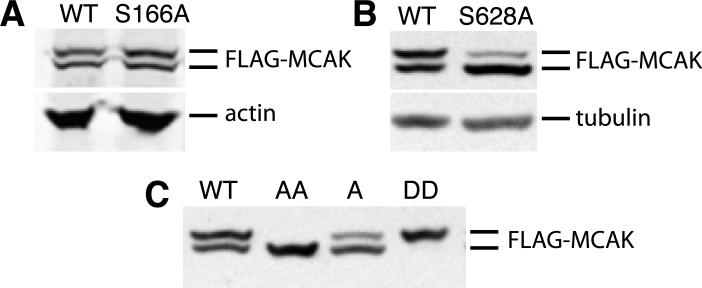
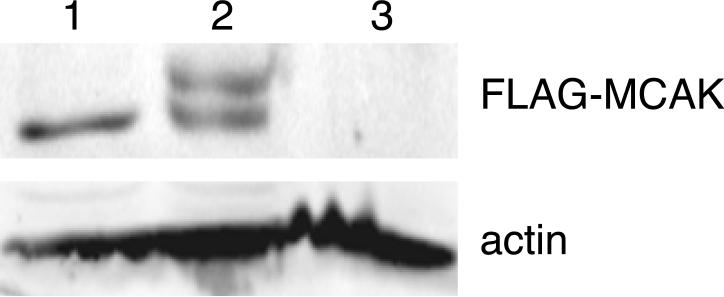
To determine whether either of the identified phosphorylation sites was responsible for altering the electrophoretic mobility of MCAK, wild-type as well as S166A and S628A mutants of Flag-MCAK were transfected into CHO cells using a tetracycline regulated expression vector, the cells were synchronized in mitosis, and lysates were compared for the presence or absence of the upper MCAK band (Fig. 2). As previously observed [Ganguly et al., 2008], wild-type FLAG-MCAK displayed approximately equal amounts of the upper and lower bands. Mutant S166A also had similar upper and lower band intensities (Fig. 2 A) but the S628A mutant exhibited a significant reduction in the intensity of the upper band (Fig. 2 B). The results suggested that phosphorylation of S628 was responsible, at least in part, for the altered mobility of MCAK on SDS gel electrophoresis.
Fig. 2. The effects of mutations on the electrophoretic mobility of MCAK.
Cells transfected with wild-type (WT) or mutant forms of FLAG-MCAK cDNA were synchronized in mitosis, lysed, and Western blotted with an antibody to the FLAG-tag. (A) WT and mutant S166A are compared. Actin was used as a loading control. (B) WT and mutant S628A are compared. Alpha-tubulin was used as a loading control. (C) WT and mutants S628A (lane A), S628A/S629A (lane AA), and S628D/S629D (lane DD) are compared.
Because there is a second serine at residue 629, we considered the possibility that preventing S628 phosphorylation might cause the kinase to phosphorylate S629, albeit at a lower efficiency. We tested this possibility by creating the S628A/S629A double mutant. Analysis of mitotic lysates revealed that the double mutant had no detectable upper band (Fig. 2 C), demonstrating that phosphorylation of S628 or S629 was necessary to retard the mobility of MCAK on SDS gels. This conclusion was further strengthened by creating the S628D/S629D phosphomimetic mutations which caused the loss of the lower MCAK band (Fig. 2 C).
Mutation of S628 and S629 Alters the Stability of MCAK
In our previous study it was found that phosphorylated, slowly migrating MCAK disappeared more quickly than the faster migrating MCAK as the cells progressed through mitosis, thus implying that the phosphorylated form of the protein might be preferentially degraded [Ganguly et al., 2008]. To determine whether phosphorylation of S628 triggers degradation, we analyzed the stability of mutant and wild type Flag- MCAK in transfected CHO cells using a “pulse-chase” experiment. FLAG-MCAK production was first induced by incubating the cells overnight without tetracycline, and the antibiotic was then added back to halt further transcription of the cDNA. Cells were sampled at various times following tetracycline addition and analyzed by western blots to determine how quickly the FLAG-MCAK protein disappeared. The results (Fig. 3) demonstrated that wild-type FLAG-MCAK was progressively depleted and largely lost by 12 h following tetracycline addition as previously reported [Ganguly et al., 2008]. The S628D/S629D double mutant designed to mimic the phosphorylated protein appeared to be even less stable and was barely detectable after only 8 h (Fig. 3A). In contrast, the S628A mutant protein disappeared at a slower rate than wild-type such that it was still detectable at 12 h, and the S628A/S629A double mutant was even more highly stabilized and prominent at 12 h.
