Abstract
Pathophysiology of the neurovascular unit (NVU) is commonly seen in neurological diseases. The typical features of NVU pathophysiology include tissue hypoxia, inflammatory and angiogenic activation, as well as initiation of complex molecular interactions between cellular (brain endothelial cells, astroctyes, pericytes, inflammatory cells, and neurons) and acellular (basal lamina) components of the NVU, jointly resulting in increased blood–brain barrier permeability, brain edema, neurovascular uncoupling, and neuronal dysfunction and damage. The evidence of important role of the brain vascular compartment in disease pathogenesis has elicited the debate whether the primary vascular events may be a cause of the neurological disease, as opposed to a mere participant recruited by a primary neuronal origin of pathology? Whereas some hereditary and acquired cerebral angiopathies could be considered a primary cause of neurological symptoms of the disease, the epidemiological studies showing a high degree of comorbidity among vascular disease and dementias, including Alzheimer's disease, as well as migraine and epilepsy, suggested that primary vascular pathology may be etiological factor causing neuronal dysfunction or degeneration in these diseases. This review focuses on recent hypotheses and evidence, suggesting that pathophysiology of the NVU may be initiating trigger for neuronal pathology and subsequent neurological manifestations of the disease.
Keywords: Alzheimer's disease, blood–brain barrier, genetic vasculopathies, migraine, neurovascular unit, seizures
Neurovascular unit anatomy to function: overview of recent progress
Although the concept of the neurovascular unit (NVU) arose from long-known and studied phenomenon of coupling between neuronal activity (energy demand) and local blood flow (energy supply), in the last decade, the term came to symbolize and promote the research into the integrated system of vascular and neuronal cells and their milieu working in concert to enable proper brain homeostasis and function. From the initial description as ‘interactions between circulating blood elements and the blood vessel wall, extracellular matrix, glia, and neurons (together, the NVU)' (Report of the Stroke Progress Research Group, 2002; http://www.ninds.nih.gov), the concept has evolved towards ‘extended NVU' that includes other cell types, notably pericytes and microglia, and specialized cellular compartments such as endothelial glycocalyx. The current cellular anatomy and organization of the NVU, described in detail in recent review articles (Abbott et al, 2006; Neuwelt et al, 2011), and shown in upper inset of Figure 1, incorporates specialized endothelium lining brain capillaries, sealed by tight junctions and demarcated from the surrounding brain cells by a continuous basement membrane (BM). Tightly apposed to brain endothelial cells (BECs) and enveloped in the BM are pericytes, whose long processes extend over the vessel wall. End-feet of perivascular astrocytes cover large domains of the outer brain capillary BM. Astrocytes also communicate with neurons acting as a liaison for endothelial–neuronal coupling. Axonal projections from neurons containing vasoactive neurotransmitters and peptides extend into the close vicinity of cerebral microvessels: perivascular neuronal ‘boutons' abut primarily on astrocytic end-feet surrounding blood vessel walls, with a smaller proportion directly contacting the vessel basal lamina (Hamel, 2006). The cellular anatomy of the NVU has recently been extended to include inflammatory circulating cells that participate in CNS (central nervous system) immune surveillance by probing and interacting with the luminal surface of brain endothelium covered by thick glycocalyx, as well as perivascular macrophages and microglia, participating in innate immune responses in the neurovascular ‘niche' (Neuwelt et al, 2011). Functional NVU adaptations to physiological and pathological stimuli are effected through complex cell–cell and cell–extracellular matrix interactions and paracrine cell–cell communication. Regulation of the local cerebral blood flow (CBF), blood–brain barrier (BBB) permeability and transport mechanisms, neuroimmune responses, and neurovascular remodeling (angiogenesis) are principal functions integrated in the NVU.
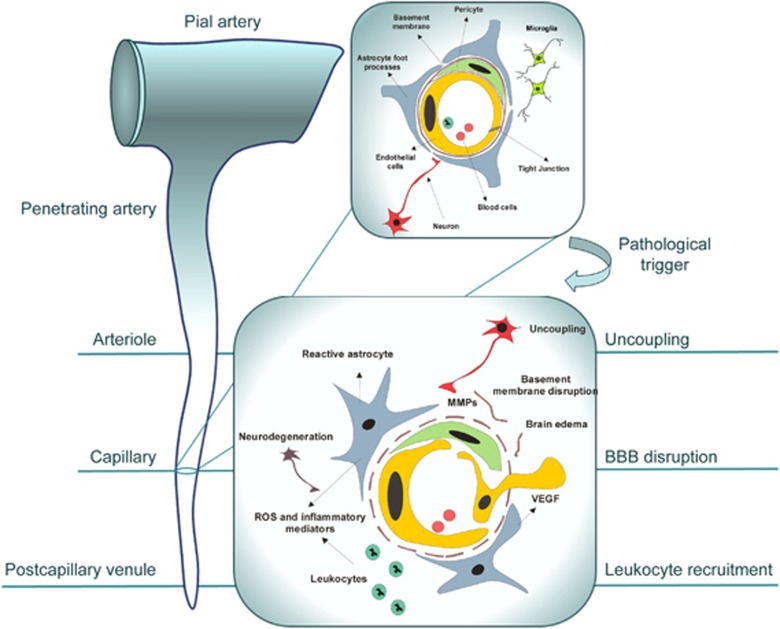
Figure 1.
Neurovascular unit (NVU) reorganization in response to pathogenic stimulus. Highly structured multicellular anatomy of the NVU (shown in the upper right inset) undergoes profound changes in response to pathogenic stimulus, such as tissue hypoxia, schematically shown in the lower inset. The subsequent ‘sequence of events' leading to neuronal injury includes the expression and release of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), by astrocytes and surrounding cells and upregulation of their receptors in brain endothelial cells, stimulating endothelial proliferation and migration with consequent disruption of tight junctions and increased blood–brain barrier (BBB) permeability. Release of metaloproteases by migrating endothelial cells and pericytes leads to proteolytic disruption of the basement membrane and additional release of pro-angiogenic breakdown products of the extracellular matrix. Accompanying influx of serum proteins and water through disrupted BBB results in vasogenic edema, which further disconnects cellular interactions within the NVU; toxic serum components cause astrocyte activation. Upregulation and secretion of inflammatory mediators from both activated astrocytes and endothelial cells stimulates the expression of adhesion molecules in endothelial cells and the recruitment of inflammatory cells into the brain. Reactive oxygen species and proteases released from leukocytes and activated perivascular cells cause oxidative injury to neurons. Secondary injury to neurons, if prolonged or repeated, could cause dissociation of neuronal projections from the NVU, uncoupling and subsequent retrograde degeneration. Some of the described events and their manifestation occur at specific sites of the brain microcirculatory tree: uncoupling at the level of arterioles, BBB disruption at the level of capillaries, and leukocyte recruitment at the level of postcapillary venules.
