Abstract
Mitochondrial dysfunction contributes to the pathophysiology of acute neurologic disorders and neurodegenerative diseases. Bioenergetic failure is the primary cause of acute neuronal necrosis, and involves excitotoxicity-associated mitochondrial Ca2+ overload, resulting in opening of the inner membrane permeability transition pore and inhibition of oxidative phosphorylation. Mitochondrial energy metabolism is also very sensitive to inhibition by reactive O2 and nitrogen species, which modify many mitochondrial proteins, lipids, and DNA/RNA, thus impairing energy transduction and exacerbating free radical production. Oxidative stress and Ca2+-activated calpain protease activities also promote apoptosis and other forms of programmed cell death, primarily through modification of proteins and lipids present at the outer membrane, causing release of proapoptotic mitochondrial proteins, which initiate caspase-dependent and caspase-independent forms of cell death. This review focuses on three classifications of mitochondrial targets for neuroprotection. The first is mitochondrial quality control, maintained by the dynamic processes of mitochondrial fission and fusion and autophagy of abnormal mitochondria. The second includes targets amenable to ischemic preconditioning, e.g., electron transport chain components, ion channels, uncoupling proteins, and mitochondrial biogenesis. The third includes mitochondrial proteins and other molecules that defend against oxidative stress. Each class of targets exhibits excellent potential for translation to clinical neuroprotection.
Keywords: biogenesis, mitophagy, Nrf2, oxidative stress, peroxisome proliferator-activated receptor-gamma coactivator 1a, protein kinase C
Introduction
Mitochondria are principal mediators of cell death that occurs during both central nervous system injury and in chronic neurodegenerative disorders; see Nagley et al (2010) and Schon and Przedborski (2011) for comprehensive reviews. Mitochondria are therefore important targets for neuroprotective interventions. The significance of mitochondrial dysfunction was previously thought to be limited to effects on mitochondrial ATP production and therefore to necrotic cell death. We now understand that mitochondrial mechanisms of cell death include mitochondrial contribution to oxidative stress, apoptosis, and other forms of programmed cell death. Mild mitochondrial injury, with maintenance of near-normal cellular ATP, results in mainly programmed cell death, while more extensive injury that causes ATP depletion shifts the form of cell death toward necrosis (Ankarcrona et al, 1995).
Several fundamentally different, albeit interactive, mechanisms contribute to mitochondrial dysfunction and potentially cell death in both acute and chronic neurologic disorders. In neurons exposed to excitotoxic levels of the neurotransmitter glutamate, excessive mitochondrial accumulation of Ca2+ triggers several forms of mitochondrial injury, mediated at least in part by Ca2+-activated proteases (calpains) and phospholipases (Volbracht et al, 2001; Chiricozzi et al, 2010), and by opening of the inner membrane permeability transition pore (PTP) (Pivovarova and Andrews, 2010; Nicholls, 2009; Figure 1). A limited extent or duration of elevated Ca2+ may not result in the extent of bioenergetic impairment necessary for evoking acute cell death but can promote caspase-independent apoptosis through calpain-mediated mitochondrial release of apoptosis inducing factor (AIF) (Polster et al, 2005). Calpains can also cleave inactive BH3 domain-only proteins, forming active peptides, e.g., truncated Bid (tBid), which bind to proapoptotic and antiapoptotic proteins, e.g., Bax and Bcl2, resulting in outer membrane permeabilization and release of cytochrome c, AIF, and other proteins that trigger either caspase-dependent or caspase-independent programmed cell death (Krajewska et al, 2004; Cabon et al, 2012). A greater mitochondrial insult, which leads to delayed neuronal Ca2+ deregulation, is more apt to induce rapid necrosis, particularly when PTP opening causes irreversible mitochondrial inner membrane depolarization and osmotic mitochondrial lysis (Pivovarova and Andrews, 2010).
Figure 1.
Mitochondrial mechanisms of neural cell death and targets for neuroprotection. The inner membrane electrical potential established by electron transport chain (ETC)-driven proton efflux is responsible both for ATP formation and for uptake of Ca2+ by the mitochondrial Ca2+ uniporter (MCU). Excessive Ca2+ uptake can activate the permeability transition pore (PTP), which releases accumulated Ca2+ and dissipates the proton gradient, thus uncoupling ATP synthesis from respiration. A critical decline in cellular ATP can cause acute, necrotic cell death. Elevated intracellular Ca2+ can also initiate mitochondria-dependent programmed cell death by activating calpain-mediated proteolysis of apoptosis inducing factor (AIF), normally bound to the inner membrane, and of Bid, normally located in the cytosol. Truncated Bid (tBid) can induce a conformation change in Bax, stimulating its oligomerization and megapore formation. Outer membrane megapores formed by Bax or Bak allow for release of intermembrane proteins, e.g., cytochrome c (C), and truncated AIF (tAIF), into the cytosol. These proteins trigger caspase-dependent and caspase-independent programmed cell death, respectively. Elevated Ca2+ promotes mitochondrial fission by at least two mechanisms that involve mitochondrial fission/fusion proteins. Membrane depolarization in response to Ca2+-induced PTP opening stimulates proteolysis of Opa-1, an inner membrane fusion protein, which then promotes mitochondrial fragmentation. Extramitochondrial Ca2+ indirectly interacts with outer membrane dynamin-related protein 1 (Drp1), which also promotes fission. Reactive O2 species (ROS) and nitrogen species produced by mitochondria and extramitochondrial sources target numerous mitochondrial molecules. Oxidative modification of proteins present in the ETC or the tricarboxylic acid (TCA) cycle inhibit aerobic energy metabolism. Oxidation of protein sulfhydryl groups also greatly increases sensitivity of PTP opening by Ca2+. Peroxidation of cardiolipin (CL) decreases the amount of membrane bound cytochrome c and therefore increases the amount available for release to the cytosol. Oxidized cardiolipin also translocates to the outer membrane, where it enhances the ability of extramitochondrial Bax to bind and cause outer membrane pores to form.
Mitochondria are also highly sensitive targets of toxic reactive oxygen species (ROS) and reactive nitrogen species, respectively, generated by either intramitochondrial or extramitochondrial reactions (Figure 1). Mitochondrial sources of ROS include the electron transport chain, matrix dehydrogenases, free iron, and monoamine oxidases (Andreyev et al, 2005; Horowitz and Greenamyre, 2010). Numerous extramitochondrial sources of ROS and reactive nitrogen species include inducible NADPH oxidase, which is particularly important in the oxidative stress associated with cellular inflammatory activities (Sorce and Krause, 2009). Mitochondrial targets for damage by ROS and reactive nitrogen species include electron transport chain components, tricarboxylic acid cycle enzymes, the mitochondria-specific phospholipid, cardiolipin, and DNA (Andreyev et al, 2005; Sparvero et al, 2010; Chaturvedi and Beal, 2008). Oxidative stress also greatly increases the sensitivity of PTP opening by Ca2+ (Greco and Fiskum, 2010b). Due to the high rate of mitochondrial ROS production and the critical impact that oxidative damage to mitochondrial metabolism has on cell survival, mitochondria possess numerous systems for ROS detoxification and for inhibiting or reversing oxidative molecular modifications (Andreyev et al, 2005). These systems include low molecular weight antioxidants, e.g., glutathione, coenzyme Q, lipoic acid, and ascorbate. Antioxidant-related proteins include manganese superoxide dismutase (SOD2), glutathione peroxidase, peroxiredoxin, glutaredoxin, thioredoxin, glutathione reductase, and thioredoxin reductase. The reactions catalyzed by many of these enzymes depend on reducing power in the form of NADPH. Therefore, the mitochondrial redox state and the levels of mitochondrial enzymes that reduce NADP+, e.g., malic enzyme and NAD(P)H transhydrogenase, are equally important in defending against mitochondrial oxidative stress.
Mitochondrial damage can contribute to the vicious cycle of bioenergetic dysfunction and oxidative stress. Mitochondrial ‘quality control' is maintained by the highly dynamic equilibrium between mitochondrial fusion and fission, segregating pathologic from healthy mitochondria, which are then targeted for disposal and recycling by mitochondrial autophagy (mitophagy) (Gottlieb and Carreira, 2010). This process together with mitochondrial biogenesis provides the highly efficient energy transducing machinery necessary for cell survival; therefore, impaired mitophagy or mitochondrial biogenesis can contribute to neuropathologies (Vosler et al, 2009).
