Abstract
Brain injury after focal ischemia evolves along two basically different pathophysiologies, depending on the severity of the primary flow reduction and the dynamics of postischemic recirculation. In permanent and gradually reversed focal ischemia as after thromboembolic occlusion, primary core injury is irreversible but the expansion of the core into the penumbra can be alleviated by hemodynamic and molecular interventions. Such alleviation can only be achieved within 3 hours after the onset of ischemia because untreated core injury expands to near maximum size during this interval. In promptly reversed transient ischemia as after mechanical vascular occlusion, primary core injury may recover but a secondary delayed injury evolves after a free interval of as long as 6 to 12 hours. This injury can be alleviated throughout the free interval but the longer window is without clinical relevance because transient mechanical vascular occlusion is not a model of naturally occurring stroke. As this difference is widely ignored in stroke research, most clinical trials have been designed with a far too long therapeutic window, which explains their failure. Transient mechanical vascular occlusion models should, therefore, be eliminated from the repertoire of preclinical stroke research.
Keywords: animal models, cell death mechanisms, drug trials, focal ischemia, neuroprotection, reperfusion, thrombolysis
Introduction
By the end of August 2011, the clinical Stroke Trials Registry of the Internet Stroke Center at Washington University School of Medicine listed 1,104 entries that reflect the enormous efforts taken to establish efficient treatments for acute ischemic stroke (Internet_Stroke_Center, 2011). The number of experimental stroke studies is even higher, but the outcomes of clinical and preclinical treatments greatly differ. Whereas most of the preclinical treatments reported substantial improvements, clinical interventions were much less successful. In fact, looking at the outcome of statistically reliable prospective phase III trials, only 3 out of 11 thrombolytic interventions, and none of 19 neuroprotection trials were positive (Tables 1 and 2).
Table 1. Phase III trials for neuroprotection of acute ischemic stroke.
| Compound | Mode of action | Study acronym | Time window | Outcome |
|---|---|---|---|---|
| Fosphenytoin | Sodium channel blocker | — | 4 Hours | Negative |
| GM-1 ganglioside | Attenuation of excitotoxicity | EST | 5 Hours | Negative |
| CGS 19755 | Glutamate antagonist | ASSIST | 6 Hours | Negative |
| Aptiganel | Glutamate antagonist | — | 6 Hours | Negative |
| Cervene | Opioid antagonist | — | 6 Hours | Negative |
| Tirilazad mesylate | Peroxidation inhibitor | RANTTAS | 6 Hours | Negative |
| Disufenton NXY-059 | Free radical trapping | SAINT | 6 Hours | Negative |
| (S)-BMS 204352 | Potassium channel opener | POST | 6 Hours | Negative |
| Enlimomab | Anti-ICAM-1 antibody | EAST | 6 Hours | Negative |
| Nimodipine | Calcium channel blocker | VENUS | 6 Hours | Negative |
| bFGF | Trophic growth factor | — | 6 Hours | Negative |
| Erythropoietin | Growth hormone | ESS | 6 Hours | Negative |
| Lubeluzole | Ion channel/NO blocker | LUB-INT | 6–8 Hours | Negative |
| Clomethiazole | GABA agonist | CLASS | 12 Hours | Negative |
| Diazepam | GABA agonist | EGASIS | 12 Hours | Negative |
| Magnesium | Ion channel blocker | IMAGES | 12 Hours | Negative |
| GM-1 ganglioside | Attenuation of excitotoxicity | IASS-H | 12 Hours | Negative |
| Citocoline | Membrane stabilizer | ECCO | 24 Hours | Negative |
| Nimodipine | Calcium channel blocker | ANS | 48 Hours | Negative |
bFGF, basic fibroblast growth factor; ICAM, intercellular adhesion molecule; NO, nitric oxide; GABA, gamma-aminobutyric acid.
Data from Stroke Trials Registry (http://www.strokecenter.org/trials).