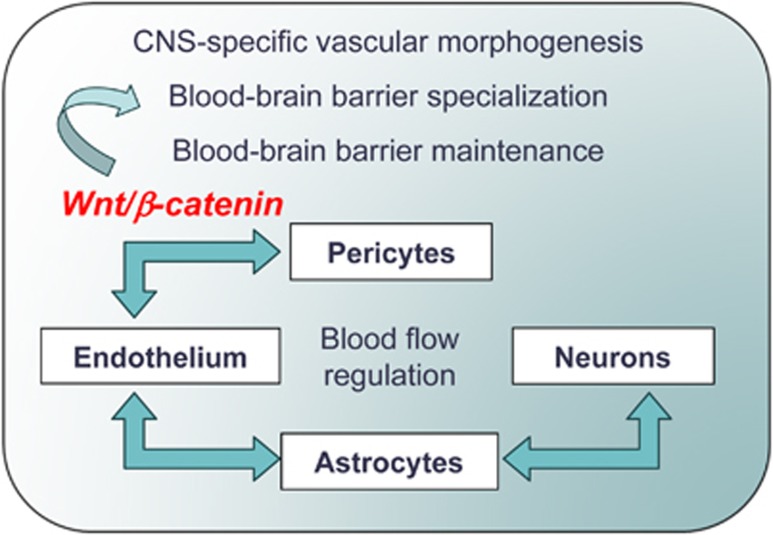
In the last decade, a significant progress has been made in the understanding of the role of different cellular constituents of NVU and their intercellular and molecular interactions and signaling during development, physiological, and pathological conditions. During development, the neuroepithelium signals to the vascular endothelium through the canonical Wnt/β-catenin pathway to induce a CNS-specific vascular system and BBB specialization (Stenman et al, 2008; Daneman et al, 2009; Liebner and Plate, 2010). The critical role of pericytes in these processes, especially in the formation and maturation of the BBB during development (Daneman et al, 2010) and its dysfunction during aging (Bell et al, 2010) has recently emerged from elegant studies in pericyte-defective Pdgfrb+/− animal models. Specialized properties of the brain endothelium have been extensively studied, leading to systematic cataloging of proteins, receptors, and transporters polarized to BEC luminal and abluminal membranes involved in ion-, metabolite-, and vesicular transport across the BBB (Uchida et al, 2011; Haqqani et al, 2011). Molecular and structural studies of tight junctions painted a picture of complex, yet dynamic and highly regulated molecular structures in which claudins (1/3, 5, and possibly 12) contribute to tight barrier properties, junctional adhesion molecules participate in the tight junction maintenance, and occludin and zonula occludens-1 in their regulation through targeted signaling (Bazzoni, 2006; Abbott et al, 2010). The ‘classical' role of perivascular astrocytes in inducing and maintaining the BBB properties of BEC (Arthur et al, 1987) has been expanded to include their multifaceted functions in water homeostasis and blood flow regulation. Perivascular astrocytic end-feet, polarized through influence of pericytes (Armulik et al, 2010), exhibit a high density of particles containing the water channel AQP4 (aquaporin 4) and the Kir4.1 K+ channel, segregated by the basal lamina components agrin and α-dystroglycan, which are involved in ion and volume regulation (Noell et al, 2011). The anatomical, pharmacological, and molecular underpinnings of the astrocyte role as interface between endothelial and neuronal responses, which mediate blood flow regulation, have recently been understood in more detail (Takano et al, 2006; Hamel, 2006; Gordon et al, 2007). Basal forebrain neurons send projection fibers to cortical microvessels and surrounding astrocytes; specific receptors for the vasoactive mediators they release exist on both microvascular endothelial and/or smooth muscle cells and on astrocytes, providing means for modulation of microvascular tone in response to changes in neuronal activity (Hamel, 2006). Increased intracellular Ca2+ in astrocytes triggers the production of AA (arachidonic acid) via a Ca2+-sensitive phospholipase A2 that could elicit either vasoconstriction via a conversion of AA to 20-hydroxyeicosatetraenoic acid or dilation from the production of prostaglandin E2 or epoxyeicosatrienoic acid; which of these two pathways is recruited appears to be regulated by the levels of NO (nitric oxide) (Gordon et al, 2007). Forebrain neurons also project to γ-aminobutyric acid-ergic interneurons, which then indirectly modulate vasoactive responses of cortical microvessels via the release of somatostatin and vasoactive intestinal peptide (Hamel, 2006). Smooth muscle cells in arterioles and pericytes in capillaries (Peppiatt et al, 2006) effect contraction or dilation in response to vasoactive stimuli released in their vicinity. The prevailing concept of the role of intercellular interactions in ‘functional specialization' of the NVU is schematically shown in Figure 2.
Figure 2.
Cellular interactions implicated in the development, maturation and functional responses of the neurovascular unit (NVU). Current understanding places pericyte–endothelial cell interactions, mediated via canonical Wnt/β-catenin signaling, at the core of central nervous system (CNS)-specific vascular morphogenesis, the blood–brain barrier (BBB) specialization and maintenance during development. Whereas astrocytes provide trophic influence involved in inducing and maintaining BBB functions, their principal role is in establishing metabolic and functional link between endothelial and neuronal compartments, resulting in neurovascular coupling and local cerebral blood flow regulation. Pericytes contribute to the blood flow regulation by responding with contraction or relaxation to vascoactive stimuli released by surrounding cells.
This accelerated understanding of molecular mechanisms governing NVU functions has aided in understanding the pathophysiology of brain diseases. It has become apparent from these studies that NVU is not simply a ‘passive responder' but rather an active participant in pathogenesis of virtually all brain pathologies, ranging from chronic neurodegenerative diseases to brain tumors and infections (for extensive list, see Neuwelt et al, 2011). This has recently elicited the debate whether primary vascular events may be a cause of the neurological disease, as opposed to being a mere participant in disease pathogenesis recruited by a principal neuronal origin of pathology? This article aims to further this debate by summarizing current hypotheses, experimental and clinical evidence in support of vascular origin for some CNS pathologies. If indeed specific CNS diseases originate from primary NVU pathology which then causes neuronal dysfunction, then, conceptually, the diagnostic and treatment strategies should be (re)focused to chiefly address/target vascular component of the disease, avoiding often insurmountable problems of drug delivery across the BBB and side effects associated with targeting neuronal compartment(s).
Neurovascular unit pathophysiology: common molecular features
The pathophysiology of the NVU, whether a source or participant in brain disease, could be scoped through the lens of common molecular/functional processes that are observed across the neurological disease spectrum. Perhaps, the most prevalent characteristics of the NVU pathophysiology detected at various degrees of interplay in many neurological diseases, where they combine with disease-specific pathologies (e.g., Aβ accumulation, fibrosis, etc.), are tissue hypoxia, inflammation, and angiogenic neurovascular remodeling.
Tissue hypoxia is often an initial trigger of a cascade of pathophysiological changes in the NVU. Tissue hypoxia could be caused by nonvascular mechanisms (e.g., altitude disease) or by acute or chronic processes affecting brain vasculature, including common cardiovascular risk factors such as atherosclerosis and hypertension. In hypoxic tissues, immediate early genes that encode many functionally different products including secreted pro-inflammatory cytokines and chemokines, cytoplasmic enzymes such as cyclo-oxygenase-2, and inducible transcription factors, are promptly induced in a protein synthesis-independent manner. Hypoxia-inducible factor-1 (HIF-1) is a transcription factor that is specifically activated by hypoxia by both modifications in protein processing and by transcriptional induction (Semenza, 2011). Hypoxia-inducible factor-1 is a heterodimeric protein composed of HIF-1α and HIF-1β (ARNT) subunits; whereas ARNT is only marginally affected by changes in oxygen tension, hypoxia markedly increases HIF-1α protein levels (Semenza, 2011). This in turn leads to upregulation of HIF-1-dependent genes that promote cell survival by increasing oxygen delivery (erythropoietin, transferrin, and heme oxygenase), glucose transport (glucose transporter-1), glycolysis (lactate dehydrogenase A), and angiogenesis (vascular endothelial growth factor (VEGF), inducible NO synthase, angiopoietin-2, fibroblast growth factor) (Semenza, 2011). The end-result of this gene induction program is tissue adaptation to hypoxia through increased blood and oxygen supply. The accompanying pathophysiology includes increased BBB permeability, water and ion redistribution and cerebrovascular oxidative stress, which, if unresolved, create secondary injury to NVU (Stanimirovic and Satoh, 2000). Vascular endothelial growth factor, a multitasking cytokine, which stimulates differentiation, survival, migration, proliferation, tubulogenesis, and vascular permeability in endothelial cells through VEGF receptors, tyrosine kinases flt-1 (VEGFR-1), KDR/flk-1 (VEGFR-2), and flt-4 (VEGFR-3), and coreceptor neuropilin-1 (Koch et al, 2011), is the major pro-angiogenic factor induced through HIF-1α activation.
Hypoxia-inducible factor-1α is an initial trigger of inflammatory gene transcription in both BEC and astrocytes; secreted cytokines then strongly amplify inflammatory response through recruitment of nuclear factor-κB in the context of ischemic injury (Zhang et al, 2006; Stanimirovic, 2001) or activator protein 1 in the context of Aβ-induced injury (Vukic et al, 2009). Two major routes employed in amplifying inflammation are propagation through receptor signaling and autocrine/paracrine recruitment of neighboring cells (Stanimirovic, 2001; Stanimirovic and Satoh, 2000). This inflammatory cascade is another typical feature of NVU pathophysiology and consists of two components, an acellular inflammatory component characterized by the production of inflammatory cytokines, chemokines, and eicosanoids by NVU cells (BEC, astrocytes, pericytes, and perivascular macrophages), and cellular inflammation characterized by the recruitment (intravascular and parenchymal) of peripheral inflammatory cells (Stanimirovic and Satoh, 2000; del Zoppo, 2010). Leukocytes are selectively recruited via a sequence of interactions with BEC adhesion molecules (Greenwood et al, 2011) governing leukocyte tethering and rolling along endothelial surface, firm adhesion (arrest), and diapedesis from the luminal to the abluminal side of BEC. Whereas initial steps of leukocyte–BEC interactions have been well understood in the context of ischemic and neuroinflammatory disease (Greenwood et al, 2011), the mechanisms of their abluminal extravasation are still poorly understood. Traditionally, leukocytes were believed to extravasate mainly through the paracellular route, leading to redistribution and a subsequent loss of the tight junction proteins occludin and zonula occludens-1 from BEC (Kebir et al, 2007). However, recent histological and electron microscopy studies demonstrated that leukocytes can also migrate through the transcellular pathway, that is, through the BEC themselves, leaving the tight junctions morphologically intact (Engelhardt and Wolburg, 2004; Wittchen, 2009). Several putative mediators released by activated leukocytes including free radicals, metalloproteinases, and eicosanoids have been implicated in endothelial injury, disruption of the BM, and transient permeabilization of the BBB (Stanimirovic, 2001; del Zoppo, 2010).
