Abstract
Placing a patient in a state of general anesthesia is crucial for safely and humanely performing most surgical and many nonsurgical procedures. How anesthetic drugs create the state of general anesthesia is considered a major mystery of modern medicine. Unconsciousness, induced by altered arousal and/or cognition, is perhaps the most fascinating behavioral state of general anesthesia. We perform a systems neuroscience analysis of the altered arousal states induced by five classes of intravenous anesthetics by relating their behavioral and physiological features to the molecular targets and neural circuits at which these drugs are purported to act. The altered states of arousal are sedation-unconsciousness, sedation-analgesia, dissociative anesthesia, pharmaco-logic non-REM sleep, and neuroleptic anesthesia. Each altered arousal state results from the anesthetic drugs acting at multiple targets in the central nervous system. Our analysis shows that general anesthesia is less mysterious than currently believed.
Keywords: dexmedetomidine, droperidol, ketamine, opioids, propofol
Introduction
The practice of administering general anesthesia, which began nearly 165 years ago, revolutionized medicine by transforming surgery from trauma and butchery into a humane therapy (Bigelow 1846, Long 1849, Fenster 2001, Boland 2009). In the United States, 21 million patients receive general anesthesia each year for surgical procedures alone (Jt. Comm. 2004). General anesthesia is also essential for conducting many nonsurgical diagnostic and therapeutic procedures. Significant improvements have been made in anesthetic drugs (Schuttler & Schwilden 2008), monitoring standards (Eichhorn et al. 1986), monitoring devices (Ali et al. 1970, 1971; Tremper & Barker 1989; Kearse et al. 1994), and delivery systems (Wuesten 2001, Sloan 2002). Nevertheless, debate continues in anesthesiology over how best to define general anesthesia (Eger 1993, Kissin 1993). How anesthetic drugs create the state of general anesthesia is considered one of the biggest mysteries of modern medicine (Kennedy & Norman 2005).
Studies of the mechanisms of general anesthesia focus on characterizing the actions of anesthetic drugs at molecular targets in the central nervous system (Hemmings et al. 2005, Grasshoff et al. 2006, Franks 2008). This work has been crucial for identifying molecular and pharmacological principles that underlie anesthetic drugs and for establishing the existence of multiple mechanisms of anesthetic action. Studies of the pharmacokinetic and pharmaco-dynamic properties of anesthetics provide the main guidelines for drug dosing (Shafer et al. 2009). Neurophysiological (Angel 1991, 1993; Gredell et al. 2004; Velly et al. 2007; Bieda et al. 2009; Breshears et al. 2010) and functional imaging studies of general anesthesia (Alkire et al. 1995, Veselis et al. 1997, Bonhomme et al. 2001, Purdon et al. 2009, Mhuircheartaigh et al. 2010) are helping to identify the neural circuits involved in creating the states of general anesthesia. Furthermore, the behavioral and physiological changes observed when anesthetics are administered in clinical practice provide important insights into their mechanisms. Deciphering the mechanisms of general anesthesia requires a systems neuroscience analysis—that is, relating the actions of the drugs at specific molecular targets in specific neural circuits to the behavioral and physiological states that comprise general anesthesia (Brown 2007).
To develop such an analysis we define general anesthesia as a drug-induced, reversible condition composed of the behavioral states of unconsciousness, amnesia, analgesia, and immobility along with physiological stability (Brown et al. 2010). Of the behavioral states, unconsciousness is perhaps the most fascinating. Patients often admit that the unknown of being unconscious is their biggest fear about undergoing general anesthesia. Unconsciousness can be achieved by altering a patient's level of arousal and/or cognition. Anesthesiologists use different drugs to alter arousal in order to create the state of general anesthesia. We review the altered arousal states of five classes of intravenous anesthetic drugs: gamma-amino butyric acid type A (GABAA) receptor agonists, opioid receptor agonists, N-methyl D-aspartate receptor (NMDA) antagonists, alpha 2 receptor agonists, and dopamine receptor antagonists. We demonstrate that each altered arousal state can be characterized in terms of its specific behavioral and physiological features, as well as the molecular targets and neural circuits in which the drugs are purported to act. For each drug, we summarize these features in a table at the end of each section.
Sedation and Unconsciousness: GABAA Receptor Agonists
Clinical Features of Altered Arousal of GABAA Agonists
The anesthetic drugs that act as agonists at GABAA receptors are used primarily to induce sedation or unconsciousness. These drugs, which include propofol, sodium thiopental, methoxital, and etomidate, belong to different drug classes. Propofol is 2,6 di-isopropyl-phenol (James & Glen 1980), sodium thiopental and methohexital are barbiturates (Russo & Bressolle 1998), and etomidate is a carboxylated imidazole derivative (Vanlersberghe & Camu 2008). All act at GABAA receptors to enhance inhibition. Their effects depend on how much and how rapidly they are administered.
Administration of small doses of a GABAA hypnotic induces sedation. If the dose is increased slowly, the patient can enter a state of paradoxical excitation or disinhibition defined by euphoria or dysphoria, incoherent speech, purposeless or defensive movements, and increased electroencephalogram (EEG) oscillations in the beta range (13–25 Hz).
The excitatory state is termed paradoxical because a drug given to induce sedation or unconsciousness results in excitation (Brown et al. 2010). Paradoxical excitation frequently occurs when GABAA agonists are administered as sedatives for colonoscopies, dental extractions, or radiological procedures (Fulton & Mullen 2000, Tung et al. 2001). Methohexitalis distinct from other hypnotics because small doses of it can induce seizures (Rockoff & Goudsouzian 1981). Therefore, methohexital is used as an induction agent during electroconvulsive therapy because the therapeutic benefit of this procedure is related to the quality of the seizure activity it induces (Avramov et al. 1995). Low-dose methohexital is also used during cortical mapping studies to help identify seizure foci (Hufnagel et al. 1992).
If the hypnotic is administered as a bolus dose over 5 to 10 seconds, as is typical for induction of general anesthesia, then within 20 to 30 seconds, the patient becomes unresponsive to verbal commands, regular breathing transitions to apnea, and ventilation by bag and mask must be started to support breathing (Brown et al. 2010). Loss of consciousness can be easily tracked during drug administration on induction by having the patient execute a cognitive task such as counting backward or performing smooth eye pursuit of the anesthesiologist's finger. Patients counting backward rarely go beyond 10 digits. With loss of consciousness, the smooth pursuit excursions decrease then stop; blinking increases; nystagmus may appear; and the eyelash, corneal and oculocephalic reflexes are lost. The pupillary response to light remains intact (Brown et al. 2010). With propofol, a concomitant loss of skeletal muscle tone occurs (Brown et al. 2010), whereas with etomidate, the loss of muscle tone may be preceded by myoclonus (Van Keulen & Burton 2003). The EEG typically shows alpha and delta oscillations or burst suppression. The vasodilatory and myocardial depressant effects of the hypnotic drugs generally cause a decrease in blood pressure that leads to a baroreceptor reflex-mediated increase in heart rate. An opioid is frequently administered prior to or during induction to help mitigate the rise in heart rate, and vasopressors may be administered to maintain blood pressure. After the anesthesiologist administers a muscle relaxant, the patient is intubated, and maintenance of general anesthesia is achieved by a combination of inhalational agents, opioids, hypnotic agents, muscle relaxants, sedatives, and cardiovascular medications with ventilation and thermoregulatory support.
Mechanisms of GABAA-Mediated Altered Arousal
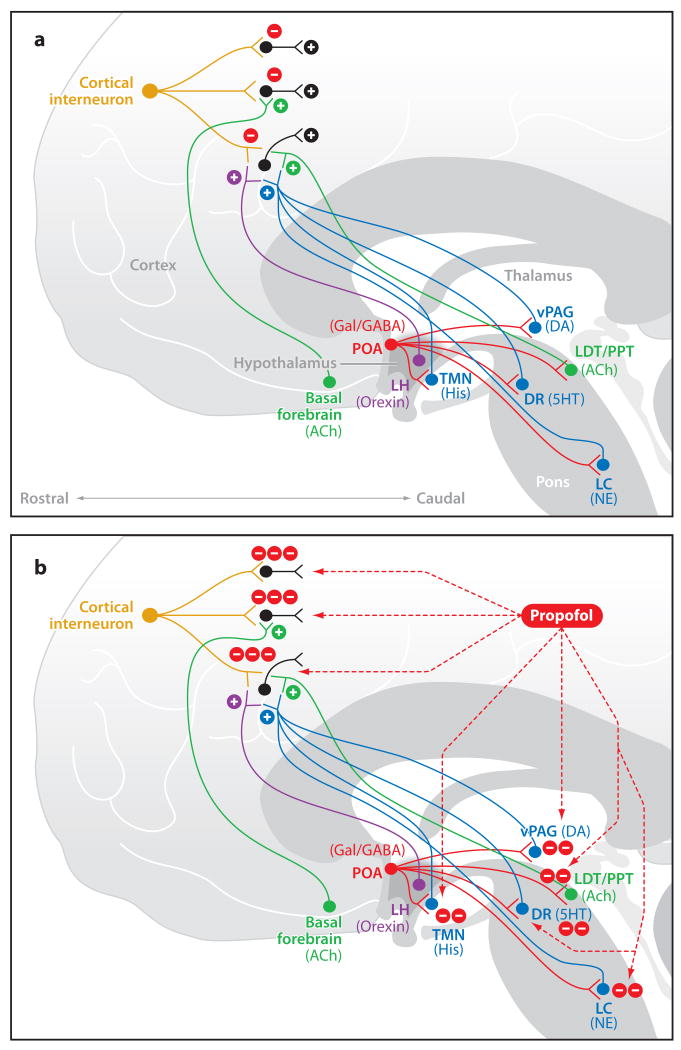
GABAA receptors are widely distributed throughout the brain (Bowery et al. 1987). Molecular studies have identified GABAA receptors as the targets of anesthetic drugs (Hemmings et al. 2005, Grasshoff et al. 2006). Binding of a GABAA hypnotic to the GABA receptors helps maintain postsynaptic chloride channels in the open position, thereby enhancing the inward chloride current, which hyperpolarizes the pyramidal neurons (Bai et al. 1999). For example, a pyramidal neuron in the cortex receives excitatory inputs from each of the major cholinergic, monoaminergic, and orexinergic arousal pathways as well as inhibitory inputs from local inhibitory interneurons (Figure 1a). During wakefulness, there is a balance between the pyramidal neuron's excitatory and inhibitory inputs. Because small numbers of inhibitory interneurons control large numbers of excitatory pyramidal neurons, hypnotic-induced enhancement of GABAA inhibition can efficiently inactivate large brain regions (Figure 1b) (Markram et al. 2004). Several neurophysiological and imaging studies support the idea that cortical sites are targets of GABAergic hypnotics (Angel 1991, Alkire et al. 1995, Velly et al. 2007, Purdon et al. 2009, Breshears et al. 2010, Ferrarelli et al. 2010).
Figure 1.
GABAergic signaling during the awake state (a) and during propofol administration (b). (a) A cortical interneuron during wakefulness mediating control of pyramidal neurons and being modulated by ascending arousal centers. Also shown are inhibitory projections from the POA to the arousal-promoting nuclei. (b) Propofol enhances GABAergic transmission in the cortex and at the inhibitory projections from the POA to the arousal centers. Abbreviations: 5HT, serotonin; ACh, acetylcholine; DA, dopamine; DR, dorsal raphe; GABA, gamma aminobutyric acid; Gal, galanin; His, histamine; LC, locus coeruleus; LDT, laterodorsal tegmental area; LH, lateral hypothalamus; NE, norepinephrine; POA, preoptic area; PPT, pedunculopontine tegmental area; TMN, tuberomamillary nucleus; vPAG, ventral periaquaductal gray.
