Abstract
Some 40 years ago it was recognized by Furchgott and colleagues that the endothelium releases a vasodilator, endothelium-derived relaxing factor (EDRF). Later on, several groups identified EDRF to be a gas, nitric oxide (NO). Since then, NO was identified as one of the most versatile and unique molecules in animal and human biology. Nitric oxide mediates a plethora of physiological functions, for example, maintenance of vascular tone and inflammation. Apart from these physiological functions, NO is also involved in the pathophysiology of various disorders, specifically those in which regulation of blood flow and inflammation has a key role. The aim of the current review is to summarize the role of NO in cerebral ischemia, the most common cause of stroke.
Keywords: acute stroke, experimental, focal ischemia, neuroprotection, nitric oxide
Introduction
In the late 1970s, it was recognized that the endothelium releases a factor that relaxes vascular smooth muscle cells thereby causing vasodilatation (Furchgott and Zawadzki, 1980). Since the chemical structure of this factor was unknown at the time, it was named EDRF (endothelium-derived relaxing factor). Later on, EDRF was identified to be a color and odorless gas, nitric oxide (NO) (Furchgott et al, 1987; Ignarro et al, 1987a, 1987b). Since then, NO, the most convincing gaseous signaling molecule in vertebrates, was increasingly recognized as one of the most versatile and unique molecules mediating such diverse physiological functions as the maintenance of vascular tone, thrombotic-thrombolytic homeostasis, cell growth, and inflammation. Apart from its physiological functions, NO has also an important role in the pathophysiology of various disorders, specifically those in which regulation of blood flow and inflammatory reactions are key pathophysiological events.
Nitric Oxide—Physiology
Nitric oxide is an uncharged gas that can easily cross biological membranes. It is, however, quickly deactivated by oxidation with a biological half-life of a few seconds thereby limiting its spatial extent in biological tissues (Gibson and Roughton, 1957; Stamler et al, 1992). Nitric oxide is synthesized by a group of three NO synthases (NOS) from L-arginine (Furchgott and Zawadzki, 1980; Palmer et al, 1988), a reaction that requires the presence of molecular oxygen, nicotinamide adenine dinucleotide phosphate (NADPH), tetrahydrobiopterin (BH4), heme, Flavin mononucleotide (FMN), and calmodulin (Bredt and Snyder, 1990). Two constitutively expressed isoforms, mainly located in endothelial cells and in neurons (eNOS and nNOS, respectively) generate low NO levels, and are constitutively expressed and calcium dependent (Knowles et al, 1989). The third isoform, inducible NOS (iNOS), is expressed in many different cell types, for example, macrophages, astrocytes, and microglia, and is calcium independent (Fleming et al, 1991; Cho et al, 1992). Once upregulated, iNOS produces large amounts of NO, which can damage or even destroy cells, for example, microorganisms or the brain tissue.
Nitric oxide toxicity usually occurs through a direct interaction with various protein moieties. This may result in the reversible formation of mixed disulfides between cysteines and glutathione, termed S-glutathiolation (Cohen and Adachi, 2006), the formation of nitrosothiols (Stamler, 1994), or protein nitrosylation (Xu et al, 1998; Matsushita et al, 2003). Toxicity may also result from formation of peroxynitrite anion (ONOO−), a strong oxidant and mediator of tissue injury after its combination with superoxide anion (Dawson and Dawson, 1996). Nitric oxide also can trigger apoptosis directly or via ONOO− (Leist et al, 1997a, 1997b).
Apart from these direct actions, NO can also promote intercellular signaling via activation of soluble guanylate cyclase and subsequent formation of cyclic guanosine monophosphate (cGMP). The best documented signaling activity using this mechanism is the migration of NO generated by the endothelium into neighboring smooth muscle cells whereupon NO relaxes vascular smooth cell through Ca2+-mediated formation of cGMP and phosphorylation of downstream kinases that ultimately impact calcium availability (Murad et al, 1987).
Endothelial NOS-derived NO has furthermore been demonstrated to have a key role in vascular remodeling and angiogenesis (Papapetropoulos et al, 1997; Rudic et al, 1998; Murohara et al, 1998).
In the brain, NO is mainly formed by nNOS and eNOS. Accordingly, it serves as a neurotransmitter and neuromodulator (Garthwaite et al, 1988; Dawson and Snyder, 1994; Dawson, 1995) and—among many other functions—is concerned with the maintenance of basal cerebral blood flow (CBF) (Tanaka, 1996). Specifically, NO has been implicated in cerebral autoregulation (Dirnagl et al, 1994; Richards et al, 1997; White et al, 2000), chemoregulation of CBF (Iadecola, 1992; Thompson et al, 1996; Lavi et al, 2003), and neurovascular coupling (Dirnagl et al, 1994).
More recent evidence suggests a role of NO in neuronal proliferation and differentiation. Tanaka et al (1994) showed that NO inhibits neural proliferation and differentiation in the developing brain; in vitro data further supported a role for NO in the differentiation of developing as well as adult neuronal cells (Peunova and Enikolopov, 1995; Viani et al, 1997). In vivo, NO negatively regulates cell proliferation in the developing (Tegenge and Bicker, 2009) as well as adult (Oh et al, 2010) brains (see Gibbs, 2003; Contestabile and Ciani, 2004 for reviews).
Neurogenesis, which in the adult brain seems restricted to the subventricular zone, the olfactory bulb, and the subgranular zone of the hippocampus (Zhao et al, 2008), persists in the adult brain and can be induced after cerebral insults (Zhao et al, 2008; Imayoshi et al, 2009). Nitric oxide exerts a dual effect on neurogenesis: While nNOS-derived NO decreases neurogenesis (Packer et al, 2003; Moreno-Lopez et al, 2004; Zhu et al, 2006), NO produced by endothelial (Reif et al, 2004) or inducible (Luo et al, 2007; Bechade et al, 2011) NOS seems to stimulate it.
Nitric Oxide—Role in Cerebral Ischemic Preconditioning
It was discovered in the early 1990s (Kitagawa et al, 1990) that exposing the brain to sublethal injury increases its tolerance for subsequent insults (Gidday, 2006). Among the stimuli investigated for induction of tolerance are sublethal ischemia and proinflammatory mediators (Stagliano et al, 1999; Dawson and Dawson, 2000; Dirnagl et al, 2003; Kunz et al, 2007). Nitric oxide seems to have a major role in mediating ischemic tolerance induced by preconditioning. While there is mounting evidence that iNOS-derived NO is a key effector of ischemic preconditioning (Park et al, 2003; Cho et al, 2005), the contribution of constitutive NOS is not fully elucidated yet (Gidday et al, 1999; Gonzalez-Zulueta et al, 2000; Atochin et al, 2003).
Nitric Oxide—Pathophysiology in Stroke
Cerebral ischemia induces multiple and distinct changes in cerebral NO content and signaling. Occlusion of the middle cerebral artery (MCAo) results in an increased production of NO by 20-fold for up to 30 minutes (Malinski et al, 1993; Kader et al, 1993) most likely through increased calcium availability and activation of nNOS (Huang et al, 1994). Thereafter, the brain tissue NO is reduced below detectable levels for up to 7 days (Malinski et al, 1993; Sugimura et al, 1998), indicating a long-lasting NO deficiency in the ischemic brain. If reperfusion occurs, NO concentration may transiently increase by 50% for about 30 minutes (Fassbender et al, 2000; Uetsuka et al, 2002). Concomitant with changes in NO levels, the activities of eNOS and nNOS increases within the first few minutes after MCAo, but decrease significantly thereafter (Kader et al, 1993). In contrast to the constitutive NOS isoforms, iNO becomes upregulated from 12 hours after MCAo for up to 7 days (Niwa et al, 2001).
In the chronic phase after cerebral ischemia, angiogenesis has been shown to occur (Beck and Plate, 2009); the extent of collateralization is a predictor for neurological outcome after stroke (Christoforidis et al, 2005). Nitric oxide has a crucial role in angiogenesis after ischemic stroke: NO donor DETA-NONOate increased angiogenesis after experimental cerebral ischemia (Zhang et al, 2003; Chen et al, 2004). Endothelial NOS-deficient mice revealed significantly impaired neovascularization after stroke, indicating that endothelial-derived NO mediates this effect (Cui et al, 2009).
Based on the time course of ischemia-induced changes in NO levels and NOS regulation in the brain and cerebral blood vessels, several strategies were suggested to manipulate the NO system for the treatment of stroke.
When the cationic amino-acid substrate L-arginine was administered intravenously after MCAo, CBF and spike activity were restored within the ischemic penumbra (Dalkara et al, 1994), and infarct size reduced (Morikawa et al, 1992, 1994). L-arginine's effect was enantiomer specific and not observed in eNOS-deficient mice. By contrast, experiments using NOS inhibitors and selective NOS isoform-deficient mice revealed that NO generated by the neuronal isoform is detrimental to tissue survival and neurological outcome (Huang et al, 1994; Hara et al, 1996; Zaharchuk et al, 1997). This also applies to the generation of NO by the inducible isoform following ischemic challenge (Iadecola et al, 1995, 1997; Zhang et al, 1996a; Zhao et al, 2003). In contrast to the detrimental functions of nNOS and iNOS enzymatic activity, eNOS and eNOS-derived NO is neuroprotective under most conditions of ischemia–reperfusion, although oxygen radical generation and enhanced injury can occur with eNOS uncoupling in the presence of BH4 deficiency, a mechanism implied in the pathophysiology of vascular diseases (Landmesser et al, 2003; Alp and Channon, 2004). Endothelial NOS knockout mice have lower postischemic CBF levels and larger infarcts than their wild-type littermates following MCAo (Lo et al, 1996; Huang et al, 1996). Interestingly, eNOS-deficient mice not only exhibit larger infarcts, they also show smaller penumbral regions (Lo et al, 1996), indicating that eNOS may be critical for maintaining and restoring penumbral and collateral blood flow. Increases in infarct size in mice deficient in eNOS phosphorylation (Atochin et al, 2007) or deficient in the expression of the α-1 subunit of the obligate heterodimer, soluble guanylate cyclase, are consistent with these findings (Atochin et al, 2010). Furthermore, deficient angiogenesis after stroke might contribute to the increased postischemic damage in eNOS null mice (Cui et al, 2009).