Based on the extensive knowledge of mitochondrial mechanisms of acute and chronic neurodegeneration, mitochondria have been targeted by many experimental neuroprotective interventions. This review focuses on three relatively new classifications of mitochondrial targets for neuroprotection: mitochondrial dynamics, mitochondrial preconditioning, and mitochondrial antioxidants.
Mitochondrial dynamics
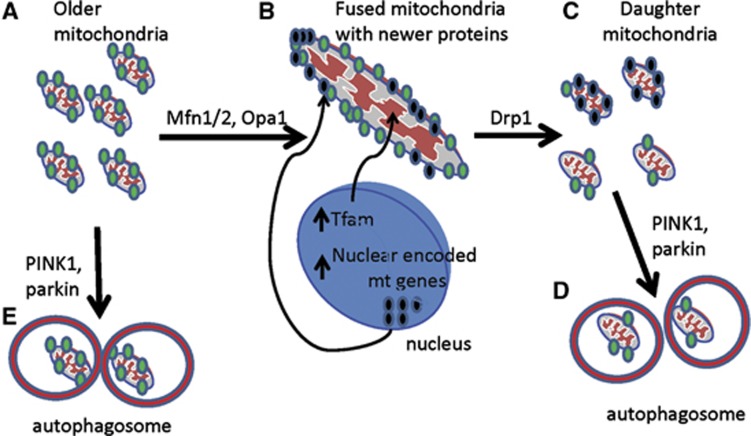
In nonpathogenic cells, mitochondria typically appear as a highly interconnected reticular network capable of mobilizing mitochondria to distant cellular regions, such as the neuronal synapse (Palmer et al, 2011). During cell division and throughout the lifespan of postmitotic cells, mitochondria undergo cycles of fusion and fission (1) to produce homogenous daughter cells providing functional biochemical capabilities, (2) to isolate damaged mitochondrial subsets for targeted degradation (mitophagy), and (3) to facilitate movement of mitochondria to various parts of the cell, including synapses (Palmer et al, 2011). Fusion and fission events are thus intimately entwined in both turnover and mobility. Although static imaging presents mitochondrial networks in most nonpathogenic quiescent cells as a reticular structure, mitochondria can be nonetheless undergoing transient (‘kiss and run') fusion rather than remaining in a completely fused network (Twig et al, 2008). The rate of fission and fusion varies among cell types, and its significance is still widely unknown. However, as part of the flux between fission and fusion, mitochondrial biogenesis and mitophagy represent a delicate balance to maintain a consistent quantity of quality mitochondria, while specifically targeting damaged organelles for degradation without depleting cellular energetic supplies (Figure 2).
Figure 2.
Biogenesis and mitophagy flux through fusion and fission. (A) Older mitochondria present in the cell may have loss of membrane potential (denoted by green dots, which could be due to older protein products of the electron transport chain). (B) These mitochondria can be fused and, in closer proximity to the nucleus, import newly synthesized nuclear-encoded gene products, such as electron transport chain subunits and the critical mitochondrial transcription factor TFAM. (C) The fused mitochondria then undergo fission to split into daughter mitochondria, which can produce a heterogenous pool of mitochondria. Some mitochondria contain enough new material to maintain membrane potential, and are then kept to replenish the pool of functional quality mitochondria. However, some daughter mitochondria are not of high enough quality, and are thus targeted for mitophagy (D). (E) Damaged mitochondria can be targeted directly for mitophagy. Drp1, dynamin-related protein 1; PINK1, PTEN-induced putative kinase protein-1; Mfn1/2, mitofusin 1/2; TFAM, transcription factor A, mitochondrial.
The molecular mechanisms of mitochondrial dynamics have become better understood in recent years, but remain elusive in understanding the full role of accessory proteins, many of which have complementary or overlapping functions. On the most basic level, fusion of mitochondria requires the two-step process of joining the double membrane (reviewed in Palmer et al, 2011). The primary proteins central to the initial fusion of the outer membrane are the GTPases mitofusin (Mfn)1 and Mfn2, which form homodimers or heterodimers in trans to allow for tethering of adjacent mitochondria. Concurrent to this, the mitochondrial phospholipase D hydrolyzes cardiolipin, which induces a structural bend and thus allows curvature of the fusing membranes (Choi et al, 2006). Several modulating proteins affecting fusion at the outer membrane have been identified, and include the proapoptotic molecules Bak and Bax. Fusion of the inner mitochondrial membrane centers on Opa-1, another dynamin-like GTPase protein. Opa-1 associates with the inner mitochondrial membrane, and is stabilized by Prohibitin and stomatin-like protein-2. Interestingly, Opa-1 differentially processed by changes in the subcellular environment, yielding distinct function in fusion/fission events. Opa-1 processing is sensitive to changes in the mitochondrial membrane potential (Ehses et al, 2009), where loss of membrane potential increases the processing of Opa-1 to shorter isoforms, which is then associated with increased fragmentation of the mitochondrial network (Palmer et al, 2011). This shift in mitochondrial morphology can be rescued by overexpression of long isoform of Opa-1. Alternatively, mitochondrial proteases may also target Opa-1 for cleavage. The specific regulation of fusion events at both the outer and inner membranes are still unclear, and are hampered by difficulty in model systems and molecular tools. Interestingly, Opa-1 has been implicated in antiapoptotic cristae remodeling, which appears to be distinct from its role in fusion (Ehses et al, 2009).
Fission of mitochondria can occur in the role of biogenesis, where intramitochondrial components are sorted and split into daughter mitochondria. However, fragmentation of mitochondria also precedes the selective targeting of mitochondria for mitophagy (Twig et al, 2008; Westermann, 2010). Dynamin-related protein 1 (Drp1) is a member of the dynamin family of GTPases, and is the major protein involved in the scission of membranes via translocation from the cytosol to the outer mitochondrial membrane for the formation of constricting rings (Palmer et al, 2011). Because Drp1 lacks a pleckstrin-homology domain, it requires a membrane receptor protein (e.g., Fis1) to associate with and polymerize at membrane. The Drp1 undergoes numerous posttranslational modifications, including phophorylation, S-nitrosylation, ubiquitylation, and sumoylation (Cho et al, 2009; Palmer et al, 2011), which are not well characterized but are likely to reveal a highly controlled mechanism for responding to the cellular environment. Several of the identified modulators of Drp1 are Ca2+ or nitric oxide (NO) sensitive, and thus support the concept that fission is a functional response to a changing cell environment (Palmer et al, 2011).
The controlled regulation of fission and fusion events in the process of mitochondrial biogenesis is critical for the uniform distribution of mtDNA and the maintenance of consistent energetic production capacity. Although fusion and fission underlie the gross structural alterations required for mitochondrial biogenesis, a bigenomic upregulation of critical transcription factors and subsequent functional proteins is also necessary to maintain fidelity of biophysically and biochemically functional mitochondria (Figure 3). A cascade of nuclear transcription factors, including but not limited to peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and nuclear respiratory factor-1 (NRF-1), are activated and upregulated, leading to the transcription of nuclear-encoded electron transport chain subunits and transcription factor A, mitochondrial (TFAM), a critical mitochondrial DNA transcription factor that is also involved in mtDNA replication. These proteins are specifically transported into the preexisting mitochondria and imported before fission and subsequent incorporation into the mitochondrial network (Ventura-Clapier et al, 2008). The resulting content of mtDNA as well as electron transport chain proteins per daughter mitochondria can vary significantly, and thus cannot be used as sole indicators of cellular mitochondrial content.
Figure 3.
Bigenomic requirement of mitochondrial biogenesis. Critical transcription factors are activated (e.g., PGC-1α) or upregulated (e.g., NRF-1), which leads to the upregulation of critical gene products for newly generated mitochondria, including the nuclear-encoded subunits of the electron transport chain, critical tRNAs, and TFAM, the major mitochondrial transcription factor necessary for new synthesis of mitochondrial-encoded proteins. TFAM also has a critical role in the replication of the mitochondrial genome. PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; NRF-1, nuclear respiratory factor-1; TFAM, transcription factor A, mitochondrial.
When newly formed daughter mitochondria have been incorporated into the mitochondrial network, mitochondria that have been damaged or that have lost membrane potential are specifically targeted for degradation via an autophagic-like process termed as mitophagy (Twig et al, 2008; Youle and Narendra, 2011). Originally discovered in yeast, the mitophagic process has recently been identified in mammalian cells, and is dependent on key proteins, such as the PINK1 (PTEN-induced putative kinase protein-1) and parkin, that are also particularly relevant to neurodegenerative diseases. However, much more detailed mechanistic work needs to be done to fully understand its role in physiologic and pathologic settings.
