Abstract
Since its introduction 16 years ago, the astrocyte–neuron lactate shuttle (ANLS) model has profoundly modified our understanding of neuroenergetics by bringing a cellular and molecular resolution. Praised or disputed, the concept has never ceased to attract attention, leading to critical advances and unexpected insights. Here, we summarize recent experimental evidence further supporting the main tenets of the model. Thus, evidence for distinct metabolic phenotypes between neurons (mainly oxidative) and astrocytes (mainly glycolytic) have been provided by genomics and classical metabolic approaches. Moreover, it has become clear that astrocytes act as a syncytium to distribute energy substrates such as lactate to active neurones. Glycogen, the main energy reserve located in astrocytes, is used as a lactate source to sustain glutamatergic neurotransmission and synaptic plasticity. Lactate is also emerging as a neuroprotective agent as well as a key signal to regulate blood flow. Characterization of monocarboxylate transporter regulation indicates a possible involvement in synaptic plasticity and memory. Finally, several modeling studies captured the implications of such findings for many brain functions. The ANLS model now represents a useful, experimentally based framework to better understand the coupling between neuronal activity and energetics as it relates to neuronal plasticity, neurodegeneration, and functional brain imaging.
Keywords: astrocytes, brain imaging, energy metabolism, lactate, neurodegeneration, neuronal–glial interaction
Introduction
Over 60 years of clinical and experimental studies of brain energy metabolism in a variety of species including humans have yielded the unquestioned evidence that, under normal physiological conditions in the adult brain, glucose is the almost exclusive energy substrate and that it is fully oxidized, resulting in a respiratory quotient close to 1 (Magistretti, 2008). Some refinements in the overall energy budget of the brain have been provided, showing for example that glucose utilization is in fact higher than that predicted by oxygen consumption in an organ with a respiratory quotient of 1. Based on stoichiometric relationships, to fully oxidize 1 mmol of the 6-carbon glucose molecule, 6 mmol of oxygen would be required. Based on oxygen consumption measurements, glucose utilization by the brain should be around 26.6 mmol/100 g of brain weight/min. Yet, glucose utilization is significantly higher in the order of 31 mmol/100 g of brain weight/min (Kety and Schmidt, 1948). The question then arises of the fate of the additional 4.4 mmol/100 g of brain weight/min. The bioenergetic reply to this question would be glycolysis, namely nonoxidative glucose utilization. If this were the case, a negative arterio-venous (A-V) difference for lactate should be measured, indicating lactate production by the brain. However, a large set of data indicate that such lactate production is marginal (Magistretti, 2008). In addition to glycolysis, and two additional metabolic pathways for glucose processing such as the pentose phosphate pathway and glycogen synthesis, other possible fates for glucose consumed by the brain in excess of oxygen consumption, may be structural, rather than energy-producing ones. Thus, glucose is a substrate for the synthesis of lipids and for amino acids, the building blocks of proteins, as well as for neurotransmitters such as glutamate, GABA (γ-aminobutyric acid), and acetylcholine. A physiological process that entails high structural demands is neuronal plasticity, as expansions of the lipid bilayer of membranes at synaptic sites and new protein synthesis are associated with such processes.
Studies at the organ level do not allow the appreciation of one of the fundamental features of brain energy metabolism, namely the fact that it is tightly coupled to synaptic activity and hence subject to spatially and temporally defined regulatory mechanisms. A major advance in the analysis of local brain glucose metabolism has been provided by Sokoloff (1981) in the 1970s with the development of the 2-deoxyglucose (2-DG) autoradiographic technique for laboratory animals, which was later applied to human with 18F-2-DG positron emission tomography (PET). While of invaluable usefulness for functional brain imaging, the spatial resolution of the 2-DG technique could not afford the identification of the cellular elements into which glucose was being predominantly taken up nor its metabolic fate after phosphorylation to 2-DG-6-phosphate. Indications that the predominant site of 2-DG uptake was in the neuropil, possibly at synaptic or perisynaptic sites, rather than the cell body of neurons, was provided by a series of elegant experiments performed by Sokoloff's laboratory (Kadekaro et al, 1987). In a striking example, providing a spatial dissociation between neuronal cell bodies and the neuropil, it was shown that stimulation of primary somatosensory fibers increased the 2-DG signal in the posterior horn of the relevant spinal cord segment, with minimal uptake into the dorsal root ganglion, where the cell bodies are localized (Kadekaro et al, 1985).
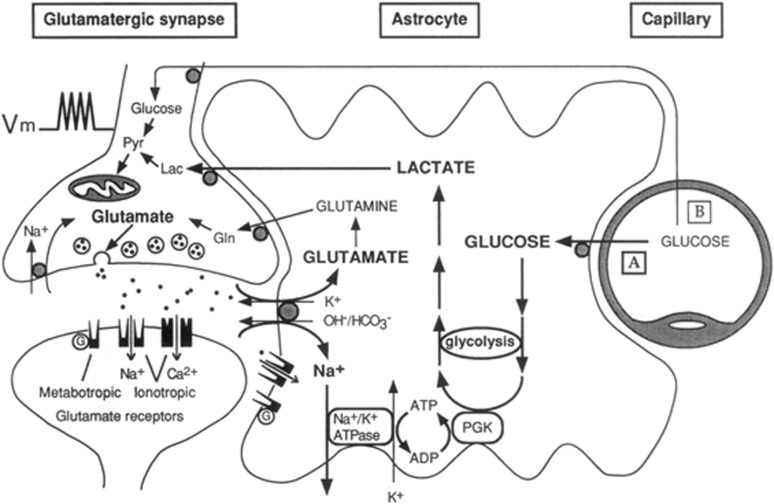
It is against this background that in the early 1990s we started a series of experiments aiming at providing a cellular resolution to the 2-DG technique and at identifying the neuronal signals that could trigger the local increases in glucose utilization associated with synaptic activity. To address these questions, two conditions were initially required: first, to have access to purified preparations of the two main cell types of the brain, namely neurons and astrocytes. Second, to test the effects of neurotransmitters released during synaptic activity on 2-DG uptake in these purified preparations. The results of this study were published in 1994 in a Proceedings of the National Academy of Sciences of the United States of America (PNAS) article entitled: ‘Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization' (Pellerin and Magistretti, 1994). This is the princeps article of the ‘Astrocyte Neuron Lactate Shuttle' (ANLS) model as the experimental data reported in the article essentially documented all the elements of the ANLS (Figure 1). Thus, (1) a neurotransmitter released at over 80% of synapses, namely glutamate, was identified as a neuronal signal able to trigger glucose uptake into astrocytes; (2) the signaling mechanism of this effect of glutamate was identified as resulting from the activation of glutamate transporters and the associated Na+ cotransport that led to the activation of the Na+–K+ ATPase, an energy-consuming process; and (3) the fate of glucose taken up in a glutamate-dependent manner was lactate, indicating activation of aerobic glycolysis. The article also pointed at the potential role of astrocyte-derived lactate as a substrate that could meet the energetic demands of active neurons. The article also provided initial evidence for a likely cellular resolution for the 2-DG technique, of interest for the interpretation of functional brain imaging studies with 18F-2-DG-PET, as more explicitly exposed later (Magistretti and Pellerin, 1996; Figure 2). Recent imaging studies combining multiple modalities such as PET, functional magnetic resonance imaging, and magnetic resonance spectroscopy confirm that the experimental data observed during activation can best be accounted for by the operation of the ANLS (Lin et al, 2010; Figley and Stroman, 2011). The relevance of the ANLS for functional brain imaging has also been the subject of modeling with, however, contrasting views notably from Jolivet et al (2009) and Mangia et al (2009). This point will be further addressed later on.
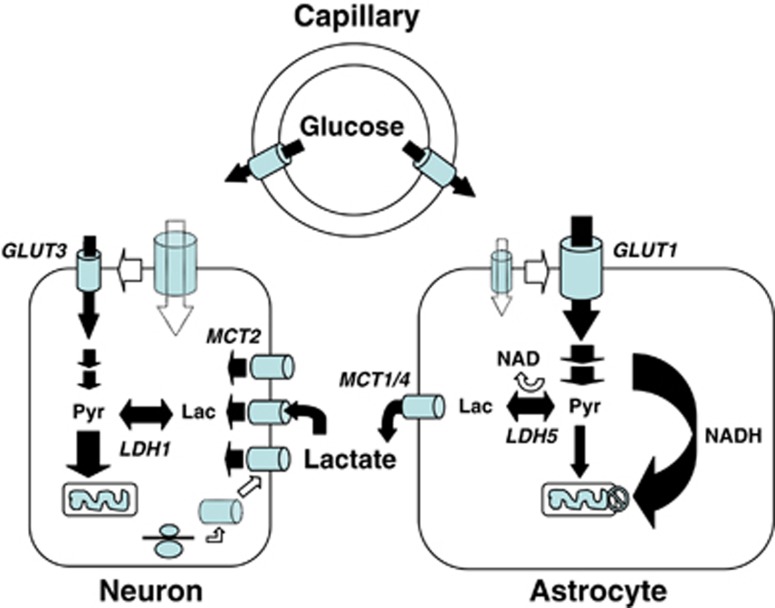
Figure 1.
The first picture of the astrocyte–neuron lactate shuttle (ANLS) model at birth on 25 October 1994. Schematic of the mechanism for glutamate-induced glycolysis in astrocytes during physiological activation. At glutamatergic synapses, glutamate depolarizes neurons by acting at specific receptor subtypes. The action of glutamate is terminated by an efficient glutamate uptake system located primarily in astrocytes. Glutamate is cotransported with Na+, resulting in an increase in [Na+]i, leading to activation of Na+/K+ ATPase. The pump, fueled by ATP provided by membrane-bound glycolytic enzymes (possibly phosphoglycerate kinase (PGK)), activates glycolysis—that is, glucose utilization and lactate production—in astrocytes. Lactate, once released, can be taken up by neurons and serve as an adequate energy substrate. For graphic clarity, only lactate uptake into presynaptic terminals is indicated. However, this process could also occur at the postsynaptic neuron. Based on recent evidence, glutamate receptors are also shown on astrocytes. This model, which summarizes in vitro experimental evidence indicating glutamate-induced glycolysis, is taken to reflect cellular and molecular events occurring during activation of a given cortical area [arrow labeled A (activation)] direct glucose uptake into neurons under basal conditions is also shown [arrow labeled B (basal conditions)]. Pyr, pyruvate; LAC, lactate; GLN, glutamine; G, guanine nucleotide binding protein. Taken from Pellerin and Magistretti (1994).