Table 2. Phase III trials for thrombolytic treatment of acute ischemic stroke.
| Compound | Mode of action | Study acronym | Time window | Outcome |
|---|---|---|---|---|
| rtPA | Thrombolysis | NIHSSG | 3 Hours | Positive |
| rtPA | Thrombolysis | ECASS III | 3–4.5 Hours | Positive |
| rtPA | Thrombolysis | ATLANTIS | 3–6 Hours | Negative |
| Streptokinase | Thrombolysis | ASK | 4 Hours | Negative |
| rtPA (alteplase) | Thrombolysis | ECASS I | 6 Hours | Negative |
| rtPA (alteplase) | Thrombolysis | ECASS II | 6 Hours | Negative |
| Ancrod | Thrombolysis | ASP II | 6 Hours | Negative |
| Streptokinase | Thrombolysis | MAST | 6 Hours | Negative |
| rPro-urokinase | Thrombolysis | PROACT II | 6 Hours | Positive |
| Urokinase | Thrombolysis | PASS | 6 Hours | Negative |
| dsPA (desmoteplase) | Thrombolysis | DIAS II | 9 Hours | Negative |
dsPA, desmodus rotundus salivary plasminogen activator; rtPA, recombinant tissue plasminogen activator.
Data from Stroke Trials Registry (http://www.strokecenter.org/trials).
The poor translational power of preclinical stroke studies has led to the establishment of the Stroke Therapy Academic Industry Roundtable who issues regularly updated recommendations to improve the quality of preclinical stroke research (Fisher et al, 2009). Important aspects of these recommendations are the precise definition of the time window of treatment, the use of both permanent and transient occlusion models, the use of relevant biomarker end points, and good laboratory practice. However, even strict adherence to these recommendations did not promote major progress in clinical translation, as reflected by the negative outcome of the SAINT (Stroke Acute Ischemia NXY-059 (Cerovive) Treatment) (Shuaib et al, 2007). In this trial the beneficial preclinical effect of cerovive, a free-radical scavenger with antioxidant properties could not be replicated under clinical conditions, although most methodological recommendations were carefully observed and although the drug provided robust neuroprotection in permanent and transient focal ischemia models.
It has been argued that the neuroprotective effect of the SAINT trial may have been flawed by inappropriate statistics, the failure to consider general physiological variables such as blood pressure, and the preferential use of young healthy instead of aged comorbid animals (Dirnagl and Macleod, 2009). Moreover, species differences in drug sensitivity or therapeutic side effects have been widely ignored in this and other trials. However, as this drug is not the only one that succeeded under preclinical and failed under clinical conditions, an alternative and potentially more basic explanation should be sought. Here, the proposition is made that a possible explanation could be the inappropriate mix-up of preclinical data derived from clinically relevant and clinically irrelevant stroke models. In fact, different models of experimental focal cerebral ischemia exhibit different pathophysiologies which respond differently to therapeutic interventions but which are of different relevance to the clinical setting (Hossmann, 2008). In the following, an attempt will be made to explain the failure of translational stroke research on the basis of the known differences between these pathophysiologies.
Injury evolution after the initiation of focal brain ischemia
Damage inflicted on the brain by focal impairment of cerebral blood supply can be differentiated into two only partly related injury modes: the alterations induced in response to the initiation of ischemia and—if applicable—those inflicted by postischemic reperfusion.
The initial response to vascular occlusion is stereotyped and can be predicted by the viability thresholds of cerebral ischemia (Heiss et al, 2004). At declining flow values, various metabolic and biological functions are sequentially suppressed until, at a threshold of ∼15% of control, energy metabolism fails and cell membranes depolarize (terminal anoxic depolarization). The central part of the ischemic territory in which blood flow declines below the threshold of terminal depolarization is the infarct core, and the more peripheral parts where various electrical and metabolic functions—but not energy metabolism and membrane polarization—are affected, is the penumbra (Astrup et al, 1981). Lesser reductions in blood flow, which do not lead to apparent functional or metabolic disturbances, are called benign oligemia and do not produce tissue injury (Kidwell et al, 2003).