In recent years, abnormalities in mitochondrial fusion/fission, biogenesis, and mitophagy have been identified in several neurodegenerative diseases, including Parkinson's, Alzheimer's, Huntington's and Charcot-Marie-Tooth diseases, as well as in nonneural injury states such as cardiac ischemia/reperfusion. Indeed, mutations of Mfn2 are associated with a subset of Charcot-Marie-Tooth (type 2A) (Zuchner et al, 2004) and Opa-1 in autosomal dominant optic atrophy (Alexander et al, 2000). Recently, PINK and parkin, proteins found to be mutated in a subset of PD patients, were recently identified as regulatory elements in directing mitophagy. Specifically, PINK and parkin appear to be highly involved in the induction of mitophagy. Disease-causing mutations lead to aberrant clearance of damaged mitochondria and defects in maintaining mitochondrial quality control (Youle and Narendra, 2011). In addition to disease-causing mutations, alterations in the posttranslational modifications of essential proteins in fission or fusion can also lead to neurodegeneration. For example, in a cellular model of Alzheimer's disease, β-amyloid protein increased mitochondrial fission in neurons. Mutation of the nitrosylation-sensitive cysteine residue of Drp1 effectively reduced the mitochondrial fragmentation as well as the neurotoxicity and synaptic damage due to β-amyloid exposure (Cho et al, 2009). Furthermore, aberrations in mitochondrial fission and fusion, mobility, and mitochondrial activity were observed in neurons cultured from transgenic mice overexpressing Aβ precursor protein (Calkins et al, 2011). In models of Huntington's disease, dominant-negative Drp1 decreased mitochondrial fragmentation and cell death in cells overexpressing mutant Huntington (mtHtt), the protein that underlies Huntington's disease (Wang et al, 2009). Additionally, PGC-1α transcriptional activity was significantly repressed by mtHtt overexpression, and subsequent overexpression of PGC-1α was neuroprotective (Cui et al, 2006). Although PGC-1α has many downstream transcriptional targets, mitochondrial biogenesis could be an important aspect to investigate.
The implications of mitochondrial dynamics in neural ischemia are still unknown, and only beginning to be investigated. Transient increases in mitochondrial proteins and mtDNA can be induced by ischemic injury, including neonatal hypoxia/ischemia (Rasbach and Schnellmann, 2007; Yin et al, 2008). Furthermore, modest changes in mitochondrial mobility and morphology were also observed in neuronal ischemia (Bertoni-Freddari et al, 2006; Yin et al, 2008; Valerio et al, 2011). The calcium- and oxidant-sensitive nature of several of the signaling molecules leading to mitochondrial dynamics underscore the need for further investigation of the role of mitochondrial responses as an organelle after neural ischemic injury.
Unfortunately, the technical assessments of mitochondrial dynamics are still lacking in reliability, applicability, and interpretation. Improvements in functional assays and end points as well as a critical sense of data interpretation are necessary to be able to accurately and consistently describe mitochondrial changes under different cellular environments. Changes in mitochondrial morphology have been observed in correlation with cell death stimuli, but most of the functional studies rely heavily on the knockdown or overexpression of singular proteins involved in fission/fusion, which can in and of itself render the cell more susceptible to cell stress. Furthermore, lack of experimental evidence of manipulation of mitochondrial dynamics may also be difficult to interpret, as compensatory mechanisms are widespread throughout the system, or morphology may be left unchanged even in the face of altered rates of fission/fusion (Arnold et al, 2011). Combined with a high degree of heterogeneity present in the molecular makeup of individual mitochondria, it is critical for current investigations into mitochondrial dynamics to use a wide range of cellular, molecular, histological, and biochemical tools to fully explore the effects of changes in cell environments.
The concept of targeting an organelle as a therapeutic strategy remains a difficult process to imagine. However, the examples of aberrant fusion and clearance of mitochondria underlying pathology in models of Parkinson's disease, Alzheimer's disease and other neurodegenerative processes give merit to the idea of the organelle itself as a therapeutic or pathogenic target. Mitochondrial fusion and biogenesis have also been implicated in neuroprotective strategies against acute neural injury. Calpain inhibition provided neuroprotection against NMDA-induced neurotoxicity and was dependent on Opa-1, an enzyme critical for mitochondrial fusion (Jahani-Asl et al, 2011) Furthermore, induction of core elements of mitochondrial biogenesis have been observed using multiple preconditioning stimuli in neurons, such as sublethal hypoxia or hypoxia/ischemia (Gutsaeva et al, 2008), hyperbaric oxygen (Gutsaeva et al, 2006), and resveratrol (Dasgupta and Milbrandt, 2007; Biala et al, 2010). These stimuli have been well documented as neuroprotective strategies against acute brain injury, including cerebral ischemia and glutamate excitotoxicity. Thus, on a similar conceptual basis as cell restoration and phagocytosis, or organ transplant and resection, the targeted clearance and replacement of a damaged organelle could theoretically have a pluripotent impact on cellular (and hence, systemic) outcomes that is lacking by therapeutic strategies targeting a single molecule.
Ischemic preconditioning and mitochondria
Ischemic preconditioning (IPC) has gained attention as a robust neuroprotective mechanism against conditions of metabolic stress in different organs, including brain (Gidday, 2006). It is defined as the ability of either a brief (‘sublethal') ischemic episode or mild stress imposed on a given organ, followed by a period of recovery, to increase an organ's resistance to injury. The cellular and subcellular mechanisms that support IPC are much better understood today, but still many of the signaling pathways and interaction among pathways remain undefined (see review Gidday, 2006). It is important to clarify that the main emphasis in this field is the elucidation of the mechanisms by which IPC affords protection against cerebral ischemia, to discover potential therapies that can emulate this condition in the clinic.
One signaling pathway identified as key in the protection of brain mitochondria in preconditioning is protein kinase C (PKC). The role of PKC isozymes on cerebral ischemia was suggested by many studies in the past; however, development of novel pharmacologic tools that distinguish among the different isozymes offered new insights into specific PKC isozymes (Bright and Mochly-Rosen, 2005). Thus, the role of PKC isozymes on mitochondrial physiology/dysfunction has only recently emerged with new in-depth investigations in the brain.
A number of excellent reviews have been written describing the biochemistry of PKCs (Bright and Mochly-Rosen, 2005). In summary, PKC is a family of at least 12 serine/threonine kinases, which can be split into three broad categories: conventional, novel, and atypical (Bright and Mochly-Rosen, 2005), and they have multiple cellular roles. The conventional PKCs (α, βI, βII, and γ) require Ca2+, diacylglycerol, and phospholipid for activation. The novel PKC isozymes (δ, ɛ, η, and θ) lack the calcium-binding region, so these subtypes are not dependent on Ca2+ for activation but may be activated by Ca2+ indirectly with diacylglycerol. Activation of isozymes of the atypical PKC group (ζ, λ, μ, and ι) is also independent of Ca2+. However, atypical PKC isozymes lack the Zn2+ finger region required for binding of diacylglycerol or phorbol ester. Instead, 3′-phosphoinositides may be the activators of atypical PKCs (Bright and Mochly-Rosen, 2005).
Among all these PKC isozymes, a number of groups have now confirmed that the novel PKC isozyme epsilon (ɛPKC) is a key signaling pathway after IPC and has an important role in protection against lethal ischemia in brain (Bright and Mochly-Rosen, 2005). In brain, IPC leads to activation of ɛPKC in hippocampus, the most vulnerable part of brain to global cerebral ischemia (Raval et al, 2007).
The ɛPKC can protect brain mitochondria either by activation of other downstream signaling pathways or by its direct phosphorylation of mitochondrial targets. For example, downstream signaling pathways ɛPKC include: the Src family of protein tyrosine kinases, the mitogen-activated protein kinase p38, the MAPK/ERK kinase MEK1/2, and the serine/threonine kinase Akt (Lange-Asschenfeldt et al, 2004; Greco et al, 2006). These signaling pathways (and potentially many others yet to be identified) evoke posttranslational modifications of existing proteins as well as transcriptional activation and de-novo protein synthesis that result in neuroprotection.
A more direct role of ɛPKC on mitochondria leading to inhibition of apoptosis was previously observed by Baines et al (2002) who showed that transgenic mice expressing activated ɛPKC formed ɛPKC-ERK ‘modules' in cardiac mitochondria. The ɛPKC has been shown to directly target mitochondria by phosphorylating subunit IV of the mitochondrial respiratory chain cytochrome c oxidase (COX) complex in cardiac myocytes (Guo et al, 2007).