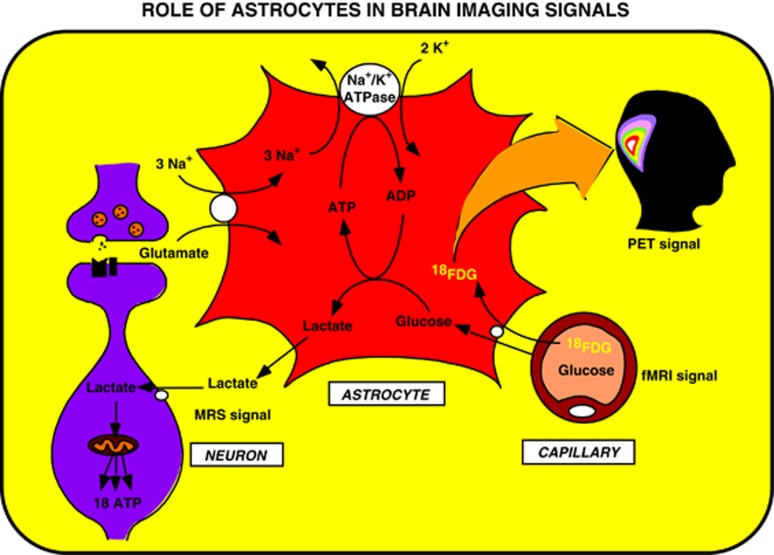
Figure 2.
Proposed concept derived from the astrocyte–neuron lactate shuttle (ANLS) model that brain imaging signals based on glucose uptake primarily reflect astrocyte metabolism. During synaptic activity, glutamate is released into the synaptic cleft. A glutamate transporter system on astrocytes ensures removal of glutamate from the synaptic cleft. The entry of Na+ cotransported with glutamate activates the Na+/K+ ATPase. Activation of the Na+/K+ ATPase is coupled with an increased glycolytic flux, hence resulting in the stimulation of glucose uptake from the capillaries. Lactate, the major end product of glycolysis, is released by astrocytes and taken up by neurons where it can enter the tricarboxylic acid (TCA) cycle and provide 18 ATP per molecule. This model implies that the activity-linked uptake of 18Fluorodeoxyglucose (18FDG) monitored with positron emission tomography (PET) reflects primarily an astrocyte-based signal. However, since neuronally released glutamate triggers the cascade of events that leads to glucose uptake, the 18FDG-PET signal faithfully reflects activation of neuronal circuits. Taken from Magistretti and Pellerin (1996). fMRI, functional magnetic resonance imaging.
The past 16 years have seen an accumulation of experimental demonstrations that imposed the ANLS as a coherent model capable to integrate a vast array of in vitro and in vivo observations related to neuroenergetics. Although it is not the purpose of this article to provide an exhaustive review of all the studies performed, it will be useful to depict the main features that form the cornerstones on which this model relies, and present some of the recent work that further support it.
Astrocytes and Neurons Exhibit Distinct Cytoarchitectural and Metabolic Features Consistent with Astrocyte–Neuron Lactate Shuttle
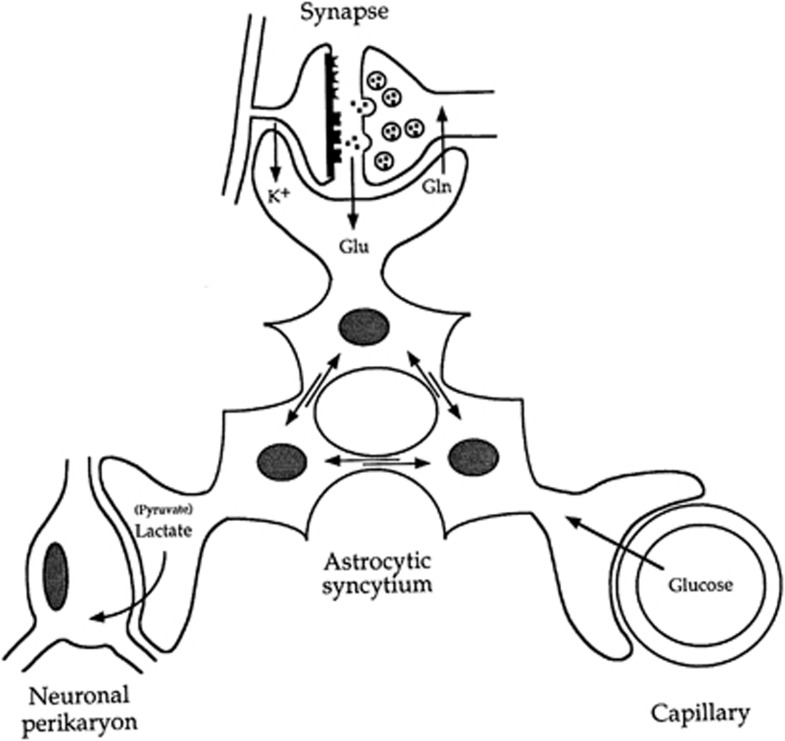
Nineteenth century's anatomists already recognized the particular cytoarchitectural relationships entertained by astrocytes with various parenchymal elements including neurons and blood vessels. Thus, Camillo Golgi and others suggested over a 100 years ago that astrocytes are likely to be involved in the transit of energy substrates from the circulation to supply neurons (Andriezen, 1893). Indeed, astrocytes send processes forming at their extremities specialized structures called end-feet abutting on cerebral blood vessels (Kacem et al, 1998). The presence of specific glucose transporters (GLUT1) at the surface of these structures (Morgello et al, 1995; Yu and Ding, 1998) argues in favor of the idea that it represents a privileged uptake site for glucose, as it leaves the circulation to enter the brain parenchyma. In parallel, other astrocytic processes ensheath synapses, providing an interface of communications between neurons and astrocytes. Such features provide the anatomical basis to implicate astrocytes in a coupling mechanism between synaptic activity and glucose utilization as we suggested in the original description of the model (Pellerin and Magistretti, 1994). A similar parallel was recently made to support the newly described role of astrocytes in coupling synaptic activity with the local regulation of blood flow (Gordon et al, 2008), although the mechanisms for neurovascular coupling appear to be quite complex and redundant, involving also, for example, the activity of GABAergic interneurons (Kocharyan et al, 2008). Quite early on, it became clear that a single astrocyte might not be the most adequate entity to host the entire metabolic coupling mechanism. Since astrocytes are not isolated but form a syncytium through their connections via gap junctions (Giaume et al, 2010), it was proposed that energy substrates produced in response to synaptic activity would transit through several astrocytes before being delivered to stimulated neurons (Magistretti et al, 1995) (Figure 3). As predicted, evidence was recently provided demonstrating that astrocytic coupling via gap junctions contributes to the energetic support of active neurons through supply of glucose-derived lactate (Rouach et al, 2008).
Figure 3.
Introduction of the concept of the importance of the astrocytic syncytium for intracellular metabolite trafficking, also known as metabolic polarity of the astrocytic syncytium. Astrocytes have various processes that make contact either with capillaries, neuronal perikarya, or synapses. In addition, they are connected with each other at gap junctions; through these junctions small molecules (MW<1,000) can be exchanged. These properties endow the astrocytic syncytium with the capacity to ensure the transfer of metabolic intermediates from areas of production to areas of demand. Gln, glutamine; Glu, glutamate. Taken from Magistretti et al (1995).
Several studies have documented clear metabolic differences between astrocytes and neurons in vitro but also in vivo. For example, astrocytes selectively express high-affinity, sodium-dependent glutamate transporters as well as the enzyme glutamine synthetase that allow them to have a critical role in glutamate recycling and thus in the maintenance of synaptic transmission (Benarroch, 2005). This makes them uniquely prone to sense and react to synaptic activity (Bergles and Jahr, 1997). Moreover, astrocytes and neurons exhibit some distinct features in relation with energy metabolism. It has been suggested for a long time that astrocytes and neurons might exhibit a different metabolic profile. From initial studies on enzymatic analyses of individually isolated cells (Hydén and Lange, 1962; Hamberger and Hydén, 1963) up to most recent transcriptomic studies (Rossner et al, 2006; Lovatt et al, 2007; Cahoy et al, 2008), all argue for a predominance of glycolytic and glycogen pathways in astrocytes and of lactate utilization in neurons. In particular, expression of key enzyme and transporter isoforms (e.g., lactate dehydrogenase (LDH) and monocarboxylate transporters) was found to be consistent with this view (Bittar et al, 1996; Pellerin et al, 2005; Laughton et al, 2007; O'Brien et al, 2007). Investigation of metabolic activity in each cell type consistently demonstrated the same phenotypic difference, despite the fact that astrocytes exhibit an oxidative and anaplerotic potential at least comparable to neurons. Extreme cases of metabolic compartmentation have been described in the retina (in the honeybee with a comparable situation in the mammalian retina) where glial cells display a prominent glycolytic activity while photoreceptors rely entirely on oxidative metabolism (Tsacopoulos et al, 1994, 1998). Although such an absolute situation is not encountered in the mammalian brain, many investigations have come to the conclusion that glycolytic activity is more important in astrocytes than in neurons (Itoh et al, 2003; Bouzier-Sore et al, 2006) even if neurons do exhibit glycolytic activity (Bittner et al, 2010). In parallel, it was recently shown that glycolytic activity is not only actively limited in neurons but its enhancement could be deleterious for neuronal survival (Herrero-Mendez et al, 2009). In contrast, enhancement of glycolytic activity (at the expense of oxidative metabolism) is favored by the low expression of an essential component of the malate–aspartate shuttle in astroglial mitochondria (Ramos et al, 2003; Xu et al, 2007). Such an important difference in glycolytic activity is likely to have a major impact on glucose utilization by each cell type. Indeed, it was repeatedly reported that glucose utilization is more important in astrocytes than in neurons (Waagepetersen et al, 1998; Bouzier-Sore et al, 2006). Consistent with this view, visualization of glucose utilization using a fluorescent deoxyglucose analog in cerebellar slices showed a strikingly more important fluorescent signal arising from Bergmann glial cells than from neighboring neurons (Barros et al, 2009). As neuronal energy demands are considerably superior to those of astroglia (Attwell and Laughlin, 2001), even if there is some disagreement on the exact proportions (Nehlig and Coles, 2007), such a finding cannot be explained if each cell type was consuming (and oxidizing) glucose in proportion with its needs. In contrast, a model in which a predominant glycolytic activity resides in astrocytes while neurons rely more heavily on oxidation, including oxidation of substrates other than glucose provided by astrocytes, can perfectly account for this observation as proposed in ANLS (Pellerin and Magistretti, 2005) (Figure 4). This review of experimental evidence is not meant to imply that astrocytes in the brain parenchyma do not display the capacity for oxidation. Indeed, as pointed out by Lovatt et al (2007), astrocytes appear to express high levels of mRNA encoding enzymes of the TCA (tricarboxylic acid) cycle. Nevertheless, functional studies presented in the same article indicate the production of lactate from labeled glucose, consistent, as noted by the authors themselves, with the ANLS. In our own studies in cultures (unpublished) and as demonstrated in vivo using 13C MR (magnetic resonance) spectroscopy (Hyder et al, 2006), a ratio of ∼4:1 for glucose-derived pyruvate oxidation between neurons and astrocytes seems likely. In such a case, if glucose consumption between neurons and astrocytes is essentially equivalent under basal, unstimulated conditions as proposed by Nehlig and Coles (2007), there is still a significant discrepancy consistent with the ANLS. This discrepancy implies a net transfer of glucose-derived carbons (e.g., as lactate) from astrocytes to neurons (Pellerin and Magistretti, 2003).
