Abstract
Anesthesia has broad actions that include changing neuronal excitability, vascular reactivity, and other baseline physiologies and eventually modifies the neurovascular coupling relationship. Here, we review the effects of anesthesia on the spatial propagation, temporal dynamics, and quantitative relationship between the neural and vascular responses to cortical stimulation. Previous studies have shown that the onset latency of evoked cerebral blood flow (CBF) changes is relatively consistent across anesthesia conditions compared with variations in the time-to-peak. This finding indicates that the mechanism of vasodilation onset is less dependent on anesthesia interference, while vasodilation dynamics are subject to this interference. The quantitative coupling relationship is largely influenced by the type and dosage of anesthesia, including the actions on neural processing, vasoactive signal transmission, and vascular reactivity. The effects of anesthesia on the spatial gap between the neural and vascular response regions are not fully understood and require further attention to elucidate the mechanism of vascular control of CBF supply to the underlying focal and surrounding neural activity. The in-depth understanding of the anesthesia actions on neurovascular elements allows for better decision-making regarding the anesthetics used in specific models for neurovascular experiments and may also help elucidate the signal source issues in hemodynamic-based neuroimaging techniques.
Keywords: animal models, awake experiments, neuroimaging
Introduction
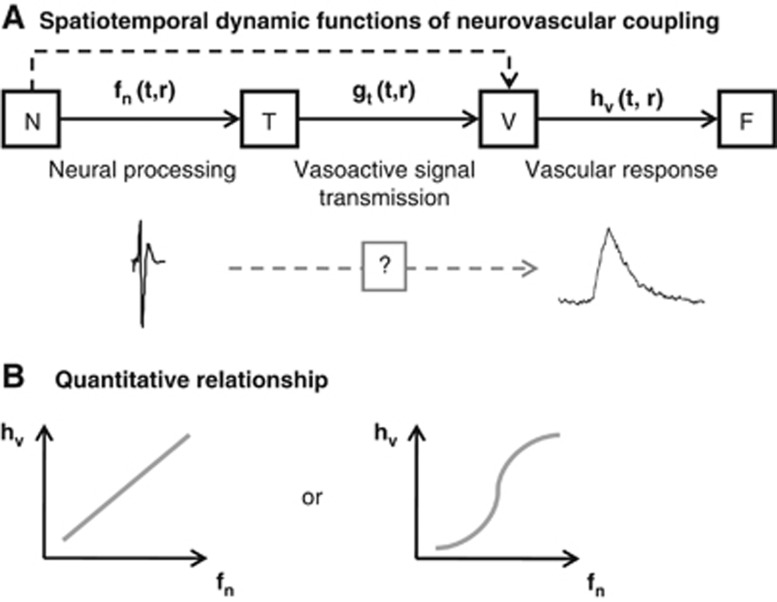
Neurovascular coupling consists of three brain cell types: neurons, supporting cells (astrocytes), and vascular cells (vascular smooth muscle, pericyte, and endothelial cells). These cells can be grouped into three conceptual components: neurons, the message senders associated with information processing; supporting cells, the potential transmission sites that mediate vasoactive signals in response to the neuronally derived messages; and vascular cells, the recipients of the signals. After evoked neural activation, vasoactive signals are transmitted directly and indirectly via supporting cells to the vascular cells, which cause redistribution of the local cerebral blood flow (CBF). The hypothetical view of the neurovascular coupling relationship is schematically shown in Figure 1. Great effort has been invested in elucidating the spatiotemporal dynamic functions of neurovascular coupling (Figure 1A), quantitative coupling relationships (Figure 1B), and the mechanisms underlying signal transmission using in-vivo animal models (for reviews, see Kleinfeld et al, 2011; Attwell et al, 2010; Iadecola, 2004; and Lauritzen, 2001).
Figure 1.
Compartmentalized neurovascular coupling relationship. Neurovascular cells are grouped into three compartments: neurons (N), supporting cells that are potential transmission sites of vasoactive signals (T), and vascular cells (V). The vasoactive signals that are released accompanying neural processing are transferred to the vascular cells directly or indirectly via the supporting cells and help coordinate local cerebral blood flow (CBF) (F). A sequential relationship is depicted (A). Each transfer function represents cortical neural processing (fn), vasoactive signal transmission (gt), and vascular reactivity (hv), which are functions of time (t) and space (r). Hemodynamic response, a final output of neurovascular function, is expressed by the convolution of these transfer functions. (B) Linear and nonlinear relationships have been observed between neural (fn) and vascular (hv) signal changes. However, their relationships to transmission function (gt) remain uncertain.
The anesthesia that has been widely used for studying neurovascular coupling in in-vivo animal models has broad action on brain cells that include changes in neural processing, vascular reactivity, and other baseline states (e.g., spontaneous neural activity, cerebral energy metabolism, and baseline CBF). These modulatory effects eventually modify the coupling relationship between neural and vascular responses, and thus, anesthesia is a potential confounder that interferes with the neurovascular coupling relationship. In this article, we review the effects of anesthesia (e.g., anesthetic agents and depths) on the (1) spatial coordination, (2) temporal dynamics, and (3) quantitative relationships between the neural and vascular responses. To help understand the underlying causes of these anesthesia effects, this review aims to provide comprehensive lists of the actions of anesthesia on (1) general physiology, (2) neurons, (3) supporting cells (vasoactive signal transmission), and (4) vascular cells. This may also aid decision-making regarding anesthetics for specific studies of neurovascular coupling in in-vivo animal models.
Why Is Anesthesia Needed?
The need for anesthesia depends on the methodology applied in neurovascular coupling studies (for reviews, see Vanzetta and Grinvald, 2008 and Villringer and Dirnagl, 1995). The electrophysiological techniques that have been used to represent neural activity as a quantitative index to compare vascular responses involves local field potentials (LFPs), and single unit and multiunit spiking activity (Lauritzen, 2001; Logothetis et al, 2001). Laminar electrodes have also been used to investigate neurovascular coupling with current source density analysis (Martindale et al, 2003). These electrophysiological techniques must fix the electrode(s) at certain locations within the brain, and thus, anesthesia has been used to minimize movement artifacts and eliminate the induced stress to the animals. For anesthesia-free neural recording, telemetry systems and linear arrays of microelectrodes have been introduced in behaving small animals (Schregardus et al, 2006) and humans (Keller et al, 2009). Electroencephalogram and magnetoencephalogram also provide less-invasive neural measurements and allow concurrent recording with cortical hemodynamics in anesthetized animals (Franceschini et al, 2008) and in conscious humans (Rosengarten and Kaps, 2010; Ou et al, 2009).
To quantify vascular responses, early studies were conducted with a locally generated hydrogen clearance method with microelectrodes to measure dynamic CBF changes elicited by cortical stimulation (Leniger-Follert and Hossmann, 1979), but this technique is invasive and may disturb cortical microcirculation in the vicinity of the measurement regions. An alternative, less-invasive method, laser-Doppler flowmetry, was introduced to monitor dynamic CBF (Dirnagl et al, 1989). This technique has become widely used to examine the temporal and quantitative neurovascular relationship in the anesthetized rodents (Matsuura et al, 2000; Matsuura and Kanno, 2001; Ances et al, 2000; Ngai et al, 1999; Akgoren et al, 1996). Some groups have shown that laser-Doppler flowmetry can also be used to monitor longitudinal changes of CBF repeatedly in awake behaving rodents (Takuwa et al, 2011; Gu et al, 2003). Using either scanning laser-Doppler flowmetry or laser speckle flowmetry, two-dimensional maps of evoked CBF have also been reported in both anesthetized (Du and Pan, 2011; Kannurpatti and Biswal, 2006; Ayata et al, 2004; Weber et al, 2004) and unanesthetized rodents (Takuwa et al, 2011). Optical coherence tomography and functional magnetic resonance imaging (fMRI) techniques provide further layer-specific hemodynamic mapping in anesthetized animal cortex (Chen et al, 2009; Jin and Kim, 2008; Maheswari et al, 2003; Silva and Koretsky, 2002). These neuroimaging techniques are noninvasive, and measurements can therefore also be acquired in awake conditions with the administration of a light sedative or paralyzing agent (Sicard et al, 2003; Peeters et al, 2001; Lahti et al, 1998). However, caution should be exercised in interpreting the data measured in waking conditions because the obtained signals might be contaminated by restraint-related stress and discomfort or enhanced arousal due to recording noises and motion artifacts (Lahti et al, 1998, 1999).
Optical intrinsic signal (OIS) imaging techniques, including near infrared spectroscopy (Fuster et al, 2005; Obrig and Villringer, 2003) and diffuse optical imaging (Franceschini et al, 2008), also allow for mapping the evoked hemodynamic changes (e.g., blood oxygenation and volume) under both anesthetized (Devor et al, 2005; Jones et al, 2001) and unanesthetized conditions (Berwick et al, 2002; Martin et al, 2002). For those techniques, waking animal experiments that require the animal to be trained to tolerate restraint in a holder and experimental set-up have been established (Berwick et al, 2002; Martin et al, 2002). Compared with other imaging methods, one major advantage that optical imaging techniques offer in neurovascular coupling studies is that they allow for imaging of neural activity on a mesoscopic scale similar to that of the hemodynamic imaging accomplished by measuring activity-induced changes in membrane potentials (Obrenovitch et al, 2009; Takashima et al, 2001; Ebner and Chen, 1995), intracellular pH (Sun et al, 2011), calcium ion concentrations (Homma et al, 2009) combined with exogenous fluorescent compounds, and endogenous fluorescent changes arising from activity-dependent cellular autofluorescence (Reinert et al, 2007; Shibuki et al, 2003). These techniques enable us to directly examine the spatial gaps between neural and vascular response regions (Weber et al, 2004). More directly, the in-vivo microscopic morphology and function of neurons, astrocytes, pericytes, and capillaries are resolved at the single-cell level with laser scanning fluorescent microscopic techniques, including confocal (Seylaz et al, 1999; Villringer et al, 1994) and two-photon excitation fluorescence microscopy (Fernández-Klett et al, 2010; Göbel and Helmchen, 2007; Takano et al, 2006; Chaigneau et al, 2003; Kleinfeld et al, 1998). In these techniques, image quality is sensitive to animal vibration, and thus, imaging is preferably conducted under anesthesia. A recent study showed that, in two-photon microscopic experiments, the image distortion was minimized to 2 to 5 μm in waking conditions by fixing animal's head to the stage and allowing the animal to move freely on a floating ball (Dombeck et al, 2007). This report indicates the feasible image resolution for examining neurovascular coupling in awake animals. Miniaturized two-photon microscopy has also been shown to capture images of cortical cells and capillary blood flow under unanesthetized conditions (Helmchen, 2002), which could be useful in future studies of cellular-scale neurovascular coupling in freely behaving animals.
Why Do We Need to Care About Anesthesia?