Based on previous findings that showed that IPC was able to improve mitochondrial functions after cerebral ischemia (Perez-Pinzon et al, 2002), a link between ɛPKC and mitochondrial neuroprotection was established. It was first established that ɛPKC translocated early on to mitochondria after IPC and that one of the main targets was the mitochondrial K+ATP channel (Raval et al, 2007; Figure 4). After IPC, selective inhibition of ɛPKC activation prevented Kir6.2 phosphorylation, a specific subunit of the mtK+ATP channel, consistent with Kir6.2 as a phosphorylation target of ɛPKC or its downstream effectors, and inhibition of this channel inhibited IPC-induced neuroprotection. In another study, ɛPKC levels were found to increase in the hippocampal synaptosomal fraction 48 hours after IPC (Dave et al, 2008). Treatment with a specific ɛPKC activating peptide 48 hours after IPC, increased the rate of oxygen consumption in the presence of substrates for complexes I, II, and IV (Dave et al, 2008). These increases in the rate of respiration were correlated with increased levels of serine and tyrosine phosphorylation of 18 kDa subunit of complex I, threonine phosphorylation of COX IV, increased mitochondrial membrane potential, and decreased H2O2 production. In addition, induction of in-vitro ischemia decreased mitochondrial cytochrome c release (Dave et al, 2008). These results suggest that after IPC, ɛPKC is readily available for activation in synaptosomal mitochondria on ischemia/reperfusion, in such a manner to increase mitochondrial respiration, reduce ROS production and cytochrome c release, hallmarks of reperfusion injury.
Figure 4.
Mitochondrial targets of ischemic preconditioning. In brain, ischemic preconditioning (IPC) activates the adenosine A1 receptor (A1AR), which then activates phospholipase C (PLC). Phosphatidyl inositol bis-phosphate is hydrolyzed by PLC forming diacylglycerol, which is a potent activator of protein kinase C isoforms, e.g., protein kinase C epsilon (ɛPKC). ɛPKC in turn binds to a mitochondrial receptors for activated C kinase (RACK), and activate the ATP-sensitive mitochondrial potassium channel (mtK+ATP). Transmembrane cycling of potassium through the mtK+ATP channel and the K+/H+ antiporter may slightly depolarize the mitochondrial inner membrane and decrease production of reactive O2 species (ROS) by the electron transport system (ETS). IPC also alters NAD+/NADH levels, activating SIRT1, which leads to a decrease of the uncoupling protein 2 (UCP2).
For many years, it was believed that one of the main causes of aging was the slow production of ROS over a long time, leading to a gradual decline in metabolism (Guarente, 2008). Although, the field of cellular aging has become much more complex, it is now accepted that the phenomenon of calorie restriction (CR) extends lifespan in many species and that this in part is due to mitochondrial modifications (Guarente, 2008). Calorie restriction is initiated by an increase in the ratio of cellular NAD+/NADH, which in turn activates a family of proteins called sirtuins (Guarente, 2008). Since CR is a type of metabolic stress that also targets mitochondria, CR proponents suggest that there may be similar pathways activated by both CR and IPC. Lending support to this hypothesis, anoxic preconditioning was found to decrease mitochondrial NAD+/NADH ratio in hippocampal slices, as measured by spectropfluorometry (reviewed in Morris et al, 2011). This finding also suggests that cytosolic NAD+/NADH was decreased after preconditioning. Since increases in cytosolic NAD+/NADH ratio activate the sirtuin 1 (SIRT1) enzyme, these findings suggested that IPC might act by activating the SIRT1 pathway (Figure 4). This hypothesis was confirmed in recent studies in brain, demonstrating that showed that resveratrol, an activator of SIRT1, emulated IPC via the SIRT1 pathway and that blockade of SIRT1 abolished IPC (Raval et al, 2006; Della-Morte et al, 2009).
There are at least seven sirtuins (SIRT 1–7) identified in mammals. SIRT1 is the most extensively studied and has been implicated in lifespan extension of organisms including mammals (Guarente, 2008). SIRT1 exerts its effect by deacetylating histones (e.g., H1, H3, and H4), as well as nonhistone targets such as TAFI68 (TATA-box binding protein)-associated factor I, MEF2 (MADS box transcription enhancer factor 2), the transcription factor NF-KB, tumor suppressor p53, a p53-related tumor suppressor p73, a DNA-repair factor, and inhibitor of Bax-mediated apoptosis Ku70 and FOXO (Forkhead box class O) transcription factors, among others (for specific references, see a recent review Morris et al, 2011).
One recent study showed that SIRT1 is present in the mitochondria where it may locally regulate mitochondrial biogenesis (Aquilano et al, 2010). However, the more conventionally recognized mitochondrial localized sirtuins are SIRT3, SIRT4, and SIRT5, which also have the ability to regulate mitochondrial dynamics and are implicated in CR, aging, and metabolic stress protection (Morris et al, 2011). Similarly to SIRT1, these mitochondrial sirtuins may augment mitochondrial activity and serve as novel targets for protection against cerebral ischemia. For a recent extensive review of these sirtuins, see Morris et al (2011).
A recent study by Dioum et al (2009) showed that SIRT1 deacetylated hypoxia inducible factor-2α (HIF-2α) that promoted expression of cytoprotective genes in culture mammalian cells. HIF-1α and HIF-2α are transcription factors that respond to low oxygen levels (Semenza, 2011). Under normal conditions, both HIFs are hydroxylated on proline residues, which then promote their degradation. Under hypoxic conditions, HIF proteins stabilize and accumulate leading to transcription of key cytoprotective pathways. HIF-1α has been linked to hypoxic preconditioning in brain (Ran et al, 2005). SIRT1 activation of HIF-2α was shown to enhance expression of mitochondrial ROS scavenger manganese SOD2 and erythropoietin (Dioum et al, 2009). Although the role of HIF-2α has not been defined in IPC in brain, one of its downstream targets, erythropoietin, has been well characterized (Zhang et al, 2006). The IPC elevation of erythropoietin was sufficient to induce a preconditioned response (Meloni et al, 2006) and to protect rat CA1 neurons against ischemia (Zhang et al, 2006).
The transcriptional coactivator PGC-1α is another well-known target of SIRT1. This pathway has been shown to mediate mitochondrial biogenesis by stimulating the expression of nuclear-coded mitochondrial proteins (Puigserver and Spiegelman, 2003). Recent studies suggest that PGC-1α and SIRT1 may also reside in mitochondria and promote mitochondrial biogenesis in HeLa cells and mouse brains via interaction with TFAM (Aquilano et al, 2010).
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha has been associated with protection against oxidative stress. Enhanced levels of PGC-1α protected hippocampal neurons against delayed cell death after transient ischemic episodes and decreased expression of manganese SOD and uncoupling protein 2 (UCP2) (Chen et al, 2010). Mice lacking PGC-1α displayed enhanced hippocampal neurodegeneration in response to oxidative stress (St-Pierre et al, 2006) and exhibited decreased antioxidant expression after ischemia (Chen et al, 2010). Therefore, PGC-1α has an important role in protection against ischemic death and ROS-induced oxidative stress.
SIRT1's neuroprotective effects against ischemia may also stem from direct modulation of mitochondrial activity. SIRT1 represses UCP2, a member of inner mitochondrial membrane proteins regulating proton electrochemical gradient, by binding directly to the UCP2 promoter (Bordone and Guarente, 2007; Guarente, 2008). A recent study showed that resveratrol treatment in vivo decreased UCP2 protein expression, which was blocked by an SIRT1 inhibitor (Della-Morte et al, 2009), suggesting that SIRT1 may regulate mitochondrial dynamics by influencing UCP2 expression. These multiple observations are compatible with SIRT1 acting through modulation of mitochondrial function.
In addition to the activation of SIRT1, resveratrol is also known to modulate the expression of several genes including COX-2 (cyclooxygenase-2) (inflammation), inducible nitric oxide synthase (NO production), endothelin-1 (vasoconstriction), and insulin-like growth factor binding protein (cell growth), among others (Morris et al, 2011). Recent studies showed that the signal transducers and activators of transcription 3 (STAT3) mediated COX-2 overexpression plays a key role in IPC-induced neuroprotection (Kim et al, 2008). STATs have been suggested to modulate mitochondrial function (Lin et al, 2011), and may be another signaling pathway involved in mitochondrial protection after IPC.