Fig. 3. The effect of mutations at S628 and S629 on the stability of MCAK.
Cells transfected with FLAG-MCAK cDNA were incubated 16 h without tetracycline to induce expression and then lysed at varying times following re-addition of tetracycline to halt further transcription. (A) Western blots with antibodies to the FLAG tag and to actin as a loading control. The blots show the decrease in FLAG-MCAK for each of the 4 indicated cell lines with time. (B) Quantification of the data from blots similar to panel A. Band intensities were determined from fluorescence emission captured by a Storm 860 scanner and expressed as FLAG-MCAK/actin ratios. The ratio at each time point was normalized to zero time set at 100%. Averages from at least 3 independent experiments are plotted along with their corresponding standard deviations.
The results were plotted in Fig. 3B and gave relative half lives for the proteins of 4.5, 5.8, 7.4, and 15 h for the DD, WT, A, and AA versions of FLAG-MCAK respectively. However, it should be noted that these numbers likely underestimate the stability of the FLAG-MCAK and of the AA mutant in particular because the amount of protein was estimated relative to actin whose synthesis was not halted by the addition of tetracycline. If one factors in the doubling time for CHO cells, this would produce a 50% decrease in the FLAG-MCAK to actin ratio in 12 h by dilution alone (i.e., by a doubling of the amount of actin). This point is further strengthened by an experiment in which FLAG-MCAK was allowed to accumulate and compared to actin in cells that were induced by tetracycline removal. In this case, the FLAG-MCAK to actin ratio remained relatively constant over a 48 h period for the WT and DD proteins that were subject to degradation; but the ratio progressively increased for the degradation-resistant AA mutant (Fig. S2). Based on these observations, we conclude that there is very little degradation of the AA mutant.
The S628A/S629A Double Mutant Prolongs Mitosis and Inhibits Cell Division
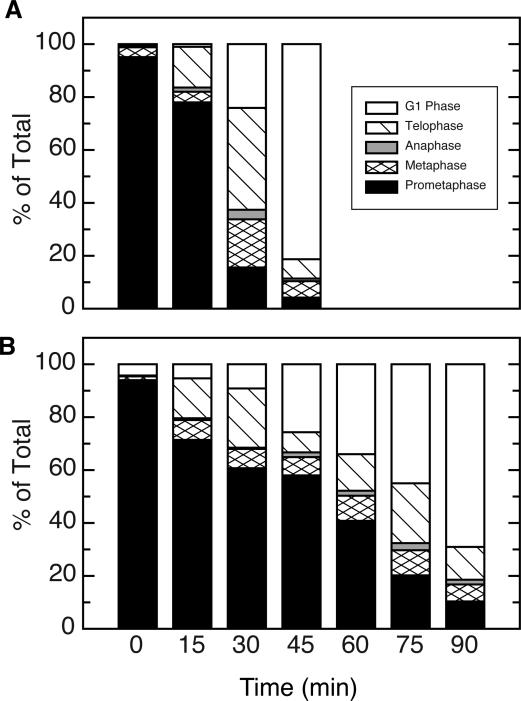
Because MCAK degradation is normally triggered at the metaphase to anaphase transition [Ganguly et al., 2008], we reasoned that the increased stability of the AA mutant could be due to blockage of the cells in prometaphase. To test this possibility, cells induced to express wild-type or mutant FLAG-MCAK were synchronized by successive thymidine and nocodazole blocks followed by shaking off mitotic cells and reversing the nocodazole block. The cells were then sampled at various times to determine their cell cycle stage. Most of the shake-off cells transfected with wild-type FLAG-MCAK (Fig. 4A) were initially in prometaphase and showed a steady progression through metaphase, anaphase, and telophase that resulted in an accumulation of G1-phase cells within 45 minutes. Cells transfected with the AA mutant (Fig. 4B) showed a similar progression, but the amount of time required to reach G1 phase was approximately 2-fold longer. The slower mitotic transit of cells producing the AA mutant protein appeared to result from a delay in the time required for cells to progress beyond prometaphase.
Fig. 4. Mitotic progression of cells expressing mutant MCAK.