Angiogenic NVU remodeling initiated by the secretion of VEGF and modulated by inflammatory milieu involves dynamic interactions of proliferating cells with the extracellular matrix mediated by specific adhesion molecules, integrins, transmembrane receptors composed of different heterodimeric combinations of α and β subunits (del Zoppo and Milner, 2006). The composition of the extracellular matrix is changed to reflect modified secretory status of extracellular protein-producing cells, including astrocytes, pericytes, and BEC, as well as its proteolysis through plasminogen activator and matrix metalloproteases cascades (Arai et al, 2009; Candelario-Jalil et al, 2009). Signaling pathways elicited in NVU cells via integrins are responsible for changes in cell morphology and migration within the given extracellular matrix environment—processes essential for both acute response to injury and late reorganization and vessel maturation (del Zoppo and Milner, 2006).
In summary, the process of NVU reorganization in response to tissue hypoxia and inflammatory activation (schematically shown in the lower inset of Figure 1) includes the disruption of interendothelial tight junctions, retraction of pericytes from the abluminal surface of the capillary, breakdown of the basal lamina with transudation of plasma, infiltration of inflammatory cells, endothelial cell proliferation and migration, and in some cases formation of new vessels through angiogenesis and remodeling. At the molecular level, this reorganization is accompanied by increased expression of endothelial cell-leukocyte adhesion receptors, loss of endothelial cell and astrocyte integrin receptors, loss of their matrix ligands, expression of members of several matrix-degrading protease families, and the appearance of receptors associated with angiogenesis and neovascularization (del Zoppo, 2010). This remodeling causes profound functional changes in the NVU unit, including BBB dysfunction, impaired neurovascular coupling, leukocyte adhesion and infiltration, pro-thrombotic conversion, angiogenesis and vasculogenesis. Although initiated as adaptive response aiming to limit injury and promote recovery, in the environment of neurological disease, this adaptive process can be either interrupted or pathologically perpetuated, leading to amplification of initial pathology.
The relationship of the NVU reorganization initiated by the noxious stimulus to subsequent acute or chronic neuronal injury will be further examined in this article using examples from experimental and clinical studies. The issue in focus will remain: can primary vascular pathophysiology elicit and sustain neuronal pathophysiology that produces neurological manifestations often identified as ‘disease,' such as for example Alzheimer's disease?
Vasculopathies and neurological phenotype
Epidemiological studies have recently provided strong evidence of cooccurrence of neurological manifestations such as migraine, dementia, and mood disorders with primary genetic vasculopathies, most notably cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL), as well as acquired small vessel disease.
CADASIL is the hereditary disease of small cerebral arteries that affects middle-aged adults and leads to disability and dementia, which accounts for 11% of lacunar stroke cases with leukoaraiosis in patients younger than 50 years (Chabriat et al, 2009; Herve and Chabriat, 2010). The disease is essentially characterized by five main symptoms: migraine with aura, subcortical ischemic events, mood disturbances, apathy, and cognitive impairment. The most frequent manifestations of CADASIL, occurring in 60% to 85% of patients, are transient ischemic attacks and subcortical ischemic strokes, in most cases in the absence of conventional vascular risk factors (Chabriat et al, 2009; Herve and Chabriat, 2010). Microscopic ultrastructural studies show a specific arteriopathy affecting mainly the small penetrating cerebral and leptomeningeal arteries characterized by a thickening of the arterial wall leading to luminal stenosis, accumulation of a nonamyloid granular osmiophilic material in the BM, and prominent morphological alterations of smooth muscle cells with their eventual disappearance (Chabriat et al, 2009; Herve and Chabriat, 2010). Clinical and neuroimaging features resemble those of sporadic small artery disease (Jouvent et al, 2008). CADASIL is autosomal dominant disease caused by mutations in NOTCH3 (Notch homolog 3), which encodes a transmembrane receptor primarily expressed in systemic arterial smooth muscle cells (Monet-Leprêtre et al, 2009). Pathogenic mutations alter the number of cysteine residues in the extracellular domain of NOTCH3, which accumulates and forms aggregates around smooth muscle cells and pericytes of brain arteries and capillaries (Monet-Leprêtre et al, 2009). Recent studies suggest that CADASIL mutations produce gain of novel function(s) of mutated protein arising from novel protein–protein interactions rather than a loss of its canonical function (Yamamoto et al, 2011). On the basis of genetic, clinical, and imaging studies, the proposed pathogenic mechanism of the disease arising from both structural and functional changes in brain arteries centers on an early decrease in the CBF with chronic subcortical ischemia. Functional studies have indicated a blunted increase in CBF response to carbon dioxide inhalation in patients with CADASIL (Pfefferkorn et al, 2001). This chronic subcortical ischemia can cause recurrent lacunar infarcts and microstructural alterations that correlate with cognitive decline (Chabriat et al, 2009; Herve and Chabriat, 2010).
By contrast, the pathophysiology of another common symptom of CADASIL, migraine with aura, is mostly unknown. Migraine with aura generally starts long before the first ischemic attacks and before changes on magnetic resonance imaging are detectable (Stam et al, 2009; Chabriat et al, 2009). Furthermore, migraine with aura is not seen in chronic, hypertension-related small artery diseases of the brain, which suggests specific mechanisms for migraine with aura in CADASIL.
Cortical spreading depolarization, a slowly propagating wave of neuronal and glial depolarization that can be evoked in the cortex, cerebellum, basal ganglia, thalamus, and hippocampus, is a putative biologic substrate for migraine aura (Charles and Brennan, 2009). Indeed, migraine visual aura and spreading depolarization share several characteristic functional magnetic resonance imaging findings including that both are associated with an initial hyperemia lasting 3 to 4.5 minutes, which is followed by mild hypoperfusion lasting 60 to 120 minutes (Charles and Brennan, 2009). The hypothesis that endothelial dysfunction may cause spreading depolarization and in consequence migraine aura was inspired by experiments in which topical brain application of the vasoconstrictive peptide, endothelin-1, potently induced spreading depolarization (Dreier et al, 2002). This concept stipulates that NVU misbalance between vasoconstrictors and vasodilators that favors vasoconstriction leads to energy compromise of the neurons and consequent spreading depolarization (Dreier, 2011; Charles and Brennan, 2009).
Experimental and epidemiological evidence suggested that hypoperfusion disorders, stroke, and microemboli reduce the threshold for the induction of spreading depolarization (Dalkara et al, 2010). Whereas migraine aura and ischemic stroke share spreading depolarization as a common element, they differ fundamentally in that the ischemic stroke is typically associated with nonspreading or persistent depression, with sudden multiple neurological deficits, whereas in migraine aura slowly spreading depression of spontaneous activity correlates with clinical presentation of slowly creeping neurological deficits that successively affect various functions such as vision, language, somatosensory, or motor function (Dreier, 2011).
Spreading depolarization, induced by acute neuronal hyperexcitability, normally stimulates the NVU to respond with marked vasodilatation and spreading hyperemia (normal neurovascular coupling) to match the increased neuronal energy demand. In the healthy tissue, spreading depolarization does not cause neuronal damage (Nedergaard and Hansen, 1988), because it is coupled with reactive hyperemia. In contrast, under pathological conditions, NVU may respond to spreading depolarization with marked vasoconstriction and spreading ischemia (inverse neurovascular coupling), leading to widespread secondary neuronal injury (Dreier et al, 1998, 2001; Shin et al, 2006). Direct recordings from the surface of the brain from patients with subarachnoid hemorrhage, traumatic or ischemic brain injuries support the high frequency of spreading depolarization waves in these patients, and indicate that they may be associated with ‘inverse' neurovascular coupling increasing the likelihood of cortical damage (Lauritzen et al, 2011; Dreier et al, 2009). Inverse neurovascular coupling and spreading ischemia may explain the clinical syndrome of migraine stroke (Dreier, 2011).