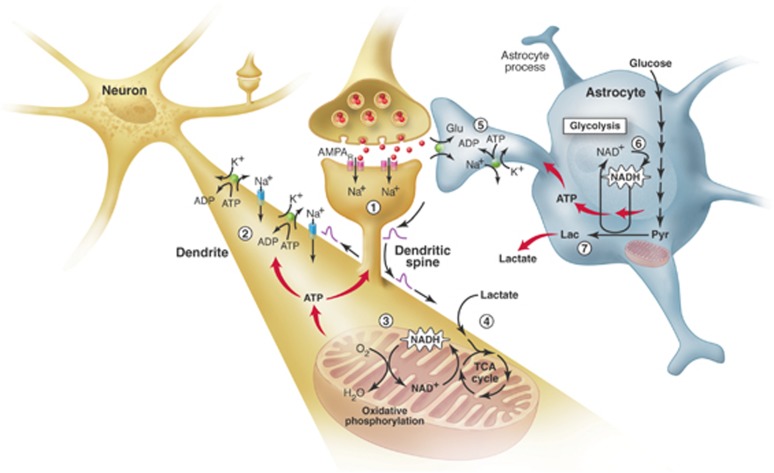
Figure 4.
The brain energetics in the limelight. Separate activation of oxidative phosphorylation (respiration) in neurons (brown) and glycolysis in astrocytes (gray), as revealed by two-photon fluorescence imaging of nicotinamide adenine dinucleotide (NADH). (1) Stimulation of excitatory (glutamatergic) neurons activates postsynaptic AMPA receptors and induces an excitatory postsynaptic potential (EPSP) in the dendritic spine of the neuron. (2) The depolarization propagates from the dendritic spine to the dendrite, where it may cause further opening of voltage-gated sodium channels and activation of the Na+/K+ ATPase, leading to an increased demand for energy (ATP). (3) In response, oxidative phosphorylation is rapidly activated, causing a decrease in mitochondrial NADH content (the so-called ‘dip' in the fluorescent signal). (4) Recovery of mitochondrial NADH in dendrites is accomplished by stimulation of the tricarboxylic acid (TCA) cycle, fueled largely by lactate from the extracellular pool. (5) In parallel, but delayed in time, glutamate reuptake in astrocytes (gray) activates the glial Na+/K+ ATPase. (6) The increased energy demand leads to a strong enhancement of glycolysis in the cytoplasm of astrocytes, as indicated by the large increase in cytosolic NADH fluorescence (the so-called ‘overshoot'). (7) To maintain the high glycolytic flux, NAD+ must be regenerated via the conversion of pyruvate to lactate through the activity of the enzyme lactate dehydrogenase. Release of lactate into the extracellular space not only replenishes the extracellular pool, but also may sustain the late phase of neuronal activation. In vivo, glucose is delivered from the blood to both the extracellular space and astrocytes (via astrocytic protrusions called end-feet that are in close contact with the blood vessel wall). AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors; Glu, glutamate; Lac, lactate; Pyr, pyruvate. Taken from Pellerin and Magistretti (2005).
In addition to constitutive differences in the metabolic phenotype of astrocytes and neurons, metabolic responses of each cell type to external stimuli are also different. While it was never reported that neurons acutely enhanced their glucose utilization when stimulated despite several attempts to reveal it, an enhancement of glucose uptake in astrocytes mainly induced by glutamate is a widely confirmed finding now (Pellerin and Magistretti, 1994; Takahashi et al, 1995; Keller et al, 1996; Bittner et al, 2010). Concomitant to glucose utilization, modifications of glucose transport capacity induced by glutamate were observed in both astrocytes and neurons. Thus, while glucose transport was even reduced in neurons on glutamate exposure, glucose transport was conversely enhanced in astrocytes (Loaiza et al, 2003; Porras et al, 2004) (Figure 5). A critical demonstration of the preferential glucose utilization by astrocytes following synaptic activation was recently provided in vivo (Chuquet et al, 2010). It was shown that whisker stimulation leads to enhanced glucose utilization in astrocytes of the somatosensory cortex as revealed by accumulation of the fluorescent deoxyglucose analog 6-NBDG. In contrast, very little increase if any was observed in parallel in neighboring neurons. As further elaborated at a later point in this article, such observations, which are consistent with the ANLS model, do not seem compatible with alternative interpretations (Chih et al, 2001b; Gandhi et al, 2009; Mangia et al, 2009).
Figure 5.
Importance of monocarboxylate transporters and the regulation of their expression/localization in lactate shuttling between astrocytes and neurons. Several unique features of astrocytes and neurons have been uncovered that suggest a partial metabolic compartmentalization and the existence of a preferential lactate transfer between the two cell types. Thus, exposure of astrocytes to glutamate was shown to directly enhance glucose transport in parallel with increased glucose utilization. Conversion of pyruvate into lactate in astrocytes is facilitated by key characteristics. Astrocytes lack a mitochondrial aspartate/glutamate carrier that reduces their capacity to transfer reducing equivalent as nicotinamide adenine dinucleotide (NADH) by the malate/aspartate shuttle in the mitochondria and regenerate NAD. To maintain the glycolytic flux, cytosolic NADH is rather converted to NAD through the reaction catalyzed by the lactate dehydrogenase isoform LDH5, preferentially expressed in astrocytes. Lactate formed is then released in the extracellular space via the high-capacity monocarboxylate transporters, MCT1 and MCT4. In contrast to astrocytes, glucose uptake in neurons is reduced by glutamate. Moreover, ascorbic acid that is shuttled from astrocytes to neurons also contributes to reduce glucose uptake in neurons. In parallel, ascorbic acid enhances lactate uptake by neurons, and lactate conversion to pyruvate is facilitated by the preferential expression of the lactate dehydrogenase isoform, LDH1. Expression of the neuronal monocarboxylate transporter, MCT2, can be enhanced through an increase in protein synthesis by various stimuli, which might contribute to long-term adaptation of energy supply to demand. GLUT, glucose transporter; Lac, lactate; LDH, lactate dehydrogenase; MCT, monocarboxylate transporter; Pyr, pyruvate. Taken from Pellerin (2008).
A Precise Molecular Description of the Astroglial-Based Coupling Mechanism Relies on Extensive In Vitro, Ex Vivo, and In Vivo Investigations
The precise mechanisms proposed to be part of the ANLS model have been the subject of several experimental investigations. First of all, the implication of glutamate transporters (and not receptors) in the glycolytic response of astrocytes has been demonstrated both in vitro and in vivo. The use of knockout mice for the glial glutamate transporters GLAST and GLT1 as well as knockdown experiments in adult animals provided a robust confirmation that activation of these transporters are a key step in the glycolytic response observed (Cholet et al, 2001; Voutsinos-Porche et al, 2003). Moreover, it was shown that only activation of glutamate transporters (and not glutamate receptors nor GABA transporters) are capable of raising intracellular sodium concentrations to such an extent that the Na+/K+ ATPase is substantially activated (Pellerin and Magistretti, 1997; Chatton et al, 2000). It was also shown that glutamate transporter activation mobilizes a specific subunit of the Na+/K+ ATPase known as the α2 subunit. Interestingly, in accordance with the prediction of the ANLS model, it was later found that the Na+/K+ ATPase α2 subunit colocalizes and specifically interacts with glial glutamate transporters (Cholet et al, 2002; Gegelashvili et al, 2007; Rose et al, 2009). Since these two proteins are located at the surface of astrocytic processes that ensheath glutamatergic (and not GABAergic) synapses, it was proposed that such a close interaction is necessary to ensure an efficient glutamate reuptake and recycling. A direct consequence of glutamate transport-mediated activation of the Na+/K+ ATPase α2 subunit should be an enhancement in ATP consumption. Indeed, it was shown that glutamate uptake in astrocytes led to a decrease in intracellular ATP concentration, which closely parallels the activity of the pump (Magistretti and Chatton, 2005). As mentioned above, it is unlikely that astrocytes act in isolation but rather respond metabolically in a coordinated manner throughout the syncytium that they establish. Indeed, it was demonstrated that astrocytes undergo waves of both intracellular Na+ rises and glucose uptake following restricted glutamate exposure (Bernardinelli et al, 2004; Charles, 2005). Such an observation concurs with the idea that metabolic neuronal supply requires a coordinated response from the astrocytic syncytium (Giaume et al, 2010).