Another restorative mechanism after stroke that is NO dependent is neurogenesis (see Zhang et al, 2005, 2008 for reviews). While iNOS-derived NO was shown to be neurotoxic in the acute phase after stroke, several studies suggest an important role of the inducible isoform for cell proliferation after experimental ischemia: iNOS-positive cells increased significantly in the periinfarct zone 1 and 3 days after focal ischemia (Sehara et al, 2006), the number of iNOS-positive cells increased with increasing survival time (Corsani et al, 2008). Treatment with iNOS inhibitor aminoguanidine prevented postischemic neurogenesis (Zhu et al, 2003); the phenomenon could not be detected in iNOS null mice, either (Zhu et al, 2003). Nitric oxide produced by nNOS exerts negative effects on neurogenesis (Packer et al, 2003; Moreno-Lopez et al, 2004; Luo et al, 2007), nNOS inhibition has been shown to increase neurogenesis after experimental ischemia (Sun et al, 2005). Neuronal NOS and iNOS therefore seem to have opposite roles in postischemic neurogenesis. There are hints that there is crosstalk between the neuronal and inducible NOS isoform: genetic deletion of nNOS or nNOS downregulation after cerebral ischemia-induced iNOS upregulation and—thus—neurogenesis while the protective effect of pharmacological nNOS inhibition was abolished in iNOS-deficient mice (Luo et al, 2007).
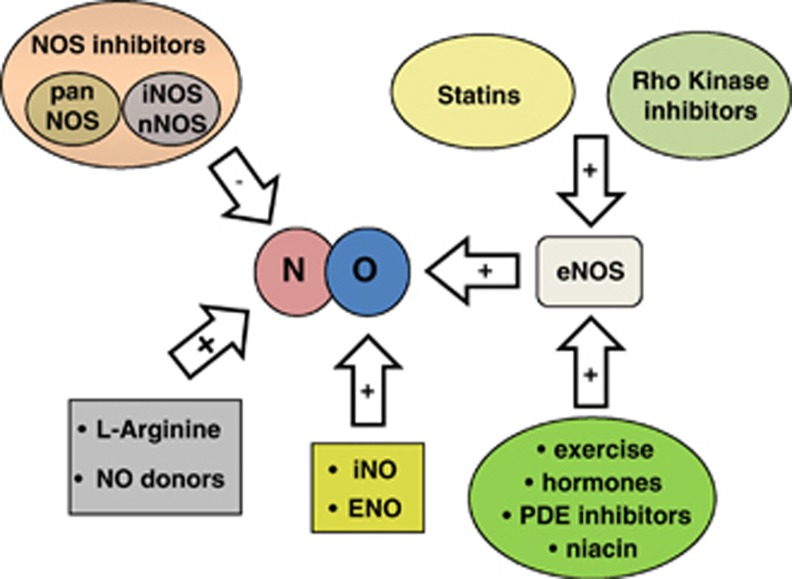
Given the ample evidence for NO-mediated neuroprotection after cerebral ischemia, several treatment strategies have been examined that increase postischemic NO content specifically generated by eNOS activity to augment CBF to the penumbra. One putative strategy is to replenish the early postischemic NO deficit by application of NO or NO precursors; another is to increase or modulate endogenous NO production/bioavailability by enhancing eNOS activity (Endres et al, 2004); for example, via the phosphatidylinositol 3-kinase (PI3-kinase)/protein B kinase (Akt) pathway (Dimmeler et al, 1999). Another potential mechanism is to directly or indirectly inhibit NOS, preferably nNOS and/or iNOS. Putative treatment strategies are summarized in Figure 1.
Figure 1.
Summary of therapeutical strategies influencing nitric oxide signaling in ischemic stroke. eNOS, endothelial nitric oxide synthases; iNOS, inducible NOS; nNOS, neuron NOS; PDE, phosphodiesterase.
Therapeutic Approaches
L-Arginine and Nitric Oxide Donors
After encouraging preclinical data following application of the NO precursor L-arginine (Morikawa et al, 1992, 1994; Dalkara et al, 1994), other NO donors, for example, nitrite, were reported to be neuroprotective in various models of transient and permanent cerebral ischemia (Willmot et al, 2005b; Jung et al, 2006). Several clinical trials were initiated. Application of L-arginine, however, failed to significantly improve outcome in stroke in preclinical and clinical trials (see Bath et al, 2002 for a meta-analysis). The main reason for the failure of translation was probably the reduction of mean arterial pressure following systemically administered NO/NO precursors. The impact on cerebral perfusion pressure was probably compensated to a greater extent in normally perfused brain tissues as compared with the area distal to occlusion. It seems likely that CBF in normally perfused brain tissue increased at the expense of blood flow in ischemic tissue, thereby further reducing the already critically low penumbral perfusion (Vorstrup et al, 1986; Lassen, 1990). By contrast, the only successful strategy delivering NO without affecting CBF was the transdermal application of glyceryl trinitrate. Glyceryl trinitrate delivered in this way even improved systemic blood flow; however, cerebral infarct size following MCAO was unchanged (Willmot et al, 2006). Transdermal application of glyceryl trinitrate is currently evaluated in a clinical trial (ENOS, efficacy of nitric oxide in stroke trial, start date July 2001 (ENOS Trial Investigators, 2006)).
Nitric Oxide Synthase Inhibitors
The excessive production of NO by brain parenchyma is a characteristic of ischemic stroke. Hence, inhibition of NOS was viewed as an alternative strategy to reducing NO availability in cells expressing the neuronal isoform. In a first approach, nonselective inhibition of NOS by Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME) reduced stroke volume after MCAo (Wei et al, 1994; Margaill et al, 1997). Further approaches using the same strategy, that is, pan-NOS inhibitors, yielded conflicting results (Willmot et al, 2005a) most likely due to their differential activity on the three different NOS isoforms (eNOS: protective, nNOS/iNOS: destructive).
More selective approaches using nNOS and iNOS inhibitors seem to be more promising. Pharmacological inhibition of nNOS with 7-nitroindazole given 5 minutes after MCAo reduced infarct volume in rats by up to 27% (Yoshida et al, 1994; Zhang et al, 1996b) and the application of aminoguanidine (Zhang et al, 1996a; Cash et al, 2001), N-3-(aminomethyl-benzyl)-acetamidine (Parmentier et al, 1999; Perez-Asensio et al, 2005), or 7-nitroindazol adduct 3-bromo-7-nitroindazole (Srinivasan and Sharma, 2012), all regarded as fairly selective iNOS inhibitors significantly reduced postischemic brain damage after transient and permanent cerebral ischemia. Similarly, noncompetitive NOS inhibitors, like the BH4 analog 4-amino-tetrahydro-L-biopterine, which putatively inhibit only newly formed NOS isoforms in vivo, that is, iNOS, reduced brain edema when applied after traumatic brain injury (Terpolilli et al, 2009). Another study reduced postischemic brain damage effectively by using antisense oligodeoxynucleotide to iNOS (Parmentier-Batteur et al, 2001).
However, NOS inhibitors currently are not in use clinically and are not evaluated in any ongoing clinical stroke trial because most are nonselective and may thus cause deleterious side effects due to their actions on eNOS. The development of highly specific inhibitors of NOS subtypes might make this therapeutic approach feasible (Salerno et al, 2002).
Statins (HMG-CoA-Reductase Inhibitors)
HMG-CoA-reductase inhibitors, so called statins, completely inhibit the conversion of HMG coenzyme A to mevalonate thereby blocking cholesterol and isoprenoid intermediate synthesis (Liao and Laufs, 2005). Isoprenoid intermediates such as farnesylpyrophosphate and geranylgeranylpyrophosphate are responsible for modulation of activity of several small GTPases such as Ras, Rho, and Rac, which are blocked by statins.
Originally developed to treat and prevent coronary heart disease, statins were found to be effective also in primary and secondary prevention of ischemic stroke in several randomized clinical trials (Amarenco et al, 2004, 2006; Kennedy et al, 2007; Ridker et al, 2008). Initially designed to treat hypercholesterinemia, it soon became evident that the beneficial effect of statins in stroke patients was independent of the serum cholesterol lowering effect of the compounds (Treasure et al, 1995; Laufs et al, 2001). Statins have a number of pleiotropic effects that contribute to vascular integrity and health of the blood vessel wall. Experimental stroke studies revealed that long-term treatment with HMG-CoA-reductase inhibitors induced eNOS upregulation (Endres et al, 1998; Laufs et al, 2000b, 2002; Amin-Hanjani et al, 2001) and blocked hypoxia induced downregulation of eNOS in rodents (Laufs et al, 1997), thus increasing NO bioavailability and CBF (Endres et al, 1998) and saving brain tissue from ischemic injury (Endres et al, 1998; Laufs et al, 2000b, 2002; Amin-Hanjani et al, 2001). The effects were dose dependent and observed with a number of HMG-CoA-reductase inhibitors. That statins mediate their neuroprotective effect through the upregulation of eNOS and consecutive improvement of penumbral blood flow was further validated by experiments showing that treatment was ineffective in mutant mice deficient in eNOS expression (Endres et al, 1998; Laufs et al, 2000b, 2002).