Different anesthetics have different action sites, which can potentially cause discrepancies in interpreting the mechanism of neurovascular coupling due to animal experiments conducted with different anesthetics. It was shown that intravenous infusion of cocaine in anesthetized rats provoked an increase in CBF under α-chloralose (25 mg/kg per hour), but the same experiments conducted under isoflurane (1.8% to 2.0%) showed decreases in CBF (Du et al, 2009). The cerebrovascular response to ethanol was shown to cause vasoconstriction under α-chloralose/urethane anesthesia (50/600 mg/kg) but vasodilation under halothane (0.5% to 1.0%) (Gordon et al, 1995). Furthermore, the role of the nitric oxide pathway in controlling CBF response to sensory stimulation was shown to be dominant in anesthetized rats (urethane, Gerrits et al, 2001; α-chloralose 40 mg/kg per hour, Nakao et al, 2001). However, when examined in awake rats, this nitric oxide pathway does not have a major role (Nakao et al, 2001). These findings indicate that particular anesthetics critically interfere with the pathway of neurovascular coupling.
Another issue concerning the use of anesthesia in neurovascular coupling experiments is that anesthesia profoundly affects the stability and reproducibility of neurovascular imaging. Austin et al (2005) showed that fMRI blood oxygen level-dependent responses to sensory stimulation varied over 6 hours of measurements in rats under α-chloralose (10 to 30 mg/kg per hour), accompanied with the varying electroencephalogram. However, in rats under urethane anesthesia (1.1 g/kg), electroencephalogram activity was shown to be stable for a prolonged time (8 to 12 hours) (Lincoln, 1969). To maintain steady electroencephalogram and fMRI responses with rats receiving medetomidine infusion (0.1 to 0.3 mg/kg per hour), the infusion rate needs to be adjusted over time due to potential pharmacokinetic changes in long-term experiments (>3 hours) (Pawela et al, 2009). Moreover, functional connectivity examined with blood oxygen level-dependent fMRI was well localized in rats anesthetized with either α-chloralose (27 mg/kg per hour) or medetomidine (0.1 mg/kg per hour) but less so under isoflurane (2%) (Williams et al, 2010). These reports indicate that choosing the appropriate anesthesia and adjusting its dosage are critical for achieving stable and reproducible experimental conditions. In the following sections, we will discuss the effects of anesthesia on the major properties of neurovascular coupling: (1) the spatial coordination of the vascular response with respect to the neurally derived map; (2) the temporal dynamics; and (3) the quantitative relationships examined under a variety of anesthesia conditions compared with those under unanesthetized conditions.
Anesthesia interference with neurovascular coupling
Spatial Coordination
Anesthesia-dependent variations in cortical mapping with OIS (630 nm) based on fingerpad stimulation were reported in the monkey somatosensory cortex (pentothal 1 to 2 mg/kg per hour versus isoflurane 0.8% to 1.5%) (Chen et al, 2001). In this study, mapping under pentothal produced focal localization, whereas maps obtained under isoflurane were less uniform and more broad. This observation was thought to occur because pentothal has suppressive actions on cortical activity due to both the potentiation of GABAergic interneurons and the suppression of the excitability of glutamatergic neurons, whereas isoflurane enhances surround inhibition (Chen et al, 2001). Although the effects of focal excitation and surround inhibition on hemodynamic regulation are not fully understood (Boorman et al, 2010; Devor et al, 2007), the observed variation of the cortical mapping could depend on the actions of the anesthesia on both the neural and vascular components. Enhanced stimulus-specific localization of the cerebral blood volume (CBV)-weighted OIS (570 nm) under waking conditions relative to anesthetized conditions was observed for orientation column mapping in the cat visual cortex (Fukuda et al, 2005). Shtoyerman et al (2000) suggested that the increased specificity for the CBV response is due to an increased vascular response and signal transmission from neurons to vessels at the active columns because of the relatively unchanged spatial properties of neural responses. With increased focal activity, one may expect that the CBF/CBV response region would expand due to the activity-dependent spread of the vasodilation region (Kannurpatti and Biswal, 2011; Masamoto et al, 2010a; Durduran et al, 2004). However, enhanced surround inhibition may also cause vasoconstriction in that region (Devor et al, 2007), leading to a sharp tuning of the CBF/CBV supply to the focal active region.
To further explore the spatial gap between vascular response regions and activated neural sites at the cellular scale of the spatial resolution, Chaigneau et al (2003, 2007) applied two-photon imaging techniques to a rodent olfactory bulb model. A series of experiments showed that no tight coupling exists between activated glomeruli, and nearby capillary flow changes depending on inducing odor stimuli in rats or mice (either urethane 1.5 g/kg or ketamine/xylazine 90/10 to 100/16 mg/kg, Jukovskaya et al, 2011; Chaigneau et al, 2007). Based on these findings, it was suggested that the spatial mismatch originates from a vascular mechanism, such as the nonspecific orientation of capillaries, which pass through several neural modules. Furthermore, this spatial mismatch may also be related to the regulatory mechanism of capillary blood flow in which control may not be localized within a single capillary. The findings further suggest a role for precapillary arterioles in controlling capillary blood flow during cortical activation (Fernández-Klett et al, 2010). Overall, anesthesia interference with spatial CBF coordination could be caused by both anesthesia-dependent modulation of neural processing, such as local balance between excitatory and inhibitory activity, and changes in vascular response sensitivity to underlying neural activity. However, the mechanisms that (1) detect focal excitatory and surrounding inhibitory activity in neighboring capillaries, (2) transport the vasoactive signals to upstream parent arteries (arterioles), and (3) control CBF balances between the active and inactive regions remain unknown.
Temporal Dynamics
The factors affecting the time delay in the vascular response relative to the onset of a neural response are the following: (1) the release of vasoactive signals from neurons, (2) the transmission of the signals, (3) the uptake of the signals by vascular cells, and (4) the action of the vascular cells. Hayton et al (1999) reported that the onset latency of somatosensory-evoked potential after electrical stimulation to rat paws was slightly changed by anesthesia: 8.2±5.0 ms for ketamine/xylazine (90/10 mg/kg), 7.5±3.5 ms for medetomidine (0.3 mg/kg), 5.4±2.6 ms for isoflurane (2%), and 6.3±2.6 ms for fentanyl/fluanisone-midazolam (0.85/27 and 13.5 mg/kg, respectively), after the onset of forelimb stimulation. Dose-dependent increases in the latency of somatosensory-evoked potential (1.8±0.8 ms per % isoflurane concentration) have also been observed in human subjects with inhaled anesthetics (0% to 1.65% isoflurane) (Sebel et al, 1986). These reports showed that the effect of anesthesia on the temporal dynamics of synaptic transmission is relatively small, indicating minimum interference with the latency of vasoactive signal release (i.e., within a couple of milliseconds).
An early study using an impedance technique suggested that the earliest onset of evoked vasodilation in response to auditory stimulation is ∼0.15 to 0.25 seconds from the onset of neural activation in conscious human brains (Sandman et al, 1984). In accordance with this observation, Nielsen and Lauritzen (2001) reported that the earliest onset latency of laser-Doppler flowmetry response measured in upper cortical layers was 0.2±0.2 seconds after infraorbital nerve stimulation in anesthetized rats (α-chloralose 45 to 60 mg/kg per hour). Short onset latencies (0.2 to 0.4 seconds) of the red blood cell speed change after sensory stimulation were also observed in the parenchymal capillaries of awake mice with two-photon microscopy (Drew et al, 2011). These observations are also in good agreement with the reported onset time of the earliest plasma volume increases (<0.5 second latency) that originate from the arterioles of the middle cortical layers (α-chloralose 40 mg/kg per hour) (Tian et al, 2010), and the CBV onset (0.35 second) that starts from middle cortical layers measured with fMRI in rats (α-chloralose 26.7 mg/kg per hour) (Hirano et al, 2011). Considering these small variations in the reported onset latency of evoked vasodilation and capillary red blood cell speed changes, it can be expected that the transmission of vasodilatory signals to vascular cells is minimally influenced by anesthesia (e.g., α-chloralose) or has minimal variations that are not detectable with current methodologies. Because the frame rates of the MRI and two-photon imaging conducted in those studies were reported to be 4 and 5 to 30 frames per second, respectively, these resolutions may be not sufficient to stably track the fast responses of the vascular reaction. Furthermore, additional tests of other cortical regions and with different anesthetics are also needed.
Moreover, the definition of the onset timing of the evoked vascular response may be biased depending on the criteria by which threshold levels for activity-induced changes are determined relative to baseline fluctuations (e.g., a threshold at twofold the standard deviation of baseline or an intersection between baseline and initial slope). Nevertheless, we found that the literature showed narrow ranges for the onset time (0.2 to 0.7 seconds) of evoked CBF in the rat somatosensory cortex regardless of the criteria used to define onset time under α-chloralose (Hirano et al, 2011; Sheth et al, 2005; Nielsen and Lauritzen, 2001; Silva et al, 2000; Matsuura et al, 1999, 2000). These findings are also in good agreement with the results measured under isoflurane anesthesia (0.4 to 0.6 seconds; Masamoto et al, 2007; Table 1). In contrast, relatively variable reports were found for the onset time of CBV changes (0.5 to 1.3 seconds) with OIS in a rat somatosensory model (α-chloralose, Chen et al, 2011; Hillman et al, 2007, versus urethane, Berwick et al, 2005). The large variations observed in the OIS results could be related to technical issues concerning spectral decomposition to calculate total hemoglobin content and the different signal-to-noise ratios of the measurements. Differing regions of interest also potentially contributed to the observed discrepancies, as it is well known that CBV changes spread along the vasculature from the activated hot spots (Chen et al, 2011; Sheth et al, 2005).
Table 1. Onset delay of hemodynamic response relative to neural response in rats.