The STATs family consist of seven members 1, 2, 3, 4, 5A, 5B, and 6 (recently reviewed in Lin et al, 2011). The STAT activation can be proapoptotic or antiapoptotic. For example, STAT1 has been shown to induce apoptosis in cardiomyocytes, whereas STAT3 protects cardiomyocytes against ischemic injury (see Lin et al, 2011 for details). In brain, phosphorylation and nuclear translocation of STAT3 in response to IPC were observed in mixed cortical/glial cocultures (Kim et al, 2008). The STAT3 was found to exert neuroprotection through the transcriptional upregulation of COX-2. Also, another study showed that erythropoietin neuroprotection against CA1 pathology after cerebral ischemia was mediated by another STAT isozyme (i.e., STAT5; Zhang et al, 2007). Some of the mechanisms by which STATs regulate mitochondrial function include: (1) STAT1 activation leads to production of ROS, loss of mitochondrial membrane potential, and upregulation of the mitochondrial adenine nucleotide translocator 3; (2) in contrast to STAT1, when STAT3 is knocked out or downregulated, impairments in complexes of the electron transport chain are observed; and (3) STAT3 appears to transcriptionally upregulate the activity of manganese SOD2 and deletion of STAT3 leads to increased production of ROS (reviewed in Lin et al, 2011).
Protection against mitochondrial oxidative stress
A wide range of experimental neuroprotective approaches have been used based on protection against mitochondrial oxidative stress. Strategic classifications include the following: (1) Inhibitors of ROS formation. (2) Exogenous antioxidants. (3) Agents that may increase reducing power necessary for ROS detoxification. (4) Stimulation of gene expression that increases mitochondrial antioxidant defenses.
There are two basic mechanisms by which the generation of superoxide by the mitochondrial electron transport chain or tricarboxylic acid cycle dehydrogenases can be regulated. The first is by controlling tissue O2 levels and the second is controlling the redox state of redox sites that are responsible for superoxide production. Chance and coworkers showed decades ago that mitochondrial ROS production continues to increase with the ambient O2 concentration up to 100% O2 (Boveris and Chance, 1973). More recently, Brookes and colleagues showed that the Km for O2 for mitochondrial ROS production varies between 0.2 and 5.0 μmol/L, depending on the site of superoxide formation within the electron transport chain (Hoffman and Brookes, 2009). Since the Km for O2 for normal mitochondrial respiration is in the range of 0.1 to 1.0 μmol/L, both of these studies strongly suggest that the presence of abnormally high, hyperoxic, levels of O2 in tissues can increase mitochondrial free radical production without significantly increasing respiration and therefore aerobic energy metabolism. An example of how avoiding hyperoxia can provide neuroprotection comes from studies comparing neurochemical and neurologic outcomes after experimental cardiac arrest, for animals artificially ventilated using either 100% O2 or 21% to 30% O2 during the first hour of reperfusion. Compared with animals receiving normoxic resuscitation, those receiving hyperoxic ventilation display elevated markers of oxidative stress, e.g., nitrotyrosine, impaired mitochondrial respiration, inhibited pyruvate dehydrogenase enzyme activity, decreased cerebral aerobic energy metabolism, increased hippocampal neuronal death, and worse neurologic outcome (Vereczki et al, 2005; Balan et al, 2006; Richards et al, 2007; Fiskum et al, 2008). These findings illustrate the concept that simple avoidance of unnecessarily high levels of O2 can defend against oxidative stress-induced mitochondrial bioenergetic dysfunction and provide neuroprotection in a clinically relevant model of acute brain injury.
Another approach toward reducing mitochondrial superoxide production is shifting the redox state of mitochondrial electron transport chain components from a relatively reduced level, which promotes one electron transfer to O2, to a more oxidized redox state, which decreases the thermodynamic force toward superoxide production. This oxidized shift in redox state is most commonly accomplished by the use of protonophore uncoupling molecules, which depolarize the mitochondrial inner membrane and inhibit oxidative stress and neuronal death in experimental models of cerebral ischemia, brain trauma, and Parkinson's disease (Korde et al, 2005). Thermodynamic inhibition of mitochondrial ROS production has also been used to explain how increased expression of mitochondrial ‘UCP2' results in cytoprotection (Negre-Salvayre et al, 1997). While there are numerous examples of neuroprotection by either activation or overexpression of UCP2 (Paradis et al, 2003), the mechanism of protection by uncoupling and inhibition of mitochondrial superoxide production is not uniformly accepted (Cannon et al, 2006). The same lack of consensus applies to the mild uncoupling mechanism by which activators of the mitochondrial ATP-regulated potassium channel, e.g., diazoxide, provide neuroprotection (Xie et al, 2010).
Numerous exogenous antioxidants have shown neuroprotection in many different animal models of acute central nervous system injury and neurodegenerative diseases (Calabrese et al, 2009), and some of these appear to act either primarily or at least partially at the mitochondrial level (Chaturvedi and Beal, 2008; Yang et al, 2009). A few of the most widely studied of these agents include the hydrophobic natural compounds ubiquinone, α-tocopherol, and estrogen, which scavenge free radicals primarily at or within membranes. While progesterone does not exhibit the antioxidant activity as estrogen, progesterone displays a membrane stabilizing effect that also serves to reduce damage caused by lipid peroxidation (Roof and Hall, 2000). In addition, both estrogen and progesterone exert antioxidant actions indirectly through their ability to stimulate the expression of mitochondrially localized gene products. These proteins include SOD2 and Bcl-2 that in turn display direct and indirect antioxidant activities, respectively (Kowaltowski et al, 2004; Tripanichkul et al, 2007; Matsuoka et al, 2010).
A number of neuroprotective antioxidants have been chemically designed specifically to target mitochondria. This approach generally involves conjugation between the antioxidant and a lipophilic cation, e.g., tetraphenylphosphonium cation. The lipophilicity promotes diffusion across the blood brain barrier and neural cell membranes while the positive charge promotes accumulation at the inner membrane or in the mitochondrial matrix, due to the very high, 180 mV negative-inside mitochondrial membrane potential. Antioxidants as diverse as ubiquinone, lipoic acid, vitamin E, and nitrone spin-traps have been targeted to mitochondria in this manner (Murphy and Smith, 2007) and have shown neuroprotection in many models including those for neurodegenerative diseases and alcohol neurotoxicity (Siler-Marsiglio et al, 2005). Both mitochondrially targeted ubiquinone and a nitrone spin-trap have shown efficacy in a neonatal rat cerebral hypoxic ischemia model (Hobbs et al, 2008), which is particularly significant since endogenous antioxidant levels in the neonatal brain and neonatal brain mitochondria are considerably lower than those present in adult brain (Bayir et al, 2006).
Increasing mitochondrial antioxidant activities by elevating mitochondrial reducing power is considered as an important mechanism of neuroprotection by several naturally occurring compounds, including ketone bodies, pyruvate, and acyl-carnitines (Ryu et al, 2004; Zanelli et al, 2005; Gasior et al, 2006; Zhao et al, 2006b; Jarrett et al, 2008; Rosca et al, 2009; Alves et al, 2009; Jones et al, 2010; Zhang et al, 2010). This mechanism of action might be particularly important during reperfusion after cerebral ischemia, when the mitochondrial redox state is hyperoxidized, ROS production is elevated, and there are increased markers of oxidative molecular modification (Perez-Pinzon et al, 1999; Fiskum et al, 2004). There is evidence for mitochondrial metabolism of both ketone bodies, e.g., β-hydroxybutyrate, and acetyl-L-carnitine after acute brain injury, which is associated with a reduction in oxidative stress and neuroprotection (Rosenthal et al, 1992; Liu et al, 1993; Prins et al, 2005; Scafidi et al, 2010, 2011). While there is less evidence that neuroprotection by exogenous pyruvate is a consequence of its oxidative metabolism, support for this mechanism comes from recent measurements of brain slice O2 consumption indicating that exogenous pyruvate significantly elevates endogenous respiration (Schuh et al, 2011). Other experiments indicate that each of these three neuroprotectants exhibit alternative mechanisms of neuroprotection, including direct scavenging of free radicals (Packer et al, 1991; Varma et al, 1998; Haces et al, 2008). Moreover, one study indicates that ketone bodies inhibit mitochondrial ROS production in neurons after glutamate excitotoxicity by decreasing mitochondrial redox state (Maalouf et al, 2007). More research is clearly necessary to elucidate the mechanisms of neuroprotection by these relatively very safe, naturally occurring substances.