Whereas pathogenesis of the third common symptom of hereditary and acquired vasculopathies, mood disorders, is far from being clear, a recent hypothesis (Shalev et al, 2009) proposed that a focal BBB breakdown triggers events within the NVU associated with dysfunction of brain astrocytes and local inflammatory response, leading to pathological synaptic plasticity, increased network connectivity with manifestations of psychiatric illness (see below). Observations of increased markers of the BBB breakdown, CSF albumin and serum S100β, inflammatory cytokines and astroglial activation, along with changes in glutamate levels, neuronal connectivity, and, in some cases, neuronal loss, are consistent with this hypothesis. This chain of events could further explain the high rate of psychiatric illness following brain injury, acute stress reactions, and vascular pathologies (e.g., systemic lupus erythematous), which are frequently associated with malfunction of the BBB (Shalev et al, 2009).
Neurovascular hypothesis of Alzheimer's disease: or is it vasculoneuronal?
Acquired cerebrovascular disease is common in several cognitive disorders, including multiinfarct dementia, poststroke dementia, mild cognitive impairment, degenerative dementias such as Alzheimer's disease (AD) and others (Iadecola et al, 2010; Iadecola, 2010; Humpel and Marksteiner, 2005). Dementia is more likely to be present when vascular and AD lesions coexist, and association between stroke and AD in elderly individuals is especially strong in patients with vascular risk factors (Jellinger, 2008; Iadecola, 2010). Pathologically, cerebral amyloid angiopathy is found in both AD and cerebral atherosclerosis and is associated with the development of cognitive deficits (Iadecola, 2010; Jellinger, 2008; Humpel and Marksteiner, 2005). These epidemiological observations and experimental studies have started challenging the common view that ‘the earliest manifestation of AD is synaptic failure,' (Selkoe, 2002) and that, consequently, the disease is inherently neuronal, and have brought about an alternative, vascular hypothesis of AD.
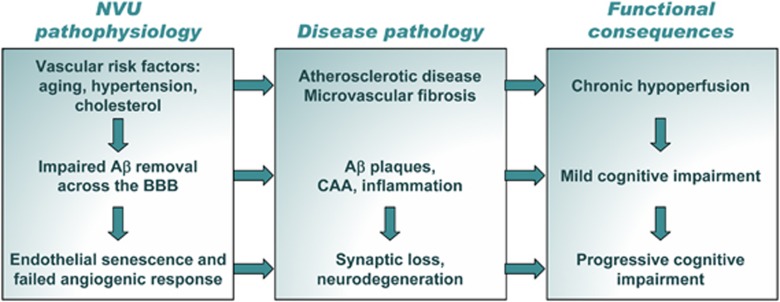
The vascular hypothesis of AD proposes that vascular risk factors including hypercholesterolemia, hypoglycemia, hypertension, etc. damage the NVU during the process of aging, leading to chronic hypoperfusion, BBB dysfunction, and common NVU pathophysiological responses described in ‘Neurovascular unit pathophysiology: common molecular features' (Figure 2) (Zlokovic, 2010; Iadecola, 2010). Dysregulation and uncoupling of NVU due to disassembly of cellular architecture and dissociation of cholinergic nerve terminals establishes vicious circle, leading to degeneration of nerve endings and retrograde death of cholinergic neurons. These processes also impair the BBB functions, diminishing its ability to clear soluble brain β-amyloid through reverse transcytosis, contributing to its accumulation and deposition in vessels and neuropil. Perpetual microglial activation during failed attempts to remove Aβ deposits, leads to chronic inflammation, oxidative stress, and further damage to NVU components. These processes amplify neurotoxicity, synaptic loss, and NVU uncoupling, leading to the clinical presentation of cognitive impairments. Importantly, not only neurons suffer degenerative process; BEC undergo accelerated senescence with permanent growth arrest, fail to respond to hypoxic conditions by mounting adaptive angiogenic response and, in contrast, undergo vascular regression (Zlokovic, 2010). The vicious cycle of vascular insufficiency and neuronal death is thus accelerated, leading to brain atrophy and severe cognitive decline. The chain of proposed events is schematically shown in Figure 3.
Figure 3.
Vascular hypothesis of neurodegeneration in Alzheimer's disease. Early triggers of homeostatic misbalance are common vascular risk factors, including age, hypertension, and cholesterol, leading to atherosclerotic disease and microvascular fibrosis. Functional consequence of these changes is chronic hypoperfusion that initiates neurovascular remodeling cascade. While the aberrant clearance of amyloid-β (Aβ) across the blood–brain barrier (BBB) initiates ‘seed' accumulation of Aβ in the brain and brain vessels, endothelial senescence, and impaired adaptive angiogenic response perpetuates chronic hypoxia, jointly leading to increased Aβ burden and clinical symptoms of mild cognitive impairment. Subsequent accelerated synapse loss and neurodegeneration in combination with progressive vascular pathology result in advanced cognitive loss characteristic of progressive disease.
Several elements of the vasculoneuronal hypothesis of AD are supported by strong experimental evidence obtained in animal models. Morphological and architectural analyses of cerebral vasculature using scanning electron microscopy of brain vascular corrosion casts (Meyer et al, 2008) showed that significant microvascular alterations, often accompanied by small amyloid deposits attached to the vessels, could be detected in APP23 transgenic mice at young ages before the appearance of parenchymal amyloid plaques. In older animals, vasculature abruptly ended at amyloid plaques, resulting in holes in microvascular and capillary network; between such holes, the surrounding vascular array appeared more dense and showed features typical of angiogenesis (Meyer et al, 2008).
Vascular insufficiency with underlying tissue hypoxia can accelerate Aβ production through upregulation of the β-site amyloid precursor protein (APP) cleaving enzyme, BACE1 (Guglielmotto et al, 2009), favoring pro-amyloidogenic processing of APP. Hypoxic activation of BACE1 is initially triggered by the release of ROS (reactive oxygen species) from mitochondria, and sustained by HIF-1α-mediated BACE1 gene induction (Guglielmotto et al, 2009). Aβ in turn stimulates ROS production in endothelial cells; ROS scavenge endothelium-derived relaxing factor (NO) thus impairing resting cerebrovascular tone and NO-dependent dilatation (Thomas et al, 1996). Cerebrovascular function in transgenic animal models overexpressing human APP can be completely rescued by the antioxidants, tempol and N-acetyl-L-cysteine, without affecting brain levels of Aβ (Nicolakakis et al, 2008).
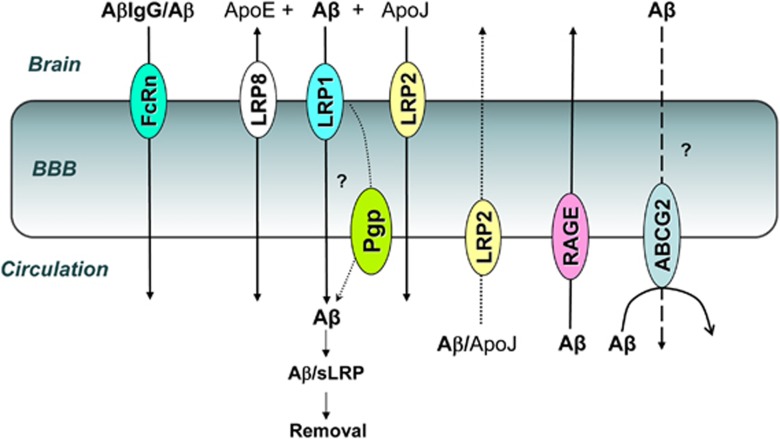
Mechanistic studies have generated a substantial body of evidence that brain accumulation of Aβ is not solely a consequence of increased production, but also of reduced brain clearance and/or increased uptake (Zlokovic et al, 2010; Deane et al, 2009). Both later processes are controlled by the polarized BBB transporters (Figure 4). Whereas systemic Aβ is taken into the brain by luminally expressed endothelial receptor for advanced glycation, its brain efflux is largely mediated by the abluminal low-density lipoprotein receptor-related protein 1 (LRP1); LRP1 expression in brain endothelium naturally declines with age in both wild-type mice and human AD patients (Zlokovic et al, 2010; Deane et al, 2009), reducing brain clearance of Aβ. In support of the role of impaired brain efflux of Aβ in AD pathogenesis, LRP1 antisense reduced BBB clearance of Aβ, increased Aβ levels in the brain and, after chronic administration, resulted in impaired hippocampal spatial learning and memory (Jaeger et al, 2009). In normal human plasma, a soluble form of LRP1 (sLRP1) is the major endogenous brain Aβ ‘sinker' that sequesters some 70% to 90% of plasma Aβ peptides (Zlokovic et al, 2010). In AD, the levels of sLRP1 and its capacity to bind Aβ are reduced (Zlokovic et al, 2010), which may increase brain Aβ burden through increased Aβ influx across the BBB. In a mouse model of AD, restoring plasma sLRP1 with recombinant LRP-IV cluster reduces brain Aβ burden and improves functional changes in CBF and behavioral responses (Zlokovic et al, 2010; Deane et al, 2009). Recent evidence also implicated other BBB transporters in Aβ trafficking between brain and circulatory compartments; luminal efflux transporter ABCG2 has been shown to prevent systemic Aβ entry into the brain (Xiong et al, 2009), whereas reduced P-glycoprotein function has been associated with reduced Aβ clearance (Cirrito et al, 2005; Hartz et al, 2010) in transgenic AD models. Efflux of Aβ by LRPs (LRP1, LRP2, and LRP8) is modulated by ApoE and ApoJ (Jaeger and Pietrzik, 2008; Bell et al, 2007). ApoJ–Aβ complex has high affinity for LRP2 (glycoprotein 330 or megalin) that mediates its influx into the brain (Zlokovic et al, 1996) (Figure 4); ApoE4 and ApoJ (clusterin) are both involved in lipid metabolism/homeostasis as components of high-density lipoprotein and are only two genes independently associated with late-onset AD by large-scale Genome-Wide Association Studies (Seshadri et al, 2010). These and other pathological and molecular studies in transgenic animal models of AD collectively suggested that NVU pathophysiology is evident early in the disease process, often preceding and potentially causing appearance of parenchymal Aβ deposits and neuronal loss.