| Onset time (seconds) | Criteria | Anesthetic (dosage) | Stimulation (pulse width, current, frequency) | Measurement | Reference |
|---|---|---|---|---|---|
| 1.8±0.2 | >2 s.d. | Ure (1.5 g/kg) | Od (2 seconds) | Two-photon MS (capillary RBC speed) | Chaigneau et al (2003) |
| 0.50±0.10 0.63±0.25 | Intersection of initial slope | Ure (1.25 g/kg) | WP (0.3 ms, 1.2 mA, 5 Hz) | OIS (parenchyma) OIS (artery) | Berwick et al (2005) |
| 0.56±0.19 | OIS (vein) | ||||
| 0.7±0.1 | Not described | Ket (6 mg/kg) | WD (2 Hz, 10 seconds) | H2 clearance | Khananashvili and Demidova (2002) |
| 0.2±0.2 | >2 s.d. | AC (45–60 mg/kg per hour) | IO (1 ms, 1.5 mA, 2 Hz) | LDF | Nielsen and Lauritzen (2001) |
| 0.34±0.06 | Intersection of initial slope | AC (35 mg/kg per hour) | Cor (1 ms, 10–15 μA, 5–50 Hz) | LDF | Matsuura et al (1999) |
| 0.4±0.1 | >2 s.d. | AC (30 mg/kg per hour) | HP (1 ms, 1.2 mA, 2–20 Hz) | LDF | Sheth et al (2005) |
| 0.52±0.06 | Intersection of initial slope | AC (45 mg/kg per hour) | HP (0.1 ms, 1.5 mA, 5 Hz) | LDF | Matsuura et al (2000) |
| 0.54±0.07 0.75±0.13 | Statistical analysis | AC (40 mg/kg per hour) | FP or HP (3 ms, 1 mA, 3 Hz) | OIS (parenchyma) OIS (artery) | Chen et al (2011) |
| <0.5 | Intersection of initial slope | AC (40 mg/kg per hour) | FP (0.3 ms, 1 mA, 3 Hz) | Two-photon MS (plasma volume) | Tian et al (2010) |
| 0.7±0.4 | >1 s.d. | AC (27 mg/kg per hour) | FP (0.3 ms, 1.5 mA, 3 Hz) | fMRI (CBF) | Silva et al (2000) |
| 0.40±0.22 0.43±0.18 | >1 s.d. | AC (27 mg/kg per hour) | BiFP (0.3 ms, 2 mA, 3 Hz) | fMRI (CBF, layers I–II) fMRI (CBF, layers III–V) | Hirano et al (2011) |
| 0.61±0.34 | fMRI (CBF, layer VI) | ||||
| 0.34±0.19 0.35±0.16 | >1 s.d. | AC (27 mg/kg per hour) | BiFP (0.3 ms, 2 mA, 3 Hz) | fMRI (CBV, layers I–II) fMRI (CBV, layers III–V) | Hirano et al (2011) |
| 0.58±0.25 | fMRI (CBV, layer VI) | ||||
| 1.3 | 30% peak | AC (30–40 mg/kg per hour) | FP (3 ms, 1.6 mA, 3 Hz) | OIS (total Hb) | Hillman et al (2007) |
| 0.4–0.6 | Intersection of initial slope | Iso (1.4%) | FP (1.0 ms, 1.0 mA, 2–20 Hz) | LDF | Masamoto et al (2007) |
AC, α-chloralose; BiFP, bilateral forepaw; CBF, cerebral blood flow; CBV, cerebral blood volume; Cor, cortex; fMRI, functional magnetic resonance imaging; FP, forepaw; HP, hindpaw; IO, infraorbital nerve; Iso, isoflurane; Ket, ketamine; LDF, laser-Doppler flowmetry; MS, microscope; Od, odor; OIS, optical intrinsic signal; RBC, red blood cell; s.d., standard deviation; Ure, urethane; WD, whisker deflection; WP, whisker pad.
Whether certain anesthetics directly interfere with the dynamics of vascular responses (e.g., the propagation speeds of the vasodilatory signals) remains incompletely understood. In rats anesthetized with urethane (1.25 g/kg), the times-to-peak of CBF responses were observed to be longer (0.6 to 1.2 seconds) than those observed under waking conditions (Martin et al, 2006). In contrast, a similar time course of evoked change was observed for arterial vascular responses measured under either awake or urethane anesthesia (1 g/kg) conditions in the mouse somatosensory cortex (Drew et al, 2011). Although the cause of the controversial results (i.e., urethane effects) remains unclear in those studies, anesthesia may affect the temporal dynamics of vascular responses in varying degrees via its actions on the intracellular calcium dynamics of smooth muscle cells and the reactivity to the vasoactive substances (Altura et al, 1980), and secondary effects via anesthesia-induced changes in systemic conditions, such as hypercapnia and hypotension.
Quantitative Relationships
The quantitative coupling relationships between evoked neural and vascular responses are largely influenced by the anesthesia. The route of this action involves anesthesia-dependent modulation of the following: (1) neural processing, (2) the vasoactive signal pathway, and (3) vascular cell reactivity. In awake conditions, larger evoked CBF/CBV changes have generally been observed than those observed in anesthetized conditions (Fukuda et al, 2005; Martin et al, 2006). Simultaneous recordings of evoked neural and vascular responses in rats have shown that the reduced hemodynamic responses under anesthesia (urethane 1.25 g/kg) were mainly due to the suppression of cortical excitability (Martin et al, 2006). The suppression of cortical activity is thought to be due to the suppression of thalamocortical inputs and suppression of cortical processing. Franceschini et al (2010) showed strong reduction of thalamocortical input under either ketamine/xylazine (20/2 mg/kg per hour) or fentanyl/droperidol (0.09/4.5 mg/kg per hour) anesthesia measured with somatosensory-evoked potential (i.e., a first positive peak, P1) in rats, whereas relatively preserved thalamocortical input was observed with α-chloralose (40 mg/kg per hour). Either pentobarbital (25 mg/kg) or propofol (50 mg/kg per hour) preserved thalamocortical inputs but reduced cortical activity, leading to the lowest hemodynamic responses among the anesthesia conditions tested (Franceschini et al, 2010). These findings strongly indicate that the reduced magnitude of cortical vascular response under general anesthesia originates from the anesthesia-dependent modulation of cortical processing.
In the rat primary somatosensory cortex, it was shown that anesthesia profoundly affects neural refractory periods with different degrees of potentiation depending on the anesthesia type and dosage (Masamoto et al, 2007, 2009). A prolonged refractory period was observed under α-chloralose (Masamoto et al, 2007; Ogawa et al, 2000), whereas short refractory periods were maintained under isoflurane and enflurane anesthesia. The latter anesthetics maintained robust hemodynamic responses to higher frequency stimulation (6 to 10 Hz; Kim et al, 2010; Masamoto et al, 2007; Sheth et al, 2003), which is in contrast to the well-known frequency dependencies in the rat somatosensory models using α-chloralose (a peak at a frequency of 1.5 to 5 Hz; Table 2). In addition, under different levels of isoflurane (0.8% to 2.2%), cortical adaptation was enhanced in a dose-dependent manner (Masamoto et al, 2009). In this condition, the evoked CBF induced by single-pulse local field potential (i.e., hemodynamic impulse response) was, however, found to increase in a dose-dependent manner (Masamoto et al, 2009). As a result, the optimum stimulus frequency that evoked the highest CBF response per given stimulus duration was shifted from high to low frequency stimulation with increases in anesthesia depths (Masamoto et al, 2009). Strong cortical adaptation under isoflurane (1.2%) was also consistently found in the rat somatosensory model with diffuse optical imaging (Franceschini et al, 2010). In this study, the highest hemodynamic response per given neural activity (P2 or N1 of somatosensory-evoked potential) was observed for either α-chloralose (40 mg/kg per hour) or isoflurane (1.2%), while a 10-fold higher CBF response to CO2 challenges (5% CO2 gas) was observed for isoflurane compared with ketamine/xylazine, propofol, or α-chloralose anesthesia (Franceschini et al, 2010). These findings suggest that separate mechanisms are involved in the vasodilatory response to neural stimulation and CO2 challenge. In conclusion, the effects of anesthesia on quantitative neurovascular coupling primarily consist of the modulation of cortical processing and, thus, vasoactive pathways, while the effects of vascular reactivity differences on evoked vascular responses are weaker and the degree of these effects depends on anesthesia type and depth. Because different stimulus frequencies (4 to 30 Hz) were shown to evoke different populations of excitatory pyramidal cells and vasoactive inhibitory interneurons (Enager et al, 2009), it is likely that this anesthesia interference with cortical processing involves actions on the variable populations of vasoactive local interneurons (Lecrux et al, 2011; Harris et al, 2010). The cellular mechanism of the anesthesia action and its involvement in neurovascular transmission must be better identified in future studies.
Table 2. Stimulation frequency dependences of hemodynamic responses in rat somatosensory cortex.
| Optimum frequency (Hz) | Anesthetic (dosage) | Stimulation (pulse width, current) | Range of frequency (Hz) | Duration (seconds) | Techniques | Reference |
|---|---|---|---|---|---|---|
| 40 | Waking | WP (0.3 ms, 0.3 mA) | 1–40 | 2 | LDF | Martin et al (2006) |
| 5 | Ure (1.25 g/kg) | WP (0.3 ms, 1.2 mA) | 1–40 | 2 | ||
| 3 | Ure (1 g/kg) | WD (1 mm) | 1–20 | 60 | H2 clearance | Moskalenko et al (1996) |
| 5 | Eto (1.5–2.1 mg/kg per hour) | WD (maximum) | 1–7 | 120 | ARG (14C-IAP) | Vogel and Kuschinsky (1996) |
| 10.5 | Ure (1.2 g/kg) | WD (5 mm) | 1.5–10.5 | 15 | LDF | Gerrits et al (1998) |
| 10 | Ure (1.2 g/kg) | WAP (15 ms) | 1–10 | 44 | 2D-LDFI | Kannurpatti and Biswal (2011) |
| 12 | AC (40 mg/kg per hour) | WAP (16.7–125 ms) | 4–30 | 30 | fMRI (BOLD) | Sanganahalli et al (2008) |
| 1.5 | FP (0.3 ms, 2 mA) | 0.5–30 | 30 | |||
| 9–15 | Ure (1.25 g/kg) | FP (0.3 ms, 1–1.2 mA) | 1–15 | 30 | fMRI (BOLD) | Huttunen et al (2008) |
| 10 | Enf (1–2%) | HP (1.0 ms, 1.0 mA) | 2–20 | 2 | OIS (570 nm) | Sheth et al (2003) |
| 12 | Iso (1.4%) | FP (1.0 ms, 1.0 mA) | 2–20 | 0.5–5 | LDF and fMRI | Masamoto et al (2007) |
| 6–8 | Iso (1.3–1.5%) | FP (1.0 ms, 1.5 mA) | 1–24 | 30 | LDF and fMRI | Kim et al (2010) |
| 9 | Me (0.1 mg/kg per hour) | FP (0.3 ms, 2 mA) | 1–18 | 20 | fMRI (BOLD) | Zhao et al (2008) |
| 8 | K/X (75/5 mg/kg)+AC (60 mg/kg) | BiFP (10 ms, 1 mA) | 1–12 | 40 | fMRI (BOLD) | van Camp et al (2006) |
| 3 | AC (27 mg/kg per hour) | BiFP (0.3 ms, 2 mA) | 1–8 | 45 | fMRI (BOLD) | Keilholz et al (2004) |
| 2 | AC (30 mg/kg per hour) | HP (1.0 ms, 0.8 mA) | 2–20 | 2 | LDF and OIS | Sheth et al (2004) |
| 5 | AC (45 mg/kg per hour) | HP (0.1 ms, 1.5 mA) | 0.2–10 | 5 | LDF | Matsuura and Kanno (2001) |
| 3 | AC (27 mg/kg per hour) | FP (0.3 ms, 1.5 mA) | 1–5 | 40 | LDF | Silva et al (1999) |
| 1.5 | AC (27 mg/kg per hour) | FP (0.3 ms, 0.5 mA) | 1.5–6 | 50 | fMRI (BOLD) | Brinker et al (1999) |
| 1.5 | AC (27 mg/kg per hour) | FP (0.3 ms, 0.5 mA) | 1.5–9 | 40 | fMRI (BOLD) | Gyngell et al (1996) |
AC, α-chloralose; ARG, autoradiography; BiFP, bilateral forepaw; BOLD, blood oxygen level-dependent; 14C-IAP, [14C]iodoantipyrine; 2D-LDFI, two-dimensional laser-Doppler flowmetry imaging; Enf, enflurane; Eto, etomidate; fMRI, functional magnetic resonance imaging; FP, forepaw; HP, hindpaw; Iso, isoflurane; K/X, ketamine/xylazine; LDF, laser-Doppler flowmetry; Me, medotomidine; OIS, optical intrinsic signal; Ure, urethane; WAP; whisker air-puff; WD, whisker deflection; WP, whisker pad.