The levels of gene products that are responsible both for detoxification of reactive O2 species and for the reducing power that drives their detoxification are controlled through transcriptional regulation using antioxidant response elements (ARE) that interact with transcriptional activating factors such as Nrf2 (Thimmulappa et al, 2002; Figure 5). One mechanism by which the Nrf2/ARE pathway of antioxidant- and other cytoprotective-gene expression is activated is oxidation of critical cysteine sulfhydryl groups located on KEAP1 (Kelch-like ECH-associated protein 1), a cytoplasmic Nrf2 binding protein. On oxidation of KEAP1, Nrf2 is released from this protein, undergoes serine phosphorylation, and translocates to the nucleus where it binds to AREs (Jaiswal, 2004).
Figure 5.
Neuroprotection by Nrf2-stimulated expression of cytoprotective genes. In unstressed cells, Nrf2 is bound to its inhibitory protein, Kelch-like ECH-associated protein 1 (KEAP 1), which targets it for proteasomal degradation. Oxidation of specific KEAP1 cysteine sulfhydryl groups in response to either oxidative stress or mixed dithiol reactions with compounds, e.g., sulforaphane, induces a conformational change in KEAP1, reducing its ability to bind Nrf2. Following serine phosphorylation of Nrf2 by kinases, e.g., protein kinase C (PKC) delta, Nrf2 can enter the nucleus where it binds to antioxidant response elements (ARE) in the promoter regions of many cytoprotective genes. Examples of gene products that contribute to neuroprotection by Nrf2 activation include those present in the cytosol, e.g., NADPH/CoQ oxidoreductase 1, and those present in the mitochondria, e.g., thioredoxin and NADP+-dependent malic enzyme. Changes in mitochondrial antioxidant-associated proteins appear responsible for inhibition of permeability transition pore (PTP) opening in brain and liver mitochondria from animals treated with Nrf2 activators, e.g., sulforaphane.
Sulforaphane, an isothiocyanate derived from a glucosinolate found in cruciferous vegetables, e.g., broccoli, forms mixed disulfide bonds with KEAP1, and is a well-studied pharmacologic activator of Nrf2-mediated gene expression (Zhang et al, 1992; Kensler et al, 2000). Sulforaphane shows neuroprotection in several rat models of acute brain injury, e.g., stroke (Zhao et al, 2006a) and head trauma (Zhao et al, 2005), in which evidence for mitochondrial PTP involvement exists (Kristian and Siesjo, 1998; Okonkwo and Povlishock, 1999). Rats fed a broccoli-enriched diet exhibit significant increases in aortic smooth muscle mitochondrial proteins that could influence PTP opening, including thioredoxin, thioredoxin reductase, glutathione reductase, glutathione, and catalase enzyme activities (Mukherjee et al, 2008). Mitochondria isolated from the livers of rats injected intraperitoneally 40 hours earlier with sulforaphane exhibit elevated immunoreactive levels of glutathione peroxidase 1, thioredoxin 2, and mitochondrial malic enzyme (Greco et al, 2011). These mitochondria are characterized by elevated peroxidase enzyme activity, increased glutathione, and resistance to NAD(P)H oxidation induced by exposure to peroxides. These investigators also found that both the liver and brain mitochondria isolated from sulforaphane-treated rats are resistant to PTP opening induced by exposure to Ca2+ plus peroxides (Greco and Fiskum, 2010a). Considering the importance of PTP opening in the pathophysiology of both acute central nervous system injury and possibly also in neurodegenerative diseases, the ability of sulforaphane and other Nrf2 activators to increase mitochondrial antioxidant proteins inhibits the opening of this mitochondrial death pore may represent a powerful new approach toward neuroprotection.
The Nrf2/ARE pathway is not the only approach toward cytoprotection through elevation of mitochondrial antioxidant-related proteins. For instance, pharmacologic activation of the STAT3 stimulates the expression of the mitochondrial SOD2 and limits ischemic neuronal death (Jung et al, 2009, 2010). Considering the fact that SOD2 immunoreactivity is not increased in brain mitochondria from rats treated with the Nrf2 activator sulforaphane, a combination of Nrf2 and STAT3 activators could provide mitochondria with a greater resistance to oxidative stress than following the administration of activator alone.
Summary and Conclusions
Mitochondria are in many ways like cells within cells, possessing their own genome, the ability to replicate, and molecular mechanisms for responding to changes in their environment. The mitochondrial presence of ∼1,000 different proteins, two very different membranes, numerous transporters for ions, metabolites, and proteins provide numerous targets for both injury and cytoprotection. Like injured cells, damaged mitochondria can poison their immediate environment, ultimately leading to the death of the host cells in which they reside. There are many strategies for protecting cells such as neurons against pathologic mitochondrial injury. This review focused on three approaches: (1) Optimization of mitochondrial dynamics through mitophagic disposal of abnormal mitochondria and biogenesis of new, healthy mitochondria. (2) Utilization of ischemic-preconditioning mechanisms such as mitochondrial protein phosphorylation and sirtuin activation that improve mitochondrial bioenergetics. (3) Pharmacologic inhibition of mitochondrial oxidative stress by inhibition of mitochondrial superoxide production, by mitochondrially targeted antioxidants, and by stimulation of antioxidant gene expression that increases endogenous mitochondrial antioxidant defenses. Overlap between these three approaches clearly exists. Agents or conditions that activate all three pathways may have particularly strong potential for neuroprotection. The possibility also exists, however, that negative interactions could limit the effectiveness of combination therapies. For instance, Nrf2 activation results in a shift in cellular redox state to a more reduced level (del et al, 2008; Greco et al, 2011), which could inhibit the effectiveness of neuroprotective approaches mediated by sirtuins, that are activated by an oxidized shift in redox state. Furthermore, considering the finding that histone deacetylase inhibitors both activate Nrf2 and protects against ischemic brain injury (Kawai et al, 2011; Wang et al, 2011), activation of sirtuin histone deacetylase activity could potentially impair Nrf2-mediated cytoprotective gene expression. A myriad of other synergistic and antagonistic interactions could exist between agents or conditions that exert mitochondria-based neuroprotection through different molecular mechanisms. These interactions are likely to be dependent on dose, timing of administration, and the neuropathologic paradigms in which these approaches are tested.
Acknowledgments
The authors appreciate the assistance of Ms Denise Brown in the preparation of this manuscript.
The authors declare no conflict of interest.
Footnotes
This study was supported by NIH NS45676, NS054147, and NS34773 to MAPP; NIH NS056118 to RAS; NIH HD16596 and US Air Force FA8650-11-2-6D04 to GF.