In addition to the eNOS-dependent effect of statins in vivo, an eNOS-independent neuroprotective effect of statins in vitro has been reported. This effect is believed to be mediated by alterations in the cholesterol containing neuronal membrane subdomains thus protecting neuronal cultures against NMDA (N-methyl-D-aspartate)-mediated excitotoxicity (Zacco et al, 2003; Bosel et al, 2005; Ponce et al, 2008). However, like all so far mentioned in vivo studies, these studies applied statins as a pretreatment underlining the role of statins treatment in the prevention rather than the treatment of stroke.
To evaluate the value of statins for the acute treatment of stroke, several studies applied HMG-CoA-reductase inhibitors after the initiation of cerebral ischemia. Surprisingly, also under these conditions, statins significantly reduced infarct size and brain edema formation (Sironi et al, 2003; Kilic et al, 2005; Nagaraja et al, 2006; Sugiura et al, 2007; Prinz et al, 2008; Mariucci et al, 2011). Interestingly, neuroprotection could be achieved by statin application as late as 2 days after the onset of MCAo (Sugiura et al, 2007). The main neuroprotective mechanisms are thought to be mediated via enhancement of eNOS activity (Mariucci et al, 2011), either by phosphorylation of eNOS at the S1177 site via the PI3 k/Akt pathway (Kureishi et al, 2000; Dimmeler et al, 2001) leading to reduction of inflammation (Sugiura et al, 2007) and induction of angiogenesis and neurogenesis (Chen et al, 2003) or via mRNA stabilization by inhibition of the Rho Kinase (ROCK) pathway (see below). This was proven by measurements of CBF, which showed that immediate postischemic CBF was not affected by acute statin treatment (Kilic et al, 2005; Berger et al, 2008), but that 5 days later—consistent with angiogenesis—CBF was improved (Berger et al, 2008).
Given their widely proven neuroprotective actions in experimental as well as in clinical trials, statins are recommended for primary as well as for secondary prevention of stroke (Heart Protection Study Collaborative Group, 2002; Adams et al, 2007). Despite these clear recommendations, it remains unclear whether HMG-CoA-reductase inhibitors indeed improve cerebral perfusion when given in the acute postischemic phase (Kennedy et al, 2007). Furthermore, most recent data suggest that statins exhibit untoward effects such as an increased risk of infection (Becker et al, 2011) and a higher incidence of hemorrhagic stroke (Collins et al, 2004; Goldstein et al, 2008; Vergouwen et al, 2008), stressing the need for further clinical evaluation of HMG-CoA-reductase inhibitors in the context of acute ischemic injury.
Rho Kinase (ROCK)—Inhibitors
There is ample evidence that the neuroprotective effect of statins/statin-induced upregulation of eNOS activity is at least in part mediated by inhibition of rho kinase (Laufs and Liao, 1998; Laufs et al, 1999, 2000a). Rho kinase (ROCK) is a serine–threonine kinase whose activity is modulated by isoprenoid intermediate geranygeranylpyrophosphate (GGPP). Rho kinase is implicated in the pathophysiology of atherosclerosis (Miyata et al, 2000), myocardial infarction, and hypertension (Uehata et al, 1997). Activation of Rho kinase in endothelial cells under hypoxic conditions (Wolfrum et al, 2004) has been related to the mechanism responsible for the downregulation of eNOS during and after ischemia (Takemoto et al, 2002; Ming et al, 2002; Wolfrum et al, 2004; Jin et al, 2006).
Rho kinase inhibitors such as fasudil or hydroxyfasudil rapidly lead to increased eNOS activity by Akt kinase-dependent phosphorylation of eNOS at S1179 (Fulton et al, 1999). This results in a significant reduction of ischemic brain damage when given before (Rikitake et al, 2005; Shin et al, 2007) but also when given after (Shin et al, 2007) experimental cerebral ischemia. The observed neuroprotective mechanism was proven to be eNOS dependent, based on the observation that ROCK inhibitors failed to improve CBF or reduce infarct volumes in eNOS null mice (Rikitake et al, 2005; Shin et al, 2007).
Apart from their well-documented neuroprotective effect in experimental stroke models, Rho kinase inhibitors lower systemic blood pressure potently, which may limit their use for the treatment of acute stroke (Uehata et al, 1997; Shimokawa, 2002; Takahara et al, 2003). Interestingly, however, the ROCK inhibitor hydroxyfasudil, which is neuroprotective, caused hypotension in wild type as well as in eNOS null mice (Shin et al, 2007), suggesting that the blood pressure lowering effect may be mediated by actions independent of eNOS activity. Therefore, the development of ROCK inhibitors with selectivity toward the cerebral circulation or toward ROCK2 (ROCKβ), the isoform most mainly expressed in the brain and heart (Nakagawa et al, 1996; Wei et al, 2001), may increase the potential of these compounds in acute stroke therapy.
Phosphodiesterase Inhibitors
Emerging evidence suggests that phosphodiesterase (PDE) inhibitors such as dipyramidol exhibit neuroprotective effects beyond their well-known antiplatelet properties. It was shown that dipyramidol augmented the neuroprotective effects of statins in an eNOS-dependent way (Kim et al, 2008). The putative mechanism involves an increase in eNOS S1177 phosphorylation, as demonstrated in spontaneously hypertensive rats (Oyama et al, 2011) based on the reduction in postischemic brain damage following pretreatment with PDE inhibitor cilostazol. While these are the first encouraging data suggesting that PDE inhibitors can also increase eNOS activity in stroke patients (Serebruany et al, 2011), clinical use of PDE inhibitors is so far restricted to secondary prevention of ischemic stroke: Two large clinical trials (ESPRIT: European/Australasian Stroke Prevention in Reversible Ischaemia Trial (Halkes et al, 2007), ESPS-2: European Stroke Prevention Study 2 (Diener et al, 1996)) proved that dipyramidole in combination with acetylsalicylic acid (ASA) is more effective in preventing secondary ischemic insults than ASA alone. Use of PDE inhibitors in the early postischemic phase is currently not recommended due to a possibly increased bleeding risk by PDE-induced platelet inhibition. However, a recently completed clinical trial with cilostazole (CAIST: Cilostazol in acute ischemic stroke treatment (Lee et al, 2011)) showed that cilostazol is comparable to aspirine in terms of safety and bleeding complications so further clinical evaluation of cilostazol mono- or combination-therapy in acute stroke is ongoing (Nakamura et al, 2011).
Other Neuroprotective Strategies Influencing the Endothelial Nitric Oxide Synthases Pathway
PI3 K/Akt pathway—S1177 phosphorylation—drugs
Niacin (vitamin D3) was shown to increase the levels of high-density lipoprotein (HDL), thus increasing levels of HDL-cholesterol (HDL-C) and lowering levels of serum triglycerides (Guyton, 1998; Elam et al, 2000). High-density lipoprotein promotes reendothelialization and stimulates migration of endothelial cells (Seetharam et al, 2006) by increasing NO concentration by activation of eNOS activity via the PI3/Akt pathway (Mineo et al, 2003; Drew et al, 2004).
After experimental ischemic stroke niacin (vitamin D3) and niacin derivative niaspan significantly improved functional outcome independently of HDL-C levels due to improved angiogenesis (Chen et al, 2007; Shehadah et al, 2010); again, this is thought to be conferred mainly by eNOS via the PI3 K/Akt pathway.
Niacin and niacin derivative niaspan are potent drugs to increase levels of HDL-cholesterin and thus help to restore vascular function and reduce occurrence of subsequent cardiovascular events without obvious side effect (Guyton et al, 2000; Grundy et al, 2002). However, a recently completed study (AIM-HIGH (Boden et al, 2011)) could not detect significant benefit of niacin treatment in addition to statins compared with statins alone in primary prevention of cardiovascular events; ischemic stroke was recorded as a secondary parameter.
Currently, a randomized double-blinded phase II study is investigating whether niaspan can improve recovery after ischemic stroke (Randomized, Controlled Trial of Extended-Release Niacin (Niaspan, Abbott Laboratories, Abbott Park, IL, USA) to Augment Subacute Ischemic Stroke Recovery, start date: April 2009, NCT00796887).
Another group of compounds known to increase levels of HDL-cholesterol are liver X receptor agonists (Brunham et al, 2006). TO901317, a synthetic liver X receptor agonist, was shown to improve neurological function after MCA occlusion in healthy but not in eNOS-deficient mice (Chen et al, 2009). The suggested neuroprotective mechanism is promotion of angiogensis via increased eNOS phosphorylation, but also antiinflammatory effects are discussed (Morales et al, 2008). Further evaluation of the mechanisms involved are necessary before clinical evaluation.
PI3 K/Akt pathway—S1177 phosphorylation—hormones
Steroids hormones when used in pharmacological doses increase CBF and improve outcome after experimental ischemic stroke (de Court et al, 1994; Bertorelli et al, 1998). This effect is due to a rapid onset of transcription-independent eNOS activity via the PI3/Akt pathway (Limbourg et al, 2002; Hafezi-Moghadam et al, 2002). In an animal model of transient cerebral ischemia, dexamethasone increased CBF and reduced infarct volume for up to 3 days after MCAo in wild type but not in eNOS knockout mice (Limbourg et al, 2002). Despite these promising data, clinical trials yielded mixed results (Patten et al, 1972; Bauer and Tellez, 1973); corticosteroids are therefore not recommended for the treatment of acute stroke (Adams et al, 2007).
Other hormones are proposed to exert their neuroprotective effect by an increase in eNOS activity: Thyroid hormone-mediated neuroprotection is mediated by the PI3/Akt pathway and eNOS phosphorylation after acute stroke (Hiroi et al, 2006). Furthermore, estrogen confers increased eNOS activity via the same mechanism (Haynes et al, 2000; Simoncini et al, 2000). While these results might add to our understanding of gender related differences in stroke incidence and outcome, it remains unclear whether hormone replacement or treatment might be a tool to acutely increase CBF after ischemic stroke. Adiponectin, an adipokine generated by visceral fat cells was shown to increase eNOS phosphorylation. Following MCAO, infarct size was larger in adiponectin-deficient mice, an effect reversed by increasing adiponectin expression in its knockout mouse (Nishimura et al, 2008). Low plasma levels of adiponectin have been implicated in obesity, hypertension, and diabetes.