Sites of action of anesthesia and neurovascular coupling
The potential target sites of anesthesia actions involved in neurovascular coupling are summarized in this section with emphasis on the anesthetics frequently used in animal experiments of neurovascular coupling and imaging, such as α-chloralose, isoflurane, pentobarbital, urethane, and medetomidine. The appropriate dosages and type of anesthetic for acute and chronic experiments in both rats and mice can be referenced in the literature (Lukasik and Gillies, 2003).
General Physiology
Alpha-chloralose is thus far the most commonly used anesthesia in neurovascular coupling imaging and physiology experiments in rodents because the early studies have shown that this agent preserves robust and stable hemodynamic and metabolic coupling to sensory stimulation (Lindauer et al, 1993; Ueki et al, 1992). The effects of α-chloralose on general physiology include respiratory depression, metabolic acidosis, and hyperreactivity (Arfors et al, 1971). The analgesic properties of this agent are questionable (Silverman and Muir, 1993), and thus, any surgical preparation must be performed with administration of another anesthetic. This procedural complication before the experiment contributes to the variable results regarding neurovascular coupling relationships in α-chloralose-anesthetized rats (Bonvento et al, 1994). In addition, the use of α-chloralose is limited to experiments with nonsurvival protocols (Silverman and Muir, 1993), which hampers the use of this animal model for wider applications, such as repeated longitudinal experiments.
Alternatively, several groups have introduced inhaled anesthetics (e.g., halothane, enflurane, and isoflurane) for rodent neurovascular coupling studies (Kim et al, 2010; Masamoto et al, 2007; Schulte and Hudetz, 2006; Sheth et al, 2003). A major advantage of inhaled anesthetics is that the fast induction and rapid recovery achieved with these anesthetics make them usable for repeated longitudinal experiments. However, a known disadvantage of these agents is that volatile anesthetics themselves are potent vasodilators and, thus, cause cerebral vasodilation, which leads to increased baseline CBF that is uncoupled from the cerebral energy metabolism (van Aken and van Hemelrijck, 1991). The effects might be more severe in mice compared with rats. Moreover, it was shown that high concentrations of isoflurane (>3%) break down the cortical blood–brain barrier (Tétrault et al, 2008), and isoflurane impairs glucose-stimulated insulin release (Tanaka et al, 2011).
Barbiturate anesthetics, including pentobarbital and thiopental, suppresses cardiac output and often cause hypotension. Respiratory depressant effects of pentobarbital have also been reported (Field et al, 1993). In contrast, urethane provides moderate depression of blood pressure and heart rate, and may cause hyperventilation (Field et al, 1993). Intraperitoneal administration of urethane (1.2 g/kg) was shown to cause hyperglycemia associated with hypothalamic activation (Reinert, 1964; Maggi and Meli, 1986). Urethane is also known as potential mutagen and carcinogen (Field and Lang, 1988). Medetomidine and xylazine are specific α2-adrenoreceptor agonists that block norepinephrine release. Due to the limited analgesic properties of these agents, surgical procedures should be performed in combination with other anesthetic agents. The α2-adrenoreceptor was also known to significantly affect cardiovascular function and depress respiratory function (Sinclair, 2003). Animals can recover after the administration of a reversible α2-antagonist, making this agent also suitable for use in repeated longitudinal imaging experiments (Pawela et al, 2009; Weber et al, 2006).
Neural Activity
The action sites at which anesthesia influences neural pathways have been shown to involve common targets, such as ligand-gated ion channels (potentiation of GABA type A and glycine receptors, and suppression of NMDA (N-methyl-D-aspartate) receptor) and presynaptic actions on calcium, potassium, and sodium channels (for reviews, see Chau, 2010; Hemmings 2009; Franks, 2008). Isoflurane was shown to predominantly reduce presynaptic excitability via sodium channel blockade (Hemmings 2009) and, thus, glutamate release (Sandstrom, 2004; Wu et al, 2004). Moreover, the inhibition of the NMDA receptor, potentiation of GABA type A receptors, and suppression of acetylcholine transmission and receptors have also been reported (Dickinson et al, 2007; Hentschke et al, 2005; Violet et al, 1997).
The action site of α-chloralose has been shown to involve the potentiation of GABA-induced currents by increasing affinity for GABA (Garrett and Gan, 1998), whereas preserved synaptic transmission and glutamate-, glycine-, and acetylcholoine-induced current were observed at low concentration of α-chloralose (Wang et al, 2008). Pentobarbital binds to GABA type A receptors and enhances GABA-mediated inhibitory neurotransmission (Curtis and Lodge, 1977). Pentobarbital and thiopental have also been shown to inhibit the release of acetylcholine, norepinephrine, and glutamate (Nicoll, 1978; Curtis and Lodge, 1977). Significant inhibition of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor by pentobarbital has also been found, but pentobarbital has small effects on NMDA, glycine, and GABA receptors (Hara and Harris, 2002). Medetomidine selectively inhibits noradrenergic neurons in the locus coeruleus and has been shown to disrupt thalamocortical transmission (Sinclair, 2003). Urethane is shown to have modest effects on multiple ligand-gated ion channels, including potentiation of GABA type A and glycine receptors, and mild inhibition of NMDA and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (Hara and Harris, 2002; Maggi and Meli, 1986; Minchin, 1981), which shows that this agent is suitable for pharmacological studies of neurotransmitter release and uptake.
Vasoactive Signal Transmission
Determining the action site on neural processing and the resultant effects on the vascular responses would provide an insight into the mechanism of the neurovascular transmission pathway. However, it is difficult to directly identify and purify the action site of anesthesia because most neurovascular transmissions share a common mechanism of neural processing. Volatile anesthetics, but not ketamine or pentobarbital, have been shown to enhance glutamate uptake by astrocytes (Miyazaki et al, 1997), and dose-dependent closure of astrocytic gap junctions has been observed with volatile anesthetics in cultured astrocytes (Mantz et al, 1993). Whether these effects of isoflurane on astrocyte function are related to the observed dose-dependent increase in hemodynamic impulse responses (Masamoto et al, 2009) remains unclear. Future studies should determine the exact contribution of the actions of anesthetics on astrocyte mechanisms using pharmacological approaches. Furthermore, experimental models with well-defined neural circuits would be useful to further determine the action site in in-vivo conditions and its contribution to the generation of the blood oxygen level-dependent fMRI signal (Krautwald and Angenstein, 2012).
Vascular Responses
Some anesthetics have been shown to directly affect vascular physiology. Isoflurane dose dependently induced the relaxation of cerebral arteries (i.e., vasodilation) via its actions on ATP-sensitive potassium channels and reduced calcium current in smooth muscle cells (Iida et al, 1998; Flynn et al, 1991, 1992). In contrast, endothelium-dependent vasodilation induced by acetylcholine was inhibited by isoflurane due to its inhibiting effects on formation of nitric oxide in endothelium (Nakamura et al, 1994; Toda et al, 1992). Autoregulatory responses, and the CBF response to CO2 inhalation, were shown to be preserved under isoflurane anesthesia (Lee et al, 1994, 1995). However, a depression of the vascular response to CO2 inhalation has been found in rats anesthetized with 2% isoflurane compared with the waking condition (Sicard et al, 2003). It is well known that pentobarbital causes a reduction of CBF relative to awake conditions (Wei et al, 1993). However, the effects of barbiturate anesthetics on cerebral vessels have been shown to be controversial; they can act as potent vasoconstrictors (Tsuji and Chiba, 1987) or vasodilators (Ogura et al, 1991). Medetomidine also causes α2-adenoreceptor-mediated vasoconstriction of cerebral arteries and results in reduced CBF (Sinclair, 2003; Ganjoo et al, 1998). Decreased sensitivity of the cerebrovascular response to arterial CO2 has been reported for α-chloralose (100 mg/kg; Sándor et al, 1977). Furthermore, we observed that the capillary diameter in the resting state was slightly larger under 45 mg/kg per hour α-chloralose (5.1±1.2 μm) than 1.4% isoflurane (4.8±1.1 μm) (Masamoto et al, 2010b), which may further contribute to the anesthesia-dependent variations of microvascular responses to physiological perturbations, such as the contribution of capillary diameter changes measured under different anesthesia conditions.
Other Considerations
As discussed above, one should consider the effect of anesthesia on baseline (prestimulus resting) conditions. It has been reported that activation-induced changes of brain activity were largely dependent on the baseline states, such as the anesthesia-dependent reduction of oxygen and glucose metabolism and unit neural activity (Hyder et al, 2002; Shulman et al, 1999). In these studies, lower baseline states induced by anesthesia have been shown to cause larger activation changes. Because the resting-state energy metabolism is known to be coupled to baseline CBF, it can therefore be expected that baseline CBF also differs depending on the anesthesia. Some previous works regarding the baseline CBF measured under a variety of anesthesia conditions in the rat cerebral cortex are summarized in Table 3. Overall, injectable anesthetics (α-chloralose and pentobarbital) reduced baseline CBF, whereas low concentration of isoflurane (1.3% to 1.5%) maintained CBF values close to those of the awake condition (Table 3). Whether these modulatory effects of anesthesia on baseline CBF affect activation-induced vascular responses remains relatively unknown (Franceschini et al, 2010). However, caution should be exercised in comparing the quantitative data examined under different baseline states with different anesthesia.
Table 3. Baseline CBF in rat cortex.
| CBF (ml/100 g per minute) | Anesthetic (dosage) | Region | Technique | Reference |
|---|---|---|---|---|
| 167±45 | Waking | Sensorimotor | ARG (14C-IAP) | Kuschinsky et al (1985) |
| 155±30 | Waking | Sensorimotor | ARG (14C-IAP) | Maekawa et al (1986) |
| 102±35 | Iso (0.7%) | |||
| 147±40 | Iso (1.4%) | |||
| 183±63 | Iso (2.1%) | |||
| 247±67 | Iso (2.8%) | |||
| 134±8 | Waking | Sensorimotor | ARG (14C-IAP) | Lenz et al (1998) |
| 132±26 | Iso (1.4%) | |||
| 153±14 | Iso (2.8%) | |||
| 151±23 | Iso (1.3–1.5%) | Somatosensory | MRI (ASL) | Kim et al (2007) |
| 146±13 | Ure (1.2 g/kg) | Somatosensory | ARG (14C-IAP) | Gerrits et al (2000) |
| 168±12 | Waking | Sensorimotor | ARG (14C-IAP) | Otsuka et al (1991) |
| 55±5 | Pen (50 mg/kg) | |||
| 180±15 | Waking | Somatosensory | ARG (14C-IAP) | Nakao et al (2001) |
| 65±5 | AC (40 mg/kg per hour) | |||
| 58±3 | AC (26.7 mg/kg per hour)/50%N2O | Cortex | MRI (CASL) | Lee et al (2001) |
| 75±9 | Morphine (60 mg/kg per hour)/70% N2O | Somatosensory | MRI | Hyder et al (2000) |
| 40±9 | AC (40 mg/kg per hour)/70% N2O | |||
| 90±20 | AC (36 mg/kg per hour)/70% N2O | Somatosensory | MRI | Smith et al (2002) |
| 60±20 | AC (46 mg/kg per hour)/70% N2O |
AC, α-chloralose; ARG, autoradiography; ASL, arterial spin labeling; CASL, continuous arterial spin labeling; CBF, cerebral blood flow; 14C-IAP, [14C]iodoantipyrine; Iso, isoflurane; MRI, magnetic resonance imaging; Pen, pentobarbital.