Figure 4.
Current understanding of amyloid-β (Aβ) transport across the blood–brain barrier (BBB). Soluble Aβ efflux from the brain across the BBB is mainly mediated by lipoprotein receptor-related protein 1 (LRP1); LRP1 expression declines in aging and Alzheimer's disease, leading to brain Aβ accumulation due to reduced clearance. Aβ complexes with ApoE and ApoJ could be eliminated from the brain via other LRP-family members, LRP8 and LRP2, respectively, but the capacity of these transporter systems and their age-dependent modulation is not known. Whereas a role for luminal P-glycoprotein (Pgp) in Aβ efflux has been proposed, molecular mechanisms and potential ‘collaboration' between LRP1 and Pgp remain uncertain. Aβ transported from the brain into circulation binds soluble LRP (sLRP) and is removed through liver degradation of the complex. Brain accumulation of Aβ could also result from increased influx of circulating Aβ; the principal receptor involved in this process is RAGE (receptor for advanced glycation). Aβ complexes with ApoJ could also be transported into the brain via LRP2 (gp330/Megalin), although the capacity of this route is significantly reduced due to receptor saturation by high circulating levels of ApoJ. Brain endothelial ABCG2 (BCRP) limits Aβ influx from peripheral compartment. Disturbed balance of Aβ influx and efflux across the BBB could be therapeutically targeted to reduce amyloid burden in the brain; for example, brain delivered anti-Aβ antibodies complex soluble brain Aβ; immunocomplexed Aβ is then eliminated by reversed transcytosis across the BBB via both LRP1 (recognizing Aβ-component of the complex), and via FcRn (recognizing Fc domain of the antibody)—later route becomes more important in aged animals, where vascular LRP1 expression declines with age.
Passive and active immunotherapy against Aβ, one of the principal avenues pursued in the development of disease-modifying treatments for AD, was founded on important elements of the ‘vascular hypothesis' described above—that the balance among circulatory and brain ‘pools' of Aβ could be modified by reducing circulatory Aβ (‘sink' effect) such that the net brain efflux of Aβ across the BBB will reduce neurotoxic Aβ load (Menéndez-González et al, 2011). In addition to LRP-1-mediated efflux, Aβ immunocomplexes with Aβ antibodies can be eliminated from the brain via a reverse-transcytosis initiated by IgG binding to the endothelial neonatal Fc receptor (FcRn) (Figure 4; Deane et al, 2005); since LRP1 expression diminishes with aging (Deane et al, 2005), the FcRn-mediated elimination of Aβ immunocomplexes may become the predominant route for reducing central Aβ load using immunotherapy. Immunotherapy strategies are currently being tested in over 10,000 patients enrolled in one of >40 ongoing clinical trials, most of which are expected to report final results within 2 years.
Nevertheless, the question whether AD is principally a vascular disease remains difficult to answer based solely on pathological and clinical evidence of association. Recent comparative studies in two animal models of AD-like pathology attempted to dissect whether cerebrovascular insufficiency/chronic brain hypoperfusion is causally linked to functional manifestations of the disease, most notably cognitive impairment (Nicolakakis et al, 2011; Nicolakakis and Hamel, 2011). In aged mice transgenically overexpressing TGFβ1 (transforming growth factor β1) in astrocytes that exhibit cerebrovascular fibrosis, chronic hypoperfusion and neurovascular uncoupling, indices of cholinergic and cognitive function remained intact (Nicolakakis et al, 2011). However, in transgenic mice overexpressing mutated human APP, along with both vascular and parenchymal Aβ deposition, aging induced cerebrovascular oxidative stress and dysfunction accompanied with cognitive deficits (Nicolakakis and Hamel, 2011). Interestingly, while the PPARγ (peroxisome proliferator-activating receptor-γ agonist), pioglitazone, prevented or reversed cerebrovascular deficits in both animal models, it failed to improve accompanying cognitive impairment (Nicolakakis and Hamel, 2011). Therefore, whereas elements of the vascular hypothesis await confirmation from clinical studies, these and other experimental studies are leaving the ‘vascular cause-neuronal consequence' debate of AD wide opened.
Evidence for blood–brain barrier dysfunction as trigger of disease
In addition to generalized or localized cerebrovascular insufficiency and hypoperfusion, recent focus has also been directed to a transient or chronic dysfunction of the BBB as a potential trigger of acute or chronic neurological symptoms. Perhaps, the two most striking examples of the BBB breakdown causing or transforming clinical presentation of neurological disease are the hemorrhagic transformation after thrombolysis in stroke patients, and iatrogenic seizures caused by the osmotic BBB disruption procedure in neuro-oncological patients (Neumann-Haefelin et al, 2002; Marchi et al, 2007). Occasional clinical reports also indicated a role for BBB breakdown in familial hemiplegic migraine-associated spreading depolarization (Dreier et al, 2005) and in pathogenesis of seizures in the cerebral hyperperfusion syndrome (Ivens et al, 2010). Whereas notion that the massive, overt BBB opening will lead to brain influx of circulating proteins, water and cells, thus causing acute neurological manifestations is not questioned, whether more subtle, transient and presumed innocuous changes in the BBB integrity could lead to evolution of neurological syndromes requires further scrutiny.
Indeed, accumulating experimental evidence support the hypothesis that primary vascular lesions and, specifically an opening of the BBB, could trigger a chain of events leading to neuronal dysfunction and damage, as well as to specific clinical syndromes, including epilepsy and dementia (Friedman et al, 2009; Marchi et al, 2007; van Vliet et al, 2007). A direct evidence that localized and transient BBB breakdown could trigger both acute epileptiform activity and chronic seizures has been recently provided by a series of studies showing that focal application of bile salts onto the rat neocortex, which opened the BBB in a spatially and temporally restricted manner (Greenwood et al, 1991), resulted in delayed appearance of a hypersynchronous epileptiform activity (Seiffert et al, 2004; Ivens et al, 2007: Tomkins et al, 2007). More recently, Marchi et al (2011) demonstrated that glucocorticoids attenuated both pilocarpine-induced seizures in animals and in pediatric drug-resistant epileptic subjects by protecting BBB integrity. The authors further suggest that because BBB dysfunction plays an etiological role in seizure disorders, that combination therapies utilizing an antiepileptic drug in conjunction with a ‘cerebrovascular' drug should be used to control seizures more effectively (Marchi et al, 2011). Interestingly, the loss of BBB integrity may also be considered as causative for specific neurological manifestations in AD in light of data showing frequent subclinical seizures, as well as BBB leakiness, in animal models of the disease (Noebels, 2011).