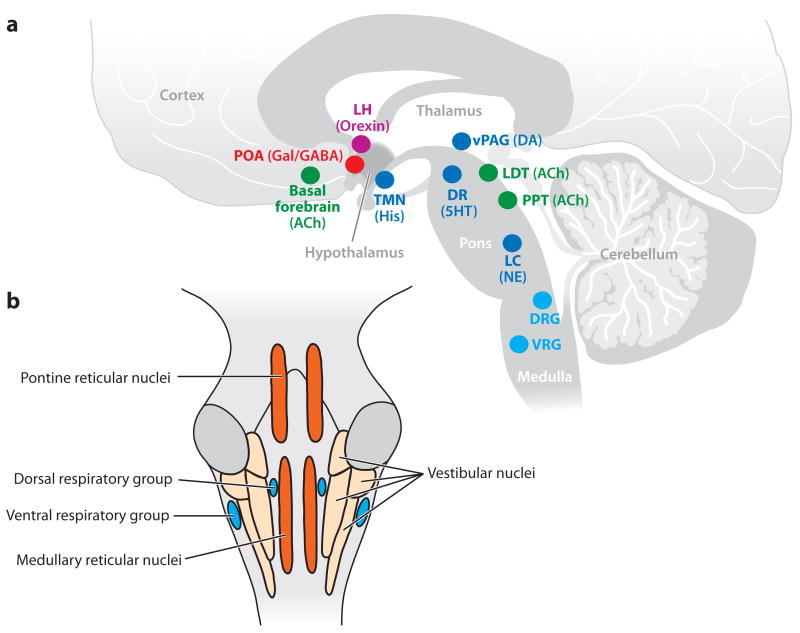
When administered as a bolus for induction of general anesthesia, the hypnotic rapidly reaches the GABAergic neurons in the respiratory centers in the pons and medulla (Feldman et al. 2003) and the arousal centers in the pons, midbrain, hypothalamus and basal forebrain (Saper et al. 2005) (Figure 2a). The clinical signs are consistent with inhibitory actions in the brainstem. Loss of the oculocephalic and corneal reflexes is a nonspecific indicator of impaired brain-stem function due to the actions of the hypnotic agent on the nuclei that control eye movements in the midbrain and pons (Posner et al. 2007). Basic science studies have shown that unconsciousness can result from direct injection of a barbiturate into the mesopontine tegmental area (Devor & Zalkind 2001). Brain injury studies show that brainstem coma typically involves bilateral lesions in either the midbrain or pontine tegementum (Parvizi & Damasio 2003). The preoptic area (POA) of the hypothalamus provides GABAergic inhibition to the principal arousal centers in the hypothalamus, midbrain, and pons (Saper et al. 2005) (Figure 1a). These GABAergic synapses onto pyramidal cells in the arousal centers are likely sites of action of the GABAergic hypnotics (Figure 1b). Inhibition of these ascending arousal centers decreases their input to the cortex and hence decreases cortical activation. The actions of the hypnotic on GABAA interneurons in the respiratory control network in the ventral medulla are most likely responsible for the apnea (Feldman et al. 2003).
Figure 2.
Sites of major nuclei that regulate arousal and respiration. (a) A sagittal diagram of a human brain with cholinergic nuclei (green), monoaminergic nuclei (dark blue), GABAergic and galanergic nuclei (red), orexin nuclei (pink), and the respiratory nuclei (light blue). (b) Sites of major respiratory and motor relay nuclei in the pons and medulla. Abbreviations: 5HT, serotonin; ACh, acetylcholine; DA, dopamine; DRG, dorsal respiratory group; GABA, gamma aminobutyric acid; Gal, galanin; His, histamine; LC, locus coeruleus; LDT, laterodorsal tegmental area; LH, lateral hypothalamus; NE, norepinephrine; POA, preoptic area; PPT, pedunculopontine tegmental area; TMN, tuberomamillary nucleus; VAG, ventral periaquaductal gray; VRG, ventral respiratory group.
The atonia observed following bolus administration of propofol may be attributed to its actions on GABAergic circuits in the spinal cord (Kungys et al. 2009) and in the pontine and medullary reticular nuclei that control the anti-gravity muscles (Brown et al. 2010) (Figure 2b). This latter hypothesis is consistent with reports of rapid atonia following inadvertent subarachnoid space and basilar artery injection of local anesthetics during interscalene blocks (Durrani & Winnie 1991, Dutton et al. 1994), direct injection of a barbiturate into the mesopontine tegmental area (Devor & Zalkind 2001), and atonia in patients suffering from pontine strokes and locked-in syndromes (Posner et al. 2007).
The output pathways from the thalamus to the cortex are regulated by major GABAergic inhibitory inputs from the thalamic reticular nucleus (Jones 2002). Most certainly, GABAergic hypnotics contribute to sedation and unconsciousness by enhancing inhibitory activity at these thalamic reticular synapses.
Table 1 summarizes the behavioral and physiological responses of the GABA agonists along with possible neural circuit mechanisms for these responses.
Table 1. Summary of the behaviors, physiological responses, and neural circuits for the actions of GABAA agonistsa.
| Drugs | Behaviors and physiological responses | Possible neural circuit mechanism | Receptors |
|---|---|---|---|
|
| |||
| Propofol, Etomidate, Thiopental, Methohexital | Unconsciousness and sedation | Potentiation of GABAergic interneurons in the cortex, the thalamic reticular nucleus, and the arsousal centers in the midbrain and pons | GABAA |
| Seizures | Potentiation of GABAergic interneurons locally in the cortex | ||
|
| |||
| Apnea | GABAergic inhibition of brain stem respiratory control areas (ventral medulla) | ||
|
| |||
| Atonia | Inhibition of the pontine and medullary reticular nuclei that control antigravity muscles and inhibition of spinal motor neurons | ||
| Myoclonus | Possible effects on primary motor pathways | ||
|
| |||
| Loss of corneal and oculocephalic reflexes | GABAergic inhibition in the brain stem of the oculomotor, abducens and trochlear nuclei, the trigeminal nuclei, and the motor nuclei of the seventh cranial nerve | ||
Abbreviations: GABAA, gamma aminobutyric acid type A.
Analgesia and Sedation: Opioid Receptor Agonists
Opioids can be divided into three categories: natural (morphine, codeine, papaverine), synthetic (methadone, meperidine, fentanyl, alfentanil, sufentanil, remifentanil), and semisynthetic (hydromorphone) (Fukuda 2010). Because the primary clinical feature of opioids is analgesia, i.e., a relief of pain, these drugs are widely used to treat postoperative pain and to provide analgesia and to serve as an adjunct to maintain unconsciousness during general anesthesia (Fukuda 2010). Opioids are frequently combined with a benzodiazepine to provide analgesia and sedation for nonsurgical procedures (Jamieson 1999, Dionne et al. 2001). An awake patient receiving an opioid to treat pain reports analgesia and sedation (Shapiro et al. 1995, Bowdle 1998). Opioids have a number of side effects, including respiratory depression, bradycardia, miosis, constipation, nausea, vomiting, euphoria, and dysphoria (Bowdle 1998).
The principal molecular targets of opioids are the μ, κ, and δ opioid receptors. These three types of G protein-coupled receptors are expressed in many brain regions, including the periaqueductal gray (PAG), the rostral ventral medulla (RVM), the basal ganglia (Burn et al. 1995), the amygdala, and the spinal cord (Stein 1995, Dowlatshahi & Yaksh 1997). Activation of the opioid receptor leads to hyperpolarization of the nerve cell membrane by inhibiting adenyl cyclase, decreasing conductance of voltage-gated calcium channels, and opening inward-rectifying potassium channels that allow potassium efflux (Fukuda 2010).
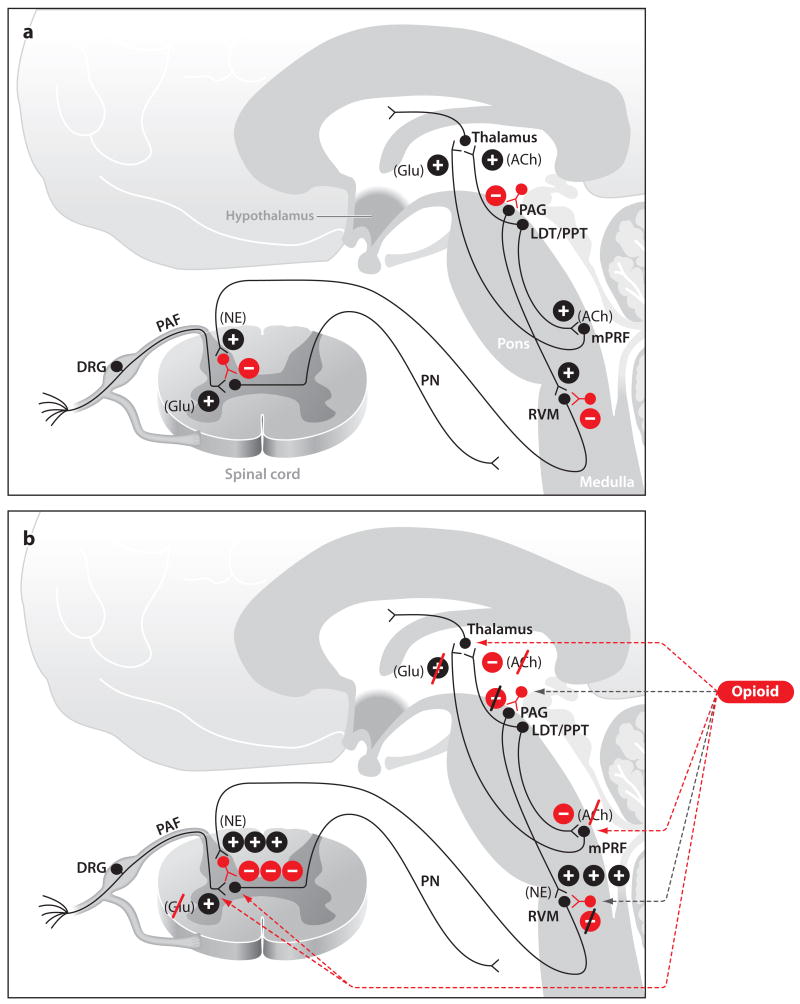
A nociceptive stimulus, such as a surgical incision, activates the free nerve endings of C-fibers and/or A-delta fibers, which make excitatory synapses onto projection neurons in the dorsal horn of the spinal cord (Figure 3a). The axons of the projection neurons cross the midline of the spinal cord and ascend in the anterolateral fasiculus to synapse in the RVM, the PAG, the thalamus, the amygdala, and the primary and secondary somatosensory cortices (Millan 2002). These are the principal components of the ascending nociceptive pathway. Nociceptive stimulation of the PAG and the RVM initiates descending pathways that modulate the nociceptive signaling through a combination of descending inhibition and descending facilitation (Millan 2002). These are the major components of the descending nociceptive pathways. These sites are also targets of the endogenous opioids, endorphins, and enkephalins (Millan 2002).
Figure 3.
Sites of opioid receptor effects during the awake state (a) and during opioid administration (b). (a) Normal cholinergic signaling during wakefulness and normal nociceptive signaling from the spinal cord to brain stem. (b) Illustrates opioid-induced decrease in arousal through a decrease in cholinergic signaling and the mechanisms of opioid-induced analgesia. Abbreviations: ACh, acetylcholine; DRG, dorsal root ganglia; Glu, glutamate; LDT, laterodorsal tegmental area; mPRF, medial pontine reticular formation; NE, norepinephrine; PAF, peripheral afferent fiber; PAG, periaquaductal gray; PN, projection neuron; PPT, pedunculopontine tegmental area; RVM, rostal ventral medulla.
Because inhibition of nociceptive processing leads to decreased arousal, the several mechanisms through which the opioids achieve their analgesic and antinociceptive effects are the principal mechanisms through which these drugs alter arousal. Opioids binding to opioid receptors in the PAG and the RVM leads to activation of the descending nociceptive pathways, which produces an overall inhibitory effect on nociceptive transmission (Millan 2002) (Figure 3b). One mechanism for activating descending inhibition is through opioid receptor mediated hyperpolarization of GABAergic inhibitory interneurons in the PAG (Heinricher & Morgan 1999). These inhibitory neurons synapse onto excitatory neurons that project to the RVM. Excitatory stimulation of the RVM by the PAG leads to increased firing of RVM off-cells and decreased firing of RVM on-cells (Heinricher & Morgan 1999). This pattern of activation in the RVM is associated with descending inhibition of nociception at the level of peripheral afferent neurons and of the projection neurons in the spinal cord.