Lactate Is a Preferential Oxidative Substrate for Neurons
The consequence of glutamate-induced glucose utilization by astrocytes is the production and release of lactate in the extracellular medium immediately surrounding neurons. Curiously, a long-held view considered lactate (and more specifically lactic acid) as a toxic waste for brain cells that must be evacuated by all means from the brain parenchyma despite the fact that it is an important energy source (Dienel and Hertz, 2001). However, evidences accumulated over >50 years have clearly established that lactate represents one of the rare alternative oxidative substrate (together with ketone bodies) for neurons (McIlwain, 1953; Pellerin, 2003). Moreover, in the presence of both substrates as it is the case physiologically, it was shown that lactate is largely preferred as an oxidative energy substrate over glucose by neurons (Itoh et al, 2003; Bouzier-Sore et al, 2003, 2006; Ivanov et al, 2011), although glucose utilization also occurs in neurons (reviewed in Mangia et al, 2009). Prominent lactate utilization by the brain and more specifically by neurons has also been confirmed in vivo (Smith et al, 2003; Serres et al, 2004; Boumezbeur et al, 2010). Lactate utilization has been shown to be essential in several neurophysiological mechanisms including control of respiration (Erlichman et al, 2008), fuel sensing for energy homeostasis (Ainscow et al, 2002; Lam et al, 2005, 2007), as well as arousal and food intake regulation (Parsons and Hirasawa, 2010). If we add to this, the recent demonstrations that lactate metabolism has a critical role in neurovascular coupling (Gordon et al, 2008), as well as in recovery from hypoxia (Schurr et al, 1997) and in neuroprotection (Bliss et al, 2004; Berthet et al, 2009), it is clear that lactate utilization is essential for the support of neuronal activity and cerebral functions.
Opening the Shuttle's Doors: Key Role of Monocarboxylate Transporters
The possibility of shuttling lactate between brain cell types is determined by the expression of specific transporters exhibiting different kinetics. Indeed, modeling studies have demonstrated the key role that monocarboxylate transporters play in regulating lactate influx and efflux (Aubert et al, 2005). In recent years, a precise description of the expression and distribution of monocarboxylate transporters in the central nervous system has been provided by immunohistochemical studies (reviewed in Pierre and Pellerin, 2005). Thus, the three main proton-dependent lactate carriers known as MCT1, MCT2, and MCT4, have been identified and extensively studied both in vivo and in vitro. In addition, a sodium-dependent monocarboxylate transporter, named sMCT1, has been detected in neurons but much less is known for the moment about its regulation and exact contribution to the overall lactate transport capacity (Martin et al, 2006).
The distribution of monocarboxylate transporters is cell specific. MCT1 is expressed on endothelial cells forming blood vessels as well as on astrocytes and oligodendrocytes (Gerhart et al, 1997; Rinholm et al, 2011). MCT2 appears predominantly present in neurons, where it is found on axons and dendrites, with a particular enrichment in dendritic spines (Bergersen et al, 2001; Pierre et al, 2002). In contrast, MCT4 is exclusively expressed by astrocytes with a polarized distribution showing a more important concentration on processes in contact with synapses (Rafiki et al, 2003; Pellerin et al, 2005). It is interesting to consider the kinetic properties of these transporters. MCT2 is a high-affinity transporter with a Km for lactate of 0.7 mmol/L. MCT1 and MCT4 have a much lower affinity for lactate (Km for lactate of 3.5 and 34 mmol/L, respectively). Their cell-specific distribution is paralleled by an enriched expression of particular LDH isoforms displaying different kinetic characteristics. Isoforms containing the LDHB subunits are more abundant in neurons while isoforms enriched in LDHA subunits are enriched in astrocytes (Bittar et al, 1996; O'Brien et al, 2007). While the main determinant of the lactate:pyruvate equilibrium is the redox state and the suggestion has been made that ‘despite changed kinetic constants, the equilibrium constant, Keq, is the same for all isoenzymes, because it is the same chemical reaction being catalyzed' (quoted from: Quistorff and Grunnet, 2011), the association of the high-affinity transporter MCT2 with LDH isoforms enriched in LDHB subunits creates kinetic conditions highly favorable for lactate uptake and utilization by neurons. The presence of LDH isoforms predominantly exhibiting LDHA subunits together with the high-capacity monocarboxylate transporters MCT1 and MCT4 rather provide optimal settings to favor lactate production and release from astrocytes. Considering the preferential metabolic profile exhibited by each cell type as described above, these characteristics reinforce the concept that lactate is shuttled preferentially from astrocytes to neurons, with varying degree depending on the activation state (Figures 6A and 6B).
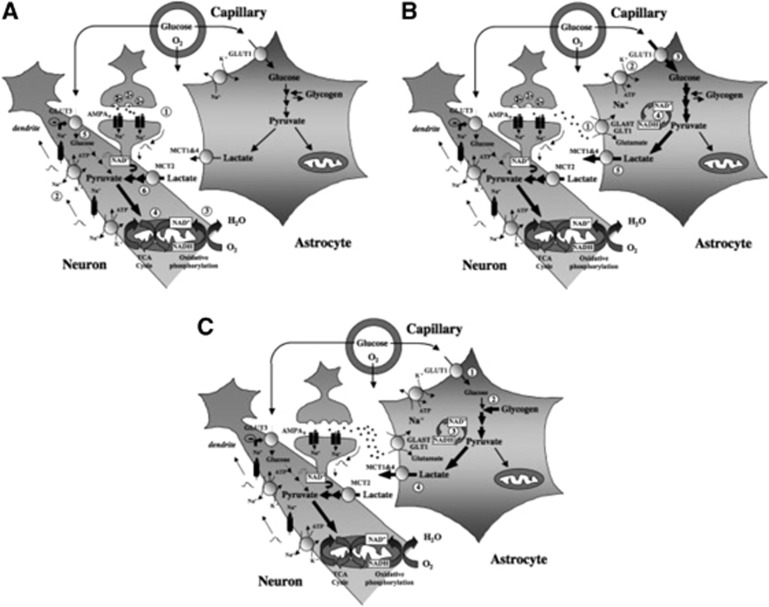
Figure 6.
Contribution of glucose- and glycogen-derived lactate to support different phases of neuronal activation. (A) Early phase. Activation of glutamatergic afferents leads to synaptic release of glutamate, AMPA receptor (AMPAR) activation, and generation of an excitatory postsynaptic potential (EPSP) caused by Na+ entry within the postsynaptic spine (1). Depolarization propagates to the dendrite and causes opening of voltage-sensitive Na+ channels, leading to further Na+ entry. Reestablishment of ion gradients is accomplished by the Na+/K+ ATPase (2), which creates considerable energy expenditure. As a consequence, oxidative phosphorylation is activated (3) and mitochondrial nicotinamide adenine dinucleotide (NADH) levels first decrease (Kasischke et al, 2004). Then, enhanced tricarboxylic acid (TCA) cycle activity will ensue (4) to supply NADH for oxidative phosphorylation and support ATP production. As pyruvate utilization in the TCA cycle increases and its cytoplasmic levels decrease, the conditions become favorable for both enhanced glucose and lactate use. Surprisingly, activation of AMPA receptors and coupled Na+ entry lead to a reduction in glucose uptake and utilization in neurons (5), thus further favoring lactate utilization as preferential oxidative substrate (6). This would cause a transient drop in extracellular lactate levels as measured in vivo. (B) Late phase. Glutamate released in the synaptic cleft is taken up by astrocytes to be recycled via the specific glutamate transporters GLAST and GLT1 (1). A large Na+ influx caused by glutamate uptake takes place and activates the Na+/K+ ATPase (2), glucose transport (3) and (4) glucose utilization in astrocytes. The enhancement of aerobic glycolysis in astrocytes first causes a large increase in cytosolic NADH that normalizes with the conversion of pyruvate into lactate and its release via monocarboxylate transporters expressed on astrocytes (mainly MCT1 and 4) (5). Such a lactate release following glutamatergic activation corresponds to the increase in extracellular lactate levels measured in vivo. Lactate produced by astrocytes during this later phase of activation not only replenishes the extracellular pool but also could help sustain neuronal energy needs as activation persists. Metabolic events occurring in the early and late phases described above constitute the so-called astrocyte–neuron lactate shuttle and its importance grows with the degree of glutamatergic activation. Such a view is supported by a series of experiments conducted in vivo. (C) Intense and prolonged stimulation. On strong and long-lasting stimulation that occurs in certain conditions, glucose utilization becomes very important, in part due to intense glutamate reuptake in astrocytes, that extracellular glucose levels are insufficient to sustain such uptake (1). In such a situation, glycogen present in the astrocyte is mobilized to provide the necessary glycosyl units (2) as previously demonstrated in vivo. Glycolysis is the predominant pathway (3) and lactate is produced (4) to maintain the high glycolytic rate. Resynthesis of glycogen will cause additional glucose uptake that might contribute to create a mismatch between glucose utilization and oxygen consumption, a phenomenon known as ‘uncoupling.' Taken from Pellerin et al (2007).