Although there is ample evidence that eNOS phosphorylation is an important mechanism of action for the hormones mentioned, one has to keep in mind that given the multifaceted and sometimes contrasting effects of hormones eNOS modulation might not be the sole mechanism of action.
Other Therapeutic Strategies Conferring Neuroprotection by Endothelial Nitric Oxide Synthases Activation
There are a variety of other pharmacological and nonpharmacological strategies that seem to exert neuroprotection mainly via modulation of eNOS activity.
Experimental studies proved that exercise increases eNOS activity, thus improving infarct volume and neurological outcome (Endres et al, 2003; Gertz et al, 2006). The mechanism may relate to increased shear stress. In line with these findings, the protective effect of prestroke exercise was absent in eNOS null mice (Gertz et al, 2006). However, there is no conclusive evidence that physical activity can be neuroprotective when started after stroke (Johansson, 2003; Gertz et al, 2006). Nevertheless, the importance of exercise in primary and secondary prevention of ischemic stroke is undoubted.
Among other drugs thought to confer stroke protection via the eNOS/cGMP pathway are the phytoalexine resveratrol, the neuro- and cardio-protectant found in red wine (Tsai et al, 2007), peroxisome proliferator-activated receptor-γ agonists (Chu et al, 2006), and angiotensin II receptor 1 antagonists (Saavedra et al, 2006; Oyama et al, 2010). These approaches, however, need to be examined more closely before clinical evaluation is feasible; as with hormones, it remains to be elucidated whether eNOS phosphorylation is the only important mechanism or whether there are other important modes of action.
Inhaled Nitric Oxide Donors
As noted above, many promising strategies that increase the availability of NO to the vessel wall have distinct problems in the context of acute cerebral ischemia. Nevertheless, a rapidly acting, easily accessible treatment is actively being sought that increases the availability of NO to acutely improve CBF—especially within the penumbra.
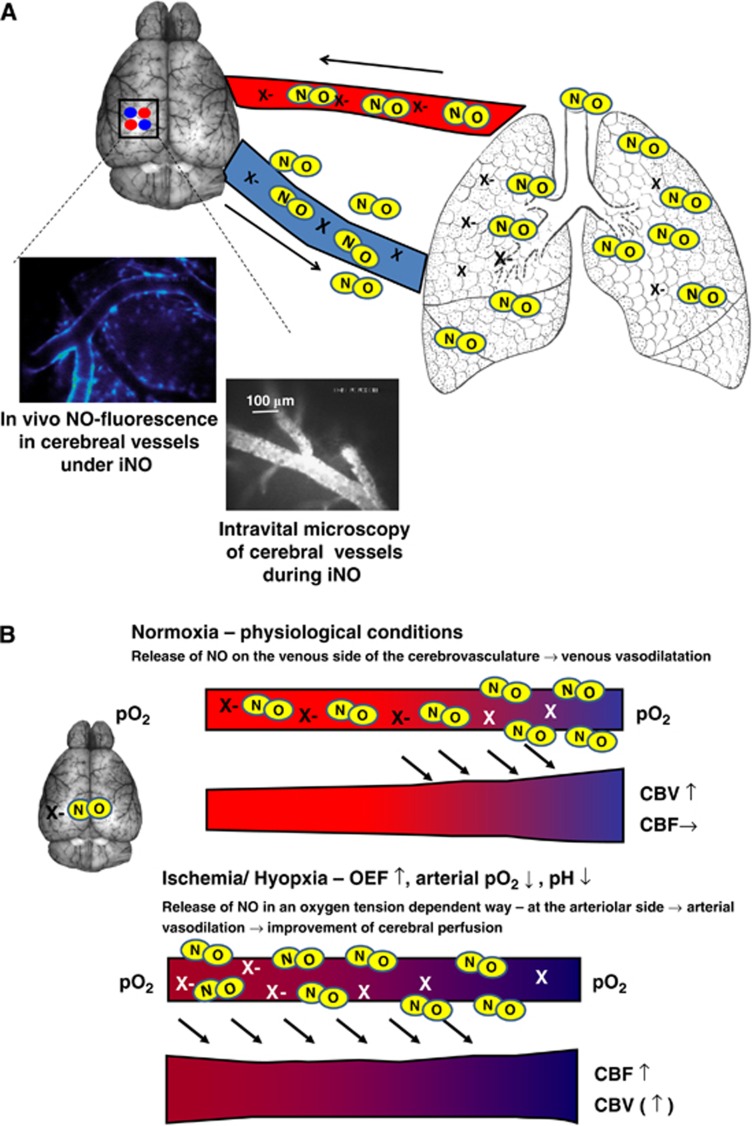
Nitric oxide inhalation (inhaled nitric oxide, iNO) has been used to treat diseases characterized by pathological pulmonary vasoconstriction, for example, pulmonary hypertension since the early 1990s. Initially, it was hypothesized that NO was rapidly inactivated in pulmonary vessels once in contact with oxyhemoglobin, thus restricting the vasodilatory effect to the lung. This was consistent with the findings that iNO therapy at moderate doses did not alter systemic vascular resistance and—thus—systemic blood pressure in experimental and clinical studies. Based on this notion and the fact that other significant adverse effects could be excluded, iNO was approved for the treatment of persistent pulmonary hypertension in neonates and later used for other pulmonary conditions (Griffiths and Evans, 2005; Kinsella and Abman, 2005). Over the ensuing decade, evidence was presented that strongly suggested that inhaled NO led to distinct extrapulmonary effects, for example, in mesenteric, renal, and cardiac vessels. Recently, there is growing evidence that iNO might be protective in reducing ischemia–reperfusion injury within mesentery (Fox-Robichaud et al, 1998), and myocardium (Nagasaka et al, 2008), thus reducing tissue damage. While experimental (Rosenberg et al, 1995; Lopes Cardozo et al, 1996; Kusuda et al, 1999) and clinical (Vavilala et al, 2001) studies could not detect changes in CBF during iNO therapy, one experimental study revealed that while it did not alter cerebral perfusion, iNO was shown to possess a cerebrovascular effect (Kuebler et al, 2003). Nitric oxide inhalation is a clinically approved treatment that has few side effects that might positively influence the outcome following ischemic stroke. Terpolilli et al (2011) recently demonstrated an effect of iNO on the cerebrovasculature using in vivo microscopy. In their studies under physiological conditions, iNO led to a significant dilatation of cerebral venules, most probably due to an oxygen-tension-dependent mechanism. Under conditions of ischemia, iNO led to arteriolar dilatation and an improvement in cerebral perfusion (see Figure 2). After experimental ischemic stroke, iNO reduced lesion size, improved CBF, cerebral metabolism, and neurological outcome, in part dependent upon improved collateral blood flow to the penumbra. There were no obvious adverse effects of this treatment. Clinical evaluation of these NO inhalation effects is planned in the near future.
Figure 2.
Nitric oxide (NO) inhalation (iNO). (A) Upon inhalation, NO is transported to the brain via the blood in a bioactive form; the nature of this NO carrier (X) is not fully elucidated yet. Among the molecules discussed are S-nitroso-hemoglobin, S-nitrosothiols, and nitrite. In the brain, inhaled NO leads to an increase of NO in the vessel wall (left inlet, in vivo NO fluorescence imaged with NO-sensitive dye DAF-FM) and to dilatation of cerebral venules (right inlet, in vivo imaging of vessel diameter using FITC-dextran). (B) NO inhalation—Putative mode of action: Bioactive NO is released via an oxygen-tension-dependent mechanism; under physiological conditions this happens in the venular compartment of the cerebrovasculature. Release of NO leads to venular dilatation and—thus—to an increase in cerebral blood volume (CBV) without influencing cerebral blood flow (CBF). During cerebral ischemia, oxygen extraction fraction (OEF) increases leading to increased oxygen desaturation, lowering of arteriolar pO2 and pH on the arteriolar side of the cerebrovasculature. Under these conditions, NO release occurs also on the arteriolar side. This induces arteriolar dilatation and, thereby, increase of CBF. Since the mechanism is restricted to ischemic vessels, normally perfused tissue is not affected. NO inhalation therefore seems to be an ideal tool to counteract regional ischemia by increasing blood flow exclusively to malperfused tissue. DAF-FM, 4-amino-5-(N-methylamino)-3′,6′-bis(acetyloxy)-2′,7′-difluoro-spiro[isobenzofuran-1(3H),9′-[9H]xanthen]-3-one, diaminofluorescein-FM diacetate; FITC, fluorescein 5-isothiocyanate.
The extrapulmonary effects of other inhaled NO donating agents such as nebulized sodium nitroprusside (Fattouch et al, 2005), inhaled ethyl nitrite (Auten et al, 2007), and inhaled nitrite (Blood et al, 2011) are being investigated in the context of pulmonary pathologies because the observed effects seem at least in part to be mediated by the same pathways as iNO. In the case of inhaled ethyl nitrite, neuroprotection after experimental subarachnoid hemorrhage has recently been demonstrated (Sheng et al, 2011), so that this might be another promising inhalative treatment strategy for cerebral ischemia.
Conclusion
Reduction in NO signaling pathways have been well documented after ischemic stroke, especially within the blood vessel wall. Many strategies have been used to restore these pathways in order to improve CBF and improve histopathological and functional outcome after cerebral ischemia. While some NO-based therapeutical approaches have been implemented into clinical guidelines for stroke prevention, most strategies for acute stroke treatment are still in the experimental stage and more research is needed to explore novel, side effect-free and specific NO-based therapeutic approaches for cerebral ischemia.