Finally, repeated longitudinal experiments are becoming increasingly more important for further understanding the biological implications and plasticity of neurovascular coupling (Brown et al, 2010; Colonnese et al, 2008). For longitudinal experiments, the same anesthetics have been repeatedly used in single animals; i.e., isoflurane (Colonnese et al, 2008; Tomita et al, 2005) and ketamine/xylazine (Brown et al, 2010). For those experiments, good recovery from anesthesia discontinuation is important in the choice of anesthetics for performing controlled experiments. Hayton et al (1999) reported that ketamine/xylazine, medetomidine, and fentanyl/fluanisone-midazolam cause losses in body weight; however, isoflurane does not have this effect. No effects on cell proliferation were found for isoflurane, propofol, medetomidine, or ketamine in young rats (Tung et al, 2008); these results are particularly important for developmental and regeneration studies with long-term imaging experiments.
Summary
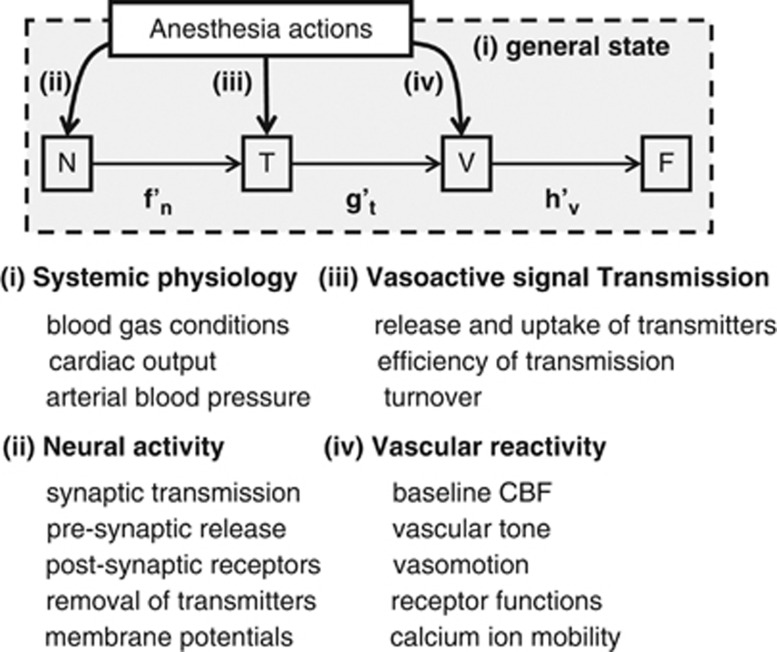
The effects of anesthesia on neurovascular coupling involve the following: (1) changes in general physiology, (2) direct interference with cortical neural processing, (3) modulation of vasoactive signal transmission, and (4) suppression of vascular cell activities (Figure 2). It is well known that anesthesia modifies the balance between focal excitation and surround inhibition in a manner dependent on the type and dosage of anesthesia. However, the resultant effects on spatial coordination of vascular responses, such as activity-dependent vasodilation and vasoconstriction, are not well understood. Propagation time, in particular that for the onset latency of vasodilation evoked by neural stimulation, is relatively preserved across anesthesia states (Table 1), but the temporal dynamics of vasodilation may vary between anesthetics. Finally, the quantitative coupling relationship of neurovascular responses is strongly influenced by anesthesia types and dosages. The fact that different anesthetics differentially modify the hemodynamic impulse response functions indicates that different anesthetics act specifically on the different cell populations that participate in the vasoactive pathways. In addition, this anesthesia interference may involve variations in baseline states, such as spontaneous neural activity, energy metabolism, and baseline CBF.
Figure 2.
Summary of the effects of anesthesia on neurovascular coupling. The effects of anesthetics involve systemic physiology (i), neural processing (ii), vasoactive signal transmission (iii), and vascular responses (iv). Depending on the type and dose of anesthetic, the anesthesia differentially modifies the individual transfer functions of neural processing (f'n), vasoactive signal transmission (g't), and vascular reactivity (h'v). CBF, cerebral blood flow.
For in-vivo rodent somatosensory models, a large amount of neurovascular physiology and imaging data have been accumulated under α-chloralose anesthesia. Some findings might be specific to this anesthetic, such as the optimum stimulus frequency (Table 2) and low baseline CBF (Table 3). For repeated longitudinal experiments, isoflurane is recommended as an alternative agent because it provides easy control, good anesthesia recovery, and robust activity-induced vascular response that is comparable to that of α-chloralose in rat somatosensory models (Masamoto et al, 2007; Franceschini et al, 2010). Intravenous injection anesthesia (e.g., α-chloralose and medetomidine) carries concerns about the stability and reproducibility for long-term experiments (>3 hours), whereas urethane provides relatively long-term stability and balanced actions on multiple neurotransmitter receptors. Although some anesthetics, such as isoflurane, pentobarbital, and medetomidine, directly affect vascular physiology (i.e., dose-dependent vasodilation or vasoconstriction) independent of neural processing, the practical effects of these actions on neurovascular signal transmission remain relatively unknown. Because specific anesthetics may influence the specific cellular mechanisms of neurovascular elements, conducting multiple tests under different anesthesia conditions is recommended to ensure the exclusion of anesthesia confounds. This approach also helps determine the mechanisms of the generation of the signal in hemodynamic-based neuroimaging techniques.
The authors declare no conflict of interest.
Footnotes
This study was partially supported by Special Coordination Funds for Promoting Science and Technology from the Japan Ministry of Education, Culture, Sports, Science and Technology (MEXT).
References
- Akgören N, Dalgaard P, Lauritzen M. Cerebral blood flow increases evoked by electrical stimulation of rat cerebellar cortex: relation to excitatory synaptic activity and nitric oxide synthesis. Brain Res. 1996;710:204–214. doi: 10.1016/0006-8993(95)01354-7. [DOI] [PubMed] [Google Scholar]
- Altura BM, Altura BT, Carella A, Turlapaty PD, Weinberg J. Vascular smooth muscle and general anesthetics. Fed Proc. 1980;39:1584–1591. [PubMed] [Google Scholar]
- Ances BM, Zarahn E, Greenberg JH, Detre JA. Coupling of neural activation to blood flow in the somatosensory cortex of rats is time-intensity separable, but not linear. J Cereb Blood Flow Metab. 2000;20:921–930. doi: 10.1097/00004647-200006000-00004. [DOI] [PubMed] [Google Scholar]
- Arfors KE, Arturson G, Malmberg P. Effect of prolonged chloralose anesthesia on acid-base balance and cardiovascular functions in dogs. Acta Physiol Scand. 1971;81:47–53. doi: 10.1111/j.1748-1716.1971.tb04876.x. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin VC, Blamire AM, Allers KA, Sharp T, Styles P, Matthews PM, Sibson NR. Confounding effects of anesthesia on functional activation in rodent brain: a study of halothane and alpha-chloralose anesthesia. Neuroimage. 2005;24:92–100. doi: 10.1016/j.neuroimage.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ayata C, Dunn AK, Gursoy-OZdemir Y, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–755. doi: 10.1097/01.WCB.0000122745.72175.D5. [DOI] [PubMed] [Google Scholar]
- Berwick J, Johnston D, Jones M, Martindale J, Redgrave P, McLoughlin N, Schiessl I, Mayhew JE. Neurovascular coupling investigated with two-dimensional optical imaging spectroscopy in rat whisker barrel cortex. Eur J Neurosci. 2005;22:1655–1666. doi: 10.1111/j.1460-9568.2005.04347.x. [DOI] [PubMed] [Google Scholar]
- Berwick J, Martin C, Martindale J, Jones M, Johnston D, Zheng Y, Redgrave P, Mayhew J. Hemodynamic response in the unanesthetized rat: intrinsic optical imaging and spectroscopy of the barrel cortex. J Cereb Blood Flow Metab. 2002;22:670–679. doi: 10.1097/00004647-200206000-00005. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Charbonné R, Corrèze JL, Borredon J, Seylaz J, Lacombe P. Is alpha-chloralose plus halothane induction a suitable anesthetic regimen for cerebrovascular research. Brain Res. 1994;665:213–221. doi: 10.1016/0006-8993(94)91340-4. [DOI] [PubMed] [Google Scholar]
- Boorman L, Kennerley AJ, Johnston D, Jones M, Zheng Y, Redgrave P, Berwick J. Negative blood oxygen level dependence in the rat: a model for investigating the role of suppression in neurovascular coupling. J Neurosci. 2010;30:4285–4294. doi: 10.1523/JNEUROSCI.6063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker G, Bock C, Busch E, Krep H, Hossmann KA, Hoehn-Berlage M. Simultaneous recording of evoked potentials and T2*-weighted MR images during somatosensory stimulation of rat. Magn Reson Med. 1999;41:469–473. doi: 10.1002/(sici)1522-2594(199903)41:3<469::aid-mrm7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Brown CE, Boyd JD, Murphy TH. Longitudinal in vivo imaging reveals balanced and branch-specific remodeling of mature cortical pyramidal dendritic arbors after stroke. J Cereb Blood Flow Metab. 2010;30:783–791. doi: 10.1038/jcbfm.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci USA. 2003;100:13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Tiret P, Lecoq J, Ducros M, Knöpfel T, Charpak S. The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J Neurosci. 2007;27:6452–6460. doi: 10.1523/JNEUROSCI.3141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau PL. New insights into the molecular mechanisms of general anaesthetics. Br J Pharmacol. 2010;161:288–307. doi: 10.1111/j.1476-5381.2010.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Bouchard MB, McCaslin AF, Burgess SA, Hillman EM. High-speed vascular dynamics of the hemodynamic response. Neuroimage. 2011;54:1021–1030. doi: 10.1016/j.neuroimage.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Friedman RM, Ramsden BM, LaMotte RH, Roe AW. Fine-scale organization of SI (area 3b) in the squirrel monkey revealed with intrinsic optical imaging. J Neurophysiol. 2001;86:3011–3029. doi: 10.1152/jn.2001.86.6.3011. [DOI] [PubMed] [Google Scholar]
- Chen Y, Aguirre AD, Ruvinskaya L, Devor A, Boas DA, Fujimoto JG. Optical coherence tomography (OCT) reveals depth-resolved dynamics during functional brain activation. J Neurosci Methods. 2009;178:162–173. doi: 10.1016/j.jneumeth.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Phillips MA, Constantine-Paton M, Kaila K, Jasanoff A. Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat Neurosci. 2008;11:72–79. doi: 10.1038/nn2017. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Lodge D. Pentobarbitone enhancement of the inhibitory action of GABA. Nature. 1977;270:543–544. doi: 10.1038/270543c0. [DOI] [PubMed] [Google Scholar]
- Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, Boas DA, Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc Natl Acad Sci USA. 2005;102:3822–3827. doi: 10.1073/pnas.0407789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Kaplan B, Jacewicz M, Pulsinelli W. Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. J Cereb Blood Flow Metab. 1989;9:589–596. doi: 10.1038/jcbfm.1989.84. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PJ, Shih AY, Kleinfeld D. Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc Natl Acad Sci USA. 2011;108:8473–8478. doi: 10.1073/pnas.1100428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Pan Y. Optical detection of brain function: simultaneous imaging of cerebral vascular response, tissue metabolism, and cellular activity in vivo. Rev Neurosci. 2011;22:695–709. doi: 10.1515/RNS.2011.053. [DOI] [PubMed] [Google Scholar]