Stable cell lines expressing WT (A) or S628A/S629A mutant forms of FLAG-MCAK (B) were induced to express the ectoptic proteins and synchronized by an overnight thymidine block, reversal, and addition of nocodazole. When the majority of cells reached mitosis, the mitotic cells were shaken off, the nocodazole was removed, and mitotic progression was monitored microscopically after staining with a membrane permeable DNA binding dye. The percentage of the total cells at each of the indicated stages of mitosis are plotted for WT (A) and the S628A/S629A mutant (B) as a function of time.
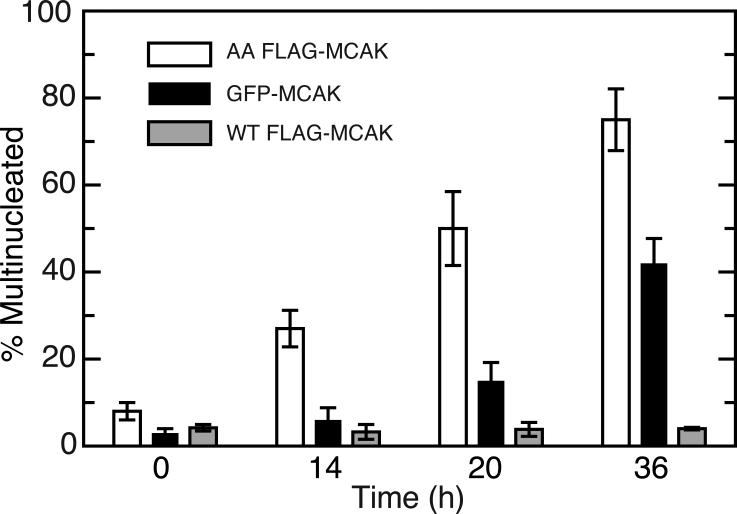
The 2-fold increase in time needed to complete mitosis was insufficient to account for the greatly increased stability of the 628A/629A FLAG-MCAK, but it did indicate that expression of the mutant protein interfered with normal mitotic progression. This toxicity of the AA mutant was further supported by the fact that, in contrast to the WT FLAG-MCAK, stable clones capable of expressing the mutant cDNA were difficult to isolate and tended to quickly lose expression even when the cells were maintained in tetracycline. Moreover, cells expressing the mutant, but not the WT FLAG-MCAK, become increasingly multinucleated when tetracycline was removed indicating that the cells experienced problems in cell division when the AA mutant protein was produced (open bars, Fig. 5).
Fig. 5. Multinucleation in cells expressing FLAG-MCAK.
Stably transfected cell lines able to produce 4 times (4X) the normal MCAK levels for FLAG-MCAK, 3X for GFP-MCAK, and 1.5X for AA FLAG-MCAK (all measured after 16 h of expression) were induced by removing tetracycline, fixed at varying times after induction, stained with DAPI and an antibody to MCAK, and viewed by immunofluorescence. The percentage of cells that were multinucleated (i.e., had micronuclei, multinuclei, or larger than diploid nuclei) at each time point are plotted. At least 300 cells were counted at each time point and the experiment was repeated 3 times. Averages with their corresponding standard deviations are shown.
Addition of a GFP Tag Stabilizes MCAK
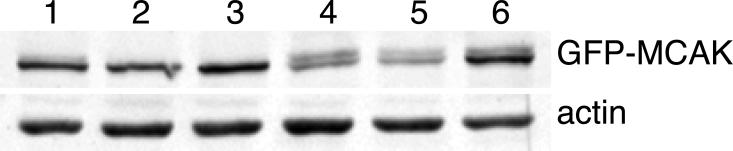
The toxicity of the 628A/629A double mutant could have resulted from a failure of the cell to degrade the mutant protein, or from other structural or functional problems arising from the amino acid substitutions. To distinguish between these possibilities, we compared the accumulation of the AA mutant with wild-type, the DD mutant, and a GFP-tagged version of wild-type MCAK (Fig. S2). As expected, the stabilized AA mutant protein, but not the wild-type or the destabilized DD mutant protein, accumulated over time. Unexpectedly, we found that GFP-MCAK also accumulated over time indicating that the addition of a large N-terminal tag is able to stabilize the protein. Like the AA mutant, and in contrast to FLAG-MCAK, GFP-MCAK also interfered with cell division (Fig. 5). The simple conclusion from these observations is that stabilization of MCAK is toxic to the cell because it accumulates to unacceptable levels. This conclusion is consistent with previous studies showing that low expression (2-4 time the endogenous MCAK level) of FLAG-MCAK is well tolerated, but 6-8 fold overexpression is toxic to CHO cells [Ganguly et al., 2011a]. However, the AA mutant appeared to exhibit toxicity beyond its ability to accumulate (compare the degree of multinucleation in cells expressing 1.5X AA mutant versus 3X GFP-MCAK in Fig. 5). Like the AA mutant, we were also not able to obtain stable clones of the DD mutant and the transiently transfected cells rapidly lost expression, indicating that modification of S628/S629 to inhibit or mimic phosphorylation produces toxic effects irrespective of their effects on protein stability. Together, these observations suggest that phosphorylation of the 628/629 sites may have direct effects on MCAK function.