Opioids applied directly to the spinal cord produce analgesia by at least two mechanisms (Figure 3b). Binding of opioids to presynaptic opioid receptors on peripheral afferent neurons inhibits adenylate cyclase and suppresses voltage-dependent calcium channels and sub-sequent release of glutamate and neuropeptides such as substance P and calcitonin (Meunier et al. 1995). Postsynaptic activation of the opioid receptors on the projection neurons in the dorsal horn initiates an increase in potassium conductance, leading to hyperpolarization of the nerve cell membrane and, hence, to decreased firing. Opioids may also produce analgesia by binding to opioid receptors on primary sensory neurons (Stein 1995).
Opioids also alter arousal through their anti-cholinergic effects. For example, during normal waking, the cholinergic projections from the lateral dorsal tegmental nucleus (LDT) and the peduculopontine tegmental nucleus (PPT) activate the medial pontine reticular formation (mPRF) and thalamus (Lydic & Baghdoyan 2005) (Figure 3a). The mPRF in turn provides excitatory glutamatergic inputs to the thalamus, which sends excitatory inputs to the cortex. The basal forebrain provides another important source of excitatory cholinergic inputs to the cortex (McCarley 2007). Fentanyl decreases arousal by decreasing acetylcholine in the mPRF, whereas morphine decreases arousal by inhibiting the neurons in the LDT, the mPRF, and the basal forebrain (Mortazavi et al. 1999, Lydic & Baghdoyan 2005) (Figure 3b).
Actions of opioids in the cortex and the limbic system also lead to altered arousal. Functional imaging studies of pain processing in humans have shown that low-dose morphine induces euphoria associated with positive blood oxygen level dependent (BOLD) signal changes in the nucleus accumbens along with a pattern of negative BOLD signal changes in the cortex similar to that seen with GABAA agonists (Becerra et al. 2006).
The altered arousal state induced by opioids does not reliably produce complete unconsciousness even when high doses are administered (Bailey et al. 1985). This is evidenced by the high incidence of awareness during cardiac surgery for which high-dose opioids had been the standard anesthetic regimen for many years because of their combined effects of analgesia, decreased arousal, and cardiovascular stability (Ranta et al. 1996, Silbert & Myles 2009).
Respiratory depression, the most clinically significant side effect of opioids, is mediated through opioid binding to μ2 receptors in the medulla (Weil et al. 1975). Reversal of respiratory depression by using an opioid antagonist also reverses analgesia as well because both work through the same mechanism (Greer & Ren 2009). Ampakines, AMPA receptor antagonists, have been shown in rats to counter fentanyl-induced respiratory depression without significantly altering analgesia and sedation (Greer & Ren 2009, Ren et al. 2009). These drugs may offer a way of maintaining adequate respiration while providing analgesia with opioids. Opioids acting at opioid receptors in the gut help explain the decreased motility and constipation seen with opioid use (Stewart et al. 1978, Greenwood-Van Meerveld et al. 2004). Nausea and emesis are due in part to binding of these drugs to opioid receptors in the chemotactic trigger zone (CMTZ) in the area postrema (Takahashi et al. 2007). Other side effects of opioids are likely to be mediated cholinergically. Bradycardia is likely due to GABAA-mediated disinhibition, leading to activation of cholinergic projections from the nucleus ambiguus to the sino-atrial node of the heart (Griffoen et al. 2004). Miosis may result from the direct or indirect anticholinergic effects of the opioids on the Edinger-Westfall nuclei in the midbrain.
Table 2 summarizes the behavioral and physiological responses of the opioid agonists along with possible neural circuit mechanisms for these responses.
Table 2. Summary of the behaviors, physiological responses, and neural circuits for the actions of opioid agonistsa.
| Drugs | Behavioral and physiological responses | Possible neural circuit mechanism | Receptors |
|---|---|---|---|
|
| |||
| Morphine, Fentanyl, Hydromorphone, Remifentanil | Analgesia, Antinociception | Activation of descending inhibitory pathways through disinhibition of GABAergic interneurons in the periacequeductal gray and rostal ventral medulla Inhibition of ascending pain pathways in the spinal cord pre-and postsynaptically in peripheral neurons and ascending projection neurons, respectively |
Opioid |
|
| |||
| Respiratory depression | μ2-opioid mediated inhibition of brain stem respiratory control areas (ventral medulla) | ||
|
| |||
| Nausea and emesis | δ-opioid receptor mediated activity in chemotactic trigger zone in area postrema | ||
|
| |||
| Sedation | Reduced arousal due to antinociception | ||
|
| |||
| Bradycardia | Activation of projections from nucleus ambiguous to sino-atrial node in heart | ||
|
| |||
| Sedation | Anticholinergic activity in lateral dorsal tegmental nucleus, pedunculopontine tegmental nucleus, median pontine reticular formation, and basal forebrain | ACh | |
|
| |||
| Miosis | Anticholinergic activity possibly in Edinger-Westfall nuclei | ||
|
| |||
| Insomnia | Reduction in brain levels of adenosine, required to initiate sleep | Adenosine | |
|
| |||
| Catalepsy | Catalepsy induced by dopaminergic antagonist activity (see Dopamine Antagonist section below) | Dopamine | |
Abbreviations: ACh, acetylcholine; GABAA, gamma aminobutyric acid type A.
Analgesia, Hallucinations, and Dissociative Anesthesia: NMDA Receptor Antagonists
Ketamine, an arylcyclohexylamine, is a congener of phencyclidines that anesthesiologists use as an analgesic and a hypnotic (Bergman 1999). It is also used as an adjunct to help maintain general anesthesia and as an alternative to opioids for the management of perioperative and neuropathic pain.
When a small dose of ketamine is administered, immediate, intense analgesia is the most apparent clinical feature (Kohrs & Durieux 1998). For this reason, low-dose ketamine is used commonly in anesthesiology to treat labor pain (Mercier & Benhamou 1998), to position a patient with lower extremity fracture for placement of a regional block, and to perform dressing changes in burn patients (Kohrs & Durieux 1998). The patient enters a dissociative state in which he/she perceives nociceptive stimuli but not as pain (Garfield et al. 1972, Kohrs & Durieux 1998, Bergman 1999). Auditory and visual hallucinations that resemble those seen in schizophrenia are common with ketamine (Seamans 2008). This is why ketamine is used as a pharmacological model of schizophrenia in animals (Olney et al. 1999, Kehrer et al. 2008) and why it is a widely used drug of abuse (Morgan et al. 2009). Respiratory function is generally preserved at sedative doses of ketamine. In contrast to the GABAA hypnotics, under ketamine, the EEG (Hayashi et al. 2007, Tsuda et al. 2007) is active, and both cerebral metabolic rates and blood flow increase (Cavazzuti et al. 1987, Strebel et al. 1995, Vollenweider et al. 1997) rather than decrease. Other key physiological effects of ketamine are horizontal nystagmus, pupillary dilation, salivation, lacrimation, tachycardia, and bronchodilation (Kohrs & Durieux 1998, Reves et al. 2009).
Ketamine's principal molecular target is the NMDA receptor, a major postsynaptic, ionotropic receptor for the excitatory neurotransmitter glutamate (Sinner & Graf 2008). These pharmacologically defined receptors have an obligatory NR1 subunit and a modulatory NR2 subunit. Channel opening requires that an agonist (glutamate or NMDA) bind to the NR2 subunit while the coagonist glycine binds to the NR1 subunit (Purves et al. 2008). Experimental evidence suggests that ketamine blocks NMDA receptors by uncompetitive binding at a location other than the glutamate or glycine sites (Kim et al. 2002).
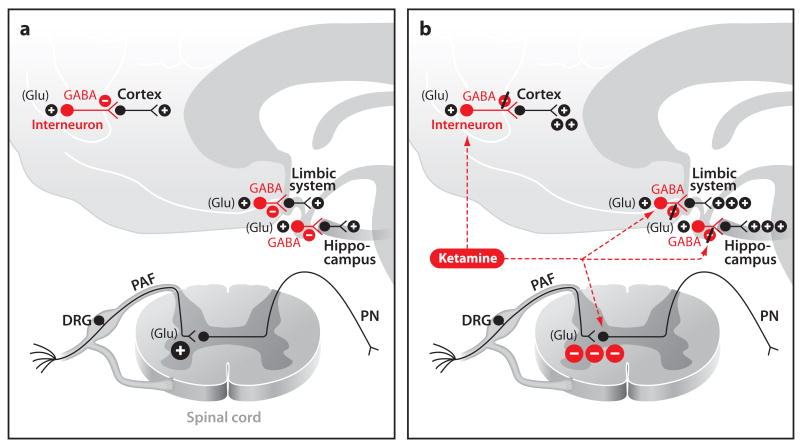
An understanding of how ketamine alters arousal is provided by strong experimental evidence that shows that ketamine binds preferentially to NMDA receptors on GABAergic inhibitory interneurons (Olney et al. 1999, Seamans 2008) (Figure 4).By selectively down-regulating GABAergic inhibition, ketamine disinhibits pyramidal neurons, leading to an altered arousal state consisting of abberant excitatory activity in multiple brain areas. Hallucinations and the dissociative state most likely result because multiple brain areas such as the cortex, the hippocampus and the limbic system are allowed to communicate through abberant activity that lacks normal spatial and temporal coordination. Benzodiazepines, GABAA agonists, are commonly administered with ketamine to help mitigate the hallucinations by possibly helping to restore some of the inhibitory activity in the affected brain regions. The increased pyramidal activity helps explain the increased EEG activity, the cerebral metabolic rate, and the cerebral blood flow seen under ketamine.
Figure 4.
NMDA receptor signaling during the awake state (a) and during ketamine administration (b). (a) Normal glutamatergic regulation of GABAergic interneuron signaling during wakefulness and normal pain pathway signaling. (b) Ketamine blockage of GABAergic interneuron inhibition and ketamine-induced mechanism of analgesia. Abbreviations: DRG, dorsal root ganglia; GABA, gamma aminobutyric acid; Glu, glutamate; PAF, peripheral afferent fiber; PN, projection neuron.
The analgesic effects of ketamine are due in part to its dissociative effects and to its blockade of NMDA receptors in the spinal cord, most notably on peripheral afferent neurons in the nociceptive pathways (Figure 4b) (Oye et al. 1992, Sinner & Graf 2008). Ketamine may also act at opioid receptors (Finck & Ngai 1982). Because certain types of neuropathic pain may be mediated in part by hyperactivity in NMDA circuits (Hocking & Cousins 2003), ketamine is widely used as a treatment for this disorder. Chronic regional pain syndrome (CRPS) is a type of neuropathic pain that may develop following trauma to an extremity that results in nerve damage (Kiefer et al. 2008). Although the initial injury may not be severe, with time, the pain associated with the injury becomes more intense and extends beyond the area that was originally affected. Sufferers from severe CRPS complain of constant burning sensations across large parts of their body.
An experimental therapy, the ketamine coma, is being used to treat severe CRPS (Kiefer et al. 2008, Becerra et al. 2009). The ketamine coma, which is not currently approved in the United States, is conducted by inducing and maintaining general anesthesia with a continuous intravenous infusion of ketamine, intubation, and mechanical ventilation for five to seven days. Following cessation of ketamine, some patients report dramatic reductions in pain symptoms that can last from a few days to several months. Although the mechanism of the effect remains unclear, it is possible that the weeklong central nervous system blockade of the NMDA receptors helps break the wind-up and central sensitization cascade believed to underlie neuropathic pain syndromes (Costigan et al. 2009). Patients are unresponsive during the ketamine coma. However, unlike the coma period resulting from a brain injury, or more standard general anesthesia, about which patients typically have no recall, patients emerging from ketamine coma recall vivid and often disturbing hallucinations (Kiefer et al. 2008).