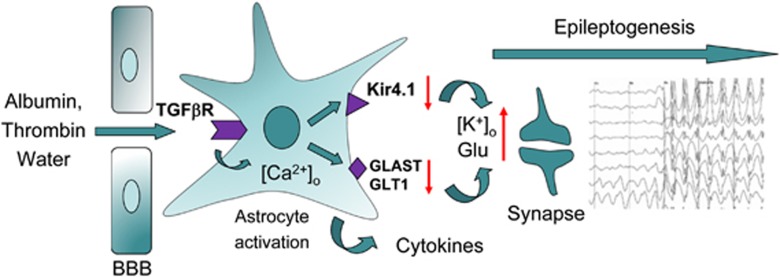
What are the mechanisms by which such BBB dysfunction leads to neuro-glial changes? The accumulating data suggest that acute increase in BBB permeability changes brain extracellular ionic environment (e.g., increase [K+]o and decrease [Ca++]o and [Mg++]o) to promote increased synchronicity and excitability of the neuronal network (Ivens et al, 2007; Marchi et al, 2007). Furthermore, when the BBB is disrupted for large-size molecules such are serum proteins, their accumulation in the brain, apart from driving water influx and edema, may affect glial and neuronal cells by triggering specific signaling pathways. For example, serum albumin has been shown to potently induce calcium signaling and DNA synthesis in astrocytes in culture (Nadal et al, 1995) and in brain slices (Nadal et al, 1998). In vivo animal studies have shown that upon local BBB disruption, albumin is selectively transported into astrocytes via TGFβ receptors, where it triggers transcriptional and functional changes, resulting in neuronal hyperexcitability (Ivens et al, 2007). Astrocytic signaling in the presence of BBB dysfunction and subsequent serum protein exposure involve transcriptional changes (David et al, 2009), which include downregulation of potassium inward rectifying channels (Kir4.1), water channels (AQP4), gap junction proteins and glutamate transporters. Functionally, these changes are associated with reduced uptake of the extracellular potassium and glutamate. During neuronal activation, such reduced capacity of astrocytes to ‘buffer' potassium and glutamate will lead to activity-dependent increase in excitability due to membrane depolarization and activation of NMDA (N-methyl-D-aspartate) receptors (David et al, 2009). Perivascular astrocytes activated in such manner also show upregulation of cytokines and chemokines, including interleukin-1β and tumor necrosis factor α, latter known as modulator of glutamate release (Bezzi et al, 2001), further potentiating glutamate-mediated NMDA currents (Viviani et al, 2003). Inflammatory process initiated by the release of cytokines from astrocytes or their diffusion from the systemic compartment through the compromised BBB, is then sustained through endothelial-leukocyte recruitment (Fabene et al, 2008) exacerbating tissue damage and excitability. Other serum proteins, normally excluded from the brain by the BBB, most notably the blood coagulation serine protease thrombin, were also shown to increase the excitability of the neuronal network (Maggio et al, 2008) and to facilitate hippocampal long-term potentiation (Han et al, 2011; Maggio et al, 2008). The proposed chain of molecular events that trigger epileptogenesis upon BBB disruption is schematically shown in Figure 5. It is important to remark that the BBB permeability changes that trigger these events may be transient and fully repaired, while the cycle of ‘epileptogenic' events continue, lowering the threshold of the local neuronal network to hypersynchronicity clinically manifested as epileptic seizures.
Figure 5.
Mechanisms involved in epileptogenesis triggered by the blood–brain barrier (BBB) permeability changes. Brain influx of serum proteins at sites of the BBB disruption, in particular that of albumin and thrombin, trigger profound astrocytic responses. Astrocytes internalize albumin via transforming growth factor (TGF)β receptors and this process triggers calcium-dependent signaling, leading to transcriptional changes; as a result, potassium inward rectifier, Kir4.1, and glutamate transporters are downregulated, whereas cytokines are upregulated and released by astrocytes. Consequently, synaptic buffering of potassium and glutamate is reduced, leading to increased excitability during neuronal activity and changed neuronal network responses leading to epileptogenesis.
In summary, accumulating experimental data suggest that BBB dysfunction is not simply an ‘epiphenomenon' or ‘biomarker' for injury, but by itself contributes to glial immune response within the exposed brain, which directly affects the function of the neuronal network and may be sufficient to cause clinical manifestation of epilepsy.
Diagnostic and therapeutic targeting of the neurovascular unit
The recognition of the importance of the NVU in etiology and pathogenesis of a variety of neurological diseases, including several that were not specifically discussed in this review such as multiple sclerosis, Parkinson's disease, and amyotrophic lateral sclerosis, led to efforts to identify informative biomarkers of brain vessel disease and to develop therapeutic strategies that target ‘vascular' component of the disease. Since brain-specific biomarkers, compartmentalized by the BBB, are not readily accessible for noninvasive detection and targeting, brain vascular compartment is particularly attractive. Vessel-specific molecular biomarkers of disease are often expressed in early stages of the disease and can be detected in situ using molecular imaging, or in the circulating compartment using ‘omics' approaches. Furthermore, functional read-outs including hemodynamics, coupling, vascular reserve and reactivity, and BBB permeability could also be used as informative disease biomarkers.
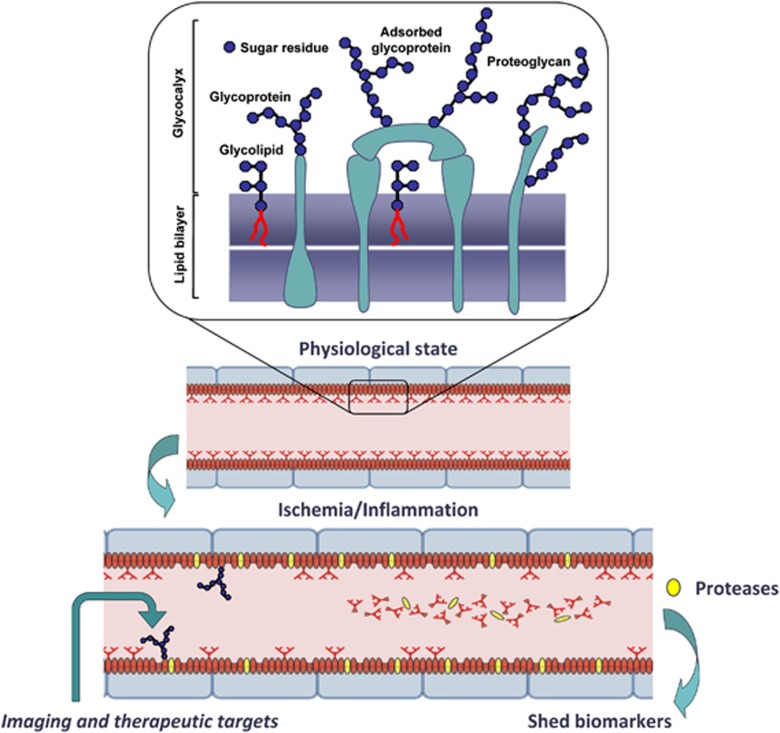
For example, specific and selective biomarkers of angiogenic brain tumor (Pen et al, 2007) identified by microarray screening have been exploited for the detection of pathological vasculature by targeted molecular imaging agents (Iqbal et al, 2010). Particularly thick (∼90 nm compared with ∼40 nm in cardiac EC) brain endothelial glycocalyx, the surface coating comprised of oligosaccharide moieties of plasmalemmal glycoproteins and glycolipids (Figure 6), has been an exceptionally rich source of vascular-specific circulating biomarkers, as it readily sheds in response to inflammatory or ischemic conditions. Not surprisingly, several identified blood biomarkers that associated to various degrees with the risk or progression of AD are ‘shed' or secreted molecules of vascular origin, including endothelial adhesion molecules, adrenomedulin, and endothelin-1 (Ewers et al, 2010). A key future challenge in multiparametric disease evaluation and staging remains meaningful integration of disease-specific cerebrovascular biomarker profiles with functional (neuro)vascular assessments using advanced imaging techniques.
Figure 6.
Brain endothelial glycocalyx as a source of circulating biomarkers and therapeutic targets for neurovascular injury. Brain endothelial cell (BEC) glycocalyx is an exceptionally thick layer composed of sugar residues decorating glycolipids, membrane and adsorbed glycoproteins and proteoglycans that cover luminal lining of BECs and participate in essential functions of the neurovascular unit (NVU) (i.e., blood–brain barrier permeability, blood flow control, interactions with inflammatory and immune cells, as a source of adsorbed growth factors, thrombogenesis, and angiogenesis). Through the activation of membrane proteases by hypoxic or inflammatory stimuli, BEC glycocalyx components (proteins, glycosylated fragments of proteins, glycosylated lipids, oligosaccharides, etc.) are promptly ‘shed' into circulatory compartment, creating a pool of unique endothelial-derived biomarkers that could be used to assess NVU and brain pathology. Luminal BEC (glyco)proteins, such are for example adhesion molecules and transporters, are systemically accessible imaging and therapeutic targets for assessing and modifying functions of the NVU in disease.
Along with advances in molecular understanding of the NVU in disease, some new targets and strategies to protect and repair damaged NVU have started to emerge. For example, nuclear receptor PPARγ has been identified as potential target to rescue cerebrovascular function in AD (Nicolakakis and Hamel, 2011). Modulation of targets implicated in Aβ transport across the BBB holds promise in controlling parenchymal pathology induced by Aβ (Zlokovic, 2010). New experimental strategies have been tested to protect BBB integrity and offset side effects of thrombolytic treatment of stroke; among others, these include inhibitors of matrix metalloproteases (Wang et al, 2008) and desmoteplase, a plasminogen activator derived from the saliva of Desmodus rotundus vampire bat, shown to antagonize tissue plasminogen activator-induced neurotoxicity by competitively blocking LRP-mediated uptake of tissue plasminogen activator into brain parenchyma at the BBB interface (López-Atalaya et al, 2007).