- Du C, Tully M, Volkow ND, Schiffer WK, Yu M, Luo Z, Koretsky AP, Benveniste H. Differential effects of anesthetics on cocaine's pharmacokinetic and pharmacodynamic effects in brain. Eur J Neurosci. 2009;30:1565–1575. doi: 10.1111/j.1460-9568.2009.06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durduran T, Burnett MG, Yu G, Zhou C, Furuya D, Yodh AG, Detre JA, Greenberg JH. Spatiotemporal quantification of cerebral blood flow during functional activation in rat somatosensory cortex using laser-speckle flowmetry. J Cereb Blood Flow Metab. 2004;24:518–525. doi: 10.1097/00004647-200405000-00005. [DOI] [PubMed] [Google Scholar]
- Ebner TJ, Chen G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Prog Neurobiol. 1995;46:463–506. doi: 10.1016/0301-0082(95)00010-s. [DOI] [PubMed] [Google Scholar]
- Enager P, Piilgaard H, Offenhauser N, Kocharyan A, Fernandes P, Hamel E, Lauritzen M. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J Cereb Blood Flow Metab. 2009;29:976–986. doi: 10.1038/jcbfm.2009.23. [DOI] [PubMed] [Google Scholar]
- Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field KJ, Lang CM. Hazards of urethane (ethyl carbamate): a review of the literature. Lab Anim. 1988;22:255–262. doi: 10.1258/002367788780746331. [DOI] [PubMed] [Google Scholar]
- Field KJ, White WJ, Lang CM. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab Anim. 1993;27:258–269. doi: 10.1258/002367793780745471. [DOI] [PubMed] [Google Scholar]
- Flynn N, Buljubasic N, Bosnjak ZJ, Kampine JP. Cerebral vascular responses to anesthetics. Adv Exp Med Biol. 1991;301:237–246. doi: 10.1007/978-1-4684-5979-1_22. [DOI] [PubMed] [Google Scholar]
- Flynn NM, Buljubasic N, Bosnjak ZJ, Kampine JP. Isoflurane produces endothelium-independent relaxation in canine middle cerebral arteries. Anesthesiology. 1992;76:461–467. doi: 10.1097/00000542-199203000-00021. [DOI] [PubMed] [Google Scholar]
- Franceschini MA, Nissilä I, Wu W, Diamond SG, Bonmassar G, Boas DA. Coupling between somatosensory evoked potentials and hemodynamic response in the rat. Neuroimage. 2008;41:189–203. doi: 10.1016/j.neuroimage.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini MA, Radhakrishnan H, Thakur K, Wu W, Ruvinskaya S, Carp S, Boas DA. The effect of different anesthetics on neurovascular coupling. Neuroimage. 2010;51:1367–1377. doi: 10.1016/j.neuroimage.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Rajagopalan UM, Homma R, Matsumoto M, Nishizaki M, Tanifuji M. Localization of activity-dependent changes in blood volume to submillimeter-scale functional domains in cat visual cortex. Cereb Cortex. 2005;15:823–833. doi: 10.1093/cercor/bhh183. [DOI] [PubMed] [Google Scholar]
- Fuster J, Guiou M, Ardestani A, Cannestra A, Sheth S, Zhou YD, Toga A, Bodner M. Near-infrared spectroscopy (NIRS) in cognitive neuroscience of the primate brain. Neuroimage. 2005;26:215–220. doi: 10.1016/j.neuroimage.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Ganjoo P, Farber NE, Hudetz A, Smith JJ, Samso E, Kampine JP, Schmeling WT. In vivo effects of dexmedetomidine on laser-Doppler flow and pial arteriolar diameter. Anesthesiology. 1998;88:429–439. doi: 10.1097/00000542-199802000-00022. [DOI] [PubMed] [Google Scholar]
- Garrett KM, Gan J. Enhancement of gamma-aminobutyric acid A receptor activity by alpha-chloralose. J Pharmacol Exp Ther. 1998;285:680–686. [PubMed] [Google Scholar]
- Gerrits RJ, Raczynski C, Greene AS, Stein EA. Regional cerebral blood flow responses to variable frequency whisker stimulation: an autoradiographic analysis. Brain Res. 2000;864:205–212. doi: 10.1016/s0006-8993(00)02142-9. [DOI] [PubMed] [Google Scholar]
- Gerrits RJ, Stein EA, Greene AS. Blood flow increases linearly in rat somatosensory cortex with increased whisker movement frequency. Brain Res. 1998;783:151–157. doi: 10.1016/s0006-8993(97)01320-6. [DOI] [PubMed] [Google Scholar]
- Gerrits RJ, Stein EA, Greene AS. Anesthesia alters NO-mediated functional hyperemia. Brain Res. 2001;907:20–26. doi: 10.1016/s0006-8993(01)02298-3. [DOI] [PubMed] [Google Scholar]
- Göbel W, Helmchen F. In vivo calcium imaging of neural network function. Physiology (Bethesda) 2007;22:358–365. doi: 10.1152/physiol.00032.2007. [DOI] [PubMed] [Google Scholar]
- Gordon EL, Meno JR, Ngai AC, Lam AM, Winn HR. Anesthetic-dependent pial arteriolar response to ethanol. J Neurosurg. 1995;83:875–877. doi: 10.3171/jns.1995.83.5.0875. [DOI] [PubMed] [Google Scholar]
- Gu W, Jiang W, Wester P. Real-time cortical cerebral blood flow follow-up in conscious, freely moving rats by laser Doppler flowmetry. Methods. 2003;30:172–177. doi: 10.1016/s1046-2023(03)00078-1. [DOI] [PubMed] [Google Scholar]
- Gyngell ML, Bock C, Schmitz B, Hoehn-Berlage M, Hossmann KA. Variation of functional MRI signal in response to frequency of somatosensory stimulation in alpha-chloralose anesthetized rats. Magn Reson Med. 1996;36:13–15. doi: 10.1002/mrm.1910360104. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Harris S, Jones M, Zheng Y, Berwick J. Does neural input or processing play a greater role in the magnitude of neuroimaging signals. Front Neuroenergetics. 2010;2:1–7. doi: 10.3389/fnene.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayton SM, Kriss A, Muller DP. Comparison of the effects of four anaesthetic agents on somatosensory evoked potentials in the rat. Lab Anim. 1999;33:243–251. doi: 10.1258/002367799780578219. [DOI] [PubMed] [Google Scholar]
- Helmchen F. Miniaturization of fluorescence microscopes using fibre optics. Exp Physiol. 2002;87:737–745. doi: 10.1113/eph8702478. [DOI] [PubMed] [Google Scholar]
- Hemmings HC., Jr Sodium channels and the synaptic mechanisms of inhaled anaesthetics. Br J Anaesth. 2009;103:61–69. doi: 10.1093/bja/aep144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschke H, Schwarz C, Antkowiak B. Neocortex is the major target of sedative concentrations of volatile anaesthetics: strong depression of firing rates and increase of GABAA receptor-mediated inhibition. Eur J Neurosci. 2005;21:93–102. doi: 10.1111/j.1460-9568.2004.03843.x. [DOI] [PubMed] [Google Scholar]
- Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, Bacskai BJ, Dale AM, Boas DA. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Stefanovic B, Silva AC. Spatiotemporal evolution of the functional magnetic resonance imaging response to ultrashort stimuli. J Neurosci. 2011;31:1440–1447. doi: 10.1523/JNEUROSCI.3986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma R, Baker BJ, Jin L, Garaschuk O, Konnerth A, Cohen LB, Zecevic D. Wide-field and two-photon imaging of brain activity with voltage- and calcium-sensitive dyes. Philos Trans R Soc Lond B Biol Sci. 2009;364:2453–2467. doi: 10.1098/rstb.2009.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen JK, Gröhn O, Penttonen M. Coupling between simultaneously recorded BOLD response and neuronal activity in the rat somatosensory cortex. Neuroimage. 2008;39:775–785. doi: 10.1016/j.neuroimage.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Hyder F, Kennan RP, Kida I, Mason GF, Behar KL, Rothman D. Dependence of oxygen delivery on blood flow in rat brain: a 7 tesla nuclear magnetic resonance study. J Cereb Blood Flow Metab. 2000;20:485–498. doi: 10.1097/00004647-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc Natl Acad Sci USA. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iida H, Ohata H, Iida M, Watanabe Y, Dohi S. Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation. Anesthesiology. 1998;89:954–960. doi: 10.1097/00000542-199810000-00020. [DOI] [PubMed] [Google Scholar]
- Jin T, Kim SG. Cortical layer-dependent dynamic blood oxygenation, cerebral blood flow and cerebral blood volume responses during visual stimulation. Neuroimage. 2008;43:1–9. doi: 10.1016/j.neuroimage.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. Neuroimage. 2001;13:1002–1015. doi: 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- Jukovskaya N, Tiret P, Lecoq J, Charpak S. What does local functional hyperemia tell about local neuronal activation. J Neurosci. 2011;31:1579–1582. doi: 10.1523/JNEUROSCI.3146-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB. Spatial extent of CBF response during whisker stimulation using trial averaged laser Doppler imaging. Brain Res. 2006;1089:135–142. doi: 10.1016/j.brainres.2006.02.114. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB. Frequency tuning in the rat whisker barrel cortex revealed through RBC flux maps. Brain Res. 2011;1417:16–26. doi: 10.1016/j.brainres.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP. Functional MRI of the rodent somatosensory pathway using multislice echo planar imaging. Magn Reson Med. 2004;52:89–99. doi: 10.1002/mrm.20114. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Cash SS, Narayanan S, Wang C, Kuzniecky R, Carlson C, Devinsky O, Thesen T, Doyle W, Sassaroli A, Boas DA, Ulbert I, Halgren E. Intracranial microprobe for evaluating neuro-hemodynamic coupling in unanesthetized human neocortex. J Neurosci Methods. 2009;179:208–218. doi: 10.1016/j.jneumeth.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khananashvili YA, Demidova AA. Dynamics of the development of microvascular reactions in the projection zones of the somatosensory cortex of the brain in rats. Neurosci Behav Physiol. 2002;32:435–442. doi: 10.1023/a:1015892513911. [DOI] [PubMed] [Google Scholar]
- Kim T, Hendrich KS, Masamoto K, Kim SG. Arterial versus total blood volume changes during neural activity-induced cerebral blood flow change: implication for BOLD fMRI. J Cereb Blood Flow Metab. 2007;27:1235–1247. doi: 10.1038/sj.jcbfm.9600429. [DOI] [PubMed] [Google Scholar]
- Kim T, Masamoto K, Fukuda M, Vazquez A, Kim SG. Frequency-dependent neural activity, CBF, and BOLD fMRI to somatosensory stimuli in isoflurane-anesthetized rats. Neuroimage. 2010;52:224–233. doi: 10.1016/j.neuroimage.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Blinder P, Drew PJ, Driscoll JD, Muller A, Tsai PS, Shih AY. A guide to delineate the logic of neurovascular signaling in the brain. Front Neuroenergetics. 2011;3:1–9. doi: 10.3389/fnene.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci USA. 1998;95:15741–15746. doi: 10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautwald K, Angenstein F. Low frequency stimulation of the perforant pathway generates anesthesia-specific variations in neural activity and BOLD responses in the rat dentate gyrus. J Cereb Blood Flow Metab. 2012;32:291–305. doi: 10.1038/jcbfm.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschinsky W, Suda S, Sokoloff L. Influence of gamma-hydroxybutyrate on the relationship between local cerebral glucose utilization and local cerebral blood flow in the rat brain. J Cereb Blood Flow Metab. 1985;5:58–64. doi: 10.1038/jcbfm.1985.8. [DOI] [PubMed] [Google Scholar]
- Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Imaging brain activity in conscious animals using functional MRI. J Neurosci Methods. 1998;82:75–83. doi: 10.1016/s0165-0270(98)00037-5. [DOI] [PubMed] [Google Scholar]
- Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn Reson Med. 1999;41:412–416. doi: 10.1002/(sici)1522-2594(199902)41:2<412::aid-mrm28>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Relationship of spikes, synaptic activity, and local changes of cerebral blood flow. J Cereb Blood Flow Metab. 2001;21:1367–1383. doi: 10.1097/00004647-200112000-00001. [DOI] [PubMed] [Google Scholar]
- Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, Lévesque M, Plaisier F, Shmuel A, Cauli B, Hamel E. Pyramidal neurons are ″neurogenic hubs″ in the neurovascular coupling response to whisker stimulation. J Neurosci. 2011;31:9836–9847. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Hudetz AG, Smith JJ, Hillard CJ, Bosnjak ZJ, Kampine JP. The effects of halothane and isoflurane on cerebrocortical microcirculation and autoregulation as assessed by laser-Doppler flowmetry. Anesth Analg. 1994;79:58–65. [PubMed] [Google Scholar]
- Lee JG, Smith JJ, Hudetz AG, Hillard CJ, Bosnjak ZJ, Kampine JP. Laser-Doppler measurement of the effects of halothane and isoflurane on the cerebrovascular CO2 response in the rat. Anesth Analg. 1995;80:696–702. doi: 10.1097/00000539-199504000-00008. [DOI] [PubMed] [Google Scholar]
- Lee SP, Duong TQ, Yang G, Iadecola C, Kim SG. Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow: implications for BOLD fMRI. Magn Reson Med. 2001;45:791–800. doi: 10.1002/mrm.1107. [DOI] [PubMed] [Google Scholar]
- Leniger-Follert E, Hossmann KA. Simultaneous measurements of microflow and evoked potentials in the somatomotor cortex of the cat brain during specific sensory activation. Pflugers Arch. 1979;380:85–89. doi: 10.1007/BF00582617. [DOI] [PubMed] [Google Scholar]
- Lenz C, Rebel A, van Ackern K, Kuschinsky W, Waschke KF. Local cerebral blood flow, local cerebral glucose utilization, and flow-metabolism coupling during sevoflurane versus isoflurane anesthesia in rats. Anesthesiology. 1998;89:1480–1488. doi: 10.1097/00000542-199812000-00026. [DOI] [PubMed] [Google Scholar]
- Lincoln DW. Correlation of unit activity in the hypothalamus with EEG patterns associated with the sleep cycle. Exp Neurol. 1969;24:1–18. doi: 10.1016/0014-4886(69)90002-8. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Villringer A, Dirnagl U. Characterization of CBF response to somatosensory stimulation: model and influence of anesthetics. Am J Physiol. 1993;264:H1223–H1228. doi: 10.1152/ajpheart.1993.264.4.H1223. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lukasik VM, Gillies RJ. Animal anaesthesia for in vivo magnetic resonance. NMR Biomed. 2003;16:459–467. doi: 10.1002/nbm.836. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Tommasino C, Shapiro HM, Keifer-Goodman J, Kohlenberger RW. Local cerebral blood flow and glucose utilization during isoflurane anesthesia in the rat. Anesthesiology. 1986;65:144–151. doi: 10.1097/00000542-198608000-00003. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia. 1986;42:109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Maheswari RU, Takaoka H, Kadono H, Homma R, Tanifuji M. Novel functional imaging technique from brain surface with optical coherence tomography enabling visualization of depth resolved functional structure in vivo. J Neurosci Methods. 2003;124:83–92. doi: 10.1016/s0165-0270(02)00370-9. [DOI] [PubMed] [Google Scholar]
- Mantz J, Cordier J, Giaume C. Effects of general anesthetics on intercellular communications mediated by gap junctions between astrocytes in primary culture. Anesthesiology. 1993;78:892–901. doi: 10.1097/00000542-199305000-00014. [DOI] [PubMed] [Google Scholar]
- Martin C, Berwick J, Johnston D, Zheng Y, Martindale J, Port M, Redgrave P, Mayhew J. Optical imaging spectroscopy in the unanaesthetised rat. J Neurosci Methods. 2002;120:25–34. doi: 10.1016/s0165-0270(02)00185-1. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural- hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage. 2006;32:33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Martindale J, Mayhew J, Berwick J, Jones M, Martin C, Johnston D, Redgrave P, Zheng Y. The hemodynamic impulse response to a single neural event. J Cereb Blood Flow Metab. 2003;23:546–555. doi: 10.1097/01.WCB.0000058871.46954.2B. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Fukuda M, Vazquez A, Kim SG. Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur J Neurosci. 2009;30:242–250. doi: 10.1111/j.1460-9568.2009.06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Obata T, Kanno I. Cerebrovascular dynamics in response to neural stimulation. Hirosaki Med J. 2010a;61:S181–S186. [Google Scholar]
- Masamoto K, Obata T, Kanno I. Intracortical microcirculatory change induced by anesthesia in rat somatosensory cortex. Adv Exp Med Biol. 2010b;662:57–61. doi: 10.1007/978-1-4419-1241-1_7. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Fujita H, Kashikura K, Kanno I. Evoked local cerebral blood flow induced by somatosensory stimulation is proportional to the baseline flow. Neurosci Res. 2000;38:341–348. doi: 10.1016/s0168-0102(00)00175-9. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Fujita H, Seki C, Kashikura K, Kanno I. Hemodynamics evoked by microelectrical direct stimulation in rat somatosensory cortex. Comp Biochem Physiol A Mol Integr Physiol. 1999;124:47–52. doi: 10.1016/s1095-6433(99)00086-0. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Kanno I. Quantitative and temporal relationship between local cerebral blood flow and neuronal activation induced by somatosensory stimulation in rats. Neurosci Res. 2001;40:281–290. doi: 10.1016/s0168-0102(01)00236-x. [DOI] [PubMed] [Google Scholar]
- Minchin MC. The effect of anaesthetics on the uptake and release of gamma-aminobutyrate and D-aspartate in rat brain slices. Br J Pharmacol. 1981;73:681–689. doi: 10.1111/j.1476-5381.1981.tb16803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki H, Nakamura Y, Arai T, Kataoka K. Increase of glutamate uptake in astrocytes: a possible mechanism of action of volatile anesthetics. Anesthesiology. 1997;86:1359–1366. doi: 10.1097/00000542-199706000-00018. [DOI] [PubMed] [Google Scholar]
- Moskalenko YE, Dowling JL, Liu D, Rovainen CM, Semernia VN, Woolsey TA. LCBF changes in rat somatosensory cortex during whisker stimulation monitored by dynamic H2 clearance. Int J Psychophysiol. 1996;21:45–59. doi: 10.1016/0167-8760(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Terasako K, Toda H, Miyawaki I, Kakuyama M, Nishiwada M, Hatano Y, Mori K. Mechanisms of inhibition of endothelium-dependent relaxation by halothane, isoflurane, and sevoflurane. Can J Anaesth. 1994;41:340–346. doi: 10.1007/BF03009915. [DOI] [PubMed] [Google Scholar]
- Nakao Y, Itoh Y, Kuang TY, Cook M, Jehle J, Sokoloff L. Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proc Natl Acad Sci USA. 2001;98:7593–7598. doi: 10.1073/pnas.121179898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai AC, Jolley MA, D'Ambrosio R, Meno JR, Winn HR. Frequency-dependent changes in cerebral blood flow and evoked potentials during somatosensory stimulation in the rat. Brain Res. 1999;837:221–228. doi: 10.1016/s0006-8993(99)01649-2. [DOI] [PubMed] [Google Scholar]
- Nicoll RA. Pentobarbital: differential postsynaptic actions on sympathetic ganglion cells. Science. 1978;199:451–452. doi: 10.1126/science.202032. [DOI] [PubMed] [Google Scholar]
- Nielsen NA, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrenovitch TP, Chen S, Farkas E. Simultaneous, live imaging of cortical spreading depression and associated cerebral blood flow changes, by combining voltage-sensitive dye and laser speckle contrast methods. Neuroimage. 2009;45:68–74. doi: 10.1016/j.neuroimage.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Obrig H, Villringer A. Beyond the visible--imaging the human brain with light. J Cereb Blood Flow Metab. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Stepnoski R, Chen W, Zhu XH, Ugurbil K. An approach to probe some neural systems interaction by functional MRI at neural time scale down to milliseconds. Proc Natl Acad Sci USA. 2000;97:11026–11031. doi: 10.1073/pnas.97.20.11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Takayasu M, Dacey RG., Jr Differential effects of pentobarbital on intracerebral arterioles and venules of rats in vitro. Neurosurgery. 1991;28:537–541. doi: 10.1097/00006123-199104000-00009. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Wei L, Acuff VR, Shimizu A, Pettigrew KD, Patlak CS, Fenstermacher JD. Variation in local cerebral blood flow response to high-dose pentobarbital sodium in the rat. Am J Physiol. 1991;261:H110–H120. doi: 10.1152/ajpheart.1991.261.1.H110. [DOI] [PubMed] [Google Scholar]
- Ou W, Nissilä I, Radhakrishnan H, Boas DA, Hämäläinen MS, Franceschini MA. Study of neurovascular coupling in humans via simultaneous magnetoencephalography and diffuse optical imaging acquisition. Neuroimage. 2009;46:624–632. doi: 10.1016/j.neuroimage.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Hudetz AG, Schulte ML, Li R, Jones SR, Cho YR, Matloub HS, Hyde JS. A protocol for use of medetomidine anesthesia in rats for extended studies using task-induced BOLD contrast and resting-state functional connectivity. Neuroimage. 2009;46:1137–1147. doi: 10.1016/j.neuroimage.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters RR, Tindemans I, De Schutter E, Van der Linden A. Comparing BOLD fMRI signal changes in the awake and anesthetized rat during electrical forepaw stimulation. Magn Reson Imaging. 2001;19:821–826. doi: 10.1016/s0730-725x(01)00391-5. [DOI] [PubMed] [Google Scholar]
- Reinert H. Urethane hyperclycaemia and hypothalamic activation. Nature. 1964;204:889–891. doi: 10.1038/204889a0. [DOI] [PubMed] [Google Scholar]
- Reinert KC, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging in the cerebellar cortex in vivo. J Neurosci Res. 2007;85:3221–3232. doi: 10.1002/jnr.21348. [DOI] [PubMed] [Google Scholar]