Recent reports that low-dose ketamine can provide immediate symptom relief in patients suffering from chronic bipolar disorders and chronic depression have stimulated significant interest in another way this drug alters arousal (Zarate et al. 2006). In contrast to standard therapies for these disorders, which may require weeks to months to produce an effect, ketamine's effects can begin within 40 minutes and last for up to 7 days. This effect may be due to a change in the AMPA to NMDA receptor activity ratio (Maeng et al. 2008), to ketamine-induced synaptogenesis (Li et al. 2010), or to the possibility that the excitatory state induced by ketamine, through its actions on inhibitory interneurons, may be providing chemically what electroconvulsive therapy (ECT) provides electrically.
Ketamine-induced nystagmus appears to be the consequence of the drug's differential effects in the frontal cortex, the cerebellum, and the vestibular nuclei (Porro et al. 1999). Lacrimation and salivation seen with ketamine possibly reflect increased parasympathetically mediated activity in the inferior and superior salivatory nuclei (Purves et al. 2008), whereas pupillary dilation, tachycardia, and bronchodilation most likely reflect increased sympathetic output from the nucleus tractus solitarius (Ogawa et al. 1993, Freeman 2008). These symptoms, we speculate, may be due to NMDA-mediated disinhibition of GABAergic interneurons in these areas that leads to coactivation of the parasympathetic and sympathetic systems.
Table 3 summarizes the behavioral and physiological responses of the ketamine along with possible neural circuit mechanisms for these responses.
Table 3. Summary of the behaviors, physiological responses, and neural circuits for the actions of ketamine (NMDA receptor antagonist)a.
| Drugs | Behavioral and physiological responses | Possible neural circuit mechanisms | Receptors |
|---|---|---|---|
|
| |||
| Ketamine | Analgesia | Blockade of NMDA-mediated nociceptive stimuli in the dorsal horn of the spinal cord | NMDA |
|
| |||
| Dissociative state hallucinations | Preferential binding to NMDA receptors on GABAergic inhibitory interneurons in cerebral cortex, hippocampus, and limbic structures, resulting in disinhibition and aberrant excitatory activity in these areas. Hallucinations are treated with benzodiazepines, which is consistent with a GABA-mediated mechanism | ||
|
| |||
| Antidepressant effect | Increased neurogenesis and synaptogenesisChange in the NMDA to AMPA receptor activity ratioNMDA-mediated cortical activation by inhibition of GABAergic inhibitory interneurons (chemical ECT induced by disinhibition) | ||
|
| |||
| Lacrimation and salivation | Increased parasympathetic stimulation of inferior and superior salivatory nuclei due to NMDA-mediated inhibition of GABAergic interneurons (disinhibition) | ||
|
| |||
| Pupillary dilation, tachycardia, bronchodilation | Increased sympathetic output from nucleus tractus solitarius due to NMDA-mediated inhibition of GABAergic interneurons (disinhibition) | ||
|
| |||
| Nystagmus | Increased activity in cortical areas generating saccades (disinhibition via NMDA inhibition of GABA interneurons) combined with decreased activity in cerebellum and brain stem reticular formation and medial vestibular nuclei (direct inhibition of NMDA neurons) | ||
aAbbreviations: AMPA, 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid; ECT, electroconvulsive therapy; GABAA, gamma aminobutyric acid type A; NMDA: N-methyl D-aspartate.
Pharmacological Non-REM Sleep: Alpha Adrenergic Receptor Agonists
Dexmedetomidine and clonidine belong to the imidazole subclass of α2 adrenergic receptor agonists (Coursin & Maccioli 2001). Dexmedetomidine is a sedative whose indicated uses are for short-term sedation (<24 h) for mechanically ventilated patients in the intensive care unit (Gerlach & Dasta 2007) and for sedation of nonintubated patients for surgical and medical procedures (Hospira 2009). It is now being used off-label as an adjunct for general anesthesia (Carollo et al. 2008, Chrysostomou & Schmitt 2008). Sedation with dexmedetomidine is noticeably different from sedation with GABAA agonists because patients are readily arousable and have little to no respiratory depression (Hsu et al. 2004).
Basic science studies have demonstrated that a primary target at which dexmedetomidine acts to alter arousal is the α2 receptors on neurons emanating from the locus coeruleus (LC) (Correa-Sales et al. 1992, Chiu etal. 1995, Mizobe et al. 1996). Binding of dexmedetomidine to this G protein–coupled receptor initiates inwardly rectifying potassium currents that allow potassium efflux and inhibition of voltage-sensitive calcium channels. The net hyperpolarization of the LC neurons leads to a decrease in norepinephrine release from these neurons (Jorm & Stamford 1993, Nacif-Coelho et al. 1994, Nelson et al. 2003).
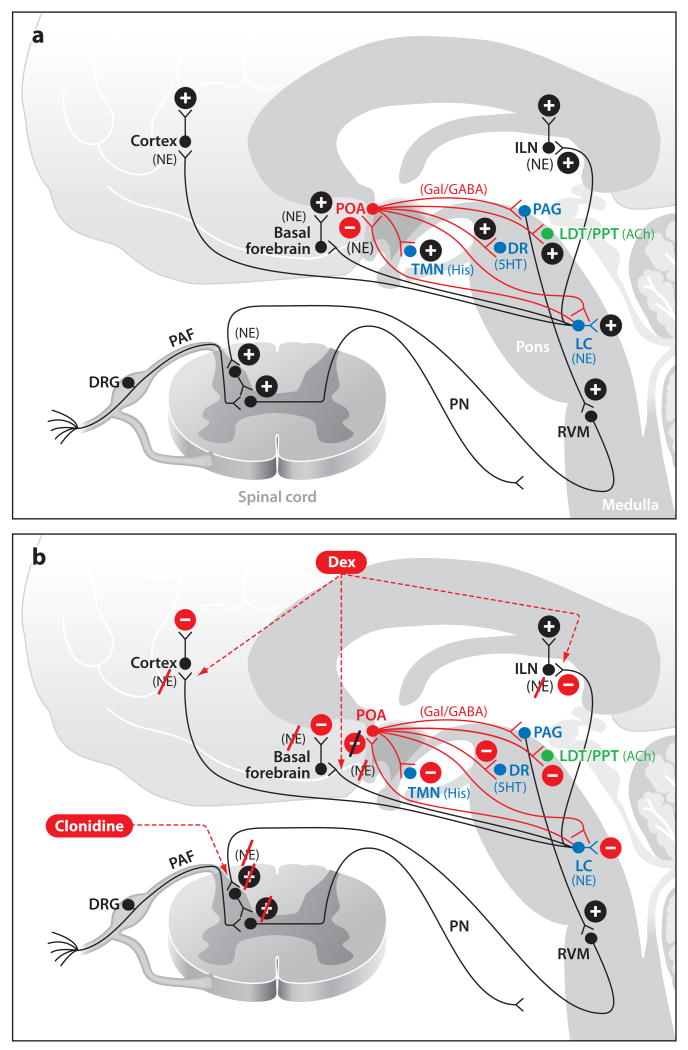
The behavioral effects of dexmedetomidine are consistent with its proposed mechanisms of action in the LC. Basic science studies suggest that, during the wake state, the LC provides norepinephrine-mediated inhibition to the POA in the hypothalamus (Figure 5a) (Osaka & Matsumura 1994, Saper et al. 2005, Lu et al. 2006). The LC also provides key adrenergically mediated excitatory inputs to the basal fore-brain, intralaminar nucleus of the thalamus, and the cortex (España & Berridge 2006). POA neurons releasing GABA and the inhibitory neurotransmitter galanin inhibit the ascending arousal centers in the midbrain, upper pons, and hypothalamus (Sherin et al. 1998, Saper et al. 2005). Thus inhibition of POA neurons promotes wakefulness. At the onset of sleep, the LC is inhibited, and its noradrenergically mediated inhibition of the POA ceases. As a consequence, the POA becomes active and its GABAergic and galaninergic neurons inhibit the ascending arousal centers to provide a possible mechanism for initiating non-REM sleep.
Figure 5.
Alpha adrenergic signaling during the awake state (a) and during dexmedetomidine or clonidine administration (b). (a) Normal adrenergic signaling from the LC during wakefulness and normal pain signaling during wakefulness from the spinal cord to the brain stem. (b) Dexmedetomidine-induced loss of consciousness through NE mediated inhibition of the POA and decreased noradrenergic signaling in the thalamus and cortex. Clonidine-induced analgesia through enhanced inhibitory activity in the descending pain pathway. Abbreviations: 5HT, serotonin; ACh, acetylcholine; DA, dopamine; DRG, dorsal root ganglia; GABAA, gamma aminobutyric acid receptor subtype A; Gal, galanin; His, histamine; ILN, intralaminar nucleus of the thalamus; LC, locus coeruleus; LDT, laterodorsal tegmental area; NE, norepinephrine; PAF, peripheral afferent fiber; PN, projection neuron; POA, preoptic area; PPT, pedunculopontine tegmental area; RVM, rostral ventral medulla; TMN, tuberomamillary nucleus; vPAG, ventral periaquaductal gray.
It is plausible that decreased norepinephrine release from the LC induced by dexmedetomidine disinhibits the POA (Nelson et al. 2002, 2003).Therefore, the disinhibited POA inhibits the ascending arousal pathways, and sedation ensues (Figure 5b). Blocking norepinephrine release from the LC would also contribute to sedation due to decreased excitatory inputs to the basal forebrain, to the intralaminar nucleus of the thalamus, and to the cortex (España & Berridge 2006). A clinical study has demonstrated a close resemblance between EEG spindles observed in dexmedetomidine-induced sedation and those observed during stage 2 non-REM sleep (Huupponen et al. 2008). Subjects were easily arousable from dexmedetomidine-induced sedation and from non-REM sleep. These basic science and clinical studies help us understand why the altered arousal state of dexmedetomidine-induced sedation closely resembles non-REM sleep.
Clonidine, injected intrathecally, is commonly used as an adjunct in postoperative pain therapy (Andrieu et al. 2009). Clonidine acts at α2 receptors in the descending nociceptive pathways in the spinal cord to enhance antinociception (Figure 5b). The α2 receptor agonist xylazine is frequently administered with ketamine to provide general anesthesia for animal surgeries (Rodrigues et al. 2006).
Table 4 summarizes the behavioral and physiological responses of the α2 agonists along with possible neural circuit mechanisms for these responses.
Table 4. Summary of the behaviors, physiological responses, and neural circuits for the actions of alpha receptor agonistsa.
| Drugs | Behavioral and physiological responses | Possible neural circuit mechanisms | Receptors |
|---|---|---|---|
|
| |||
| Dexmedetomidine | Sedation mimicking non-REM sleep | Hyperpolarization of LC reducing inhibitory output to POA, allowing POA to inhibit multiple ascending arousal systems (PPT/LDT, vPAG, TMN, Raphe, LC) by GABA and galanin projectionsReduction of NE-mediated activation of the cortex, the ILN of the thalamus, and the BF | α2 adrenergic |
|
| |||
| Clonidine | Analgesia | Inhibition of descending pain facilitation pathway achieved by blocking presynaptic NE release in excitatory interneuron in the spinal cord | |
Abbreviations: BF, basal forebrain; GABAA, gamma aminobutyric acid type A; ILN, intralaminar nucleus; LC, locus coeruleus; LDT, laterodorsal tegmental area; NE, norepinephrine; PPT, pedunculopontine tegmental area; TMN, tuberomamillary nucleus; POA preoptic area; vPAG, ventral periaquaductal gray.
Neuroleptic Anesthesia: Dopamine Receptor Antagonists
The butyrophenones, haloperidol and droperidol, are dopamine antagonists that have been used in anesthesiology (Hardman 2001) as sedatives, anesthetic adjuncts, and antiemetics. The altered arousal state induced by these drugs is insufficient for their use as sole anesthetic agents. Butyrophenones have been used in combination with opioids—haloperidol with phenoperidine and droperidol with fentanyl—to create a state of neuroleptic anesthesia characterized by unresponsiveness with eyes open, analgesia, and decreased mobility (catalepsy) with maintenance of ventilation and cardiovascular stability (Corssen et al. 1964). Neuroleptic anesthesia is no longer used because not infrequently patients complained of feeling locked in: being aware of what transpired during the surgery with substantial pinned-up emotion that they could not express (Klausen et al. 1983, Linnemann et al. 1993, Klafta et al. 1995).