In neuroinflammatory diseases, such as multiple sclerosis, endothelial-expressed molecules are targeted to inhibit brain infiltration of inflammatory cells. While intercellular adhesion molecule-1 and vascular cell adhesion molecule 1 are ‘prototypical' of such brain endothelial targets, novel targets compartmentalized in endothelial membrane subdomains such as lipid rafts (Cayrol et al, 2008) have been implicated in recruitment of specific T-cell subsets. Antibodies against some of these targets, such as activated leukocyte cell adhesion molecule 1, have shown efficacy in suppressing neurological symptoms in experimental allergic encephalomyelitis model(s) of neuroimmune demyelination (Cayrol et al, 2008). Notwithstanding a long history of vascular therapeutic targeting in the area of tumor angiogenesis, these examples demonstrate an emerging spectrum of therapeutic targets and experimental therapeutics identified specifically to protect or modify functions of the NVU. It remains to be seen whether this emerging arsenal of experimental strategies will translate into efficacious clinical therapeutics confirming that NVU, if not origin of neurological disease, is targetable participant that is inextricably linked to disease pathogenesis.
After-Word
The title of this review would have us debate whether it is the blood vessel or the neuron that determines the essence of the specific neurological phenotype (i.e., clinical presentation of the disease)—headache, seizure, dementia, or other. Although we attempted to summarize evidence supporting vascular contribution to some neurological phenotypes, it appears that the field remains ‘trapped' between neurovascular (initiating event occurs at the level of neuron and ‘mobilizes' vascular response) and vasculoneuronal (vascular event triggers neuronal pathology) intellectual seesaw. Perhaps, the term ‘NVU' had it in mind to avoid this debate altogether and instead focus our attention on the importance of interplay between vascular and neuronal systems in pathogenesis of the disease, and, by extension, the necessity to target both for disease prevention, treatment, and management.
The authors declare no conflict of interest.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009;276:4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987;433:155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Bazzoni G. Endothelial tight junctions: permeable barriers of the vessel wall. Thromb Haemost. 2006;95:36–42. [PubMed] [Google Scholar]
- Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer's amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol, R Wosik K, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani A, Stanimirovic D, Prat A. ALCAM promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. CADASIL. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- Charles A, Brennan K. Cortical spreading depression--new insights and persistent questions. Cephalalgia. 2009;29:1115–1124. doi: 10.1111/j.1468-2982.2009.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara T, Nozari A, Moskowitz MA. Migraine aura pathophysiology: the role of blood vessels and microembolisation. Lancet Neurol. 2010;9:309–317. doi: 10.1016/S1474-4422(09)70358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D, Friedman A. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis. J Neurosci. 2009;29:10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, LaRue B, Guo H, Wu Z, Holtzman DM, Zlokovic BV. IgG-assisted age-dependent clearance of Alzheimer's amyloid beta peptide by the blood-brain barrier neonatal Fc receptor. J Neurosci. 2005;25:11495–11503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Ann NY Acad Sci. 2010;1207:46–49. doi: 10.1111/j.1749-6632.2010.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Jurkat-Rott K, Petzold GC, Tomkins O, Klingebiel R, Kopp UA, Lehmann-Horn F, Friedman A, Dichgans M. Opening of the blood-brain barrier preceding cortical edema in a severe attack of FHM type II. Neurology. 2005;64:2145–2147. doi: 10.1212/01.WNL.0000176298.63840.99. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Kleeberg J, Petzold G, Priller J, Windmüller O, Orzechowski H-D, Lindauer U, Heinemann U, Einhäupl KM, Dirnagl U. Endothelin-1 potently induces Leão's cortical spreading depression in vivo in the rat: a model for an endothelial trigger of migrainous aura. Brain. 2002;125:102–112. doi: 10.1093/brain/awf007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Körner K, Ebert N, Görner A, Rubin I, Back T, Lindauer U, Wolf T, Villringer A, Einhäupl KM, Lauritzen M, Dirnagl U. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induce cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab. 1998;18:978–990. doi: 10.1097/00004647-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Petzold G, Tille K, Lindauer U, Arnold G, Heinemann U, Einhäupl KM, Dirnagl U. Ischaemia triggered by spreading neuronal activation is inhibited by vasodilators in rats. J Physiol (Lond) 2001;531 (Pt 2:515–526. doi: 10.1111/j.1469-7793.2001.0515i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Wolburg H. Mini-review: transendothelial migration of leukocytes: through the front door or around the side of the house. Eur J Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- Ewers M, Mielke MM, Hampel H. Blood-based biomarkers of microvascular pathology in Alzheimer's disease. Exp Gerontol. 2010;45:75–79. doi: 10.1016/j.exger.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, Bach S, Angiari S, Benati D, Chakir A, Zanetti L, Schio F, Osculati A, Marzola P, Nicolato E, Homeister JW, Xia L, Lowe JB, McEver RP, Osculati F, Sbarbati A, Butcher EC, Constantin G. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85:142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Adu J, Davey AJ, Abbott NJ, Bradbury MW. The effect of bile salts on the permeability and ultrastructure of the perfused, energy-depleted, rat blood-brain barrier. J Cereb Blood Flow Metab. 1991;11:644–654. doi: 10.1038/jcbfm.1991.116. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G, Tamagno E, Tabaton M. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. J Neurochem. 2009;108:1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Han KS, Mannaioni G, Hamill CE, Lee J, Junge CE, Lee CJ, Traynelis SF. Activation of protease activated receptor 1 increases the excitability of the dentate granule neurons of hippocampus. Mol Brain. 2011;4:32. doi: 10.1186/1756-6606-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqqani AS, Hill JJ, Mullen J, Stanimirovic DB. Methods to study glycoproteins at the blood-brain barrier using mass spectrometry. Methods Mol Biol. 2011;686:337–353. doi: 10.1007/978-1-60761-938-3_16. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Miller DS, Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease. Mol Pharmacol. 2010;77:715–723. doi: 10.1124/mol.109.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé D, Chabriat H. CADASIL. J Geriatr Psychiatry Neurol. 2010;23:269–276. doi: 10.1177/0891988710383570. [DOI] [PubMed] [Google Scholar]
- Humpel C, Marksteiner J. Cerebrovascular damage as a cause for Alzheimer's disease. Curr Neurovasc Res. 2005;2:341–347. doi: 10.2174/156720205774322610. [DOI] [PubMed] [Google Scholar]
- Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Hachinski V, Rosenberg GA. Vascular cognitive impairment: introduction. Stroke. 2010;41 (10 Suppl:S127–S128. doi: 10.1161/STROKEAHA.110.595488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal U, Albaghdadi H, Luo Y, Arbabi M, Desvaux C, Veres T, Stanimirovic D, Abulrob A. Molecular imaging of glioblastoma multiforme using anti-insulin-like growth factor-binding protein-7 single-domain antibodies. Br J Cancer. 2010;103:1606–1616. doi: 10.1038/sj.bjc.6605937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivens S, Gabriel S, Greenberg G, Friedman A, Shelef I. Blood-brain barrier breakdown as a novel mechanism underlying cerebral hyperperfusion syndrome. J Neurol. 2010;257:615–620. doi: 10.1007/s00415-009-5384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130 (Pt 2:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Hwang MC, Farr SA, Murphy MP, Fleegal-DeMotta MA, Lynch JL, Robinson SM, Niehoff ML, Johnson SN, Kumar VB, Banks WA. Testing the neurovascular hypothesis of Alzheimer's disease: LRP1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J Alzheimers Dis. 2009;17:553–570. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger S, Pietrzik CU. Functional role of lipoprotein receptors in Alzheimer's disease. Curr Alzheimer Res. 2008;5:15–25. doi: 10.2174/156720508783884675. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. The pathology of ‘vascular dementia': a critical update. J Alzheimers Dis. 2008;4:107–123. doi: 10.3233/jad-2008-14110. [DOI] [PubMed] [Google Scholar]