With ongoing ischemia time, the thresholds of energy failure and anoxic depolarization increase, and the core expands into the penumbra. The rate of expansion is fasted at the onset of ischemia but then it gradually declines until—between 3 and 6 hours—the penumbra has disappeared and the core reaches its maximum volume (Hossmann, 2008). The main reason for infarct core expansion is the progressing mismatch between penumbral blood flow and metabolism, induced among others by the high workload of peri-infarct spreading depolarizations, progressing mitochondrial insufficiency and a functional reduction of peri-ischemic blood flow, also referred to as spreading ischemia (Petzold et al, 2003).
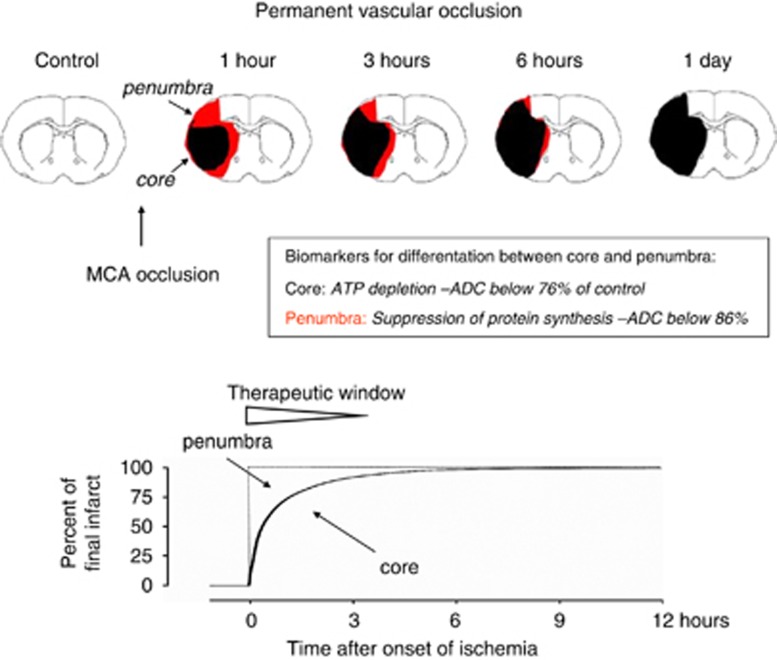
A reversal of energy failure in the infarct core is possible only if blood flow is fully restored within the revival time of the ischemic brain, which under normothermic conditions is in the range of 1 hour (see below). In the absence of reperfusion, core injury is irreversible, and therapeutic interventions are restricted to the alleviation of infarct core expansion. In accordance with the temporal dynamics of infarct expansion, the therapeutic potential is greatest during the first ‘golden' hour of ischemia (Saver et al, 2010) where the volume of the penumbra amounts up to 50% of the ischemic territory. After longer intervals, the volume of salvageable tissue rapidly declines until, after >3 hours, potential improvements are only marginal (Figure 1).
Figure 1.
Schematic representation of infarct evolution during permanent middle cerebral artery (MCA) occlusion. Core and penumbra are differentiated by biochemical and magnetic resonance imaging according to established viability thresholds of brain ischemia. With ongoing ischemia, the core expands into the penumbra until—within ∼3 hours—infarct reaches its maximum volume. This time is the therapeutic window during which infarct expansion, and hence final infarct volume, can be alleviated.
Within the time window of infarct core expansion any intervention that is able to reduce the flow/metabolism mismatch is likely to reduce the final infarct volume, as compared with untreated animals. Examples of such treatments are the suppression of spreading depolarizations (to reduce the functional workload of the penumbra; Lauritzen et al, 2011), hypothermia (to reduce the metabolic rate of brain tissue; van der Worp et al, 2010), or the improvement of collateral blood supply to the ischemic territory (Liebeskind, 2010). If initiated during the first hour of ischemia, each of these approaches is able to reduce the infarct volume by up to 50 to 60%. In contrast, the same interventions have little—if any—effect when applied after longer than 3 hours.
Thus, the therapeutic responsiveness of permanent focal ischemia is greatest during the first hour of ischemia and does not exceed the limit of 3 hours.