The authors declare no conflict of interest.
References
- Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- Amarenco P, Bogousslavsky J, Callahan A, III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- Amarenco P, Labreuche J, Lavallee P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35:2902–2909. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001;32:980–986. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- Atochin DN, Clark J, Demchenko IT, Moskowitz MA, Huang PL. Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases. Stroke. 2003;34:1299–1303. doi: 10.1161/01.STR.0000066870.70976.57. [DOI] [PubMed] [Google Scholar]
- Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atochin DN, Yuzawa I, Li Q, Rauwerdink KM, Malhotra R, Chang J, Brouckaert P, Ayata C, Moskowitz MA, Bloch KD, Huang PL, Buys ES. Soluble guanylate cyclase alpha1beta1 limits stroke size and attenuates neurological injury. Stroke. 2010;41:1815–1819. doi: 10.1161/STROKEAHA.109.577635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auten RL, Mason SN, Whorton MH, Lampe WR, Foster WM, Goldberg RN, Li B, Stamler JS, Auten KM. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med. 2007;176:291–299. doi: 10.1164/rccm.200605-662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath PM, Willmot M, Leonardi-Bee J, Bath FJ.2002Nitric oxide donors (nitrates), L-arginine, or nitric oxide synthase inhibitors for acute strokeCochrane Database Syst Rev CD000398 [DOI] [PubMed]
- Bauer RB, Tellez H. Dexamethasone as treatment in cerebrovascular disease. 2. A controlled study in acute cerebral infarction. Stroke. 1973;4:547–555. doi: 10.1161/01.str.4.4.547. [DOI] [PubMed] [Google Scholar]
- Bechade C, Pascual O, Triller A, Bessis A. Nitric oxide regulates astrocyte maturation in the hippocampus: involvement of NOS2. Mol Cell Neurosci. 2011;46:762–769. doi: 10.1016/j.mcn.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- Becker K, Tanzi P, Kalil A, Shibata D, Cain K.2011Early statin use is associated with increased risk of infection after stroke J Stroke Cerebrovasc Dise-pub ahead of print, 22 July 2011; doi: 10.1016/j.jstrokecerebovasdis.2011.06.008 [DOI] [PMC free article] [PubMed]
- Berger C, Xia F, Maurer MH, Schwab S. Neuroprotection by pravastatin in acute ischemic stroke in rats. Brain Res Rev. 2008;58:48–56. doi: 10.1016/j.brainresrev.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Bertorelli R, Adami M, Di Santo E, Ghezzi P. MK 801 and dexamethasone reduce both tumor necrosis factor levels and infarct volume after focal cerebral ischemia in the rat brain. Neurosci Lett. 1998;246:41–44. doi: 10.1016/s0304-3940(98)00221-3. [DOI] [PubMed] [Google Scholar]
- Blood AB, Schroeder HJ, Terry MH, Merrill-Henry J, Bragg SL, Vrancken K, Liu T, Herring JL, Sowers LC, Wilson SM, Power GG. Inhaled nitrite reverses hemolysis-induced pulmonary vasoconstriction in newborn lambs without blood participation. Circulation. 2011;123:605–612. doi: 10.1161/CIRCULATIONAHA.110.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- Bosel J, Gandor F, Harms C, Synowitz M, Harms U, Djoufack PC, Megow D, Dirnagl U, Hortnagl H, Fink KB, Endres M. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92:1386–1398. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham LR, Kruit JK, Pape TD, Parks JS, Kuipers F, Hayden MR. Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ Res. 2006;99:672–674. doi: 10.1161/01.RES.0000244014.19589.8e. [DOI] [PubMed] [Google Scholar]
- Cash D, Beech JS, Rayne RC, Bath PM, Meldrum BS, Williams SC. Neuroprotective effect of aminoguanidine on transient focal ischaemia in the rat brain. Brain Res. 2001;905:91–103. doi: 10.1016/s0006-8993(01)02508-2. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Jiang H, Roberts C, Zhang C, Lu M, Kapke A, Feldkamp CS, Chopp M. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Roberts C, Chopp M. eNOS mediates TO90317 treatment-induced angiogenesis and functional outcome after stroke in mice. Stroke. 2009;40:2532–2538. doi: 10.1161/STROKEAHA.108.545095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005:21–28. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1992;176:599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Park EM, Zhou P, Frys K, Ross ME, Iadecola C. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:493–501. doi: 10.1038/sj.jcbfm.9600058. [DOI] [PubMed] [Google Scholar]
- Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- Chu K, Lee ST, Koo JS, Jung KH, Kim EH, Sinn DI, Kim JM, Ko SY, Kim SJ, Song EC, Kim M, Roh JK. Peroxisome proliferator-activated receptor-gamma-agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemia. Brain Res. 2006;1093:208–218. doi: 10.1016/j.brainres.2006.03.114. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Adachi T. Nitric-oxide-induced vasodilatation: regulation by physiologic s-glutathiolation and pathologic oxidation of the sarcoplasmic endoplasmic reticulum calcium ATPase. Trends Cardiovasc Med. 2006;16:109–114. doi: 10.1016/j.tcm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Ciani E. Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem Int. 2004;45:903–914. doi: 10.1016/j.neuint.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Corsani L, Bizzoco E, Pedata F, Gianfriddo M, Faussone-Pellegrini MS, Vannucchi MG. Inducible nitric oxide synthase appears and is co-expressed with the neuronal isoform in interneurons of the rat hippocampus after transient ischemia induced by middle cerebral artery occlusion. Exp Neurol. 2008;211:433–440. doi: 10.1016/j.expneurol.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Zhang C, Roberts C, Chen J. Role of endothelial nitric oxide synthetase in arteriogenesis after stroke in mice. Neuroscience. 2009;159:744–750. doi: 10.1016/j.neuroscience.2008.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara T, Morikawa E, Panahian N, Moskowitz MA. Blood flow-dependent functional recovery in a rat model of focal cerebral ischemia. Am J Physiol. 1994;267:H678–H683. doi: 10.1152/ajpheart.1994.267.2.H678. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson VL. Nitric oxide: role in neurotoxicity. Clin Exp Pharmacol Physiol. 1995;22:305–308. doi: 10.1111/j.1440-1681.1995.tb02005.x. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Neuronal ischaemic preconditioning. Trends Pharmacol Sci. 2000;21:423–424. doi: 10.1016/s0165-6147(00)01560-1. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Wagner KR, Xi G, Myers RE. Efficacious experimental stroke treatment with high-dose methylprednisolone. Stroke. 1994;25:487–492. doi: 10.1161/01.str.25.2.487. [DOI] [PubMed] [Google Scholar]
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Niwa K, Lindauer U, Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol. 1994;267:H296–H301. doi: 10.1152/ajpheart.1994.267.1.H296. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Drew BG, Fidge NH, Gallon-Beaumier G, Kemp BE, Kingwell BA. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci USA. 2004;101:6999–7004. doi: 10.1073/pnas.0306266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam MB, Hunninghake DB, Davis KB, Garg R, Johnson C, Egan D, Kostis JB, Sheps DS, Brinton EA. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial disease multiple intervention trial. JAMA. 2000;284:1263–1270. doi: 10.1001/jama.284.10.1263. [DOI] [PubMed] [Google Scholar]
- Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schrock H, Nickenig G, Kuschinsky W, Dirnagl U, Laufs U. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54:582–590. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27:283–289. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- ENOS Trial Investigators Glyceryl trinitrate versus control, and continuing versus stopping temporarily prior antihypertensive therapy, in acute stroke: rationale and design of the efficacy of nitric oxide in Stroke (ENOS) trial (ISRCTN99414122) Int J Stroke. 2006;1:245–249. doi: 10.1111/j.1747-4949.2006.00059.x. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Fatar M, Ragoschke A, Picard M, Bertsch T, Kuehl S, Hennerici M. Subacute but not acute generation of nitric oxide in focal cerebral ischemia. Stroke. 2000;31:2208–2211. doi: 10.1161/01.str.31.9.2208. [DOI] [PubMed] [Google Scholar]
- Fattouch K, Sbraga F, Bianco G, Speziale G, Gucciardo M, Sampognaro R, Ruvolo G. Inhaled prostacyclin, nitric oxide, and nitroprusside in pulmonary hypertension after mitral valve replacement. J Card Surg. 2005;20:171–176. doi: 10.1111/j.0886-0440.2005.200383w.x. [DOI] [PubMed] [Google Scholar]
- Fleming I, Gray GA, Schott C, Stoclet JC. Inducible but not constitutive production of nitric oxide by vascular smooth muscle cells. Eur J Pharmacol. 1991;200:375–376. doi: 10.1016/0014-2999(91)90602-m. [DOI] [PubMed] [Google Scholar]
- Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Carvalho MH, Khan MT, Matsunaga K. Evidence for endothelium-dependent vasodilation of resistance vessels by acetylcholine. Blood Vessels. 1987;24:145–149. doi: 10.1159/000158689. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, Ji S, Milosevic M, Harms C, Bohm M, Dirnagl U, Laufs U, Endres M. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- Gibbs SM. Regulation of neuronal proliferation and differentiation by nitric oxide. Mol Neurobiol. 2003;27:107–120. doi: 10.1385/MN:27:2:107. [DOI] [PubMed] [Google Scholar]