- Jouvent E, Mangin JF, Porcher R, Viswanathan A, O'Sullivan M, Guichard JP, Dichgans M, Bousser MG, Chabriat H. Cortical changes in cerebral small vessel diseases: a 3D MRI study of cortical morphology in CADASIL. Brain. 2008;131 (Pt 8:2201–2208. doi: 10.1093/brain/awn129. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Plate KH. Differentiation of the brain vasculature: the answer came blowing by the Wnt. J Angiogenes Res. 2010;2:1. doi: 10.1186/2040-2384-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Atalaya JP, Roussel BD, Ali C, Maubert E, Petersen KU, Berezowski V, Cecchelli R, Orset C, Vivien D. Recombinant Desmodus rotundus salivary plasminogen activator crosses the blood-brain barrier through a low-density lipoprotein receptor-related protein-dependent mechanism without exerting neurotoxic effects. Stroke. 2007;38:1036–1043. doi: 10.1161/01.STR.0000258100.04923.84. [DOI] [PubMed] [Google Scholar]
- Maggio N, Shavit E, Chapman J, Segal M. Thrombin induces long-term potentiation of reactivity to afferent stimulation and facilitates epileptic seizures in rat hippocampal slices: toward understanding the functional consequences of cerebrovascular insults. J Neurosci. 2008;28:732–736. doi: 10.1523/JNEUROSCI.3665-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, Hallene K, Diglaw T, Franic L, Najm I, Janigro D. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granata T, Freri E, Ciusani E, Ragona F, Puvenna V, Teng Q, Alexopolous A, Janigro D. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS One. 2011;6:e18200. doi: 10.1371/journal.pone.0018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menéndez-González M, Pérez-Piñera P, Martínez-Rivera M, Muñiz AL, Vega JA. Immunotherapy for Alzheimer's disease: rational basis in ongoing clinical trials. Curr Pharm Des. 2011;17:508–520. doi: 10.2174/138161211795164112. [DOI] [PubMed] [Google Scholar]
- Meyer EP, Ulmann-Schuler A, Staufenbiel M, Krucker T. Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer's disease. Proc Natl Acad Sci USA. 2008;105:3587–3592. doi: 10.1073/pnas.0709788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monet-Leprêtre M, Bardot B, Lemaire B, Domenga V, Godin O, Dichgans M, Tournier-Lasserve E, Cohen-Tannoudji M, Chabriat H, Joutel A. Distinct phenotypic and functional features of CADASIL mutations in the Notch3 ligand binding domain. Brain. 2009;132 (Pt 6:1601–1612. doi: 10.1093/brain/awp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Fuentes E, Pastor J, McNaughton PA. Plasma albumin is a potent trigger of calcium signals and DNA synthesis in astrocytes. Proc Natl Acad Sci USA. 1995;92:1426–1430. doi: 10.1073/pnas.92.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Sul JY, Valdeolmillos M, McNaughton PA. Albumin elicits calcium signals from astrocytes in brain slices from neonatal rat cortex. J Physiol. 1998;509 (Pt 3:711–716. doi: 10.1111/j.1469-7793.1998.711bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Hansen AJ. Spreading depression is not associated with neuronal injury in the normal brain. Brain Res. 1988;449:395–398. doi: 10.1016/0006-8993(88)91062-1. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin C, Brinker G, Uhlenküken U, Pillekamp F, Hossmann KA, Hoehn M. Prediction of hemorrhagic transformation after thrombolytic therapy of clot embolism: an MRI investigation in rat brain. Stroke. 2002;33:1392–1398. doi: 10.1161/01.str.0000014619.59851.65. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnár Z, O'Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolakakis N, Aboulkassim T, Aliaga A, Tong XK, Rosa-Neto P, Hamel E. Intact memory in TGF-β1 transgenic mice featuring chronic cerebrovascular deficit: recovery with pioglitazone. J Cereb Blood Flow Metab. 2011;31:200–211. doi: 10.1038/jcbfm.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer's disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolakakis N, Hamel E. Neurovascular function in Alzheimer's disease patients and experimental models. J Cereb Blood Flow Metab. 2011;31:1354–1370. doi: 10.1038/jcbfm.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noebels J. A perfect storm: converging paths of epilepsy and Alzheimer's dementia intersect in the hippocampal formation. Epilepsia. 2011;52 (Suppl 1:39–46. doi: 10.1111/j.1528-1167.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell S, Wolburg-Buchholz K, Mack AF, Beedle AM, Satz JS, Campbell KP, Wolburg H, Fallier-Becker P. Evidence for a role of dystroglycan regulating the membrane architecture of astroglial endfeet. Eur J Neurosci. 2011;33:2179–2186. doi: 10.1111/j.1460-9568.2011.07688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pen A, Moreno MJ, Martin J, Stanimirovic DB. Molecular markers of extracellular matrix remodeling in glioblastoma vessels: microarray study of laser-captured glioblastoma vessels. Glia. 2007;55:559–572. doi: 10.1002/glia.20481. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn T, von Stuckrad-Barre S, Herzog J, Gasser T, Hamann GF, Dichgans M. Reduced cerebrovascular CO(2) reactivity in CADASIL: a transcranial Doppler sonography study. Stroke. 2001;32:17–21. doi: 10.1161/01.str.32.1.17. [DOI] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith AV, Carassquillo MM, Lambert JC, Harold D, Schrijvers EM, Ramirez-Lorca R, Debette S, Longstreth WT, Jr, Janssens AC, Pankratz VS, Dartigues JF, Hollingworth P, Aspelund T, Hernandez I, Beiser A, Kuller LH, Koudstaal PJ, Dickson DW, Tzourio C, Abraham R, Antunez C, Du Y, Rotter JI, Aulchenko YS, Harris TB, Petersen RC, Berr C, Owen MJ, Lopez-Arrieta J, Varadarajan BN, Becker JT, Rivadeneira F, Nalls MA, Graff-Radford NR, Campion D, Auerbach S, Rice K, Hofman A, Jonsson PV, Schmidt H, Lathrop M, Mosley TH, Au R, Psaty BM, Uitterlinden AG, Farrer LA, Lumley T, Ruiz A, Williams J, Amouyel P, Younkin SG, Wolf PA, Launer LJ, Lopez OL, van Duijn CM, Breteler MM. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev H, Serlin Y, Friedman A. Breaching the blood-brain barrier as a gate to psychiatric disorder. Cardiovasc Psychiatry Neurol. 2009;2009:278531. doi: 10.1155/2009/278531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- Stam AH, Haan J, van den Maagdenberg AM, Ferrari MD, Terwindt GM. Migraine and genetic and acquired vasculopathies. Cephalalgia. 2009;29:1006–1017. doi: 10.1111/j.1468-2982.2009.01940.x. [DOI] [PubMed] [Google Scholar]
- Stanimirovic DB.2001Inflammatory activation of brain cells by hypoxia: transcription factors and signaling pathways Inflammation and Stroke(Feuerstein G, ed),Basel: Birkhauser Verlag AG; 101–111. [Google Scholar]
- Stanimirovic DB, Satoh K. Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol. 2000;10:113–126. doi: 10.1111/j.1750-3639.2000.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- Tomkins O, Friedman O, Ivens S, Reiffurth C, Major S, Dreier JP, Heinemann U, Friedman A. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25:367–377. doi: 10.1016/j.nbd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117:333–345. doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, da Costa Araújo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130 (Pt 2:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukic V, Callaghan D, Walker D, Lue L-F, Liu Q-Y, Stanimirovic D, Zhang W. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer's brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. 2009;34:95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rosell A, Lo EH. Targeting extracellular matrix proteolysis for hemorrhagic complications of tPA stroke therapy. CNS Neurol Disord Drug Targets. 2008;7:235–242. doi: 10.2174/187152708784936635. [DOI] [PubMed] [Google Scholar]
- Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–2545. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Callaghan D, Jones A, Bai J, Rasquinha I, Smith C, Pei K, Walker D, Lue LF, Stanimirovic D, Zhang W. ABCG2 is upregulated in Alzheimer's brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood--brain barrier for Abeta(1-40) peptides. J Neurosci. 2009;29:5463–5475. doi: 10.1523/JNEUROSCI.5103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Craggs L, Baumann M, Kalimo H, Kalaria RN. Review: molecular genetics and pathology of hereditary small vessel diseases of the brain. Neuropathol Appl Neurobiol. 2011;37:94–113. doi: 10.1111/j.1365-2990.2010.01147.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Petrovic JM, Callaghan D, Jones A, Cui H, Howlett C, Stanimirovic D. Evidence that hypoxia-inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1beta (IL-1beta) in astrocyte cultures. J Neuroimmunol. 2006;174:63–73. doi: 10.1016/j.jneuroim.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer's amyloid β-peptide elimination from the brain. J Neurochem. 2010;15:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Martel CL, Matsubara E, McComb JG, Zheng G, McCluskey RT, Frangione B, Ghiso J. Glycoprotein 330/megalin: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci USA. 1996;93:4229–4234. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]