Energy substrate utilization can be limited by the transport capacity. It was previously shown that this could be the case for neuronal lactate utilization since MCT2 overexpression in cultured neurons using viral vectors promoted lactate utilization in these cells when they were stimulated with kainate (Bliss et al, 2004). It became of interest to determine whether the expression of monocarboxylate transporters could be regulated in the brain cells. Indeed, it was found that MCT2 expression in neurons was under regulation. Noradrenaline (NA), insulin, insulin-like growth factor-1 (IGF-1), and brain-derived neurotrophic factor (BDNF) were all shown to enhance MCT2 expression in cultured neurons (Chenal and Pellerin, 2007; Chenal et al, 2008; Robinet and Pellerin, 2010). The mechanism involves a stimulation of translation via the phosphatidylinositol 3-kinase/serine-threonine protein kinase from Akt virus/mammalian target of rapamycin (PI3K/Akt/mTOR/S6) pathway. Moreover, it was shown that such an activation occurs at the synaptic level (Robinet and Pellerin, 2010). In addition to changes in the overall expression levels, evidence was provided that the amount of MCT2 proteins present at the cell surface can be modified (Figure 5). It was shown that translocation of MCT2 from an endogenous pool to the cell surface can be induced in cultured cortical neurons by exposing them to a combination of glutamate and glycine (Pierre et al, 2009). Quite importantly, it was shown that a doubling of MCT2 expression at the cell surface leads to an increase of ∼80% in lactate transport (Pierre et al, 2009). Thus, changes in expression and localization of MCT2 induced by neuroactive signals cause significant modifications of neuronal energetics. Since such adaptations appear to occur quite rapidly (<1 minute measured with static approaches), it is likely that they represent the most appropriate process by which neurons could adapt their substrate supply and concomitant energy production to face changing needs coming with fluctuating activity. It is expected that in the case of synaptic plasticity, changes in synaptic efficacy will be accompanied by adaptations in energy supply. The AMPA type glutamate receptor subunit GluR2 is known to participate to the mechanism of synaptic plasticity at glutamatergic synapses (Isaac et al, 2007). Observations that MCT2 not only interacts with GluR2 but modifies its subcellular distribution and expression levels support the idea that energy supply might be tightly regulated as part of the mechanism of synaptic plasticity (Maekawa et al, 2009; Pierre et al, 2009).
Glycogen: the Astrocytic Energy Reserve Essential for Neurons
One of the defining features of the ANLS is the postulated central role of lactate transfer from astrocytes to neurons. In addition to glutamate-stimulated aerobic glycolysis, another astrocyte-based metabolic pathway can produce lactate, namely glycogenolysis. Glycogen is almost exclusively localized in astrocytes (Magistretti, 2008). Glycogen is not found in adult neurons, except occasionally in large neurons in the brainstem or in the peripheral nervous system (Sotelo and Palay, 1968), despite the fact that quite surprisingly, glycogen synthase—the enzyme responsible for glycogen synthesis—is present in many neurons. However, two active mechanisms operate to inhibit glycogen storage in neurons. First, a proteasome-dependent mechanism, involving the malin–laforin complex, is active in neurons to permanently degrade glycogen synthase (Vilchez et al, 2007). Second, activity of the residual glycogen synthase is inhibited by phosphorylation. The reasons for such a complex regulation are unknown, but certainly vital for neurons: indeed, manipulations aimed at allowing glycogen synthase activity and hence glycogen synthesis in neurons, lead to neuronal glycogen accumulation and apoptosis. For example, a loss-of-function mutation in the malin–laforin complex, characterized by accumulation of glycogen-containing deposits in neurons, results in progressive myoclonus epilepsy, also known as Lafora disease.
As mentioned earlier, in neurons, glycolysis is reduced to a minimum, due to the constitutive proteasome-dependent degradation of the glycolysis-promoting enzyme 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase, isoform 3, PFKFB3 (Herrero-Mendez et al, 2009). In these cells, glucose metabolism is predominantly directed to the oxidative branch of the pentose phosphate pathway, resulting in production of reducing equivalents necessary to maintain the protective antioxidant status of neurons. Removal of PFKFB3 inhibition, resulting in increased glycolysis leads to oxidative stress and apoptotic death of neurons. Taken together, these data indicate that neurons can simply not afford to process glucose through the glycogen cycle (Magistretti and Allaman, 2007); furthermore, upregulation of glucose processing through glycolysis in neurons can lead to death, thus providing a metabolic contraindication for these cells to produce lactate. As reviewed earlier, this is in stark contrast to the metabolic phenotype of astrocytes in which glycogenolysis and glycolysis, both leading to lactate production (Dringen, 2000; Pellerin and Magistretti, 1994), are the preferred metabolic pathways for glucose. These considerations imply that the ANLS can also be operated by promoting glycogenolysis in astrocytes in response to signals released by active neurons (Figure 6C).
A decade before the glutamate-stimulated aerobic glycolysis was described, it had been shown that a restricted number of neurotransmitters/modulators could promote glycogenolysis (Magistretti et al, 1981; Magistretti and Morrison, 1988). The glycogenolytic agents included NA, vasoactive intestinal peptide (VIP), and adenosine. This metabolic action of VIP, which in the neocortex is contained in locally acting bipolar neurons and hence restricted to cortical columnar modules, was contrasted to that of NA, which given the horizontal orientation of noradrenergic intracortical fibers that span horizontally across functionally distinct cortical areas (Magistretti and Morrison, 1988), would be in a position to prime metabolically the neocortex globally (Magistretti and Morrison, 1988) (Figure 7). Later on, the existence of ‘metabolic hotspots' was described in the neocortex as a result of the synergistic interaction between VIP and NA at the levels of cortical columns (Magistretti and Schorderet, 1984).
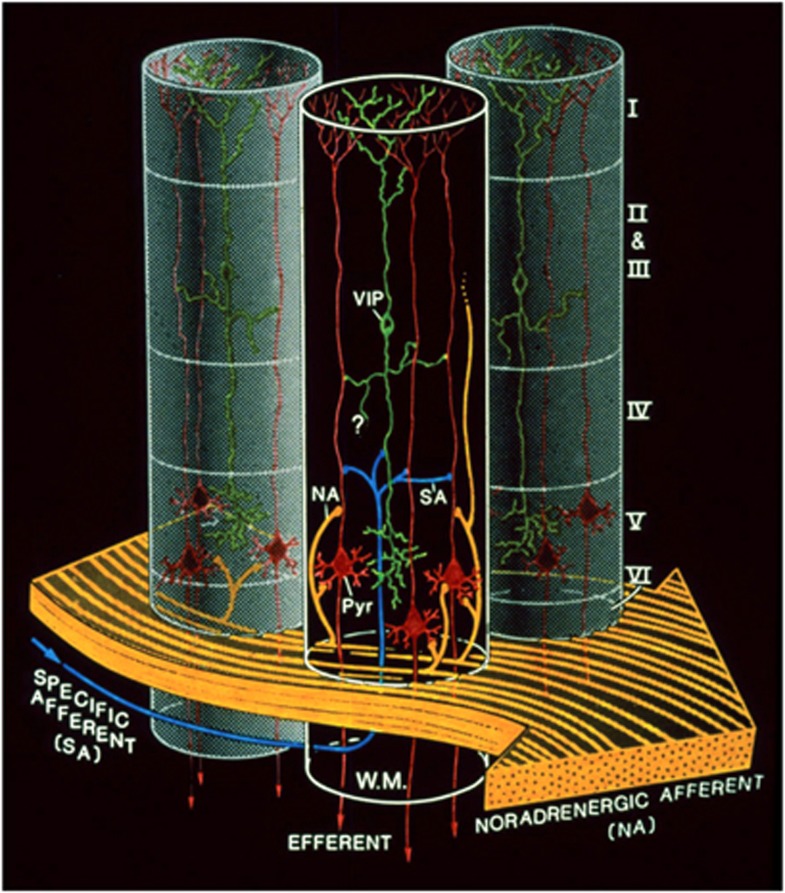
Figure 7.
Complementary spatial domains in which vasoactive intestinal peptide (VIP)-containing bipolar neurons and noradrenergic fibers exert their glycolytic effects. Such an arrangement would allow for the establishment of temporary columnar ‘hotspots.' VIP, VIP-containing bipolar cell; NA, noradrenergic afferents; Pyr, pyramidal cell furnishing major efferent projections; SA, specific afferent (i.e., from thalamus or from other cortical areas); WM, subcortical white matter. Cortical layers denoted by roman numerals. Note tangential orientation of NA fibers, and radially restricted domain of VIP-containing neuron. The concomitant activation of noradrenergic fibers (large, yellow arrow, bottom) by unexpected sensory stimuli and of a group of VIP-containing intracortical neurons by specific thalamic inputs (small, blue arrow, bottom left) would lead to a drastic increase in cAMP levels within a discrete volume of somatosensory cortex, delineated here by the cylinder with dark background. Adjoining gray-background cylinders represent nonactivated cortical volumes. For graphic clarity, only the VIP-containing (green) and the pyramidal cells (red) have been represented here. In particular, the principal target cells of the thalamocortical afferents, that is, the small stellate cells in layer IV, have been omitted. However, any cell with the capacity for dendritic reception in layer IV may receive thalamic inputs. Furthermore, also for graphic clarity, only one VIP neuron per cylinder has been drawn, in representation of a group of VIP neurons. This is a heuristic model, and as such, certain details of the synaptic circuitry depicted in the diagram (e.g., direct thalamocortical input to VIP-containing cells) are suggested by presently available data, but have not been demonstrated definitively. However, all aspects of the model are potentially testable. Taken from Magistretti and Morrison (1988).
In summary, lactate can be produced by astrocytes following two kinds of neuronal signals: glutamate-stimulated aerobic glycolysis and glycogenolysis triggered by VIP, NA, or adenosine (Magistretti and Morrison, 1988; Magistretti et al, 1986). As far as glycogenolysis is concerned, its function in neurometabolic coupling has been considered to be a sort of general mechanism that would provide additional energy substrates to match increases in neuronal activity (Magistretti, 2008). Thus, stimulation of the whisker-to-barrel pathway was shown to result in glycogenolysis in the barrel field of layer IV somatosensory cortex (Swanson et al, 1992). Maintenance of sustained axonal spiking in mouse optic nerve was shown to depend on adequate glycogen content and lactate shuttling between astrocytes and the axonal fibers (Tekkök et al, 2005). Very recently, a specific role of astrocytic glycogenolysis and the ensuing astrocyte–neuron lactate transfer has been demonstrated for synaptic plasticity in a learning paradigm (Suzuki et al, 2011).