To determine whether GFP was able to stabilize MCAK because it interfered with phosphorylation of S628/S629, cells were synchronized by successive thymidine and nocodazole blocks, and mitotic cell lysates were examined on Western blots using an antibody to MCAK (Fig. 6). The results revealed a slower migrating form of GFP-MCAK that appeared during prometaphase and persisted through telophase and into the next cell cycle, indicating that the presence of GFP at the N-terminus did not prevent phosphorylation of residues S628/S629. Moreover, the extent of phosphorylation of GFP-MCAK appeared to be similar to FLAG-MCAK in prometaphase cells blocked in nocodazole (Fig. 2 and see [Ganguly et al., 2008], Fig. 3A, lane 5). GFP-MCAK thus provides a good control for discriminating between the effects of MCAK stabilization and other changes that may result from blocking the phosphorylation of MCAK.
Fig. 6. Phosphorylation of GFP-MCAK.
A stable cell line expressing GFP-MCAK was synchronized and the protein was detected on Western blots using an antibody to MCAK. An antibody to actin was also included as a loading control. Lane 1, unsynchronized cells; lane 2, cells blocked in thymidine overnight (cells in S-phase); lane 3, 4 h after release from thymidine (cells in late S-phase); lane 4, cells blocked in nocodazole (cells in prometaphase); lane 5, 30 min following release from nocodazole (cells mostly in telophase); lane 6, 2 h after release from nocodazole (cells in G1). Note the presence of a slower migrating GFP-MCAK band during and after mitosis (lanes 3-6).
Phosphorylation of S628/S629 is Required to Release FLAG-MCAK From Centromeres
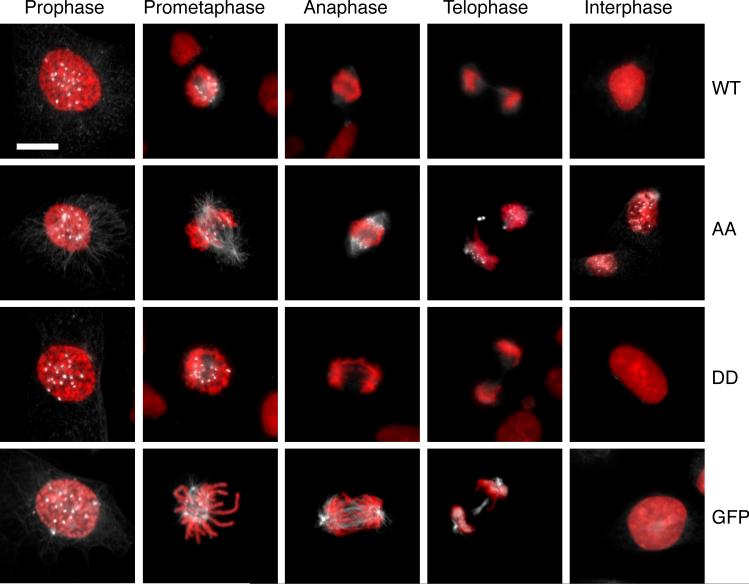
To determine why the AA mutant might be causing mitotic defects at low expression levels, we examined its localization. We previously reported that FLAG-MCAK is found at the same sites as the endogenous protein [Ganguly et al., 2008]. During interphase it was found associated with centrosomes, diffusely localized in the cytoplasm and nucleus, and weakly associated with cellular microtubules. When cells entered prophase, there was a dramatic reorganization of the FLAG-MCAK to mitotic centromeres and spindle poles as well as continued diffuse cytoplasmic and weak spindle microtubule localization. FLAG-MCAK fluorescence remained strong during prometaphase but was greatly reduced once the cells progressed beyond metaphase. Centromere staining continued to be weak during anaphase and telophase (Fig. 7).