- Rosengarten B, Kaps M. A simultaneous EEG and transcranial Doppler technique to investigate the neurovascular coupling in the human visual cortex. Cerebrovasc Dis. 2010;29:211–216. doi: 10.1159/000267840. [DOI] [PubMed] [Google Scholar]
- Sandman CA, O'Halloran JP, Isenhart R. Is there an evoked vascular response. Science. 1984;224:1355–1357. doi: 10.1126/science.6729458. [DOI] [PubMed] [Google Scholar]
- Sándor P, Nyáry I, Reivich M, Kovách AG. Comparative effects of chloralose anesthesia and Sernylan analgesia on cerebral blood flow, CO2 responsiveness, and brain metabolism in the baboon. Stroke. 1977;8:432–436. doi: 10.1161/01.str.8.4.432. [DOI] [PubMed] [Google Scholar]
- Sandstrom DJ. Isoflurane depresses glutamate release by reducing neuronal excitability at the Drosophila neuromuscular junction. J Physiol. 2004;558:489–502. doi: 10.1113/jphysiol.2004.065748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanganahalli BG, Herman P, Hyder F. Frequency-dependent tactile responses in rat brain measured by functional MRI. NMR Biomed. 2008;21:410–416. doi: 10.1002/nbm.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schregardus DS, Pieneman AW, Ter Maat A, Jansen RF, Brouwer TJ, Gahr ML. A lightweight telemetry system for recording neuronal activity in freely behaving small animals. J Neurosci Methods. 2006;155:62–71. doi: 10.1016/j.jneumeth.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Schulte ML, Hudetz AG. Functional hyperemic response in the rat visual cortex under halothane anesthesia. Neurosci Lett. 2006;394:63–68. doi: 10.1016/j.neulet.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Sebel PS, Ingram DA, Flynn PJ, Rutherfoord CF, Rogers H. Evoked potentials during isoflurane anaesthesia. Br J Anaesth. 1986;58:580–585. doi: 10.1093/bja/58.6.580. [DOI] [PubMed] [Google Scholar]
- Seylaz J, Charbonné R, Nanri K, Von Euw D, Borredon J, Kacem K, Méric P, Pinard E. Dynamic in vivo measurement of erythrocyte velocity and flow in capillaries and of microvessel diameter in the rat brain by confocal laser microscopy. J Cereb Blood Flow Metab. 1999;19:863–870. doi: 10.1097/00004647-199908000-00005. [DOI] [PubMed] [Google Scholar]
- Sheth S, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Evaluation of coupling between optical intrinsic signals and neuronal activity in rat somatosensory cortex. Neuroimage. 2003;19:884–894. doi: 10.1016/s1053-8119(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron. 2004;42:347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou MW, Walker MA, Toga AW. Spatiotemporal evolution of functional hemodynamic changes and their relationship to neuronal activity. J Cereb Blood Flow Metab. 2005;25:830–841. doi: 10.1038/sj.jcbfm.9600091. [DOI] [PubMed] [Google Scholar]
- Shibuki K, Hishida R, Murakami H, Kudoh M, Kawaguchi T, Watanabe M, Watanabe S, Kouuchi T, Tanaka R. Dynamic imaging of somatosensory cortical activity in the rat visualized by flavoprotein autofluorescence. J Physiol. 2003;549:919–927. doi: 10.1113/jphysiol.2003.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtoyerman E, Arieli A, Slovin H, Vanzetta I, Grinvald A. Long-term optical imaging and spectroscopy reveal mechanisms underlying the intrinsic signal and stability of cortical maps in V1 of behaving monkeys. J Neurosci. 2000;20:8111–8121. doi: 10.1523/JNEUROSCI.20-21-08111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. Stimulated changes in localized cerebral energy consumption under anesthesia. Proc Natl Acad Sci USA. 1999;96:3245–3250. doi: 10.1073/pnas.96.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23:472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci USA. 2002;99:15182–15187. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J Cereb Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Iadecola C, Kim SG. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J Cereb Blood Flow Metab. 2000;20:201–206. doi: 10.1097/00004647-200001000-00025. [DOI] [PubMed] [Google Scholar]
- Silverman J, Muir WW., III A review of laboratory animal anesthesia with chloral hydrate and chloralose. Lab Anim Sci. 1993;43:210–216. [PubMed] [Google Scholar]
- Sinclair MD. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can Vet J. 2003;44:885–897. [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wang Y, Chen S, Luo W, Li P, Luo Q. Simultaneous monitoring of intracellular pH changes and hemodynamic response during cortical spreading depression by fluorescence-corrected multimodal optical imaging. Neuroimage. 2011;57:873–884. doi: 10.1016/j.neuroimage.2011.05.040. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Takashima I, Kajiwara R, Iijima T. Voltage-sensitive dye versus intrinsic signal optical imaging: comparison of optically determined functional maps from rat barrel cortex. Neuroreport. 2001;12:2889–2894. doi: 10.1097/00001756-200109170-00027. [DOI] [PubMed] [Google Scholar]
- Takuwa H, Autio J, Nakayama H, Matsuura T, Obata T, Okada E, Masamoto K, Kanno I. Reproducibility and variance of a stimulation-induced hemodynamic response in barrel cortex of awake behaving mice. Brain Res. 2011;1369:103–111. doi: 10.1016/j.brainres.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kawano T, Tsutsumi YM, Kinoshita M, Kakuta N, Hirose K, Kimura M, Oshita S. Differential effects of propofol and isoflurane on glucose utilization and insulin secretion. Life Sci. 2011;88:96–103. doi: 10.1016/j.lfs.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Tétrault S, Chever O, Sik A, Amzica F. Opening of the blood-brain barrier during isoflurane anaesthesia. Eur J Neurosci. 2008;28:1330–1341. doi: 10.1111/j.1460-9568.2008.06443.x. [DOI] [PubMed] [Google Scholar]
- Tian P, Teng IC, May LD, Kurz R, Lu K, Scadeng M, Hillman EM, De Crespigny AJ, D'Arceuil HE, Mandeville JB, Marota JJ, Rosen BR, Liu TT, Boas DA, Buxton RB, Dale AM, Devor A. Cortical depth-specific microvascular dilation underlies laminar differences in blood oxygenation level-dependent functional MRI signal. Proc Natl Acad Sci USA. 2010;107:15246–15251. doi: 10.1073/pnas.1006735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H, Nakamura K, Hatano Y, Nishiwada M, Kakuyama M, Mori K. Halothane and isoflurane inhibit endothelium-dependent relaxation elicited by acetylcholine. Anesth Analg. 1992;75:198–203. doi: 10.1213/00000539-199208000-00008. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Kubis N, Calando Y, Tran Dinh A, Méric P, Seylaz J, Pinard E. Long-term in vivo investigation of mouse cerebral microcirculation by fluorescence confocal microscopy in the area of focal ischemia. J Cereb Blood Flow Metab. 2005;25:858–867. doi: 10.1038/sj.jcbfm.9600077. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Chiba S. Mechanism of vascular responsiveness to barbiturates in isolated and perfused canine basilar arteries. Neurosurgery. 1987;21:161–166. doi: 10.1227/00006123-198708000-00005. [DOI] [PubMed] [Google Scholar]
- Tung A, Herrera S, Fornal CA, Jacobs BL. The effect of prolonged anesthesia with isoflurane, propofol, dexmedetomidine, or ketamine on neural cell proliferation in the adult rat. Anesth Analg. 2008;106:1772–1777. doi: 10.1213/ane.0b013e31816f2004. [DOI] [PubMed] [Google Scholar]
- Ueki M, Mies G, Hossmann KA. Effect of alpha-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand. 1992;36:318–322. doi: 10.1111/j.1399-6576.1992.tb03474.x. [DOI] [PubMed] [Google Scholar]
- Van Aken H, van Hemelrijck J. Influence of anesthesia on cerebral blood flow and cerebral metabolism: an overview. Agressologie. 1991;32:303–306. [PubMed] [Google Scholar]
- Van Camp N, Verhoye M, Van der Linden A. Stimulation of the rat somatosensory cortex at different frequencies and pulse widths. NMR Biomed. 2006;19:10–17. doi: 10.1002/nbm.986. [DOI] [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A. Coupling between neuronal activity and microcirculation: implications for functional brain imaging. HFSP J. 2008;2:79–98. doi: 10.2976/1.2889618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: basis of functional neuroimaging. Cerebrovasc Brain Metab Rev. 1995;7:240–276. [PubMed] [Google Scholar]
- Villringer A, Them A, Lindauer U, Einhäupl K, Dirnagl U. Capillary perfusion of the rat brain cortex. An in vivo confocal microscopy study. Circ Res. 1994;75:55–62. doi: 10.1161/01.res.75.1.55. [DOI] [PubMed] [Google Scholar]
- Violet JM, Downie DL, Nakisa RC, Lieb WR, Franks NP. Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology. 1997;86:866–874. doi: 10.1097/00000542-199704000-00017. [DOI] [PubMed] [Google Scholar]
- Vogel J, Kuschinsky W. Decreased heterogeneity of capillary plasma flow in the rat whisker-barrel cortex during functional hyperemia. J Cereb Blood Flow Metab. 1996;16:1300–1306. doi: 10.1097/00004647-199611000-00026. [DOI] [PubMed] [Google Scholar]
- Wang K, Zheng C, Wu C, Gao M, Liu Q, Yang K, Ellsworth K, Xu L, Wu J. alpha-Chloralose diminishes gamma oscillations in rat hippocampal slices. Neurosci Lett. 2008;441:66–71. doi: 10.1016/j.neulet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Weber B, Burger C, Wyss MT, von Schulthess GK, Scheffold F, Buck A. Optical imaging of the spatiotemporal dynamics of cerebral blood flow and oxidative metabolism in the rat barrel cortex. Eur J Neurosci. 2004;20:2664–2670. doi: 10.1111/j.1460-9568.2004.03735.x. [DOI] [PubMed] [Google Scholar]
- Weber R, Ramos-Cabrer P, Wiedermann D, van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage. 2006;29:1303–1310. doi: 10.1016/j.neuroimage.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Wei L, Otsuka T, Acuff V, Bereczki D, Pettigrew K, Patlak C, Fenstermacher J. The velocities of red cell and plasma flows through parenchymal microvessels of rat brain are decreased by pentobarbital. J Cereb Blood Flow Metab. 1993;13:487–497. doi: 10.1038/jcbfm.1993.63. [DOI] [PubMed] [Google Scholar]
- Williams KA, Magnuson M, Majeed W, LaConte SM, Peltier SJ, Hu X, Keilholz SD. Comparison of alpha-chloralose, medetomidine and isoflurane anesthesia for functional connectivity mapping in the rat. Magn Reson Imaging. 2010;28:995–1003. doi: 10.1016/j.mri.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Sun JY, Evers AS, Crowder M, Wu LG. Isoflurane inhibits transmitter release and the presynaptic action potential. Anesthesiology. 2004;100:663–670. doi: 10.1097/00000542-200403000-00029. [DOI] [PubMed] [Google Scholar]
- Zhao F, Zhao T, Zhou L, Wu Q, Hu X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage. 2008;39:248–260. doi: 10.1016/j.neuroimage.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]