Effect of reperfusion on injury evolution
Investigations in the early seventies of brain resuscitation after circulatory arrest revealed that brain injury—except selective vulnerability—can be reversed after complete normothermic ischemia of as long as 1 hour, provided brain reperfusion is promptly restored after the ischemic impact (Hossmann and Kleihues, 1973). Inhomogeneous or protracted reperfusion, in contrast, causes irreversible brain injury after ischemia as short as 8 to 10 minutes. The reason is a multifocal deficiency in postischemic reoxygenation, which promotes postischemic lactacidosis, pericapillary glial swelling, and the evolution of a no-reflow phenomenon.
The most important single pathophysiological variable determining prompt or protracted reperfusion is local blood perfusion pressure (Fischer and Ames, 1972). As blood viscosity increases with the severity and duration of ischemia, reperfusion requires increasingly elevated blood pressure levels, whereas hypotensive pressure fails to reperfuse the tissue even after a few minutes of circulatory arrest. This is the reason why surgically induced isolated cerebrocirculatory arrest followed by hypertensive reperfusion causes much less brain injury than the same duration of naturally occurring cardiac arrest where postischemic blood pressure is greatly reduced until heart function recovers.
In experimental models of transient focal ischemia, a systematic investigation of the effect of focal reperfusion pressure has not been performed but there is incidental evidence of a similar pressure–reperfusion relationship. After transient mechanical occlusion, as induced by reversible vascular clipping or intraluminal filament insertion, release of vascular occlusion results in prompt reperfusion due to instantaneous restoration of normal reperfusion pressure (Figure 2A). Spontaneous or rtPA accelerated reversal of thromboembolic occlusion, in contrast, causes prolonged reperfusion deficits (Figure 2B). Obviously, this delay is due to the slow increase in postischemic reperfusion pressure, which prevents prompt, homogeneous restoration of microcirculation.
Figure 2.
Dynamics of postischemic recirculation after transient mechanical (A) and recombinant tissue plasminogen activator (rtPA)-treated thromboembolic occlusion (B). Reversal of mechanical occlusion results in prompt recirculation followed by postischemic hyperemia. Recirculation initiated by thrombolysis of clot embolism, in contrast, is only gradually restored and returns to normal after much longer intervals. The different hemodynamics of the two types of ischemia explain that core injury is only reversed after transient mechanical occlusion but not after thrombolytic reperfusion (see Figures 3 and 4; data from Kilic et al, 1999).
In analogy to global brain ischemia, the different postischemic hemodynamic profiles are reflected by differences in functional recovery. Protracted reperfusion, as achieved by spontaneous or thrombolytic reversal of thromboembolic occlusion, is usually unable to restore energy metabolism in the core region of the evolving infarct. Even topical application of high dose of rtPA as soon as 15 minutes after clot embolism did not reverse the shift of the apparent diffusion coefficient (ADC), a reliable biomarker of energy failure (Busch et al, 1998). Successful recovery has been reported only after partial or very brief periods of thromboembolic occlusion, which is in line with the clinical reversal of short lasting transient ischemic attacks.