Fig. 7. Localization of MCAK.
Cells expressing the indicated forms of MCAK were fixed and stained with an antibody to MCAK (white) and with DAPI (red). Cells in various stages of mitosis and in interphase are shown. Bar = 10 μm.
The AA mutant protein displayed a similar localization pattern during the early stages of mitosis, but did not disappear following metaphase and, strikingly, remained associated with centromeres even in interphase cells (Fig. 7). In contrast to the AA mutant, the DD mutant produced a staining pattern similar to the wild-type protein, indicating that mutation of the 628/629 site per se was insufficient to cause retention of the protein on centromeres. We therefore reasoned that the continued presence of the AA mutant FLAG-MCAK on the centromeres during interphase might have simply resulted from its failure to be degraded, or from an inability of the phosphorylation-resistant protein to be released. To distinguish between these possibilities, we examined the localization of GFP-MCAK and found that it also remained attached to centromeres during anaphase and telophase indicating that simply stabilizing the protein was at least partially responsible for its continued binding during the latter stages of mitosis. However, in contrast to the AA mutant, GFP-MCAK was absent from centromeres during interphase (Fig. 7). Our tentative conclusion from these observations is that phosphorylation of S628/S629 is not only required for degradation of MCAK, but it may also be required to release MCAK from centromeres.
Phosphorylation of S628 Occurs During Prometaphase
The binding of MCAK to centromeres occurs during prophase and has been shown to require phosphorylation by Aurora B kinase [Andrews et al., 2004; Lan et al., 2004]. Because degradation of the protein predominantly occurs at the metaphase/anaphase transition and is not dependent on Aurora B, we reasoned that phosphorylation of S628 should occur later in mitosis. To test this possibility, we synchronized CHO cells and used a shake-off procedure to isolate mitotic cells. DNA staining revealed that the cells remaining on the dish were approximately 70% in prophase with the remainder in interphase, whereas the cells that were removed from the dish by shaking were predominantly (85-90 %) in prometaphase. SDS gel electrophoresis of the cell lysates (Fig. 8) showed that an upper (S628 phosphorylated) band was not detected in the prophase cells (lane 1), but was prominent in the prometaphase population (lane 2). By the time the cells reached telophase (lane 3), both bands were missing consistent with degradation of the protein at the metaphase to anaphase transition. The results support a model in which phosphorylation by Aurora B occurs early in mitosis to target MCAK to centromeres, and phosphorylation of S628 occurs later in mitosis to allow removal of MCAK from centromeres and target it for degradation.
Fig. 8. Phosphorylation of S628 during mitosis.
Cells expressing WT FLAG-MCAK were synchronized using an overnight thymidine block. Tetracycline was then added during the following steps to prevent further transcription of the cDNA. The cells were reversed from thymidine for 4 h followed by nocodazole treatment. When the majority of cells reached mitosis, mitotic cells were shaken off and released from the nocodazole block. Proteins were analyzed on Western blots with antibodies to FLAG and actin (as a loading control). Lane 1, cells remaining on the dish after the mitotic shake-off. Approximately 70% of these cells were in prophase. Lane 2, cells in nocodazole. Greater than 90% of the cells were in prometaphase. Lane 3, 30 min after reversal of the nocodazole block. The majority of the cells were in telophase or early G1. Note the presence of a slower migrating band during prometaphase indicating phosphorylation at S628, and the absence of MCAK in cells that have reached telophase.