The butyrophenones, used almost exclusively now as antiemetics (Apfel et al. 2009), can cause Parkisonian symptoms (bradykinesia, tremor, muscle rigidity, blunted affect) at both low (Rivera et al. 1975, Melnick 1988) and high (Lieberman et al. 2008) doses. In the United States, droperidol carries a controversial black label warning from the US FDA because of rare reports of cardiac dysrhythmias following its use in high doses (FDA 2001, Dershwitz 2003). Use of droperidol at any dose requires perioperative electrocardiogram monitoring.
Basic science studies haveshown that the butyrophenones are dopamine receptor antagonists. The five types of dopamine receptors are all 7-transmembrane, G protein–coupled receptors that are divided into two classes: D1 and D2 (Girault & Greengard 2004). The D1 receptors mediate excitation by activating G-proteins that stimulate cyclic AMP (cAMP) synthesis. In contrast, the D2 receptors mediate inhibition by activation of G-proteins that inhibit cAMP synthesis, suppress Ca2+ currents, and activate receptor-operated potassium currents.
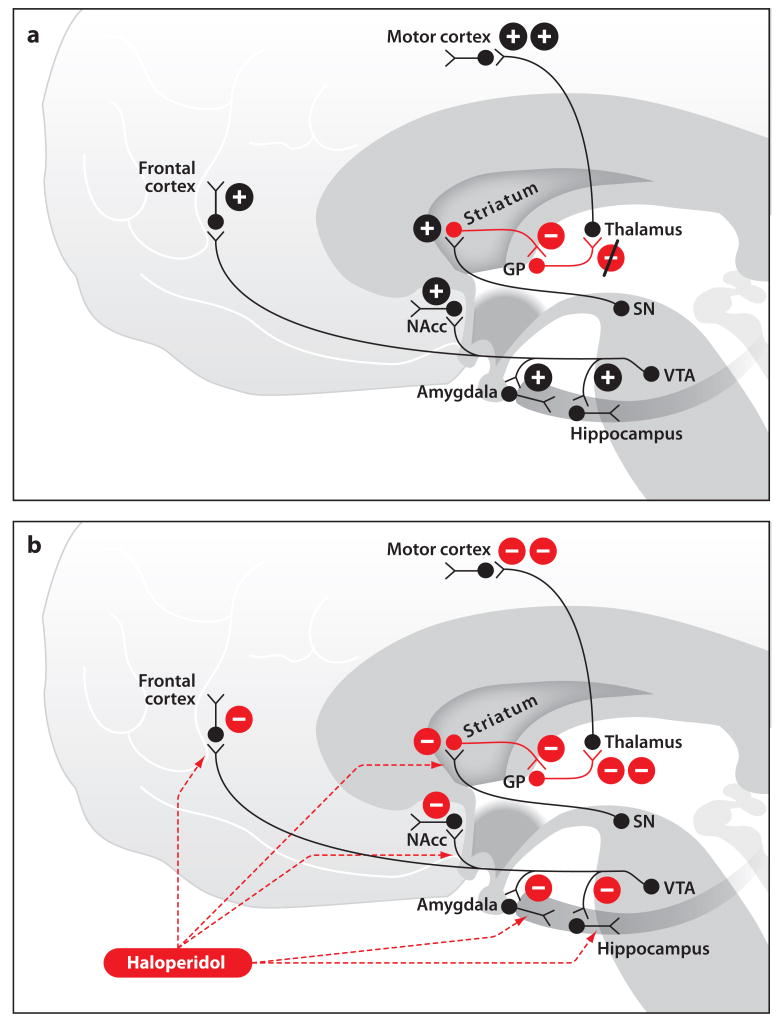
The brain has three principal dopaminergic pathways (Figure 6a). The nigrostrial pathway, which projects from the pars compacta of the substantia nigra to the striatum, is a component of the basal ganglia that is crucial for movement control (Graybiel 1991, Graybiel et al. 1994). Patients suffering from Parkinson's disease have slow movements, a resting tremor, rigidity in all extremities, and minimal facial expressions due to a lack of dopamine production in the substantia nigra (Obeso et al. 2008). The mesolimbic pathway that projects from the ventral tegmen-tum to the nucleus accumbens, amygdala, and hippocampus plays a key role in processing reward, motivation, emotion, and reinforcement. The mesocortical pathway, which projects from the ventral tegmentum to the frontal cortex, complements the function of the mesolimbic pathway and aids in cognition (Obeso et al. 2008).
Figure 6.
Dopamine signaling during the awake state (a) and during haloperidol administration (b). (a) Normal dopamine signaling during wakefulness. (b) Haloperidol effects on dopamine signaling. Abbreviations: GP, globus pallidus; NAcc, nucleus accumbens; SN, substantia nigra; VTA, ventral tegmental area.
The cataleptic state induced by the butyrophenones can be attributed to their binding to D1 and D2 receptors in the striatum, leading to a net increase in inhibitory output from the globus pallidus to the thalamus and, as a consequence, to reduced excitation of the motor cortex (Lieberman et al. 2008) (Figure 6b). Although, the mechanism may not be as simple as stated, these results are consistent with the direct- and indirect-pathway models of Parkinson's disease. The muted affect and emotion observed in patients receiving a butyrophenone may be due to loss of facial muscle control induced by the drugs' dopamine antagonistic actions in the nigrostriatal pathways (Tickle-Degnen & Lyons 2004, Lieberman et al. 2008). Sedation most likely results from the action of the drugs in the mesolimbic and mesocortical pathways (Figure 6b). The antiemetic effects of the butyrophenones are most likely multifactorial:blocking dopamine receptors in the CMTZ of the area postrema (Apfel 2009), blocking histaminergic inputs to the nucleus of the tractus solitarius (NTS), blocking serotinergic inputs to the NTS and CMTZ, and blocking cholinergic effects in the dorsal motor nucleus of the vagus (Peroutka & Synder 1980 1982; Scuderi 2003).
Although not broadly appreciated in anesthesiology, the opioids are perhaps the most widely used dopaminergic antagonist in clinical practice. Basic science studies have shown that opioid binding to μ and κ opioid receptors in the striatum and substantia nigra inhibit dopamine release (Havemann et al. 1982, Burn et al. 1995). This decrease in striatal dopamine levels can contribute to a state of decreased mobility similar to that seen in Parkinson's disease. This observation offers insight into why awareness has been more likely under high-dose fentanyl anesthesia. Fentanyl binds to opioid receptors in the rostral ventral medulla to provide both analgesia (Yaksh 1997) and activation of parasympathetic outflow (Griffioen et al. 2004). In high dose, its antidopaminergic effects are more likely to be present also. This combination of analgesia, catalepsy, parasympathetic activation (sympathetic quiescence), and muted stress response is likely to be part of the drug-induced locked-in state about which patients who received Innovar complained (Klafta et al. 1995). Hence, if high-dose fentanyl is used with few or no additional anesthetic agents that have cortical effects, a patient can be comfortable, immobile, show little to no stress response, yet remain aware.
Table 5 summarizes the behavioral and physiological responses of the dopaminergic antagonist along with possible neural circuit mechanisms for these responses.
Table 5. Summary of the behaviors, physiological responses, neural circuits, and receptors for the actions of dopamine antagonists.
| Drugs | Behavioral and physiological responses | Possible neural circuit mechanisms | Receptors |
|---|---|---|---|
|
| |||
| Haloperidol, Droperidol | Antinausea, antiemesis | Blockade of D2 receptors in chemotactic trigger zone in area postrema | Dopamine |
|
| |||
| Catalepsy, tremor, rigidity | Blockade of D1 and D2 receptors in striatum leading to a net increase in inhibition from globus pallidus to thalamus, leading to reduced activity in motor cortex | ||
|
| |||
| Loss of affect and emotional expression | Loss of facial muscle control due to antidopaminergic activity in nigrostriatal pathway and decreased affect from actions in mesolimbic and mesocortical pathways | ||
|
| |||
| Sedation | Combined inhibition of arousal, mesolimbic and mesocortical dopaminergic pathways | ||
|
| |||
| Antinausea, antiemesis | Antihistaminergic activity in nucleus tractus solitarius | Histamine | |
|
| |||
| Antinausea, antiemesis | Antiserotinergic activity in nucleus tractus solitarius and chemotactic trigger zone | Serotonin | |
Implications and Future Directions
Five altered states of arousal induced by intravenous anesthetic drugs can be understood by analyzing the behavioral and physiological effects of the drugs in relation to the molecular targets in specific neural circuits at which they are believed to act. In each case, we can suggest how the altered arousal state is created by the drug's actions at multiple sites in the central nervous system. Hence, these states are neither mysterious nor indecipherable. This systems neuroscience paradigm suggests a framework for improving research, practice, and education in anesthesiology and for relating general anesthesia to other altered states of arousal.
Anesthesiology: Research, Practice, and Education
Further clinical and basic systems neuroscience studies of general anesthesia's altered arousal states that use the latest functional neuroimaging, neurophysiological, behavioral and molecular techniques will have important implications for anesthesiology and for neuroscience. A clearer understanding of the molecular targets and the neural circuits is needed to design novel site-specific anesthetic drugs that produce desired behavioral and physiological changes in a controlled manner while obviating side effects.
For example, postoperative nausea and vomiting remain two of the most vexing side effects following general anesthesia. Postoperative cognitive dysfunction is now recognized as a major concern following surgery with general anesthesia for at least 30% of patients (Monk et al. 2008). For the elderly, the incidence can be higher than 40% (Monk et al. 2008, Price et al. 2008). Although the mechanisms are multifactorial and some are not related to the anesthetic drugs, evidence is accruing to suggest how anesthetics contribute to postoperative cognitive dysfunction.
Opioids are strongly associated with postoperative delirium, in part because of their anticholinergic effects (Marcantonio et al. 1994), which may be worst in the elderly (Hshieh et al. 2008, Campbell et al. 2009). Similarly, prolonged exposure of intensive care unit patients to benzodiazepines is associated with delirium, prolonged intensive care unit and hospital stays, and increased mortality (Pandharipande et al. 2007). These findings suggest that prolonged exposure to the nonphysiological inhibitory state created by GABAA agonists in the central nervous system may be deleterious. In contrast, the recently demonstrated benefits of dexmedetomidine as an intensive care unit sedative compared with lorazepam can be attributed, in part, to the fact that the sedative actions of the former are more site specific (Pandharipande et al. 2007).
How certain anesthetics may contribute to the high prevalence of postoperative delirium in children (Vlajkovic & Sindjelic 2007) and the potentially adverse effect of repeated anesthetic exposures in children on central nervous system development are becoming increasingly important concerns of the United States Food and Drug Administration and the International Anesthesia Research Society (Int. Anesth. Res. Soc. 2010). Ketamine and other NMDA antagonists contribute to apoptosis in the newborn brains of animals, leading to recent recommendations to reconsider the use of these drugs in neonates (Anand 2007, Mellon et al. 2007). Similarly, benzodiazepines are frequently given as sedatives to children who require multiple reconstructive surgeries following burn injuries (Stoddard et al. 2002), yet the same drugs are used in experimental models of nervous system development to probe the role of GABA in excitatory-inhibitory balance (Hensch 2004).
Successful use of systems neuroscience concepts in the daily practice of anesthesiology will require more in-depth training of anesthesiologists in neuroanatomy, neurophysiology, and neuropharmacology to bridge the current gap between clinical management of general anesthesia and the understanding of the neuro-physiological and molecular mechanisms that underlie those management decisions. This improved understanding will allow more astute interpretations of findings from clinical examinations of patients under general anesthesia, which when coupled with results from ongoing systems neuroscience studies, should lead to improved, neurophysiologically based approaches to producing general anesthesia and to monitoring the states of the brain under general anesthesia. In this way, anesthesiologists can avoid even rare though potentially traumatic events, such as awareness under general anesthesia (Avidan et al. 2008, Errando et al. 2008).