References
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- Alves E, Binienda Z, Carvalho F, Alves CJ, Fernandes E, de Lourdes BM, Tavares MA, Summavielle T. Acetyl-L-carnitine provides effective in vivo neuroprotection over 3,4-methylenedioximethamphetamine-induced mitochondrial neurotoxicity in the adolescent rat brain. Neuroscience. 2009;158:514–523. doi: 10.1016/j.neuroscience.2008.10.041. [DOI] [PubMed] [Google Scholar]
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, Ciriolo MR. Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J Biol Chem. 2010;285:21590–21599. doi: 10.1074/jbc.M109.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold B, Cassady SJ, VanLaar VS, Berman SB. Integrating multiple aspects of mitochondrial dynamics in neurons: age-related differences and dynamic changes in a chronic rotenone model. Neurobiol Dis. 2011;41:189–200. doi: 10.1016/j.nbd.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- Balan IS, Fiskum G, Hazelton J, Cotto-Cumba C, Rosenthal RE. Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke. 2006;37:3008–3013. doi: 10.1161/01.STR.0000248455.73785.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayir H, Kochanek PM, Kagan VE. Oxidative stress in immature brain after traumatic brain injury. Dev Neurosci. 2006;28:420–431. doi: 10.1159/000094168. [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Casoli T, Di SG, Solazzi M, Perna E, De AC. Reactive structural dynamics of synaptic mitochondria in ischemic delayed neuronal death. Ann NY Acad Sci. 2006;1090:26–34. doi: 10.1196/annals.1378.003. [DOI] [PubMed] [Google Scholar]
- Biala A, Tauriainen E, Siltanen A, Shi J, Merasto S, Louhelainen M, Martonen E, Finckenberg P, Muller DN, Mervaala E. Resveratrol induces mitochondrial biogenesis and ameliorates Ang II-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Press. 2010;19:196–205. doi: 10.3109/08037051.2010.481808. [DOI] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Sirtuins and beta-cell function. Diabetes Obes Metab. 2007;9 (Suppl 2:23–27. doi: 10.1111/j.1463-1326.2007.00769.x. [DOI] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36:2781–2790. doi: 10.1161/01.STR.0000189996.71237.f7. [DOI] [PubMed] [Google Scholar]
- Cabon L, Galan-Malo P, Bouharrour A, Delavallee L, Brunelle-Navas MN, Lorenzo HK, Gross A, Susin SA. BID regulates AIF-mediated caspase-independent necroptosis by promoting BAX activation. Cell Death Differ. 2012;19:245–256. doi: 10.1038/cdd.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Mancuso C, Barone E, Calafato S, Bates T, Rizzarelli E, Kostova AT. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front Biosci. 2009;14:376–397. doi: 10.2741/3250. [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum Mol Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Shabalina IG, Kramarova TV, Petrovic N, Nedergaard J. Uncoupling proteins: a role in protection against reactive oxygen species—or not. Biochim Biophys Acta. 2006;1757:449–458. doi: 10.1016/j.bbabio.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, Beal MF. Mitochondrial approaches for neuroprotection. Ann NY Acad Sci. 2008;1147:395–412. doi: 10.1196/annals.1427.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SD, Lin TK, Yang DI, Lee SY, Shaw FZ, Liou CW, Chuang YC. Protective effects of peroxisome proliferator-activated receptors gamma coactivator-1alpha against neuronal cell death in the hippocampal CA1 subfield after transient global ischemia. J Neurosci Res. 2010;88:605–613. doi: 10.1002/jnr.22225. [DOI] [PubMed] [Google Scholar]
- Chiricozzi E, Fernandez-Fernandez S, Nardicchi V, Almeida A, Bolanos JP, Goracci G. Group IIA secretory phospholipase A2 (GIIA) mediates apoptotic death during NMDA receptor activation in rat primary cortical neurons. J Neurochem. 2010;112:1574–1583. doi: 10.1111/j.1471-4159.2010.06567.x. [DOI] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006;8:1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, DeFazio RA, Raval AP, Torraco A, Saul I, Barrientos A, Perez-Pinzon MA. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci. 2008;28:4172–4182. doi: 10.1523/JNEUROSCI.5471-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del VC, Reyes JM, Park CY, Gao X, Mori K, Chuck RS, Gehlbach PL. Demonstration by redox fluorometry that sulforaphane protects retinal pigment epithelial cells against oxidative stress. Invest Ophthalmol Vis Sci. 2008;49:2606–2612. doi: 10.1167/iovs.07-0960. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187:1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G, Danilov CA, Mehrabian Z, Bambrick LL, Kristian T, McKenna MC, Hopkins I, Richards EM, Rosenthal RE. Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Ann NY Acad Sci. 2008;1147:129–138. doi: 10.1196/annals.1427.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36:347–352. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]
- Gasior M, Rogawski M, Hartman A. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299:C203–C210. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco S, Storelli C, Marsigliante S. Protein kinase C (PKC)-delta/-epsilon mediate the PKC/Akt-dependent phosphorylation of extracellular signal-regulated kinases 1 and 2 in MCF-7 cells stimulated by bradykinin. J Endocrinol. 2006;188:79–89. doi: 10.1677/joe.1.06433. [DOI] [PubMed] [Google Scholar]
- Greco T, Fiskum G. Brain mitochondria from rats treated with sulforaphane are resistant to redox-regulated permeability transition. J Bioenerg Biomembr. 2010a;42:491–497. doi: 10.1007/s10863-010-9312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T, Fiskum G. Neuroprotection through stimulation of mitochondrial antioxidant protein expression. J Alzheimers Dis. 2010b;20 (Suppl 2:427–437. doi: 10.3233/JAD-2010-100519. [DOI] [PubMed] [Google Scholar]
- Greco T, Shafer J, Fiskum G. Sulforaphane inhibits mitochondrial permeability transition and oxidative stress. Free Rad Biol Med. 2011;12:2164–2171. doi: 10.1016/j.freeradbiomed.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Mitochondria—a nexus for aging, calorie restriction, and sirtuins. Cell. 2008;132:171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Nguyen T, Ogbi M, Tawfik H, Ma G, Yu Q, Caldwell RW, Johnson JA. Protein kinase C-epsilon coimmunoprecipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardioprotection. Am J Physiol Heart Circ Physiol. 2007;293:H2219–H2230. doi: 10.1152/ajpheart.01306.2006. [DOI] [PubMed] [Google Scholar]
- Gutsaeva DR, Carraway MS, Suliman HB, Demchenko IT, Shitara H, Yonekawa H, Piantadosi CA. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci. 2008;28:2015–2024. doi: 10.1523/JNEUROSCI.5654-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsaeva DR, Suliman HB, Carraway MS, Demchenko IT, Piantadosi CA. Oxygen-induced mitochondrial biogenesis in the rat hippocampus. Neuroscience. 2006;137:493–504. doi: 10.1016/j.neuroscience.2005.07.061. [DOI] [PubMed] [Google Scholar]
- Haces ML, Hernandez-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol. 2008;211:85–96. doi: 10.1016/j.expneurol.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Hobbs CE, Murphy MP, Smith RA, Oorschot DE. Neonatal rat hypoxia-ischemia: effect of the anti-oxidant mitoquinol, and S-PBN. Pediatr Int. 2008;50:481–488. doi: 10.1111/j.1442-200X.2008.02705.x. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Brookes PS. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J Biol Chem. 2009;284:16236–16245. doi: 10.1074/jbc.M809512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MP, Greenamyre JT. Mitochondrial iron metabolism and its role in neurodegeneration. J Alzheimers Dis. 2010;20 (Suppl 2:S551–S568. doi: 10.3233/JAD-2010-100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahani-Asl A, Pilon-Larose K, Xu W, MacLaurin JG, Park DS, McBride HM, Slack RS. The mitochondrial inner membrane GTPase, optic atrophy 1 (Opa1), restores mitochondrial morphology and promotes neuronal survival following excitotoxicity. J Biol Chem. 2011;286:4772–4782. doi: 10.1074/jbc.M110.167155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Jarrett SG, Milder JB, Liang LP, Patel M. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem. 2008;106:1044–1051. doi: 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Prog Lipid Res. 2010;49:61–75. doi: 10.1016/j.plipres.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41:172–179. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci. 2009;29:7003–7014. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Garduno L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Curphey TJ, Maxiutenko Y, Roebuck BD. Chemoprotection by organosulfur inducers of phase 2 enzymes: dithiolethiones and dithiins. Drug Metabol Drug Interact. 2000;17:3–22. doi: 10.1515/dmdi.2000.17.1-4.3. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Raval AP, Perez-Pinzon MA. Preconditioning mediated by sublethal oxygen-glucose deprivation-induced cyclooxygenase-2 expression via the signal transducers and activators of transcription 3 phosphorylation. J Cereb Blood Flow Metab. 2008;28:1329–1340. doi: 10.1038/jcbfm.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korde AS, Pettigrew LC, Craddock SD, Maragos WF. The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J Neurochem. 2005;94:1676–1684. doi: 10.1111/j.1471-4159.2005.03328.x. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Fenton RG, Fiskum G. Bcl-2 family proteins regulate mitochondrial reactive oxygen production and protect against oxidative stress. Free Radic Biol Med. 2004;37:1845–1853. doi: 10.1016/j.freeradbiomed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Krajewska M, Rosenthal RE, Mikolajczyk J, Stennicke HR, Wiesenthal T, Mai J, Naito M, Salvesen GS, Reed JC, Fiskum G, Krajewski S. Early processing of Bid and caspase-6, -8, -10, -14 in the canine brain during cardiac arrest and resuscitation. Exp Neurol. 2004;189:261–279. doi: 10.1016/j.expneurol.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Kristian T, Siesjo BK. Calcium in ischemic cell death. Stroke. 1998;29:705–718. doi: 10.1161/01.str.29.3.705. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt C, Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon protein kinase C mediated ischemic tolerance requires activation of the extracellular regulated kinase pathway in the organotypic hippocampal slice. J Cereb Blood Flow Metab. 2004;24:636–645. doi: 10.1097/01.WCB.0000121235.42748.BF. [DOI] [PubMed] [Google Scholar]