Discussion
MCAK plays important roles in spindle assembly and function. Its location at mitotic centromeres and kinetochores has been linked to the correction of inappropriate microtubule attachments that would otherwise lead to missegregation of chromosomes [Kline-Smith et al., 2004]. At spindle poles, the presence of MCAK-like proteins in Drosophila has been implicated in the depolymerization of spindle microtubules to produce a flux of tubulin subunits involved in the poleward movement of sister chromatids [Rogers et al., 2004]. More recently, MCAK has been shown to catalyze the detachment of microtubules from spindle poles and to mediate the effects of tubulin binding drugs on cell division [Ganguly et al., 2011a; Ganguly et al., 2011b]. The importance of MCAK has been further highlighted by in vitro antibody depletion experiments and in vivo inhibitory RNA studies that resulted in altered spindle morphology as well as an elevated frequency of lagging chromosomes that in turn led to increased cell death or multinucleation [Ganguly et al., 2011b; Kline-Smith et al., 2004; Kline-Smith and Walczak, 2002; Walczak et al., 1996]. Overexpression of MCAK has similarly been shown to cause defects in cell division that lead to cell death or multinucleation [Ganguly et al., 2011a; Holmfeldt et al., 2004; Kline-Smith and Walczak, 2002]. The isolation of multiple stably transfected cell lines with tetracycline regulated expression has further shown that small increases in MCAK production (2-4 fold) are well tolerated, but 6-8 fold overexpression is toxic [Ganguly et al., 2011a]. It is thus critical that cells be able to regulate their levels of MCAK and the way this has been accomplished is to synthesize and accumulate new protein during each cell cycle and then degrade it during mitosis [Ganguly et al., 2008].
The current studies demonstrate that the degradation of MCAK is controlled by phosphorylation of residues S628/S629. Although mutation of both residues was necessary to fully stabilize the protein due to secondary phosphorylation of S629 when residue S628 is mutated, phosphorylation of S628 alone is probably sufficient to trigger degradation because S629 phosphorylation was not detected in the wild-type protein by mass spectrometry analysis. The possibility that the S628A/S629A double mutant was stabilized because of changes in structure rather than because phosphorylation was inhibited is unlikely because a S628D/S629D phosphomimetic mutant was degraded even more efficiently than the wild-type protein.
The identified phosphorylation site shares homology among mammalian, avian, and amphibian species (Fig. S1) but the homology doesn't extend to Leishmania LmjKIN13-1, an MCAK-like protein that also undergoes cell cycle dependent degradation [Dubessay et al., 2006]. Thus, the phosphorylation site appears to be conserved among vertebrates, but it may have diverged considerably from the site in lower organisms. A scan of databases using the sequence around S628 did not reveal a clear consensus for any kinase, and our initial use of inhibitors to identify the kinase responsible for phosphorylation have thus far been unsuccessful. Similarly, MCAK does not have obvious D-box, KEN-box, or other consensus sites for destruction [Glotzer et al., 1991; Pfleger and Kirschner, 2000; Song and Rape, 2011] and so the sequence that serves as the actual degradation signal is not yet known. S628/S629 is located in the C-terminal region of MCAK, an area that has been implicated in dimerization [Ems-McClung et al., 2007; Maney et al., 2001], but there is no evidence that mutation of this site produces any changes in the quaternary structure of the protein. On the other hand, there is considerable evidence from domain mapping studies to suggest that the C-terminal region is able to interact functionally, and perhaps physically, with the N-terminal or neck region of the protein [Ems-McClung et al., 2007]. This view is consistent with our observation that addition of GFP to the N-terminus is able to stabilize the protein even though it doesn't appear to affect phosphorylation of S628/S629. The ability of changes in the N-terminus as well as C-terminal region to affect the stability of the protein suggests the possibility that phosphorylation of the S628/S629 site might act by altering N-C interactions.
Production of the AA mutant proved to be toxic. Stable clones were difficult to isolate and cells rapidly lost expression of the cDNA even though its transcription was under tetracycline regulation, suggesting that even the small amount of mutant production resulting from leakiness of the tetracycline block was enough to cause toxicity. Part of this toxicity likely resulted from the accumulation of the mutant protein to unacceptable levels as evidenced by the observation that a GFP-tagged version of the wild-type protein also was stabilized, accumulated with time, and exhibited toxicity. However, the observation that delayed mitotic progression and defective cytokinesis (as indicated by the presence of multinucleated cells) was evident with low production and at early times after induction of the AA mutant suggests that the AA mutations may also be able to more directly interfere with the function of the protein. It thus appeared that failure to degrade MCAK could cause eventual toxicity because of protein accumulation, but that the failure to phosphorylate the AA mutant was causing more immediate problems.