Thus, pharmacological inhibition of glycogenolysis in the hippocampus with the glycogen phosphorylase inhibitor DAB (Walls et al, 2008), results in loss of long-term memory in an inhibitory avoidance learning paradigm. A similar result is obtained by disrupting the expression of the astrocyte-specific lactate transporter MCT4. These memory-inhibiting effects are fully rescued by local administration of lactate but not by equicaloric glucose. Disruption of the expression of the neuron-specific lactate transporter MCT2 also leads to amnesia, which however is not rescued by exogenous lactate, indicating that lactate import into neurons is necessary for long-term memory formation. In keeping with the behavioral effects observed, the in vivo induction of long-term potentiation and of genes know to be required for memory formation such as phospho-CREB, Arc and phospho-cofilin is prevented by inhibition of glycogenolysis and MCT4 expression in a lactate-reversible manner (Suzuki et al, 2011). These data provide the first in vivo evidence for the existence of the ANLS, as they show that astrocyte–neuron lactate transfer is required for long-term memory formation (Suzuki et al, 2011). Similar results have been obtained in a different (working memory) learning paradigm (Newman and Gold, 2010).
Some Controversies
While there is a considerable support, both theoretical and experimental, for the existence of an activity-dependent transfer of lactate between astrocytes and neurons (see preceding paragraphs), there are still a few diverging views that dispute some aspects of the ANLS. Thus, one argument derived from modeling studies suggests that the glucose transport capacity is larger in neurons than in astrocytes (DiNuzzo et al, 2010a; Mangia et al, 2009; Simpson et al, 2007), a prediction suggested to be inconsistent with ANLS. This theoretical conclusion is at odds with the observation that haploinsufficient mice for the neuron-specific glucose transporter GLUT3 do not present any neurologic or brain energy metabolism phenotype, and in particular do not display any decrease in brain glucose utilization as determined by fluorodeoxyglucose micro-PET (Schmidt et al, 2008; Stuart et al, 2011). In contrast, haploinsufficient mice for the glucose transporter GLUT1, which is absent in neurons, present a severe neurologic phenotype of impaired motor activity and coordination, microencephaly and frequent seizures (Wang et al, 2006).
Furthermore, in a recent article (Jolivet et al, 2010), we have pinpointed some major flaws in the modeling work proposed by DiNuzzo et al (2010a) and Simpson et al (2007). Thus for example, the biological data used for the modeling concerning the density of glucose transporters in astrocytes and neurons were obtained by measuring cytochalasin B binding in cultures. Using such data obtained from semiquantitative analysis of transporter density in cultures to model quantitative in vivo properties of transport, appears to be inappropriate. In addition, the proposed modeling used to contradict the ANLS, addresses only the overall glucose and lactate transport capacities, and not, the physiologically relevant issue, of metabolic fluxes. Indeed, transport capacity per se is not equivalent to metabolic flux and does not allow to predict effective glucose and lactate utilization.
Using an elegant cell-specific neurochemical technique, Gandhi et al (2009) have shown that when raising artificially extracellular lactate concentrations up to 40 mmol/L in brain slices, astrocytes display a higher transport and distribution capacity for lactate compared with neurons. From this observation, they conclude that a neuron-to-astrocyte shuttling is more likely than the opposite. The results of these experiments are not surprising: indeed astrocytes, as shown by the authors and many other investigators, are tightly coupled through gap junctions, a cytological organization that produces a large ‘sink' for lactate disposal when compared with single, isolated neurons. Thus, increasing artificially extracellular lactate, quite expectedly will result in its uptake into both cell types, but with a significant higher capacity in astrocytes because of the considerable greater size of the astrocytic network compared with isolated neurons. Furthermore, and possibly more importantly, experiments were carried out in the absence of stimulated neuronal activity, thus resulting in conditions in which the energetic needs of neurons are not engaged. Under conditions in which the ANLS would be operational, the source of extracellular lactate is the astrocytes, a fact that would determine the directionality of the lactate flux, as shown for example by the study of Rouach et al (2008).
The fact that lactate could be a valuable energy substrate capable to sustain neuronal activity in brain slices was recognized already 60 years ago by McIlwain (1953), and later confirmed by various authors including in particular Schurr et al (1988) and Izumi et al (1997). This role of lactate as a substrate in slices maintained ex vivo has been challenged by some authors (Wada et al, 1998; Chih et al, 2001a; Allen et al, 2005). A recent study by Ivanov et al (2011) may well have resolved this methodological issue, by demonstrating that under appropriate oxygenation conditions lactate, not only is an adequate energy substrate but it sustains oxidative activity even more than glucose.
Another modeling study by DiNuzzo et al (2010b), commented by Dienel (2010) has suggested that glycogenolysis in astrocytes provides a mechanism to preserve glucose availability for neurons, rather than providing lactate to them. Experimental data obtained in vivo described in Suzuki et al (2011) and reviewed in the preceding paragraphs are in contradiction with the mathematical model proposed.
Finally, a critique that has been raised, particularly at the early times of the formulation of the ANLS model, was that it was largely based on in vitro data. As reviewed in the preceding paragraph ‘A precise molecular description of the astroglial-based coupling mechanism relies on extensive in vitro, ex vivo, and in vivo investigations,' this claim does not correspond to experimental data obtained to date by several groups independently. Thus, in a recent, highly relevant and visible paper with strong experimental data (Boumezbeur et al, 2010), a significant cerebral metabolic rate of blood-borne (serum) lactate was established. Highly relevant results for the ANLS hypothesis are that (1) the majority of plasma lactate is indeed metabolized in neurons, (2) lactate transport capacities in the central nervous system are not limiting for an ANLS mechanism, and (3) plasma lactate can contribute 10% to cerebral energy metabolism under basal conditions and up to 60% under supraphysiological conditions (i.e., elevated plasma lactate with saturation of lactate transport). The relevance of these data to the ANLS have been extensively discussed by Figley (2011), in particular pointing to the fact that Boumezbeur's in vivo data were in contradiction with Gandhi et al (2009) ex vivo results. Finally, very recently, Wyss et al (2011) demonstrated in vivo that lactate is an energy source for neurons.
Conclusion
The ANLS model has now reached maturity. It is ready to settle in different domains of neurosciences well beyond the restricted circle of brain energy metabolism aficionados. Moreover, it bears a heuristic value to approach fundamental functions such as neuroprotection and memory (Figure 8). In doing so, it repositions neuroenergetics as a central player in brain function and information processing. After a healthy (and hectic at times!) development, the ANLS is ready to engage in exciting new ventures.
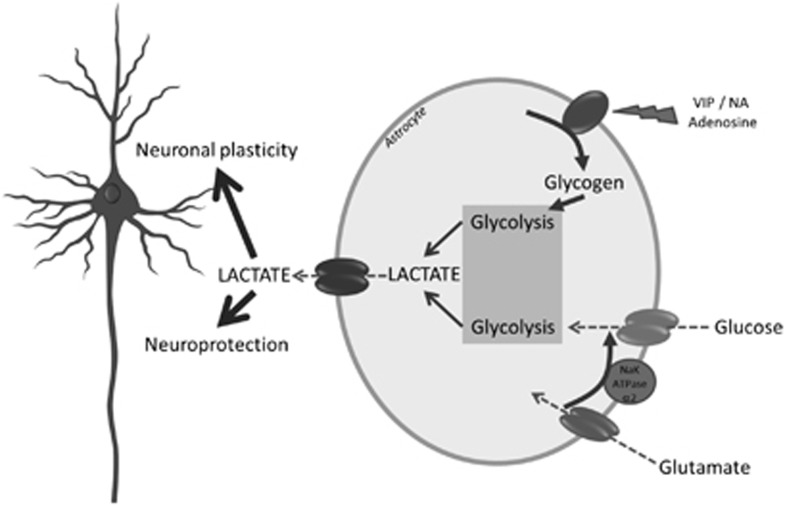
Figure 8.
Roles of glucose- and glycogen-derived lactate in neuroprotection and neuronal plasticity. It is suggested that under different levels of brain activation, glutamatergic as well as specific neuromodulatory systems (e.g., noradrenergic) will be activated. Glutamate reuptake in astrocytes will trigger glucose-derived lactate production and release. In parallel, neuromodulators such as vasoactive intestinal peptide (VIP), noradrenaline (NA), or adenosine will stimulate glycogenolysis, leading as well to lactate production and release. Such lactate provision to neurons by astrocytes turns out to be essential for the establishment of memory via support of synaptic plasticity processes as well as for neuroprotection of neurons under certain stress conditions (e.g., excitotoxicity).
Acknowledgments
The authors thank Dr Sylvain Lengacher for preparing Figure 8.
Footnotes
Research in LP's and PJM's laboratories was supported by Swiss FNS Grant nos. 31003A-125063 and 3100AO-108336, respectively.