- Gibson WH, Roughton FJ. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J Physiol. 1957;136:507–524. doi: 10.1113/jphysiol.1957.sp005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Shah AR, Maceren RG, Wang Q, Pelligrino DA, Holtzman DM, Park TS. Nitric oxide mediates cerebral ischemic tolerance in a neonatal rat model of hypoxic preconditioning. J Cereb Blood Flow Metab. 1999;19:331–340. doi: 10.1097/00004647-199903000-00011. [DOI] [PubMed] [Google Scholar]
- Goldstein LB, Amarenco P, Szarek M, Callahan A, III, Hennerici M, Sillesen H, Zivin JA, Welch KM. Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology. 2008;70:2364–2370. doi: 10.1212/01.wnl.0000296277.63350.77. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zulueta M, Feldman AB, Klesse LJ, Kalb RG, Dillman JF, Parada LF, Dawson TM, Dawson VL. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc Natl Acad Sci USA. 2000;97:436–441. doi: 10.1073/pnas.97.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683–2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Vega GL, McGovern ME, Tulloch BR, Kendall DM, Fitz-Patrick D, Ganda OP, Rosenson RS, Buse JB, Robertson DD, Sheehan JP. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med. 2002;162:1568–1576. doi: 10.1001/archinte.162.14.1568. [DOI] [PubMed] [Google Scholar]
- Guyton JR. Effect of niacin on atherosclerotic cardiovascular disease. Am J Cardiol. 1998;82:18U–23U. doi: 10.1016/s0002-9149(98)00767-x. [DOI] [PubMed] [Google Scholar]
- Guyton JR, Blazing MA, Hagar J, Kashyap ML, Knopp RH, McKenney JM, Nash DT, Nash SD. Extended-release niacin versus gemfibrozil for the treatment of low levels of high-density lipoprotein cholesterol. Niaspan-Gemfibrozil Study Group. Arch Intern Med. 2000;160:1177–1184. doi: 10.1001/archinte.160.8.1177. [DOI] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8:473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurol. 2007;6:115–124. doi: 10.1016/S1474-4422(06)70685-8. [DOI] [PubMed] [Google Scholar]
- Hara H, Huang PL, Panahian N, Fishman MC, Moskowitz MA. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J Cereb Blood Flow Metab. 1996;16:605–611. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(11)61125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, Moskowitz MA, Cheng SY, Liao JK. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci USA. 2006;103:14104–14109. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Does nitric oxide mediate the increases in cerebral blood flow elicited by hypercapnia. Proc Natl Acad Sci USA. 1992;89:3913–3916. doi: 10.1073/pnas.89.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol. 1995;268:R286–R292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987a;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987b;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Kageyama R. Continuous neurogenesis in the adult brain. Dev Growth Differ. 2009;51:379–386. doi: 10.1111/j.1440-169X.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- Jin HG, Yamashita H, Nagano Y, Fukuba H, Hiji M, Ohtsuki T, Takahashi T, Kohriyama T, Kaibuchi K, Matsumoto M. Hypoxia-induced upregulation of endothelial small G protein RhoA and Rho-kinase/ROCK2 inhibits eNOS expression. Neurosci Lett. 2006;408:62–67. doi: 10.1016/j.neulet.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Johansson BB. Environmental influence on recovery after brain lesions—experimental and clinical data. J Rehabil Med. 2003;41 (Suppl:11–16. doi: 10.1080/16501960310010089. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- Kader A, Frazzini VI, Solomon RA, Trifiletti RR. Nitric oxide production during focal cerebral ischemia in rats. Stroke. 1993;24:1709–1716. doi: 10.1161/01.str.24.11.1709. [DOI] [PubMed] [Google Scholar]
- Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961–969. doi: 10.1016/S1474-4422(07)70250-8. [DOI] [PubMed] [Google Scholar]
- Kilic U, Bassetti CL, Kilic E, Xing H, Wang Z, Hermann DM. Post-ischemic delivery of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor rosuvastatin protects against focal cerebral ischemia in mice via inhibition of extracellular-regulated kinase-1/-2. Neuroscience. 2005;134:901–906. doi: 10.1016/j.neuroscience.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Kim HH, Sawada N, Soydan G, Lee HS, Zhou Z, Hwang SK, Waeber C, Moskowitz MA, Liao JK. Additive effects of statin and dipyridamole on cerebral blood flow and stroke protection. J Cereb Blood Flow Metab. 2008;28:1285–1293. doi: 10.1038/jcbfm.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella JP, Abman SH. Inhaled nitric oxide therapy in children. Paediatr Respir Rev. 2005;6:190–198. doi: 10.1016/j.prrv.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K. ‘Ischemic tolerance' phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci USA. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler WM, Kisch-Wedel H, Kemming GI, Meisner F, Bruhn S, Koehler C, Flondor M, Messmer K, Zwissler B. Inhaled nitric oxide induces cerebrovascular effects in anesthetized pigs. Neurosci Lett. 2003;348:85–88. doi: 10.1016/s0304-3940(03)00722-5. [DOI] [PubMed] [Google Scholar]
- Kunz A, Park L, Abe T, Gallo EF, Anrather J, Zhou P, Iadecola C. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci. 2007;27:7083–7093. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuda S, Shishida N, Miyagi N, Hirabayashi M, Kim TJ. Cerebral blood flow during treatment for pulmonary hypertension. Arch Dis Child Fetal Neonatal Ed. 1999;80:F30–F33. doi: 10.1136/fn.80.1.f30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen NA. Pathophysiology of brain ischemia as it relates to the therapy of acute ischemic stroke. Clin Neuropharmacol. 1990;13 (Suppl 3:S1–S8. doi: 10.1097/00002826-199013003-00001. [DOI] [PubMed] [Google Scholar]
- Laufs U, Endres M, Liao JK. [Regulation of endothelial NO production by Rho GTPase] Med Klin (Munich) 1999;94:211–218. doi: 10.1007/BF03044857. [DOI] [PubMed] [Google Scholar]
- Laufs U, Endres M, Stagliano N, Amin-Hanjani S, Chui DS, Yang SX, Simoncini T, Yamada M, Rabkin E, Allen PG, Huang PL, Bohm M, Schoen FJ, Moskowitz MA, Liao JK. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J Clin Invest. 2000a;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- Laufs U, Gertz K, Dirnagl U, Bohm M, Nickenig G, Endres M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res. 2002;942:23–30. doi: 10.1016/s0006-8993(02)02649-5. [DOI] [PubMed] [Google Scholar]
- Laufs U, Gertz K, Huang P, Nickenig G, Bohm M, Dirnagl U, Endres M. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000b;31:2442–2449. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol. 2001;88:1306–1307. doi: 10.1016/s0002-9149(01)02095-1. [DOI] [PubMed] [Google Scholar]
- Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation. 2003;107:1901–1905. doi: 10.1161/01.CIR.0000057973.99140.5A. [DOI] [PubMed] [Google Scholar]
- Lee YS, Bae HJ, Kang DW, Lee SH, Yu K, Park JM, Cho YJ, Hong KS, Kim DE, Kwon SU, Lee KB, Rha JH, Koo J, Han MG, Lee SJ, Lee JH, Jung SW, Lee BC, Kim JS. Cilostazol in Acute Ischemic Stroke Treatment (CAIST Trial): a randomized double-blind non-inferiority trial. Cerebrovasc Dis. 2011;32:65–71. doi: 10.1159/000327036. [DOI] [PubMed] [Google Scholar]
- Leist M, Fava E, Montecucco C, Nicotera P. Peroxynitrite and nitric oxide donors induce neuronal apoptosis by eliciting autocrine excitotoxicity. Eur J Neurosci. 1997a;9:1488–1498. doi: 10.1111/j.1460-9568.1997.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Leist M, Volbracht C, Kuhnle S, Fava E, Ferrando-May E, Nicotera P. Caspase-mediated apoptosis in neuronal excitotoxicity triggered by nitric oxide. Mol Med. 1997b;3:750–764. [PMC free article] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbourg FP, Huang Z, Plumier JC, Simoncini T, Fujioka M, Tuckermann J, Schutz G, Moskowitz MA, Liao JK. Rapid nontranscriptional activation of endothelial nitric oxide synthase mediates increased cerebral blood flow and stroke protection by corticosteroids. J Clin Invest. 2002;110:1729–1738. doi: 10.1172/JCI15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Hara H, Rogowska J, Trocha M, Pierce AR, Huang PL, Fishman MC, Wolf GL, Moskowitz MA. Temporal correlation mapping analysis of the hemodynamic penumbra in mutant mice deficient in endothelial nitric oxide synthase gene expression. Stroke. 1996;27:1381–1385. doi: 10.1161/01.str.27.8.1381. [DOI] [PubMed] [Google Scholar]
- Lopes Cardozo RH, de Beaufort AJ, Gesink BJ, Moison RM, van de BM, Berger HM, van Bel F. Inhalation of nitric oxide: effect on cerebral hemodynamics and activity, and antioxidant status in the newborn lamb. Biol Neonate. 1996;69:284–292. doi: 10.1159/000244322. [DOI] [PubMed] [Google Scholar]
- Luo CX, Zhu XJ, Zhou QG, Wang B, Wang W, Cai HH, Sun YJ, Hu M, Jiang J, Hua Y, Han X, Zhu DY. Reduced neuronal nitric oxide synthase is involved in ischemia-induced hippocampal neurogenesis by up-regulating inducible nitric oxide synthase expression. J Neurochem. 2007;103:1872–1882. doi: 10.1111/j.1471-4159.2007.04915.x. [DOI] [PubMed] [Google Scholar]