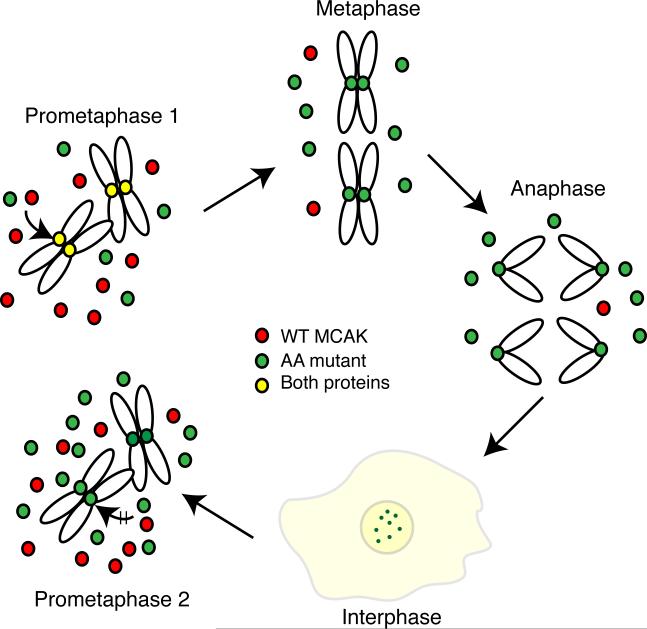
A potential cause for the enhanced toxicity of the AA mutant compared to the stabilized GFP-MCAK may be traced to its ability to remain associated with mitotic centromeres throughout the cell cycle. As summarized in Fig. 9, newly synthesized AA mutant (green) does not bind to centromeres until cells enter mitosis, whereupon it is phosphorylated by Aurora B kinase and competes with endogenous WT MCAK (red) for binding to centromeres (colored yellow to indicate the presence of both WT and AA mutant). Following metaphase, the WT protein is released and degraded, but the AA mutant persists at centromeres (now colored green) and remains attached throughout the cell cycle and into the next mitosis. We speculate that “old” AA mutant protein carried over from the previous mitosis may interfere more strongly with spindle assembly and function because there is now more mutant protein relative to WT protein, the presence of the AA mutant at the centromere may prevent the binding of newly synthesized MCAK, and the “old” AA mutant protein may retain some of the phosphorylations that were carried out during the previous mitosis. The presence of such modified AA mutant might then interfere with the regulation that normally takes place during mitotic progression, a situation that doesn't occur with WT MCAK because of its degradation after metaphase. This scenario does not rule out the possibility that there may also be other more direct effects on MCAK function caused by preventing the phosphorylation of S628/S629.
Fig. 9. Model showing the effects of the S628A/S629A mutations on MCAK function.
After the induction of the AA mutant, cells enter the first mitosis (prometaphase 1) with naive endogenous WT (red) and ectopic AA mutant (green) proteins that require Aurora B kinase phosphorylation before they can bind to centromeres (yellow to show the presence of both endogenous and AA mutant). Once cells attain a stable metaphase configuration, the WT protein is degraded because it is phosphorylated on residue 628 leaving only the AA mutant protein on centromeres. The AA mutant protein persists through anaphase, telophase, and into the next cell cycle where it, but not the newly synthesized WT protein, remains bound to centromeres. Diffuse staining is also seen for both WT and mutant proteins (light yellow color). As cells enter the next mitosis (prometaphase 2), the amount of mutant protein is higher relative to the WT protein because of its failure to be degraded during the previous mitosis. We propose that toxicity results from the increased total amount of MCAK as well as the centromeric localization of modified AA mutant protein carried over from the previous cell cycle that may disrupt the binding of newly synthesized naive MCAK and interfere with the phosphorylations that normally regulate MCAK activity during mitosis.
In summary, we identified a phosphorylation site that controls the cell cycle degradation of MCAK and demonstrated that failure to phosphorylate this site stabilizes the protein and prevents its removal from centromeres. We further showed that adding GFP to the N-terminus of MCAK also stabilizes the protein and causes eventual toxicity without inhibiting the phosphorylation of S628/S629 and without preventing the removal of the protein from centromeres. The results demonstrate the importance of cell cycle degradation of MCAK and suggest that phosphorylation of the site that controls degradation may also regulate the removal of MCAK from centromeres.
Supplementary Material
Acknowledgments
We thank Dr. Jeff Frost of our department for helpful advice on phosphorylation and Dr. David Hawke at the M.D. Anderson Cancer Center for assistance with interpreting the mass spectrometry data. These studies were supported by the National Institutes of Health Grant CA85935 to FC.