General Anesthesia and Other Altered Arousal States
We began our systems neuroscience analysis by defining general anesthesia as changes in behavioral states with maintenance of homeostasis because there is not a universally accepted definition of general anesthesia. This is due in part to the difficulty anesthesiologists have of being able to state accurately their well-formed empirical understanding of this condition. For example, confusion arises because anesthesiologists use the term sleep as a nonthreatening description of general anesthesia when speaking with patients. A level of general anesthesia appropriate for surgery is not sleep but rather a coma (Brown et al. 2010). However, like sleep, general anesthesia is reversible and can allow dreaming (Leslie & Skrzypek 2007, Errando et al. 2008, Samuelsson et al. 2008). The concept of coma is less comforting and harder for patients to understand than the notion of sleep. Despite several descriptions of the differences between sleep and general anesthesia (Lydic & Baghdoyan 2005, Brown et al. 2010), uses of the term sleep to describe the altered arousal states of general anesthesia appear in anesthesiology (Reves et al. 2009), medical (Gawande et al. 2008), and legal (Dershwitz & Henthorn 2008) articles.
Using the systems neuroscience framework to define altered arousal states in terms of behavioral and physiological responses, molecular targets and neural circuits can facilitate cross-disciplinary communication and research on how altered arousal states induced by anesthetic agents relate to the fundamental questions of consciousness (Crick & Koch 2003, Mashour 2006) and how they compare with other altered arousal states such as sleep (Lydic & Baghdoyan 2005, McCarley 2007, Alkire et al. 2008, Franks 2008), sleep aided by pharmacologic agents (NIH Consens. Dev. Conf. 1984), the stages of coma (Giacino et al. 2002), schizophrenia (Olney et al. 1999), seizures (Blumenfeld & Taylor 2003), locked-in states (Posner et al. 2007), drug-induced high or paradoxical excitation (Brown et al. 2010), meditation (Lazar et al. 2005), hypnosis (Vanhaudenhuyse et al. 2009), hibernation (Revel et al. 2007), and suspended animation (Blackstone et al. 2005). Many of these altered arousal states have analogsin pharmacologic states induced by anesthetic drugs.
Summary
General anesthesia is a nonphysiological, drug-controlled condition created so that surgical and medical therapies can be provided safely and humanely. The fundamental question for anesthesiology research is how can this state be created by making physiologically sound, reversible manipulations of the neural circuits in the central nervous system? The systems neuroscience paradigm we used to analyze general anesthesia–induced states of altered arousal offers an integrated framework for formulating and answering this question.
Acknowledgments
Research was supported by the Massachusetts General Hospital Department of Anesthesia, Critical Care and Pain Medicine and by the National Institutes of Health (NIH) Director's Pioneer Award (DP1OD003646 to E.N.B), the NIH New Innovator Award (DP2OD006454 to P.L.P.), the NIH K25 (NS057580 to P.L.P.), and the NIH-sponsored Training Program in Sleep, Circadian and Respiratory Neurobiology (HL07901 to C.J.V.). Figures 1b, 3b, 4b, and 5b were redrawn from Brown et al. (2010). We thank Kirsten Fraser and Helena Yardley for research assistance in preparing the manuscript.
Glossary
- Sedation
a pharmacologically induced state of decreased movement, anxiolysis, decreased arousal with slow and/or incoherent responses to verbal commands
- Hypnotic
any anesthetic agent used to induce unconsciousness in a patient
- Electroencephalogram (EEG) oscillations
EEG oscillatory patterns are characterized in terms of 5 different frequency bands delta (0–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (13–25 Hz), gamma (40–80 Hz)
- Induction
initiation of general anesthesia achieved usually by administering a bolus dose of a hypnotic to render a patient unconscious
- Smooth pursuit
the ability of the eyes to track closely a moving object with an uninterrupted change in gaze
- Burst suppression
an EEG oscillation pattern composed of high-frequency activity interspersed with periods of isoelectricity (flatlines)
- Myoclonus
brief involuntary twitching in a muscle or group of muscles
- Maintenance
sustaining a state of general anesthesia sufficient for a patient to receive surgical or medical procedures
- Interscalene block
regional anesthesia for the brachial plexus achieved by injecting a local anesthetic through a needle inserted between the anterior and middle interscalene muscles at C6
Footnotes
Disclosure Statement: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Emery N. Brown, Email: enb@neurostat.mit.edu.
Patrick L. Purdon, Email: patrickp@nmr.mgh.harvard.edu.
Christa J. Van Dort, Email: vandortc@mit.edu.
Literature Cited
- Ali HH, Utting JE, Gray TC. Stimulus frequency in the detection of neuromuscular block in humans. Br J Anaesth. 1970;42(11):967–78. doi: 10.1093/bja/42.11.967. [DOI] [PubMed] [Google Scholar]
- Ali HH, Utting JE, Gray TC. Quantitative assessment of residual antidepolarizing block. II. Br J Anaesth. 1971;43(5):478–85. doi: 10.1093/bja/43.5.478. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Haier RJ, Barker SJ, Shah NK, Wu JC, Kao YJ. Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography. Anesthesiology. 1995;82(2):393–403. doi: 10.1097/00000542-199502000-00010. discussion 27A. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322(5903):876–80. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KJ. Anesthetic neurotoxicity in newborns: Should we change clinical practice? Anesthesiology. 2007;107(1):2–4. doi: 10.1097/01.anes.0000268484.05444.35. [DOI] [PubMed] [Google Scholar]
- Andrieu G, Roth B, Ousmane L, Castaner M, Petillot P, et al. The efficacy of intrathecal morphine with or without clonidine for postoperative analgesia after radical prostatectomy. Anesth Analg. 2009;108(6):1954–57. doi: 10.1213/ane.0b013e3181a30182. [DOI] [PubMed] [Google Scholar]
- Angel A. The G. L. Brown lecture. Adventures in anaesthesia. Exp Physiol. 1991;76(1):1–38. doi: 10.1113/expphysiol.1991.sp003471. [DOI] [PubMed] [Google Scholar]
- Angel A. Central neuronal pathways and the process of anaesthesia. Br J Anaesth. 1993;71(1):148–63. doi: 10.1093/bja/71.1.148. [DOI] [PubMed] [Google Scholar]
- Apfel CC. Post-operative nausea and vomiting. 2010. pp. 2729–56. See Miller et al. 2010. [Google Scholar]
- Apfel CC, Cakmakkaya OS, Frings G, Kranke P, Malhotra A, et al. Droperidol has comparable clinical efficacy against both nausea and vomiting. Br J Anaesth. 2009;103(3):359–63. doi: 10.1093/bja/aep177. [DOI] [PubMed] [Google Scholar]
- Avidan MS, Zhang L, Burnside BA, Finkel KJ, Searleman AC, et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358(11):1097–108. doi: 10.1056/NEJMoa0707361. [DOI] [PubMed] [Google Scholar]
- Avramov MN, Husain MM, White PF. The comparative effects of methohexital, propofol, and etomidate for electroconvulsive therapy. Anesth Analg. 1995;81(3):596–602. doi: 10.1097/00000539-199509000-00031. [DOI] [PubMed] [Google Scholar]
- Bai D, Pennefather PS, MacDonald JF, Orser BA. The general anesthetic propofol slows deactivation and desensitization of GABA(A) receptors. J Neurosci. 1999;19(24):10635–46. doi: 10.1523/JNEUROSCI.19-24-10635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey PL, Wilbrink J, Zwanikken P, Pace NL, Stanley TH. Anesthetic induction with fentanyl. Anesth Analg. 1985;64(1):48–53. [PubMed] [Google Scholar]
- Becerra L, Harter K, Gonzalez RG, Borsook D. Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg. 2006;103(1):208–16. doi: 10.1213/01.ane.0000221457.71536.e0. [DOI] [PubMed] [Google Scholar]
- Becerra L, Schwartzman RJ, Kiefer RT, Rohr P, Moulton EA, et al. CNS measures of pain responses pre- and post-anesthetic ketamine in a patient with complex regional pain syndrome. Pain Med. 2009 doi: 10.1111/j.1526-4637.2009.00559.x. [DOI] [PubMed] [Google Scholar]
- Benthuysen JL, Smith NT, Sanford TJ, Head N, Dec-Silver H. Physiology of alfentanil-induced rigidity. Anesthesiology. 1986;64(4):440–46. doi: 10.1097/00000542-198604000-00005. [DOI] [PubMed] [Google Scholar]
- Bergman SA. Ketamine: review of its pharmacology and its use in pediatric anesthesia. Anesth Prog. 1999;46(1):10–20. [PMC free article] [PubMed] [Google Scholar]
- Bieda MC, Su H, Maciver MB. Anesthetics discriminate between tonic and phasic gamma-aminobutyric acid receptors on hippocampal CA1 neurons. Anesth Analg. 2009;108(2):484–90. doi: 10.1213/ane.0b013e3181904571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow H. Insensibility during surgical operations produced by inhalation. Boston Med Surg J. 1846;XXXV(16):309–17. [PMC free article] [PubMed] [Google Scholar]
- Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Taylor J. Why do seizures cause loss of consciousness? Neuroscientist. 2003;9(5):301–10. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- Boland F. The First Anesthetic: The Story of Crawford Long. Athens: Univ. Ga. Press; 2009. [Google Scholar]
- Bonhomme V, Fiset P, Meuret P, Backman S, Plourde G, et al. Propofol anesthesia and cerebral blood flow changes elicited by vibrotactile stimulation: a positron emission tomography study. J Neurophysiol. 2001;85(3):1299–308. doi: 10.1152/jn.2001.85.3.1299. [DOI] [PubMed] [Google Scholar]
- Bowdle TA. Adverse effects of opioid agonists and agonist-antagonists in anaesthesia. Drug Saf. 1998;19(3):173–89. doi: 10.2165/00002018-199819030-00002. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20(2):365–83. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Breshears JD, Roland JL, Sharma M, Gaona CM, Freudenburg ZV, et al. Stable and dynamic cortical electrophysiology of induction and emergence with propofol anesthesia. Proc Natl Acad Sci USA. 2010;107:21170–75. doi: 10.1073/pnas.1011949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN. A systems neuroscience approach for the study of general anesthesia. NIH Director's Pioneer Award Symp; Bethesda, MD. 2007. [Google Scholar]
- Brown EN, Lydic R, Schiff ND. General anesthesia, sleep and coma. N Engl J Med. 2010;363(27):2638–50. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn DJ, Rinne JO, Quinn NP, Lees AJ, Marsden CD, Brooks DJ. Striatal opioid receptor binding in Parkinson's disease, striatonigral degeneration and Steele-Richardson-Olszewski syndrome, a [11C]diprenorphine PET study. Brain. 1995;118(Pt. 4):951–58. doi: 10.1093/brain/118.4.951. [DOI] [PubMed] [Google Scholar]
- Campbell N, Boustani M, Limbil T, Ott C, Fox C, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4(1):225–33. doi: 10.2147/cia.s5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008;21(4):457–61. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- Cavazzuti M, Porro CA, Biral GP, Benassi C, Barbieri GC. Ketamine effects on local cerebral blood flow and metabolism in the rat. J Cereb Blood Flow Metab. 1987;7(6):806–11. doi: 10.1038/jcbfm.1987.138. [DOI] [PubMed] [Google Scholar]
- Chiu TH, Chen MJ, Yang YR, Yang JJ, Tang FI. Action of dexmedetomidine on rat locus coeruleus neurones: intracellular recording in vitro. Eur J Pharmacol. 1995;285(3):261–68. doi: 10.1016/0014-2999(95)00417-j. [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22(3):977–90. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol. 2008;4(5):619–27. doi: 10.1517/17425255.4.5.619. [DOI] [PubMed] [Google Scholar]
- Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76(6):948–52. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- Corssen G, Domino EF, Sweet RB. Neuroleptanalgesia and anesthesia. Anesth Analg. 1964;43:748–63. [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001;7(4):221–26. doi: 10.1097/00075198-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. A framework for consciousness. Nat Neurosci. 2003;6(2):119–26. doi: 10.1038/nn0203-119. [DOI] [PubMed] [Google Scholar]
- Dershwitz M. There should be a threshold dose for the FDA black-box warning on droperidol. Anesth Analg. 2003;97(5):1542–43. doi: 10.1213/01.ANE.0000077672.77618.22. author reply p. 1543. [DOI] [PubMed] [Google Scholar]
- Dershwitz M, Henthorn TK. The pharmacokinetics and pharmacodynamics of thiopental as used in lethal injection. Fordham Urban Law J. 2008;35:931–56. [Google Scholar]
- Devor M, Zalkind V. Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain. 2001;94(1):101–12. doi: 10.1016/S0304-3959(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Dionne RA, Yagiela JA, Moore PA, Gonty A, Zuniga J, Beirne OR. Comparing efficacy and safety of four intravenous sedation regimens in dental outpatients. J Am Dent Assoc. 2001;132(6):740–51. doi: 10.14219/jada.archive.2001.0271. [DOI] [PubMed] [Google Scholar]
- Dowlatshahi P, Yaksh TL. Differential effects of two intraventricularly injected alpha 2 agonists, ST-91 and dexmedetomidine, on electroencephalogram, feeding, and electromyogram. Anesth Analg. 1997;84(1):133–38. doi: 10.1097/00000539-199701000-00025. [DOI] [PubMed] [Google Scholar]
- Durrani Z, Winnie AP. Brainstem toxicity with reversible locked-in syndrome after intrascalene brachial plexus block. Anesth Analg. 1991;72(2):249–52. doi: 10.1213/00000539-199102000-00020. [DOI] [PubMed] [Google Scholar]
- Dutton RP, Eckhardt WF, 3rd, Sunder N. Total spinal anesthesia after interscalene blockade of the rachial plexus. Anesthesiology. 1994;80(4):939–41. doi: 10.1097/00000542-199404000-00028. [DOI] [PubMed] [Google Scholar]
- Eger EI. What is general anesthetic action? Anesth Analg. 1993;77(2):408–9. doi: 10.1213/00000539-199308000-00050. [DOI] [PubMed] [Google Scholar]
- Eichhorn JH, Cooper JB, Cullen DJ, Maier WR, Philip JH, Seeman RG. Standards for patient monitoring during anesthesia at Harvard Medical School. JAMA. 1986;256(8):1017–20. [PubMed] [Google Scholar]
- Errando CL, Sigl JC, Robles M, Calabuig E, Garcia J, et al. Awareness with recall during general anaesthesia: a prospective observational evaluation of 4001 patients. Br J Anaesth. 2008;101(2):178–85. doi: 10.1093/bja/aen144. [DOI] [PubMed] [Google Scholar]
- España RA, Berridge CW. Organization of noradrenergic efferents to arousal-related basal forebrain structures. J Comp Neurol. 2006;496(5):668–83. doi: 10.1002/cne.20946. [DOI] [PubMed] [Google Scholar]
- FDA. Inapsine (droperidol) Dear Healthcare Professional Letter Dec 2001. 2001 http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm173778.htm.