References
- Ainscow EK, Mirshamsi S, Tang T, Ashford ML, Rutter GA. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K(+) channels. J Physiol. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Káradóttir R, Attwell D. A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J Neurosci. 2005;25:848–859. doi: 10.1523/JNEUROSCI.4157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriezen WL. On a system of fibre-like cells surrounding the blood vessels of the brain of man and mammals, and its physiological significance. Int Monatsschr Anat Physiol. 1893;10:532–540. [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R, Magistretti PJ, Pellerin L. Brain lactate kinetics: modeling evidence for neuronal lactate uptake upon activation. Proc Natl Acad Sci USA. 2005;102:16448–16453. doi: 10.1073/pnas.0505427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros LF, Courjaret R, Jakoby P, Loaiza A, Lohr C, Deitmer JW. Preferential transport and metabolism of glucose in Bergmann glia over Purkinje cells: a multiphoton study of cerebellar slices. Glia. 2009;57:962–970. doi: 10.1002/glia.20820. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Bergersen L, Waerhaug O, Helm J, Thomas M, Laake P, Davies AJ, Wilson MC, Halestrap AP, Ottersen OP. A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp Brain Res. 2001;136:523–534. doi: 10.1007/s002210000600. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bernardinelli Y, Magistretti PJ, Chatton JY. Astrocytes generate Na+-mediated metabolic waves. Proc Natl Acad Sci USA. 2004;101:14937–14942. doi: 10.1073/pnas.0405315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet C, Lei H, Thevenet J, Gruetter R, Magistretti PJ, Hirt L. Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1780–1789. doi: 10.1038/jcbfm.2009.97. [DOI] [PubMed] [Google Scholar]
- Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J Cereb Blood Flow Metab. 1996;16:1079–1089. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Bittner CX, Loaiza A, Ruminot I, Larenas V, Sotelo-Hitschfeld T, Gutiérrez R, Córdova A, Valdebenito R, Frommer WB, Barros LF. High resolution measurement of the glycolytic rate. Front Neuroenerg. 2010;2.pii:26. doi: 10.3389/fnene.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TM, Ip M, Cheng E, Minami M, Pellerin L, Magistretti P, Sapolsky RM. Dual-gene, dual-cell type therapy against an excitotoxic insult by bolstering neuroenergetics. J Neurosci. 2004;24:6202–6208. doi: 10.1523/JNEUROSCI.0805-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30:13983–13991. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci. 2006;24:1687–1694. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A.2005Reaching out beyond the synapse: glial intercellular waves coordinate metabolism Sci Signal 2005pe6doi: 10.1126/stke.270200pe6(http://www.stke.org/cgi/content/full/sigtrans;2005/270/pe6 ) [DOI] [PubMed] [Google Scholar]
- Chatton JY, Marquet P, Magistretti PJ. A quantitative analysis of L-glutamate-regulated Na+ dynamics in mouse cortical astrocytes: implications for cellular bioenergetics. Eur J Neurosci. 2000;12:3843–3853. doi: 10.1046/j.1460-9568.2000.00269.x. [DOI] [PubMed] [Google Scholar]
- Chenal J, Pellerin L. Noradrenaline enhances the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of PI3K/Akt and the mTOR/S6K pathway. J Neurochem. 2007;102:389–397. doi: 10.1111/j.1471-4159.2007.04495.x. [DOI] [PubMed] [Google Scholar]
- Chenal J, Pierre K, Pellerin L. Insulin and IGF-1 enhance the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin pathway. Eur J Neurosci. 2008;27:53–65. doi: 10.1111/j.1460-9568.2007.05981.x. [DOI] [PubMed] [Google Scholar]
- Chih CP, He J, Sly TS, Roberts EL., Jr Comparison of glucose and lactate as substrates during NMDA-induced activation of hippocampal slices. Brain Res. 2001a;893:143–154. doi: 10.1016/s0006-8993(00)03306-0. [DOI] [PubMed] [Google Scholar]
- Chih CP, Lipton P, Roberts EL., Jr Do active cerebral neurons really use lactate rather than glucose. Trends Neurosci. 2001b;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Cholet N, Pellerin L, Magistretti PJ, Hamel E. Similar perisynaptic glial localization for the Na+,K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex. 2002;12:515–525. doi: 10.1093/cercor/12.5.515. [DOI] [PubMed] [Google Scholar]
- Cholet N, Pellerin L, Welker E, Lacombe P, Seylaz J, Magistretti P, Bonvento G. Local injection of antisense oligonucleotides targeted to the glial glutamate transporter GLAST decreases the metabolic response to somatosensory activation. J Cereb Blood Flow Metab. 2001;21:404–412. doi: 10.1097/00004647-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Chuquet J, Quilichini P, Nimchinsky EA, Buzsáki G. Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J Neurosci. 2010;30:15298–15303. doi: 10.1523/JNEUROSCI.0762-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Astrocytes are ‘good scouts': being prepared also helps neighboring neurons. J Cereb Blood Flow Metab. 2010;30:1893–1894. doi: 10.1038/jcbfm.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neurosci Res. 2001;66:824–838. doi: 10.1002/jnr.10079. [DOI] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Changes in glucose uptake rather than lactate shuttle take center stage in subserving neuroenergetics: evidence from mathematical modeling. J Cereb Blood Flow Metab. 2010a;30:586–602. doi: 10.1038/jcbfm.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Glycogenolysis in astrocytes supports blood-borne glucose channeling not glycogen-derived lactate shuttling to neurons: evidence from mathematical modeling. J Cereb Blood Flow Metab. 2010b;30:1895–1904. doi: 10.1038/jcbfm.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Hewitt A, Damon TL, Hart M, Kurascz J, Li A, Leiter JC. Inhibition of monocarboxylate transporter 2 in the retrotrapezoid nucleus in rats: a test of the astrocyte-neuron lactate-shuttle hypothesis. J Neurosci. 2008;28:4888–4896. doi: 10.1523/JNEUROSCI.5430-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figley CR. Lactate transport and metabolism in the human brain: implications for the astrocyte-neuron lactate shuttle hypothesis. J Neurosci. 2011;31:4768–4770. doi: 10.1523/JNEUROSCI.6612-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figley CR, Stroman PW. The role(s) of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals. Eur J Neurosci. 2011;33:577–588. doi: 10.1111/j.1460-9568.2010.07584.x. [DOI] [PubMed] [Google Scholar]
- Gandhi GK, Cruz NF, Ball KK, Dienel GA. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem. 2009;111:522–536. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili M, Rodriguez-Kern A, Sung L, Shimamoto K, Gegelashvili G. Glutamate transporter GLAST/EAAT1 directs cell surface expression of FXYD2/gamma subunit of Na, K-ATPase in human fetal astrocytes. Neurochem Int. 2007;50:916–920. doi: 10.1016/j.neuint.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol. 1997;273:E201–E213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger A, Hydén H. Inverse enzymatic changes in neurons and glia during increased function and hypoxia. J Cell Biol. 1963;16:521–525. doi: 10.1083/jcb.16.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Mendez A, Almeida A, Fernández E, Maestre C, Moncada S, Bolaños JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Hydén H, Lange PW. A kinetic study of the neuronglia relationship. J Cell Biol. 1962;13:233–237. doi: 10.1083/jcb.13.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci USA. 2003;100:4879–4884. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Mukhtarov M, Bregestovski P, Zilberter Y. Lactate effectively covers energy demands during neuronal network activity in neonatal hippocampal slices. Front Neuroenerg. 2011;3:2. doi: 10.3389/fnene.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Benz AM, Katsuki H, Zorumski CF. Endogenous monocarboxylates sustain hippocampal synaptic function and morphological integrity during energy deprivation. J Neurosci. 1997;17:9448–9457. doi: 10.1523/JNEUROSCI.17-24-09448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet R, Allaman I, Pellerin L, Magistretti PJ, Weber B. Comment on recent modeling studies of astrocyte-neuron metabolic interactions. J Cereb Blood Flow Metab. 2010;30:1982–1986. doi: 10.1038/jcbfm.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet R, Magistretti PJ, Weber B. Deciphering neuron-glia compartmentalization in cortical energy metabolism. Front Neuroenerg. 2009;1:4. doi: 10.3389/neuro.14.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Kadekaro M, Crane AM, Sokoloff L. Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proc Natl Acad Sci USA. 1985;82:6010–6013. doi: 10.1073/pnas.82.17.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekaro M, Vance WH, Terrell ML, Gary H, Jr, Eisenberg HM, Sokoloff L. Effects of antidromic stimulation of the ventral root on glucose utilization in the ventral horn of the spinal cord in the rat. Proc Natl Acad Sci USA. 1987;84:5492–5495. doi: 10.1073/pnas.84.15.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Keller JN, Steiner MR, Mattson MP, Steiner SM. Lysophosphatidic acid decreases glutamate and glucose uptake by astrocytes. J Neurochem. 1996;67:2300–2305. doi: 10.1046/j.1471-4159.1996.67062300.x. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure, and normal values. J Clin Invest. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocharyan A, Fernandes P, Tong XK, Vaucher E, Hamel E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J Cereb Blood Flow Metab. 2008;28:221–231. doi: 10.1038/sj.jcbfm.9600558. [DOI] [PubMed] [Google Scholar]
- Lam TK, Gutierrez-Juarez R, Pocai A, Bhanot S, Tso P, Schwartz GJ, Rossetti L. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med. 2007;13:171–180. doi: 10.1038/nm1540. [DOI] [PubMed] [Google Scholar]
- Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- Laughton JD, Bittar P, Charnay Y, Pellerin L, Kovari E, Magistretti PJ, Bouras C. Metabolic compartmentalization in the human cortex and hippocampus: evidence for a cell- and region-specific localization of lactate dehydrogenase 5 and pyruvate dehydrogenase. BMC Neurosci. 2007;8:35. doi: 10.1186/1471-2202-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A-L, Fox PT, Hardies J, Duong TQ, Gao J-H. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci USA. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa F, Tsuboi T, Fukuda M, Pellerin L. Regulation of the intracellular distribution, cell surface expression, and protein levels of AMPA receptor GluR2 subunits by the monocarboxylate transporter MCT2 in neuronal cells. J Neurochem. 2009;109:1767–1778. doi: 10.1111/j.1471-4159.2009.06100.x. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ.2008Brain energy metabolism Fundamental Neuroscience(Squire L, Berg D, Bloom FE, eds), 3rd ed,San Diego, CA: Academic Press; 271–293. [Google Scholar]