- Malinski T, Bailey F, Zhang ZG, Chopp M. Nitric oxide measured by a porphyrinic microsensor in rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1993;13:355–358. doi: 10.1038/jcbfm.1993.48. [DOI] [PubMed] [Google Scholar]
- Margaill I, Allix M, Boulu RG, Plotkine M. Dose- and time-dependence of L-NAME neuroprotection in transient focal cerebral ischaemia in rats. Br J Pharmacol. 1997;120:160–163. doi: 10.1038/sj.bjp.0700889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariucci G, Taha E, Tantucci M, Spaccatini C, Tozzi A, Ambrosini MV. Intravenous administration of pravastatin immediately after middle cerebral artery occlusion reduces cerebral oedema in spontaneously hypertensive rats. Eur J Pharmacol. 2011;660:381–386. doi: 10.1016/j.ejphar.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J Biol Chem. 2003;278:9142–9149. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata K, Shimokawa H, Kandabashi T, Higo T, Morishige K, Eto Y, Egashira K, Kaibuchi K, Takeshita A. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol. 2000;20:2351–2358. doi: 10.1161/01.atv.20.11.2351. [DOI] [PubMed] [Google Scholar]
- Morales JR, Ballesteros I, Deniz JM, Hurtado O, Vivancos J, Nombela F, Lizasoain I, Castrillo A, Moro MA. Activation of liver X receptors promotes neuroprotection and reduces brain inflammation in experimental stroke. Circulation. 2008;118:1450–1459. doi: 10.1161/CIRCULATIONAHA.108.782300. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa E, Huang Z, Moskowitz MA. L-arginine decreases infarct size caused by middle cerebral arterial occlusion in SHR. Am J Physiol. 1992;263:H1632–H1635. doi: 10.1152/ajpheart.1992.263.5.H1632. [DOI] [PubMed] [Google Scholar]
- Morikawa E, Moskowitz MA, Huang Z, Yoshida T, Irikura K, Dalkara T. L-arginine infusion promotes nitric oxide-dependent vasodilation, increases regional cerebral blood flow, and reduces infarction volume in the rat. Stroke. 1994;25:429–435. doi: 10.1161/01.str.25.2.429. [DOI] [PubMed] [Google Scholar]
- Murad F, Waldman S, Molina C, Bennett B, Leitman D. Regulation and role of guanylate cyclase-cyclic GMP in vascular relaxation. Prog Clin Biol Res. 1987;249:65–76. [PubMed] [Google Scholar]
- Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner JM. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- Nagaraja TN, Knight RA, Croxen RL, Konda KP, Fenstermacher JD. Acute neurovascular unit protection by simvastatin in transient cerebral ischemia. Neurol Res. 2006;28:826–830. doi: 10.1179/174313206X153914. [DOI] [PubMed] [Google Scholar]
- Nagasaka Y, Fernandez BO, Garcia-Saura MF, Petersen B, Ichinose F, Bloch KD, Feelisch M, Zapol WM. Brief periods of nitric oxide inhalation protect against myocardial ischemia-reperfusion injury. Anesthesiology. 2008;109:675–682. doi: 10.1097/ALN.0b013e318186316e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Tsuruta S, Uchiyama S. Cilostazol combined with aspirin prevents early neurological deterioration in patients with acute ischemic stroke: a pilot study. J Neurol Sci. 2011;313:22–26. doi: 10.1016/j.jns.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Shin HK, Moskowitz MA, Ouchi N. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- Niwa M, Inao S, Takayasu M, Kawai T, Kajita Y, Nihashi T, Kabeya R, Sugimoto T, Yoshida J. Time course of expression of three nitric oxide synthase isoforms after transient middle cerebral artery occlusion in rats. Neurol Med Chir (Tokyo) 2001;41:63–72. doi: 10.2176/nmc.41.63. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Heo JI, Kho YJ, Kim JH, Kang HJ, Park SH, Kim HS, Shin JY, Kim MJ, Kim SC, Park JB, Kim J, Lee JY. Nitric oxide is an essential mediator for neuronal differentiation of rat primary cortical neuron cells. Exp Neurobiol. 2010;19:83–89. doi: 10.5607/en.2010.19.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama N, Yagita Y, Kawamura M, Sugiyama Y, Terasaki Y, Omura-Matsuoka E, Sasaki T, Kitagawa K. Cilostazol, not aspirin, reduces ischemic brain injury via endothelial protection in spontaneously hypertensive rats. Stroke. 2011;42:2571–2577. doi: 10.1161/STROKEAHA.110.609834. [DOI] [PubMed] [Google Scholar]
- Oyama N, Yagita Y, Sasaki T, Omura-Matsuoka E, Terasaki Y, Sugiyama Y, Sakoda S, Kitagawa K. An angiotensin II type 1 receptor blocker can preserve endothelial function and attenuate brain ischemic damage in spontaneously hypertensive rats. J Neurosci Res. 2010;88:2889–2898. doi: 10.1002/jnr.22441. [DOI] [PubMed] [Google Scholar]
- Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci USA. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KM, Byun JY, Kramers C, Kim JI, Huang PL, Bonventre JV. Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem. 2003;278:27256–27266. doi: 10.1074/jbc.M301778200. [DOI] [PubMed] [Google Scholar]
- Parmentier S, Bohme GA, Lerouet D, Damour D, Stutzmann JM, Margaill I, Plotkine M. Selective inhibition of inducible nitric oxide synthase prevents ischaemic brain injury. Br J Pharmacol. 1999;127:546–552. doi: 10.1038/sj.bjp.0702549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier-Batteur S, Bohme GA, Lerouet D, Zhou-Ding L, Beray V, Margaill I, Plotkine M. Antisense oligodeoxynucleotide to inducible nitric oxide synthase protects against transient focal cerebral ischemia-induced brain injury. J Cereb Blood Flow Metab. 2001;21:15–21. doi: 10.1097/00004647-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Patten BM, Mendell J, Bruun B, Curtin W, Carter S. Double-blind study of the effects of dexamethasone on acute stroke. Neurology. 1972;22:377–383. doi: 10.1212/wnl.22.4.377. [DOI] [PubMed] [Google Scholar]
- Perez-Asensio FJ, Hurtado O, Burguete MC, Moro MA, Salom JB, Lizasoain I, Torregrosa G, Leza JC, Alborch E, Castillo J, Knowles RG, Lorenzo P. Inhibition of iNOS activity by 1400 W decreases glutamate release and ameliorates stroke outcome after experimental ischemia. Neurobiol Dis. 2005;18:375–384. doi: 10.1016/j.nbd.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Peunova N, Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature. 1995;375:68–73. doi: 10.1038/375068a0. [DOI] [PubMed] [Google Scholar]
- Ponce J, de la Ossa NP, Hurtado O, Millan M, Arenillas JF, Davalos A, Gasull T. Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol-mediated effect in neuroprotection. Stroke. 2008;39:1269–1275. doi: 10.1161/STROKEAHA.107.498923. [DOI] [PubMed] [Google Scholar]
- Prinz V, Laufs U, Gertz K, Kronenberg G, Balkaya M, Leithner C, Lindauer U, Endres M. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39:433–438. doi: 10.1161/STROKEAHA.107.492470. [DOI] [PubMed] [Google Scholar]
- Reif A, Schmitt A, Fritzen S, Chourbaji S, Bartsch C, Urani A, Wycislo M, Mossner R, Sommer C, Gass P, Lesch KP. Differential effect of endothelial nitric oxide synthase (NOS-III) on the regulation of adult neurogenesis and behaviour. Eur J Neurosci. 2004;20:885–895. doi: 10.1111/j.1460-9568.2004.03559.x. [DOI] [PubMed] [Google Scholar]
- Richards HK, Kozniewska E, Czosnyka M, Pickard JD. Changes in transcranial Doppler flow velocity waveform following inhibition of nitric oxide synthesis. Experimental study in anaesthetised rabbits. Acta Neurochir (Wien) 1997;139:63–69. doi: 10.1007/BF01850870. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AA, Kinsella JP, Abman SH. Cerebral hemodynamics and distribution of left ventricular output during inhalation of nitric oxide. Crit Care Med. 1995;23:1391–1397. doi: 10.1097/00003246-199508000-00013. [DOI] [PubMed] [Google Scholar]
- Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM, Benicky J, Zhou J. Mechanisms of the anti-ischemic effect of Angiotensin II AT(1) receptor antagonists in the brain. Cell Mol Neurobiol. 2006;26:1099–1111. doi: 10.1007/s10571-006-9009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno L, Sorrenti V, Di Giacomo C, Romeo G, Siracusa MA. Progress in the development of selective nitric oxide synthase (NOS) inhibitors. Curr Pharm Des. 2002;8:177–200. doi: 10.2174/1381612023396375. [DOI] [PubMed] [Google Scholar]
- Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens RL, Marcel YL, Rader DJ, Shaul PW. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. 2006;98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- Sehara Y, Hayashi T, Deguchi K, Nagotani S, Zhang H, Shoji M, Abe K. Distribution of inducible nitric oxide synthase and cell proliferation in rat brain after transient middle cerebral artery occlusion. Brain Res. 2006;1093:190–197. doi: 10.1016/j.brainres.2006.03.092. [DOI] [PubMed] [Google Scholar]
- Serebruany V, Sani Y, Eisert C, Schevchuck A, Fong A, Hanley D. Effects of Aggrenox and aspirin on plasma endothelial nitric oxide synthase and oxidised low-density lipoproteins in patients after ischaemic stroke. The AGgrenox versus aspirin therapy evaluation (AGATE) biomarker substudy. Thromb Haemost. 2011;105:81–87. doi: 10.1160/TH10-05-0316. [DOI] [PubMed] [Google Scholar]