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–66. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster J. Ether Day: The Strange Tale of America's Greatest Medical Discovery and the Haunted Men Who Made It. New York: Harper Collins; 2001. [Google Scholar]
- Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci USA. 2010;107(6):2681–86. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck AD, Ngai SH. Opiate receptor mediation of ketamine analgesia. Anesthesiology. 1982;56(4):291–97. doi: 10.1097/00000542-198204000-00011. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9(5):370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med. 2008;358(6):615–24. doi: 10.1056/NEJMcp074189. [DOI] [PubMed] [Google Scholar]
- Fukuda K. Opioids. 2010. pp. 769–824. See Miller et al. 2010. [Google Scholar]
- Fulton SA, Mullen KD. Completion of upper endoscopic procedures despite paradoxical reaction to midazolam: a role for flumazenil? Am J Gastroenterol. 2000;95(3):809–11. doi: 10.1111/j.1572-0241.2000.01866.x. [DOI] [PubMed] [Google Scholar]
- Garfield JM, Garfield FB, Stone JG, Hopkins D, Johns LA. A comparison of psychologic responses to ketamine and thiopental–nitrous oxide–halothane anesthesia. Anesthesiology. 1972;36(4):329–38. doi: 10.1097/00000542-197204000-00006. [DOI] [PubMed] [Google Scholar]
- Gawande A, Denno DW, Truog RD, Waisel DM. Physicians and execution-highlights froma dicussion of lethal injection. N Engl J Med. 2008;358(5):448–51. doi: 10.1056/NEJMp0800378. [DOI] [PubMed] [Google Scholar]
- Gerlach AT, Dasta JF. Dexmedetomidine: an updated review. Ann Pharmacother. 2007;41(2):245–52. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58(3):349–53. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61(5):641–44. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Glass PS, Shafer SL, Reves JG. Intravenous drug delivery systems. 2010. pp. 825–58. See Miller et al. 2010. [Google Scholar]
- Grasshoff C, Drexler B, Rudolph U, Antkowiak B. Anaesthetic drugs: linking molecular actions to clinical effects. Curr Pharm Des. 2006;12(28):3665–79. doi: 10.2174/138161206778522038. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Basal ganglia—input, neural activity, and relation to the cortex. Curr Opin Neurobiol. 1991;1(4):644–51. doi: 10.1016/s0959-4388(05)80043-1. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265(5180):1826–31. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Gredell JA, Turnquist PA, Maciver MB, Pearce RA. Determination of diffusion and partition coefficients of propofol in rat brain tissue: implications for studies of drug action in vitro. Br J Anaesth. 2004;93(6):810–17. doi: 10.1093/bja/aeh272. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Gardner CJ, Little PJ, Hicks GA, Dehaven-Hudkins DL. Preclinical studies of opioids and opioid antagonists on gastrointestinal function. Neurogastroenterol Motil. 2004;16(Suppl. 2):46–53. doi: 10.1111/j.1743-3150.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Ren J. Ampakine therapy to counter fentanyl-induced respiratory depression. Respir Physiol Neurobiol. 2009;168(1–2):153–57. doi: 10.1016/j.resp.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Griffioen KJ, Venkatesan P, Huang ZG, Wang X, Bouairi E, et al. Fentanyl inhibits GABAergic neu-rotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res. 2004;1007:109–15. doi: 10.1016/j.brainres.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman's: The Pharmacological Basis of Therapeutics. 13th New York: McGraw Hill; 2001. [Google Scholar]
- Havemann U, Turski L, Kuschinsky K. Role of opioid receptors in the substantia nigra in morphine-induced muscular rigidity. Life Sci. 1982;31(20–21):2319–22. doi: 10.1016/0024-3205(82)90146-1. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Tsuda N, Sawa T, Hagihira S. Ketamine increases the frequency of electroencephalographic bicoherence peak on the alpha spindle area induced with propofol. Br J Anaesth. 2007;99(3):389–95. doi: 10.1093/bja/aem175. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM. Supraspinal mechanisms of opioid analgesia. In: Stein C, editor. Opioids in Pain Control: Basic and Clinical Aspects. Cambridge, UK: Cambridge Univ. Press; 1999. pp. 46–69. [Google Scholar]
- Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26(10):503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–79. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730–39. doi: 10.1213/01.ANE.0000086618.28845.9B. [DOI] [PubMed] [Google Scholar]
- Hospira. Precedex dosing guidelines. 2009 http://www.precedex.com/wp-content/uploads/2010/02/Dosing_Guide.pdf.
- Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63(7):764–72. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, et al. Dexmedetomidine pharmaco-dynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101(5):1066–76. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- Hufnagel A, Burr W, Elger CE, Nadstawek J, Hefner G. Localization of the epileptic focus during methohexital-induced anesthesia. Epilepsia. 1992;33(2):271–84. doi: 10.1111/j.1528-1157.1992.tb02316.x. [DOI] [PubMed] [Google Scholar]
- Huupponen E, Maksimow A, Lapinlampi P, Sarkela M, Saastamoinen A, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52(2):289–94. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- Int. Anesth. Res. Soc. (IARS) SmartTots. 2010 from http://www.iars.org/smarttots/default.asp.
- James R, Glen JB. Synthesis, biological evaluation, and preliminary structure-activity considerations of a series of alkylphenols as intravenous anesthetic agents. J Med Chem. 1980;23:1350–57. doi: 10.1021/jm00186a013. [DOI] [PubMed] [Google Scholar]
- Jamieson J. Anesthesia and sedation in the endoscopy suite? (influences and options) Curr Opin Anaesthesiol. 1999;12(4):417–23. doi: 10.1097/00001503-199908000-00004. [DOI] [PubMed] [Google Scholar]
- Jones EG. Thalamic circuitry and thalamocortical synchrony. Philos Trans R Soc Lond B Biol Sci. 2002;357(1428):1659–73. doi: 10.1098/rstb.2002.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. Br J Anaesth. 1993;71(3):447–49. doi: 10.1093/bja/71.3.447. [DOI] [PubMed] [Google Scholar]
- Jt. Comm. Sentinel event alert: preventing, and managing the impact of anesthesia awareness. 2004 http://www.jointcommission.org/sentinel_event_alert_issue_32_preventing_and_managing_the_impact_of_anesthesia_awareness/ [PubMed]
- Kearse LA, Jr, Manberg P, Chamoun N, deBros F, Zaslavsky A. Bispectral analysis of the electroencephalogram correlates with patient movement to skin incision during propofol/nitrous oxide anesthesia. Anesthesiology. 1994;81(6):1365–70. doi: 10.1097/00000542-199412000-00010. [DOI] [PubMed] [Google Scholar]
- Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D, Norman C. What don't we know? Science. 2005;309(5731):75–102. doi: 10.1126/science.309.5731.75. [DOI] [PubMed] [Google Scholar]
- Kiefer RT, Rohr P, Ploppa A, Dieterich HJ, Grothusen J, et al. Efficacy of ketamine in anesthetic dosage for the treatment of refractory complex regional pain syndrome: an open-label phase II study. Pain Med. 2008;9(8):1173–201. doi: 10.1111/j.1526-4637.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- Kim A, Kerchner G, Choi D. Blocking excitotoxicity. In: Marcoux FW, Choi DW, editors. CNS Neuroprotection. New York: Springer; 2002. pp. 3–36. [Google Scholar]
- Kissin I. General anesthetic action: an obsolete notion? Anesth Analg. 1993;76(2):215–18. doi: 10.1213/00000539-199302000-00002. [DOI] [PubMed] [Google Scholar]
- Klafta JM, Zacny JP, Young CJ. Neurological and psychiatric adverse effects of anaesthetics: epidemiology and treatment. Drug Saf. 1995;13(5):281–95. doi: 10.2165/00002018-199513050-00002. [DOI] [PubMed] [Google Scholar]
- Klausen NO, Wiberg-Jorgensen F, Jorgensen B. Psychomimetic reactions after low-dose ketamine infusion. Comparison with neuroleptanaesthesia. Br J Anaesth. 1983;55(4):297–301. doi: 10.1093/bja/55.4.297. [DOI] [PubMed] [Google Scholar]
- Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87(5):1186–93. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- Kungys G, Kim J, Jinks SL, Atherley RJ, Antognini JF. Propofol produces immobility via action in the ventral horn of the spinal cord by a GABAergic mechanism. Anesth Analg. 2009;108(5):1531–37. doi: 10.1213/ane.0b013e31819d9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–97. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie K, Skrzypek H. Dreaming during anaesthesia in adult patients. Best Pract Res Clin Anaesthesiol. 2007;21(3):403–14. doi: 10.1016/j.bpa.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60(3):358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann MU, Guldager H, Nielsen J, Ibsen M, Hansen RW. Psychomimetic reactions after neurolept and propofol anaesthesia. Acta Anaesthesiol Scand. 1993;37(1):29–32. doi: 10.1111/j.1399-6576.1993.tb03593.x. [DOI] [PubMed] [Google Scholar]
- Long C. An account of the first use of sulphur ether by inhalation as an anaesthetic in surgical operations. South Med Surg J. 1849;5:705–13. [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103(6):1268–95. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Marcantonio ER, Juarez G, Goldman L, Mangione CM, Ludwig LE, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272(19):1518–22. [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mashour GA. Integrating the science of consciousness and anesthesia. Anesth Analg. 2006;103(4):975–82. doi: 10.1213/01.ane.0000232442.69757.4a. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8(4):302–30. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104(3):509–20. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- Melnick BM. Extrapyramidal reactions to low-dose droperidol. Anesthesiology. 1988;69(3):424–26. doi: 10.1097/00000542-198809000-00027. [DOI] [PubMed] [Google Scholar]
- Mercier FJ, Benhamou D. Promising non-narcotic analgesic techniques for labour. Baillieres Clin Obstet Gynaecol. 1998;12(3):397–407. doi: 10.1016/s0950-3552(98)80074-6. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;3776549:532–35. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, Tracey I. Cortical and sub-cortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miller RD, Eriksson LI, Fleischer LA, Wiener-Kronish JP, Young WL. Miller's Anesthesia. Philadelphia: Churchhill Livingstone; 2010. [Google Scholar]
- Mizobe T, Maghsoudi K, Sitwala K, Tianzhi G, Ou J, Maze M. Antisense technology reveals the alpha2A adrenoceptor to be the subtype mediating the hypnotic response to the highly selective agonist, dexmedetomidine, in the locus coeruleus of the rat. J Clin Invest. 1996;98(5):1076–80. doi: 10.1172/JCI118887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. Ketamine use, cognition and psychological wellbeing: a comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction. 2009;104(1):77–87. doi: 10.1111/j.1360-0443.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- Mortazavi S, Thompson J, Baghdoyan HA, Lydic R. Fentanyl and morphine, but not remifentanil, inhibit acetylcholine release in pontine regions modulating arousal. Anesthesiology. 1999;90(4):1070–77. doi: 10.1097/00000542-199904000-00021. [DOI] [PubMed] [Google Scholar]