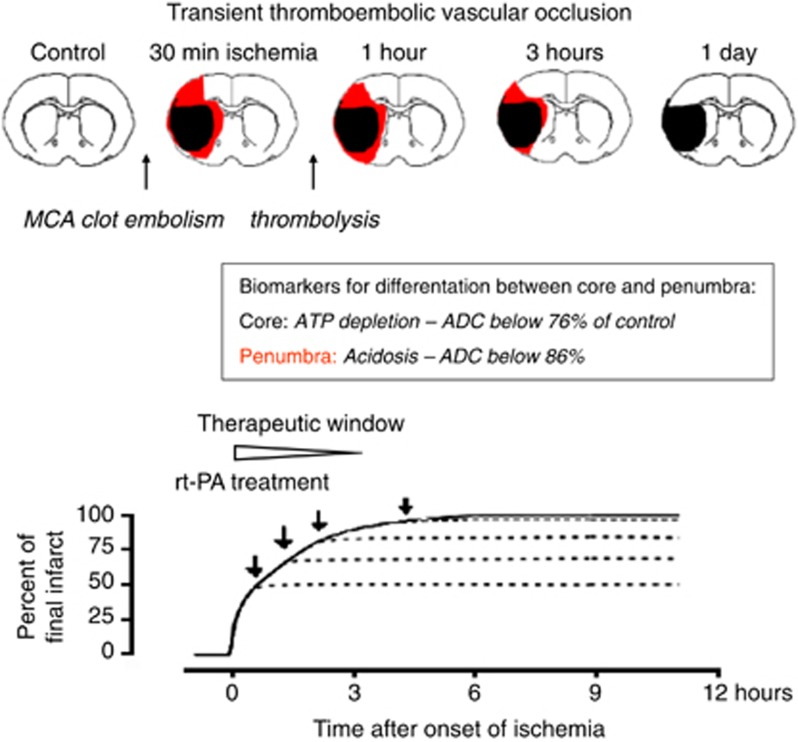
The failure of metabolic recovery in the infarct core is not equivalent to therapeutic inefficiency. Different from the core region, the penumbra benefits not only from prompt but also from protracted improvement of local blood perfusion pressure (Figure 3). The reason is the lower blood viscosity in vessels with persisting perfusion which in combination with ischemia-induced vasodilatation prevents the fatal increase in microvascular resistance. As a result, the flow-metabolism mismatch steadily improves until blood flow returns to normal and the penumbra is not longer at risk of injury.
Figure 3.
Infarct evolution after gradually reversed transient ischemia. Schematic representation of middle cerebral artery (MCA) clot embolism followed by intraarterial infusion of recombinant tissue plasminogen activator (rtPA). Thrombolytic reperfusion does not reverse metabolic failure in the infarct core but it alleviates infarct expansion by improving penumbral blood flow. The therapeutic window is a function of the infarct expansion before the initiation of thrombolysis and is limited to ∼3 hours, as in permanent focal ischemia (see Figure 1).
In transient mechanical occlusion models with prompt recirculation, suppression of energy metabolism in the infarct core is partially or even completely reversible, depending on the duration of ischemia (Lust et al, 2002). After up to 1 hour vascular occlusion, restoration of energy metabolism has been documented throughout the core region, in accordance with the previously established revival time of the brain after global circulatory arrest (Figure 4). After 90 to 120 minutes ischemia, metabolism recovers only in the peripheral parts of the ischemic territory, which can be explained by the later onset of energy failure in these regions. After 2 to 3 hours ischemia, the duration of energy failure exceeds brain revival time even in the most peripheral parts of the expanding infarct, and infarct size approaches that of permanent vascular occlusion.
Figure 4.
Infarct evolution after promptly reversed transient ischemia. Schematic representation of increasing durations of middle cerebral artery (MCA) intraluminal filament insertion. The instantaneous reversal of blood circulation after withdrawal of the filament results in prompt reoxygenation of the ischemic tissue and, depending on the duration of ischemia, promotes partial or even complete recovery of energy metabolism in the infarct core. After a free interval of up to 6 to 12 hours secondary delayed cell death evolves in the area of primary energy failure. Secondary delayed cell death can be alleviated by a multitude of molecular interventions throughout the interval between primary and secondary damage. The therapeutic window is, therefore, much longer than after permanent or gradually reversed vascular occlusion. However, this extension is of limited clinical relevance because promptly reversed focal ischemia is not a model of naturally occurring stroke.
A unique pathophysiological feature of transient mechanical vascular occlusion is the high sensitivity of the areas with primarily restored energy metabolism to delayed secondary injury, a phenomenon referred to as reperfusion or reoxygenation injury (Li and Jackson, 2002). The interval between primary recovery and secondary injury depends on the duration of ischemia. After 15 minutes transient focal ischemia, secondary injury may be delayed for as long as 3 weeks (Du et al, 1996). After 30 minutes ischemia, delayed injury has been observed after 72 hours (Endres et al, 1998), and after 1 hour ischemia the interval shortened to 3–6 hours (Hata et al, 2000). Interestingly, delayed injury occurs only in areas in which energy metabolism was suppressed before recirculation but not in the penumbra. This suggests that the manifestation of reoxygenation injury depends on the prior exposure of the tissue to transient anoxic depolarization.