- Magistretti PJ, Allaman I. Glycogen: a Trojan horse for neurons. Nat Neurosci. 2007;10:1341–1342. doi: 10.1038/nn1107-1341. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Chatton JY. Relationship between L-glutamate-regulated intracellular Na+ dynamics and ATP hydrolysis in astrocytes. J Neural Transm. 2005;112:77–85. doi: 10.1007/s00702-004-0171-6. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Hof PR, Martin JL. Adenosine stimulates glycogenolysis in mouse cerebral cortex: a possible coupling mechanism between neuronal activity and energy metabolism. J Neurosci. 1986;6:2558–2562. doi: 10.1523/JNEUROSCI.06-09-02558.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Morrison JH. Noradrenaline- and vasoactive intestinal peptide-containing neuronal systems in neocortex: functional convergence with contrasting morphology. Neuroscience. 1988;24:367–378. doi: 10.1016/0306-4522(88)90338-7. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Morrison JH, Shoemaker WJ, Sapin V, Bloom FE. Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: a possible regulatory mechanism for the local control of energy metabolism. Proc Natl Acad Sci USA. 1981;78:6535–6539. doi: 10.1073/pnas.78.10.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol Psychiatry. 1996;1:445–452. [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Martin J-L.1995Brain energy metabolism: an integrated cellular perspective Psychopharmacology: The Fourth Generation of Progress(Bloom FE, Kupfer DJ, eds),New York, NY: Raven Press; 657–670. [Google Scholar]
- Magistretti PJ, Schorderet M. VIP and noradrenaline act synergistically to increase cyclic AMP in cerebral cortex. Nature. 1984;308:280–282. doi: 10.1038/308280a0. [DOI] [PubMed] [Google Scholar]
- Mangia S, Simpson IA, Vannucci SJ, Carruthers A. The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem. 2009;109 (Suppl 1:55–62. doi: 10.1111/j.1471-4159.2009.06003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PM, Gopal E, Ananth S, Zhuang L, Itagaki S, Prasad BM, Smith SB, Prasad PD, Ganapathy V. Identity of SMCT1 (SLC5A8) as a neuron-specific Na+-coupled transporter for active uptake of L-lactate and ketone bodies in the brain. J Neurochem. 2006;98:279–288. doi: 10.1111/j.1471-4159.2006.03878.x. [DOI] [PubMed] [Google Scholar]
- McIlwain H. Substances which support respiration and metabolic response to electrical impulses in human cerebral tissues. J Neurol Neurosurg Psychiatr. 1953;16:257–266. doi: 10.1136/jnnp.16.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S, Uson RR, Schwartz EJ, Haber RS. The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia. 1995;14:43–54. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Coles JA. Cellular pathways of energy metabolism in the brain: is glucose used by neurons or astrocytes. Glia. 2007;55:1238–1250. doi: 10.1002/glia.20376. [DOI] [PubMed] [Google Scholar]
- Newman LA, Gold PE.2010The role of hippocampal astrocytic glycogen in working memory in rats Soc Neurosci Abstr407.11
- O'Brien J, Kla KM, Hopkins IB, Malecki EA, McKenna MC. Kinetic parameters and lactate dehydrogenase isozyme activities support possible lactate utilization by neurons. Neurochem Res. 2007;32:597–607. doi: 10.1007/s11064-006-9132-9. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Hirasawa M. ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: implications for brain energetics during arousal. J Neurosci. 2010;30:8061–8070. doi: 10.1523/JNEUROSCI.5741-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L. Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem Int. 2003;43:331–338. doi: 10.1016/s0197-0186(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Pellerin L. Brain energetics (thought needs food) Curr Opin Clin Nutr Metab Care. 2008;11:701–705. doi: 10.1097/MCO.0b013e328312c368. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bergersen LH, Halestrap AP, Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res. 2005;79:55–64. doi: 10.1002/jnr.20307. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake stimulates Na+,K+-ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J Neurochem. 1997;69:2132–2137. doi: 10.1046/j.1471-4159.1997.69052132.x. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. How to balance the brain energy budget while spending glucose differently. J Physiol. 2003;546:325. doi: 10.1113/jphysiol.2002.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroscience. Let there be (NADH) light. Science. 2005;305:50–52. doi: 10.1126/science.1100428. [DOI] [PubMed] [Google Scholar]
- Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:586–595. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Pierre K, Chatton JY, Parent A, Repond C, Gardoni F, Di Luca M, Pellerin L. Linking supply to demand: the neuronal monocarboxylate transporter MCT2 and the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptor GluR2/3 subunit are associated in a common trafficking process. Eur J Neurosci. 2009;29:1951–1963. doi: 10.1111/j.1460-9568.2009.06756.x. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Porras OH, Loaiza A, Barros LF. Glutamate mediates acute glucose transport inhibition in hippocampal neurons. J Neurosci. 2004;24:9669–9673. doi: 10.1523/JNEUROSCI.1882-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistorff B, Grunnet N. High brain lactate is not caused by a shift in the lactate dehydrogenase A/B ratio. Proc Natl Acad Sci USA. 2011;108:E21. doi: 10.1073/pnas.1017750108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiki A, Boulland JL, Halestrap AP, Ottersen OP, Bergersen L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. 2003;122:677–688. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Ramos M, del Arco A, Pardo B, Martínez-Serrano A, Martínez-Morales JR, Kobayashi K, Yasuda T, Bogónez E, Bovolenta P, Saheki T, Satrústegui J. Developmental changes in the Ca2+-regulated mitochondrial aspartate-glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Brain Res Dev Brain Res. 2003;143:33–46. doi: 10.1016/s0165-3806(03)00097-x. [DOI] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci. 2011;31:538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinet C, Pellerin L. Brain-derived neurotrophic factor enhances the expression of the monocarboxylate transporter 2 through translational activation in mouse cultured cortical neurons. J Cereb Blood Flow Metab. 2010;30:286–298. doi: 10.1038/jcbfm.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR. Glutamate transporter coupling to Na,K-ATPase. J Neurosci. 2009;29:8143–8155. doi: 10.1523/JNEUROSCI.1081-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossner MJ, Hirrlinger J, Wichert SP, Boehm C, Newrzella D, Hiemisch H, Eisenhardt G, Stuenkel C, von Ahsen O, Nave KA. Global transcriptome analysis of genetically identified neurons in the adult cortex. J Neurosci. 2006;26:9956–9966. doi: 10.1523/JNEUROSCI.0468-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Richter M, Montag D, Sartorius T, Gawlik V, Hennige AM, Scherneck S, Himmelbauer H, Lutz SZ, Augustin R, Kluge R, Ruth P, Joost HG, Schürmann A. Neuronal functions, feeding behavior, and energy balance in Slc2a3+/- mice. Am J Physiol Endocrinol Metab. 2008;295:E1084–E1094. doi: 10.1152/ajpendo.90491.2008. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation. J Neurochem. 1997;69:423–426. doi: 10.1046/j.1471-4159.1997.69010423.x. [DOI] [PubMed] [Google Scholar]
- Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. 1988;240:1326–1328. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- Serres S, Bezancon E, Franconi JM, Merle M. Ex vivo analysis of lactate and glucose metabolism in the rat brain under different states of depressed activity. J Biol Chem. 2004;279:47881–47889. doi: 10.1074/jbc.M409429200. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate: a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab. 2003;23:658–664. doi: 10.1097/01.WCB.0000063991.19746.11. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J Cereb Blood Flow Metab. 1981;1:7–36. doi: 10.1038/jcbfm.1981.4. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Palay SL. The fine structure of the lateral vestibular nucleus in the rat. I. Neurons and neuroglial cells. J Cell Biol. 1968;36:151–179. [PubMed] [Google Scholar]
- Stuart CA, Ross IR, Howell ME, McCurry MP, Wood TG, Ceci JD, Kennel SJ, Wall J. Brain glucose transporter (Glut3) haploinsufficiency does not impair mouse brain glucose uptake. Brain Res. 2011;1384:15–22. doi: 10.1016/j.brainres.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MM, Sagar SM, Sharp FR. Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience. 1992;51:451–461. doi: 10.1016/0306-4522(92)90329-z. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Driscoll BF, Law MJ, Sokoloff L. Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci USA. 1995;92:4616–4620. doi: 10.1073/pnas.92.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkök SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J Neurosci Res. 2005;81:644–652. doi: 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Veuthey AL, Saravelos SG, Perrottet P, Tsoupras G. Glial cells transform glucose to alanine, which fuels the neurons in the honeybee retina. J Neurosci. 1994;14:1339–1351. doi: 10.1523/JNEUROSCI.14-03-01339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Poitry-Yamate CL, MacLeish PR, Poitry S. Trafficking of molecules and metabolic signals in the retina. Prog Retin Eye Res. 1998;17:429–442. doi: 10.1016/s1350-9462(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Vilchez D, Ros S, Cifuentes D, Pujadas L, Vallès J, García-Fojeda B, Criado-García O, Fernández-Sánchez E, Medraño-Fernández I, Domínguez J, García-Rocha M, Soriano E, Rodríguez de Córdoba S, Guinovart JJ. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci. 2007;10:1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–286. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Bakken IJ, Larsson OM, Sonnewald U, Schousboe A. Comparison of lactate and glucose metabolism in cultured neocortical neurons and astrocytes using 13C-NMR spectroscopy. Dev Neurosci. 1998;20:310–320. doi: 10.1159/000017326. [DOI] [PubMed] [Google Scholar]
- Wada H, Okada Y, Uzuo T, Nakamura H. The effects of glucose, mannose, fructose and lactate on the preservation of neural activity in the hippocampal slices from the guinea pig. Brain Res. 1998;788:144–150. doi: 10.1016/s0006-8993(97)01532-1. [DOI] [PubMed] [Google Scholar]
- Walls AB, Sickmann HM, Brown A, Bouman SD, Ransom B, Schousboe A, Waagepetersen HS. Characterization of 1,4-dideoxy-1,4-imino-d-arabinitol (DAB) as an inhibitor of brain glycogen shunt activity. J Neurochem. 2008;105:1462–1470. doi: 10.1111/j.1471-4159.2008.05250.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- Wyss MT, Jolivet R, Buck A, Magistretti PJ, Weber B. In vivo evidence for lactate as a neuronal energy source. J Neurosci. 2011;31:7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ola MS, Berkich DA, Gardner TW, Barber AJ, Palmieri F, Hutson SM, LaNoue KF. Energy sources for glutamate neurotransmission in the retina: absence of the aspartate/glutamate carrier produces reliance on glycolysis in glia. J Neurochem. 2007;101:120–131. doi: 10.1111/j.1471-4159.2006.04349.x. [DOI] [PubMed] [Google Scholar]
- Yu S, Ding WG. The 45 kDa form of glucose transporter 1 (GLUT1) is localized in oligodendrocyte and astrocyte but not in microglia in the rat brain. Brain Res. 1998;797:65–72. doi: 10.1016/s0006-8993(98)00372-2. [DOI] [PubMed] [Google Scholar]