- Shehadah A, Chen J, Zacharek A, Cui Y, Ion M, Roberts C, Kapke A, Chopp M. Niaspan treatment induces neuroprotection after stroke. Neurobiol Dis. 2010;40:277–283. doi: 10.1016/j.nbd.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Reynolds JD, Auten RL, Demchenko IT, Piantadosi CA, Stamler JS, Warner DS. Pharmacologically augmented S-nitrosylated hemoglobin improves recovery from murine subarachnoid hemorrhage. Stroke. 2011;42:471–476. doi: 10.1161/STROKEAHA.110.600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2002;39:319–327. doi: 10.1097/00005344-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Shin HK, Salomone S, Potts EM, Lee SW, Millican E, Noma K, Huang PL, Boas DA, Liao JK, Moskowitz MA, Ayata C. Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab. 2007;27:998–1009. doi: 10.1038/sj.jcbfm.9600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L, Cimino M, Guerrini U, Calvio AM, Lodetti B, Asdente M, Balduini W, Paoletti R, Tremoli E. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arterioscler Thromb Vasc Biol. 2003;23:322–327. doi: 10.1161/01.atv.0000044458.23905.3b. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Sharma SS. 3-Bromo-7-nitroindazole attenuates brain ischemic injury in diabetic stroke via inhibition of endoplasmic reticulum stress pathway involving CHOP. Life Sci. 2012;90:154–160. doi: 10.1016/j.lfs.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Stagliano NE, Perez-Pinzon MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 1999;19:757–761. doi: 10.1097/00004647-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Sugimura T, Sako K, Tohyama Y, Yonemasu Y. Consecutive in vivo measurement of nitric oxide in transient forebrain ischemic rat under normothermia and hypothermia. Brain Res. 1998;808:313–316. doi: 10.1016/s0006-8993(98)00822-1. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Yagita Y, Sasaki T, Todo K, Terasaki Y, Ohyama N, Hori M, Kitagawa K. Postischemic administration of HMG CoA reductase inhibitor inhibits infarct expansion after transient middle cerebral artery occlusion. Brain Res. 2007;1181:125–129. doi: 10.1016/j.brainres.2007.08.069. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Neuronal nitric oxide synthase and ischemia-induced neurogenesis. J Cereb Blood Flow Metab. 2005;25:485–492. doi: 10.1038/sj.jcbfm.9600049. [DOI] [PubMed] [Google Scholar]
- Takahara A, Sugiyama A, Satoh Y, Yoneyama M, Hashimoto K. Cardiovascular effects of Y-27632, a selective Rho-associated kinase inhibitor, assessed in the halothane-anesthetized canine model. Eur J Pharmacol. 2003;460:51–57. doi: 10.1016/s0014-2999(02)02929-1. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Is nitric oxide really important for regulation of the cerebral circulation? Yes or no. Keio J Med. 1996;45:14–27. doi: 10.2302/kjm.45.14. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yoshida S, Yano M, Hanaoka F. Roles of endogenous nitric oxide in cerebellar cortical development in slice cultures. Neuroreport. 1994;5:2049–2052. doi: 10.1097/00001756-199410270-00015. [DOI] [PubMed] [Google Scholar]
- Tegenge MA, Bicker G. Nitric oxide and cGMP signal transduction positively regulates the motility of human neuronal precursor (NT2) cells. J Neurochem. 2009;110:1828–1841. doi: 10.1111/j.1471-4159.2009.06279.x. [DOI] [PubMed] [Google Scholar]
- Terpolilli NA, Kim SW, Thal SC, Kataoka H, Zeisig V, Nitzsche B, Klaesner B, Zhu C, Schwarzmaier S, Meissner L, Mamrak U, Engel DC, Drzezga A, Patel RP, Blomgren K, Barthel H, Boltze J, Kuebler W, Plesnila N.2011Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles Circ Rese-pub ahead of print, 29 December 2011; doi: 10.1161/CIRCRESAHA.111.253419 [DOI] [PubMed]
- Terpolilli NA, Zweckberger K, Trabold R, Schilling L, Schinzel R, Tegtmeier F, Plesnila N. The novel nitric oxide synthase inhibitor 4-amino-tetrahydro-L-biopterine prevents brain edema formation and intracranial hypertension following traumatic brain injury in mice. J Neurotrauma. 2009;26:1963–1975. doi: 10.1089/neu.2008.0853. [DOI] [PubMed] [Google Scholar]
- Thompson BG, Pluta RM, Girton ME, Oldfield EH. Nitric oxide mediation of chemoregulation but not autoregulation of cerebral blood flow in primates. J Neurosurg. 1996;84:71–78. doi: 10.3171/jns.1996.84.1.0071. [DOI] [PubMed] [Google Scholar]
- Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW, Liu HY, Zhang FB, Huang SS. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg. 2007;46:346–353. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Uetsuka S, Fujisawa H, Yasuda H, Shima H, Suzuki M. Severe cerebral blood flow reduction inhibits nitric oxide synthesis. J Neurotrauma. 2002;19:1105–1116. doi: 10.1089/089771502760342009. [DOI] [PubMed] [Google Scholar]
- Vavilala MS, Roberts JS, Moore AE, Newell DW, Lam AM. The influence of inhaled nitric oxide on cerebral blood flow and metabolism in a child with traumatic brain injury. Anesth Analg. 2001;93:351–353. doi: 10.1097/00000539-200108000-00023. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB. Statin treatment and the occurrence of hemorrhagic stroke in patients with a history of cerebrovascular disease. Stroke. 2008;39:497–502. doi: 10.1161/STROKEAHA.107.488791. [DOI] [PubMed] [Google Scholar]
- Viani P, Giussani P, Riboni L, Bassi R, Tettamanti G. Behaviour of nitric oxide synthase in rat cerebellar granule cells differentiating in culture. FEBS Lett. 1997;408:131–134. doi: 10.1016/s0014-5793(97)00406-7. [DOI] [PubMed] [Google Scholar]
- Vorstrup S, Brun B, Lassen NA. Evaluation of the cerebral vasodilatory capacity by the acetazolamide test before EC-IC bypass surgery in patients with occlusion of the internal carotid artery. Stroke. 1986;17:1291–1298. doi: 10.1161/01.str.17.6.1291. [DOI] [PubMed] [Google Scholar]
- Wei HM, Chi OZ, Liu X, Sinha AK, Weiss HR. Nitric oxide synthase inhibition alters cerebral blood flow and oxygen balance in focal cerebral ischemia in rats. Stroke. 1994;25:445–449. doi: 10.1161/01.str.25.2.445. [DOI] [PubMed] [Google Scholar]
- Wei L, Roberts W, Wang L, Yamada M, Zhang S, Zhao Z, Rivkees SA, Schwartz RJ, Imanaka-Yoshida K. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development. 2001;128:2953–2962. doi: 10.1242/dev.128.15.2953. [DOI] [PubMed] [Google Scholar]
- White RP, Vallance P, Markus HS. Effect of inhibition of nitric oxide synthase on dynamic cerebral autoregulation in humans. Clin Sci (Lond) 2000;99:555–560. [PubMed] [Google Scholar]
- Willmot M, Ghadami A, Whysall B, Clarke W, Wardlaw J, Bath PM. Transdermal glyceryl trinitrate lowers blood pressure and maintains cerebral blood flow in recent stroke. Hypertension. 2006;47:1209–1215. doi: 10.1161/01.HYP.0000223024.02939.1e. [DOI] [PubMed] [Google Scholar]
- Willmot M, Gibson C, Gray L, Murphy S, Bath P. Nitric oxide synthase inhibitors in experimental ischemic stroke and their effects on infarct size and cerebral blood flow: a systematic review. Free Radic Biol Med. 2005a;39:412–425. doi: 10.1016/j.freeradbiomed.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Willmot M, Gray L, Gibson C, Murphy S, Bath PM. A systematic review of nitric oxide donors and L-arginine in experimental stroke; effects on infarct size and cerebral blood flow. Nitric Oxide. 2005b;12:141–149. doi: 10.1016/j.niox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Limmroth V, Irikura K, Moskowitz MA. The NOS inhibitor, 7-nitroindazole, decreases focal infarct volume but not the response to topical acetylcholine in pial vessels. J Cereb Blood Flow Metab. 1994;14:924–929. doi: 10.1038/jcbfm.1994.123. [DOI] [PubMed] [Google Scholar]
- Zacco A, Togo J, Spence K, Ellis A, Lloyd D, Furlong S, Piser T. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003;23:11104–11111. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharchuk G, Hara H, Huang PL, Fishman MC, Moskowitz MA, Jenkins BG, Rosen BR. Neuronal nitric oxide synthase mutant mice show smaller infarcts and attenuated apparent diffusion coefficient changes in the peri-infarct zone during focal cerebral ischemia. Magn Reson Med. 1997;37:170–175. doi: 10.1002/mrm.1910370204. [DOI] [PubMed] [Google Scholar]
- Zhang F, Casey RM, Ross ME, Iadecola C. Aminoguanidine ameliorates and L-arginine worsens brain damage from intraluminal middle cerebral artery occlusion. Stroke. 1996a;27:317–323. doi: 10.1161/01.str.27.2.317. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–416. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Chopp M. Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology. 2008;55:345–352. doi: 10.1016/j.neuropharm.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Reif D, Macdonald J, Tang WX, Kamp DK, Gentile RJ, Shakespeare WC, Murray RJ, Chopp M. ARL 17477, a potent and selective neuronal NOS inhibitor decreases infarct volume after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1996b;16:599–604. doi: 10.1097/00004647-199607000-00009. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ross ME, Iadecola C. L-Arginine increases ischemic injury in wild-type mice but not in iNOS-deficient mice. Brain Res. 2003;966:308–311. doi: 10.1016/s0006-8993(02)04223-3. [DOI] [PubMed] [Google Scholar]
- Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23:223–229. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XJ, Hua Y, Jiang J, Zhou QG, Luo CX, Han X, Lu YM, Zhu DY. Neuronal nitric oxide synthase-derived nitric oxide inhibits neurogenesis in the adult dentate gyrus by down-regulating cyclic AMP response element binding protein phosphorylation. Neuroscience. 2006;141:827–836. doi: 10.1016/j.neuroscience.2006.04.032. [DOI] [PubMed] [Google Scholar]