Obviously, the pathomechanisms of transient ischemia with prompt or protracted reperfusion are basically different. Protracted reperfusion does not prevent primary necrotic core injury, and from a mechanistic viewpoint differs from permanent ischemia only by the alleviation of infarct expansion into the penumbra but not in respect to the flow threshold-dependent induction of primary tissue necrosis. The dominating injury mechanism is, therefore, primary anoxic-ischemic cell death, as during permanent focal ischemia.
Prompt reperfusion with primary recovery of energy metabolism, in contrast, initiates a cascade of secondary events usually referred to as the molecular ischemic injury cascade. The various molecular mechanisms associated with this cascade are operationally defined as those postischemic disturbances that respond to specific pharmacological or genetic interventions with reduction of the final infarct size. Using this simplistic approach, a great number of molecular injury pathways have been discovered, comprising so different pathomechanisms as calcium, glutamate, zinc or tPA toxicity, free-radical induced injury, apoptosis, inhibition of protein synthesis or various inflammatory and stress responses, to name only a few (for reviews, see Doyle et al, 2008; Lo et al, 2005; Neumar, 2000). The common denominator of these injury pathways is the evolution of tissue damage in the absence of circulatory and hence anoxic-ischemic disturbances. The dominant injury mechanism of transient ischemia with prompt recirculation is, therefore, secondary molecular (nonischemic) cell death.
Translation of preclinical stroke studies
The two pathophysiologies of focal brain ischemia—primary anoxic-ischemic and secondary molecular cell death—should not be confounded when preclinical data are translated into clinical stroke trials. Obviously, reliable predictions can only be made on the basis of clinically relevant experimental models, such as permanent or gradually reversed vascular occlusion. Promptly reversed vascular occlusion, as induced by transient vascular clipping or reversible intraluminal filament insertion, replicates cerebrovascular surgery or interventional thrombectomy but not naturally occurring stroke and, therefore, is mal-suited for clinical translation.
The inappropriate association of the latter pathophysiology with stroke is widely ignored in experimental stroke research. A PubMed search for experimental studies covering the terms ‘neuroprotection' and ‘middle cerebral artery occlusion' revealed that 64% of 749 treatments were performed using clinically irrelevant promptly reversed vascular occlusions and only 36% were performed in models of permanent or gradually reversed vascular occlusion. The consequences of this misconception are dramatic. Whereas the pathophysiology of permanent or gradually reversed vascular occlusion predicts a therapeutic window of <3 hours, that of promptly reversed ischemia may respond to treatment throughout the free interval between primary recovery and secondary injury, i.e., for considerably longer duration. If these long intervals are inappropriately translated to clinical stroke trials, therapeutic failure is unavoidable.
A meta-analysis of the NXY-59 preclinical studies revealed that the design of the SAINT trial was, in fact, heavily biased by the use of such inappropriate models. As reviewed by Bath et al (2009), 15 out of 26 preclinical treatment studies were performed in clinically irrelevant transient mechanical vascular occlusion models, the delays of successful treatments ranging from 15 to 300 minutes (median 180 minutes). Eleven out of 26 studies were performed using more appropriate permanent or thromboembolic occlusion models with delays ranging from 0 to 240 minutes (median 24.5 minutes). According to the two pathophysiologies described above, the preclinical data suggest that clinical stroke would benefit from the drug when applied during the time window of permanent focal ischemia, i.e., during the initial 30 minutes, but not during the window of promptly reversed transient ischemia which provided neuroprotection for up to 5 hours. The maximum treatment delay of the clinical trial, however, was set to 6 hours and, therefore, greatly exceeded the experimentally predictable window of therapeutic responsiveness.