Abbreviations
- CHO
Chinese hamster ovary
- MCAK
mitotic centromere associated kinesin
- MEM
minimum essential medium
- DAPI
4'-6-Diamidino-2-phenylindole
References
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- Cabral F, Sobel ME, Gottesman MM. CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered β-tubulin. Cell. 1980;20:29–36. doi: 10.1016/0092-8674(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Civelekoglu-Scholey G, Scholey JM. Mitotic force generators and chromosome segregation. Cell Mol Life Sci. 2010;67:2231–2250. doi: 10.1007/s00018-010-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Dubessay P, Blaineau C, Bastien P, Tasse L, Van Dijk J, Crobu L, Pages M. Cell cycle-dependent expression regulation by the proteasome pathway and characterization of the nuclear targeting signal of a Leishmania major Kin-13 kinesin. Mol Microbiol. 2006;59:1162–1174. doi: 10.1111/j.1365-2958.2005.05013.x. [DOI] [PubMed] [Google Scholar]
- Ems-McClung SC, Hertzer KM, Zhang X, Miller MW, Walczak CE. The interplay of the N- and C-terminal domains of MCAK control microtubule depolymerization activity and spindle assembly. Mol Biol Cell. 2007;18:282–294. doi: 10.1091/mbc.E06-08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ems-McClung SC, Walczak CE. Kinesin-13s in mitosis: Key players in the spatial and temporal organization of spindle microtubules. Semin Cell Dev Biol. 2010;21:276–282. doi: 10.1016/j.semcdb.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Yang H, Cabral F. Overexpression of mitotic centromere-associated kinesin stimulates microtubule detachment and confers resistance to Paclitaxel. Mol Cancer Ther. 2011a;10:929–937. doi: 10.1158/1535-7163.MCT-10-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Yang H, Pedroza M, Bhattacharya R, Cabral F. Mitotic centromere associated kinesin (MCAK) mediates paclitaxel resistance. J Biol Chem. 2011b;286:36378–36384. doi: 10.1074/jbc.M111.296483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Bhattacharya R, Cabral F. Cell cycle dependent degradation of MCAK: evidence against a role in anaphase chromosome movement. Cell Cycle. 2008;7:3187–3193. doi: 10.4161/cc.7.20.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garay ML, Chang L, Blade K, Menick DR, Cabral F. A β-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J Biol Chem. 1999;274:23875–23882. doi: 10.1074/jbc.274.34.23875. [DOI] [PubMed] [Google Scholar]
- Helenius J, Brouhard G, Kalaidzidis Y, Diez S, Howard J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature. 2006;441:115–119. doi: 10.1038/nature04736. [DOI] [PubMed] [Google Scholar]
- Holmfeldt P, Stenmark S, Gullberg M. Differential functional interplay of TOGp/XMAP215 and the KinI kinesin MCAK during interphase and mitosis. EMBO J. 2004;23:627–637. doi: 10.1038/sj.emboj.7600076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol Biol Cell. 2004;15:1146–1159. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Smith SL, Walczak CE. The microtubule destabilizing kinesin XKCM1 regulates microtubule dynamic instability in cells. Mol Biol Cell. 2002;13:2718–2731. doi: 10.1091/mbc.E01-12-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy AS, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L. A standardized kinesin nomenclature. J Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney T, Wagenbach M, Wordeman L. Molecular dissection of the microtubule depolymerizing activity of mitotic centromere-associated kinesin. J Biol Chem. 2001;276:34753–34758. doi: 10.1074/jbc.M106626200. [DOI] [PubMed] [Google Scholar]
- Miki H, Okada Y, Hirokawa N. Analysis of the kinesin superfamily: insights into structure and function. Trends Cell Biol. 2005;15:467–476. doi: 10.1016/j.tcb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes & Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- Rogers GC, Rogers SL, Schwimmer TA, Ems-McClung SC, Walczak CE, Vale RD, Scholey JM, Sharp DJ. Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature. 2004;427:364–370. doi: 10.1038/nature02256. [DOI] [PubMed] [Google Scholar]
- Shimo A, Tanikawa C, Nishidate T, Lin ML, Matsuda K, Park JH, Ueki T, Ohta T, Hirata K, Fukuda M, Nakamura Y, Katagiri T. Involvement of kinesin family member 2C/mitotic centromere-associated kinesin overexpression in mammary carcinogenesis. Cancer Sci. 2008;99:62–70. doi: 10.1111/j.1349-7006.2007.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Rape M. Substrate-specific regulation of ubiquitination by the anaphase-promoting complex. Cell Cycle. 2011;10:52–56. doi: 10.4161/cc.10.1.14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.