- Nacif-Coelho C, Correa-Sales C, Chang LL, Maze M. Perturbation of ion channel conductance alters the hypnotic response to the alpha 2-adrenergic agonist dexmedetomidine in the locus coeruleus of the rat. Anesthesiology. 1994;81(6):1527–34. doi: 10.1097/00000542-199412000-00029. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5(10):979–84. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428–36. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- NIH Consens. Dev. Conf. Drugs and insomnia. The use of medications to promote sleep. JAMA. 1984;251(18):2410–14. [PubMed] [Google Scholar]
- Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temino B, et al. The basal gangliainParkinson's disease: current concepts and unexplained observations. Ann Neurol. 2008;64(Suppl. 2):S30–46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Uemura M, Kataoka Y, Ol K, Inokuchi T. Effects of ketamine on cardiovascular responses mediated by N-methyl-D-aspartate receptor in the rat nucleus tractus solitarius. Anesthesiology. 1993;78(1):163–67. doi: 10.1097/00000542-199301000-00022. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33(6):523–33. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Osaka T, Matsumura H. Noradrenergic inputs to sleep-related neurons in the preoptic area from the locus coeruleus and the ventrolateral medulla in the rat. Neurosci Res. 1994;19(1):39–50. doi: 10.1016/0168-0102(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260(3):1209–13. [PubMed] [Google Scholar]
- Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, et al. Effect of sedation with dexmedetomidine versus lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–53. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126(Pt. 7):1524–36. doi: 10.1093/brain/awg166. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Synder SH. Relationship of neuroleptic drug effects at brain dopamine, serotonin, alpha-adrenergic, and histamine receptors to clinical potency. Am J Psychiatry. 1980;137(12):1518–22. doi: 10.1176/ajp.137.12.1518. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH. Antiemetics: neurotransmitter receptor binding predicts therapeutic actions. Lancet. 1982;1(8273):658–59. doi: 10.1016/s0140-6736(82)92206-1. [DOI] [PubMed] [Google Scholar]
- Porro CA, Biral GP, Benassi C, Cavazzuti M, Baraldi P, et al. Neural circuits underlying ketamine-induced oculomotor behavior in the rat: 2-deoxyglucose studies. Exp Brain Res. 1999;124(1):8–16. doi: 10.1007/s002210050594. [DOI] [PubMed] [Google Scholar]
- Posner J, Saper C, Schiff ND, Plum F. Diagnosis of Stupor and Coma. Oxford, UK: Oxford Univ. Press; 2007. [Google Scholar]
- Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline inolder adults after noncardiac surgery. Anesthesiology. 2008;108(1):8–17. doi: 10.1097/01.anes.0000296072.02527.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon PL, Pierce ET, Bonmassar G, Walsh J, Harrell PG, et al. Simultaneous electroencephalography and functional magnetic resonance imaging of general anesthesia. Ann N Y Acad Sci. 2009;1157:61–70. doi: 10.1111/j.1749-6632.2008.04119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Hall WC, Lamantia A, McNamara JO, White LE. Neuroscience. Sunderland, MA: Sinauer; 2008. [Google Scholar]
- Ranta S, Jussila J, Hynynen M. Recall of awareness during cardiac anaesthesia: influence of feedback information to the anaesthesiologist. Acta Anaesthesiol Scand. 1996;40(5):554–60. doi: 10.1111/j.1399-6576.1996.tb04487.x. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, Funk GD, Greer JJ. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology. 2009;110(6):1364–70. doi: 10.1097/ALN.0b013e31819faa2a. [DOI] [PubMed] [Google Scholar]
- Revel FG, Herwig A, Garidou ML, Dardente H, Menet JS, et al. The circadian clock stops ticking during deep hibernation in the European hamster. Proc Natl Acad Sci USA. 2007;104(34):13816–20. doi: 10.1073/pnas.0704699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reves JG, Glass PS, Lubarsky DA, McEvoy MD, Martinez-Ruiz R. Intravenous anesthetics. 2010. pp. 719–68. See Miller et al. 2010. [Google Scholar]
- Rivera VM, Keichian AH, Oliver RE. Persistent Parkinsonism following neuroleptanalgesia. Anesthesiology. 1975;42(5):635–37. doi: 10.1097/00000542-197505000-00034. [DOI] [PubMed] [Google Scholar]
- Rockoff MA, Goudsouzian NG. Seizures induced by methohexital. Anesthesiology. 1981;54(4):333–35. doi: 10.1097/00000542-198104000-00015. [DOI] [PubMed] [Google Scholar]
- Rodrigues SF, de Oliveira MA, Martins JO, Sannomiya P, de Cassia Tostes R, et al. Differential effects of chloral hydrate- and ketamine/xylazine-induced anesthesia by the s.c. route. Life Sci. 2006;79(17):1630–37. doi: 10.1016/j.lfs.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Russo H, Bressolle F. Pharmacodynamics and pharmacokinetics of thiopental. Clin Pharmacokinet. 1998;35(2):95–134. doi: 10.2165/00003088-199835020-00002. [DOI] [PubMed] [Google Scholar]
- Samuelsson P, Brudin L, Sandin RH. Intraoperative dreams reported after general anaesthesia are not early interpretations of delayed awareness. Acta Anaesthesiol Scand. 2008;52(6):805–9. doi: 10.1111/j.1399-6576.2008.01634.x. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Schuttler J, Schwilden H. Modern Anesthetics: Handbook of Experimental Pharmacology. Berlin: Springer; 2008. [Google Scholar]
- Scuderi PE. Pharmacology of antiemetics. Int Anesthesiol Clin. 2003;41(4):41–66. doi: 10.1097/00004311-200341040-00006. [DOI] [PubMed] [Google Scholar]
- Seamans J. Losing inhibition with ketamine. Nat Chem Biol. 2008;4(2):91–93. doi: 10.1038/nchembio0208-91. [DOI] [PubMed] [Google Scholar]
- Shafer S, Flood P, Schwinn D. Basic principles of pharmacology. 2010. pp. 479–514. See Miller et al. 2010. [Google Scholar]
- Shapiro BA, Warren J, Egol AB, Greenbaum DM, Jacobi J, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Society of Critical Care Medicine. Crit Care Med. 1995;23(9):1596–600. doi: 10.1097/00003246-199509000-00021. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18(12):4705–21. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert BS, Myles PS. Is fast-track cardiac anesthesia now the global standard of care? Anesth Analg. 2009;108(3):689–91. doi: 10.1213/ane.0b013e318193c439. [DOI] [PubMed] [Google Scholar]
- Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008;182:313–33. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- Sloan I. The new anesthetic machines. Clin J Anaesth. 2002;47:201–4. doi: 10.1007/BF03018912. [DOI] [PubMed] [Google Scholar]
- Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332(25):1685–90. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- Stewart JJ, Weisbrodt NW, Burks TF. Central and peripheral actions of morphine on intestinal transit. J Pharmacol Exp Ther. 1978;205(3):547–55. [PubMed] [Google Scholar]
- Stoddard FJ, Sheridan RL, Saxe GN, King BS, King BH, et al. Treatment of pain in acutely burned children. J Burn Care Rehabil. 2002;23(2):135–56. doi: 10.1097/00004630-200203000-00012. [DOI] [PubMed] [Google Scholar]
- Strebel S, Kaufmann M, Maitre L, Schaefer HG. Effects of ketamine on cerebral blood flow velocity in humans. Influence of pretreatment with midazolam or esmolol. Anaesthesia. 1995;50(3):223–28. doi: 10.1111/j.1365-2044.1995.tb04561.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tsuchida D, Pappas TN. Central effects of morphine on GI motility in conscious dogs. Brain Res. 2007;1166:29–34. doi: 10.1016/j.brainres.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Tickle-Degnen L, Lyons KD. Practitioners' impressions of patients with Parkinson's disease: the social ecology of the expressive mask. Soc Sci Med. 2004;58(3):603–14. doi: 10.1016/s0277-9536(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Tremper KK, Barker SJ. Pulse oximetry. Anesthesiology. 1989;70(1):98–108. doi: 10.1097/00000542-198901000-00019. [DOI] [PubMed] [Google Scholar]
- Tsuda N, Hayashi K, Hagihira S, Sawa T. Ketamine, an NMDA-antagonist, increases the oscillatory frequencies of alpha-peaks on the electroencephalographic power spectrum. Acta Anaesthesiol Scand. 2007;51(4):472–81. doi: 10.1111/j.1399-6576.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- Tung A, Tadimeti L, Caruana-Montaldo B, Atkins PM, Mion LC, et al. The relationship of sedation to deliberate self-extubation. J Clin Anesth. 2001;13(1):24–29. doi: 10.1016/s0952-8180(00)00237-3. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Boly M, Laureys S, Faymonville M. Neurophysiological correlates of hypnotic analgesia. Contemp Hypn. 2009;26(1):15–23. [Google Scholar]
- Van Keulen SG, Burton JH. Myoclonus associated with etomidate for ED procedural sedation and analgesia. Am J Emerg Med. 2003;21(7):556–58. doi: 10.1016/j.ajem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Vanlersberghe C, Camu F. Etomidate and other non-barbiturates. Handb Exp Pharmacol. 2008;182:267–82. doi: 10.1007/978-3-540-74806-9_13. [DOI] [PubMed] [Google Scholar]
- Velly LJ, Rey MF, Bruder NJ, Gouvitsos FA, Witjas T, et al. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107(2):202–12. doi: 10.1097/01.anes.0000270734.99298.b4. [DOI] [PubMed] [Google Scholar]
- Veselis RA, Reinsel RA, Beattie BJ, Mawlawi OR, Feshchenko VA, et al. Midazolam changes cerebral blood flow in discrete brain regions: an H2(15)O positron emission tomography study. Anesthesiology. 1997;87(5):1106–17. doi: 10.1097/00000542-199711000-00015. [DOI] [PubMed] [Google Scholar]
- Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104(1):84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997;7(1):25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- Weil JV, McCullough RE, Kline JS, Sodal IE. Diminished ventilatory response to hypoxia and hyper-capnia after morphine in normal man. N Engl J Med. 1975;292(21):1103–6. doi: 10.1056/NEJM197505222922106. [DOI] [PubMed] [Google Scholar]
- Wuesten R, Van Aken H, Glass PS, Buerkle H. Assessment of depth of anesthesia and postoperative respiratory recovery after remifentanil- versus alfentanil-based total intravenous anesthesia in patients undergoing ear-nose-throat surgery. Anesthesiology. 2001;94(2):211–17. doi: 10.1097/00000542-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Pharmacology and mechanisms of opioid analgesic activity. Acta Anaesthesiol Scand. 1997;41(1 Pt. 2):94–111. doi: 10.1111/j.1399-6576.1997.tb04623.x. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]