The inappropriate selection of a much too long window of stroke treatment is not unique to the NXY-059 (SAINT) trial. In fact, none of the presently completed phase III neuroprotection trials had a treatment window shorter than 4 hours, and the great majority used windows of 6 hours or even longer intervals (Table 1). It is, therefore, not surprising that all these trials were negative.
This situation differs from the many reports about the beneficial effect of thrombolysis where the NIH (National Institutes of Health)-approved time window is 3 hours. The reason for selecting a shorter window was not a better insight into the pathophysiology of gradually reversed focal ischemia but the concern about hemorrhagic complications. When clinical studies revealed that the risk of bleeding was overrated, extensions of the treatment window have been attempted. But similarly to pharmacological neuroprotection, most thrombolysis trials initiated after later than 3 hours proved to be inefficient (Table 2). The only exceptions are the Pro-Urokinase for Acute Cerebral Thromboembolism (PROACT) II and the European Cooperative Acute Stroke Study (ECASS) III trials with windows of 6 hours and 3 to 4.5 hours, respectively. However, in these trials patients with severe strokes were excluded. The beneficial effects can, therefore, be explained by the preponderance of smaller infarcts in which expansion to maximum size takes a longer time.
The here presented opinion about the different translational power of different stroke models is based on the tacit assumption that drug responsiveness and the dynamics of injury evolution are similar in humans and laboratory animals. The basic differences in anatomy, physiology, genetics, and metabolic rates question the validity of this assumption but there is no hard evidence to the contrary. In fact, the therapeutic window of thrombolysis is remarkably similar in rodents and stroke patients, indicating that the flow/metabolism mismatch responsible for primary infarct expansion is of similar magnitude. This is probably different for the molecular injury cascade associated with secondary brain damage after transient mechanical vascular occlusion but this pathophysiology is not of relevance for naturally occurring stroke. It is, therefore, not unreasonable to predict that the window of therapeutic opportunity defined in the clinically more relevant models of permanent or gradually reversed vascular occlusion can be successfully translated to the clinical situation.
Conclusions
The overall conclusion of this analysis is as follows. The dominant pathophysiology of clinical stroke is primary anoxic-ischemic cell damage in the infarct core, followed by time-limited expansion into the penumbra. Relevant preclinical models are permanent or gradually reversed vascular occlusion, and the therapeutic window is similar to 3 hours, in accordance with the time it takes until the ischemic core expands to maximum volume (Figures 1 and 3).
An entirely different pathophysiology is transient metabolic failure followed by secondary delayed molecular cell death. This pathophysiology depends on the prompt, unobstructed recirculation of the ischemic core, as obtained by the reversal of mechanical vascular occlusion. The therapeutic window of this kind of injury may well extend beyond 3 to 6 hours in accordance with the free interval between primary recovery and secondary damage. However, as this pathophysiology is uncommon in naturally occurring stroke, the longer window is not translatable to the clinical setting.
The mix-up of the two therapeutic windows associated with the two pathophysiologies is one of the reasons for the much too long treatment delays in all completed class III neuroprotection trials and explains their poor outcome (Table 1). Similarly, most thrombolysis studies failed when treatment was delayed beyond the 3-hour limit (Table 2). It is fair to assume that these failures could have been prevented when only clinically relevant stroke models were used to define the therapeutic window of clinical trials. Fortunately, experimental data collected in clinically relevant stroke models are not lost. Careful meta-analysis of these data may, therefore, be of greater benefit than the ongoing accumulation of new data using inappropriate models. Transient mechanical vascular occlusion as the widely used reversible intraluminal filament occlusion is such an inappropriate model that should be eliminated from the repertoire of preclinical stroke research.
The author declares no conflict of interest.
References
- Astrup J, Symon L, Siesjö BK. Thresholds in cerebral ischemia—The ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Bath PMW, Gray LJ, Bath AJG, Buchan A, Miyata T, Green AR. Effects of NXY-059 in experimental stroke: an individual animal meta-analysis. Br J Pharmacol. 2009;157:1157–1171. doi: 10.1111/j.1476-5381.2009.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch E, Krüger K, Allegrini PR, Kerskens CM, Gyngell ML, Hoehn-Berlage M, Hossmann K-A. Reperfusion after thrombolytic therapy of embolic stroke in the rat: magnetic resonance and biochemical imaging. J Cereb Blood Flow Metab. 1998;18:407–418. doi: 10.1097/00004647-199804000-00009. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Macleod MR. Stroke research at a road block: the streets from adversity should be paved with meta-analysis and good laboratory practice. Br J Pharmacol. 2009;157:1154–1156. doi: 10.1111/j.1476-5381.2009.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Hu R, Csernansky CA, Hsu CY, Choi DW. Very delayed infarction after mild focal cerebral ischemia: a role for apoptosis. J Cereb Blood Flow Metab. 1996;16:195–201. doi: 10.1097/00004647-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Endres H, Namura S, Skimizusasamata M, Waeber C, Zhang L, Gomezisla T, Hyman BT, Moskowitz MA. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- Fischer EG, Ames A. Studies on mechanisms of impairment of cerebral circulation following ischemia: effect of hemodilution and perfusion pressure. Stroke. 1972;3:538–542. doi: 10.1161/01.str.3.5.538. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata R, Maeda K, Hermann D, Mies G, Hossmann K-A. Evolution of brain infarction after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2000;20:937–946. doi: 10.1097/00004647-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Sobesky J, Hesselmann V. Identifying thresholds for penumbra and irreversible tissue damage. Stroke. 2004;35:2671–2674. doi: 10.1161/01.STR.0000143329.81997.8a. [DOI] [PubMed] [Google Scholar]
- Hossmann K-A. Cerebral ischemia: models, methods and outcomes. Neuropharmacology. 2008;55:257–270. doi: 10.1016/j.neuropharm.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Hossmann K-A, Kleihues P. Reversibility of ischemic brain damage. Arch Neurol. 1973;29:375–384. doi: 10.1001/archneur.1973.00490300037004. [DOI] [PubMed] [Google Scholar]
- Internet_Stroke_Center 2011. Stroke Trials Registry of the Internet Stroke Center at Washington University School of Medicine http://www.strokecenter.org/trials
- Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- Kilic E, Hermann DM, Hossmann K-A. Recombinant tissue plasminogen activator reduces infarct size after reversible thread occlusion of middle cerebral artery in mice. Neuroreport. 1999;10:107–111. doi: 10.1097/00001756-199901180-00021. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol—Cell Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- Liebeskind DS. Reperfusion for acute ischemic stroke: arterial revascularization and collateral therapeutics. Curr Opin Neurol. 2010;23:36–45. doi: 10.1097/WCO.0b013e328334da32. [DOI] [PubMed] [Google Scholar]
- Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal—How brain cells die after stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- Lust WD, Taylor C, Pundik S, Selman WR, Ratcheson RA. Ischemic cell death: dynamics of delayed secondary energy failure during reperfusion following focal ischemia. Metab Brain Dis. 2002;17:113–121. doi: 10.1023/a:1015420222334. [DOI] [PubMed] [Google Scholar]
- Neumar RW. Molecular mechanisms of ischemic neuronal injury. Ann Emerg Med. 2000;36:483–506. doi: 10.1067/mem.2000.110995. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Einhäupl KM, Dirnagl U, Dreier JP. Ischemia triggered by spreading neuronal activation is induced by endothelin-1 and hemoglobin in the subarachnoid space. Ann Neurol. 2003;54:591–598. doi: 10.1002/ana.10723. [DOI] [PubMed] [Google Scholar]
- Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, Schwamm LH. The ‘Golden Hour' and acute brain ischemia presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41:1431–1439. doi: 10.1161/STROKEAHA.110.583815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener H, Ashwood T, Wasiewski WW, Emeribe U. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Macleod MR, Kollmar R, European Stroke Research Network for Hypothermia Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials. J Cereb Blood Flow Metab. 2010;30:1079–1093. doi: 10.1038/jcbfm.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]