- Lin HW, Thompson JW, Morris KC, Perez-Pinzon MA. Signal transducers and activators of transcription: STATs-mediated mitochondrial neuroprotection. Antioxid Redox Signal. 2011;14:1853–1861. doi: 10.1089/ars.2010.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rosenthal RE, Starke-Reed P, Fiskum G. Inhibition of postcardiac arrest brain protein oxidation by acetyl-L-carnitine. Free Radic Biol Med. 1993;15:667–670. doi: 10.1016/0891-5849(93)90171-p. [DOI] [PubMed] [Google Scholar]
- Maalouf M, Sullivan P, Davis L, KIM DY, RHO JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka A, Kizuka F, Lee L, Tamura I, Taniguchi K, Asada H, Taketani T, Tamura H, Sugino N. Progesterone increases manganese superoxide dismutase expression via a cAMP-dependent signaling mediated by noncanonical Wnt5a pathway in human endometrial stromal cells. J Clin Endocrinol Metab. 2010;95:E291–E299. doi: 10.1210/jc.2010-0619. [DOI] [PubMed] [Google Scholar]
- Meloni BP, Tilbrook PA, Boulos S, Arthur PG, Knuckey NW. Erythropoietin preconditioning in neuronal cultures: signaling, protection from in vitro ischemia, and proteomic analysis. J Neurosci Res. 2006;83:584–593. doi: 10.1002/jnr.20755. [DOI] [PubMed] [Google Scholar]
- Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011;31:1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Gangopadhyay H, Das DK. Broccoli: a unique vegetable that protects mammalian hearts through the redox cycling of the thioredoxin superfamily. J Agric Food Chem. 2008;56:609–617. doi: 10.1021/jf0728146. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- Nagley P, Higgins GC, Atkin JD, Beart PM. Multifaceted deaths orchestrated by mitochondria in neurones. Biochim Biophys Acta. 2010;1802:167–185. doi: 10.1016/j.bbadis.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim Biophys Acta. 2009;1787:1416–1424. doi: 10.1016/j.bbabio.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo DO, Povlishock JT. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J Cereb Blood Flow Metab. 1999;19:443–451. doi: 10.1097/00004647-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Packer L, Valenza M, Serbinova E, Starke-Reed P, Frost K, Kagan V. Free radical scavenging is involved in the protective effect of L-propionyl-carnitine against ischemia-reperfusion injury of the heart. Arch Biochem Biophys. 1991;288:533–537. doi: 10.1016/0003-9861(91)90231-7. [DOI] [PubMed] [Google Scholar]
- Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal. 2011;23:1534–1545. doi: 10.1016/j.cellsig.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Paradis E, Clavel S, Bouillaud F, Ricquier D, Richard D. Uncoupling protein 2: a novel player in neuroprotection. Trends Mol Med. 2003;9:522–525. doi: 10.1016/j.molmed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Basit A, Dave KR, Busto R, Veauvy C, Saul I, Ginsberg MD, Sick TJ. Effect of the first window of ischemic preconditioning on mitochondrial dysfunction following global cerebral ischemia. Mitochondrion. 2002;2:181–189. doi: 10.1016/s1567-7249(02)00070-3. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Sick TJ, Rosenthal M. Mechanism(s) of mitochondrial hyperoxidation after global cerebral ischemia. Adv Exp Med Biol. 1999;471:175–180. doi: 10.1007/978-1-4615-4717-4_21. [DOI] [PubMed] [Google Scholar]
- Pivovarova NB, Andrews SB. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 2010;277:3622–3636. doi: 10.1111/j.1742-4658.2010.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- Prins ML, Fujima LS, Hovda DA. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res. 2005;82:413–420. doi: 10.1002/jnr.20633. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Ran R, Xu H, Lu A, Bernaudin M, Sharp FR. Hypoxia preconditioning in the brain. Dev Neurosci. 2005;27:87–92. doi: 10.1159/000085979. [DOI] [PubMed] [Google Scholar]
- Rasbach KA, Schnellmann RG. Signaling of mitochondrial biogenesis following oxidant injury. J Biol Chem. 2007;282:2355–2362. doi: 10.1074/jbc.M608009200. [DOI] [PubMed] [Google Scholar]
- Raval AP, Dave KR, DeFazio RA, Perez-Pinzon MA. epsilonPKC phosphorylates the mitochondrial K(+) (ATP) channel during induction of ischemic preconditioning in the rat hippocampus. Brain Res. 2007;1184:345–353. doi: 10.1016/j.brainres.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- Richards EM, Fiskum G, Rosenthal RE, Hopkins I, McKenna MC. Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke. 2007;38:1578–1584. doi: 10.1161/STROKEAHA.106.473967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Rosca MG, Lemieux H, Hoppel CL. Mitochondria in the elderly: is acetylcarnitine a rejuvenator. Adv Drug Deliv Rev. 2009;61:1332–1342. doi: 10.1016/j.addr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal RE, Williams R, Bogaert YE, Getson PR, Fiskum G. Prevention of postischemic canine neurological injury through potentiation of brain energy metabolism by acetyl-L-carnitine. Stroke. 1992;23:1312–1317. doi: 10.1161/01.str.23.9.1312. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Kim SU, McLarnon JG. Blockade of quinolinic acid-induced neurotoxicity by pyruvate is associated with inhibition of glial activation in a model of Huntington's disease. Exp Neurol. 2004;187:150–159. doi: 10.1016/j.expneurol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Scafidi S, Fiskum G, Lindauer SL, Bamford P, Shi D, Hopkins I, McKenna MC. Metabolism of acetyl-L-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J Neurochem. 2010;114:820–831. doi: 10.1111/j.1471-4159.2010.06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafidi S, Racz J, Hazelton J, McKenna MC, Fiskum G. Neuroprotection by acetyl-L-carnitine after traumatic injury to the immature rat brain. Dev Neurosci. 2011;32:480–487. doi: 10.1159/000323178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh RA, Clerc P, Hwang H, Mehrabian Z, Bittman K, Chen H, Polster BM. Adaptation of microplate-based respirometry for hippocampal slices and analysis of respiratory capacity. J Neurosci Res. 2011;89:1979–1988. doi: 10.1002/jnr.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Pan Q, Paiva M, Madorsky I, Khurana NC, Heaton MB. Mitochondrially targeted vitamin E and vitamin E mitigate ethanol-mediated effects on cerebellar granule cell antioxidant defense systems. Brain Res. 2005;1052:202–211. doi: 10.1016/j.brainres.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- Sparvero LJ, Amoscato AA, Kochanek PM, Pitt BR, Kagan VE, Bayir H. Mass-spectrometry based oxidative lipidomics and lipid imaging: applications in traumatic brain injury. J Neurochem. 2010;115:1322–1336. doi: 10.1111/j.1471-4159.2010.07055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- Tripanichkul W, Sripanichkulchai K, Duce JA, Finkelstein DI. 17Beta-estradiol reduces nitrotyrosine immunoreactivity and increases SOD1 and SOD2 immunoreactivity in nigral neurons in male mice following MPTP insult. Brain Res. 2007;1164:24–31. doi: 10.1016/j.brainres.2007.05.076. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, Bertolotti P, Delbarba A, Perego C, Dossena M, Ragni M, Spano P, Carruba MO, De Simoni MG, Nisoli E. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. J Neurochem. 2011;116:1148–1159. doi: 10.1111/j.1471-4159.2011.07171.x. [DOI] [PubMed] [Google Scholar]
- Varma SD, Devamanoharan PS, Ali AH. Prevention of intracellular oxidative stress to lens by pyruvate and its ester. Free Radic Res. 1998;28:131–135. doi: 10.3109/10715769809065799. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab. 2005;26:821–835. doi: 10.1038/sj.jcbfm.9600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volbracht C, Fava E, Leist M, Nicotera P. Calpain inhibitors prevent nitric oxide-triggered excitotoxic apoptosis. Neuroreport. 2001;12:3645–3648. doi: 10.1097/00001756-200112040-00008. [DOI] [PubMed] [Google Scholar]
- Vosler PS, Graham SH, Wechsler LR, Chen J. Mitochondrial targets for stroke: focusing basic science research toward development of clinically translatable therapeutics. Stroke. 2009;40:3149–3155. doi: 10.1161/STROKEAHA.108.543769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhu X, Kim Y, Li J, Huang S, Saleem S, Li RC, Xu Y, Dore S, Cao W. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med. 2011;52:928–936. doi: 10.1016/j.freeradbiomed.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lim PJ, Karbowski M, Monteiro MJ. Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum Mol Genet. 2009;18:737–752. doi: 10.1093/hmg/ddn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- Xie J, Duan L, Qian X, Huang X, Ding J, Hu G. K(ATP) channel openers protect mesencephalic neurons against MPP+-induced cytotoxicity via inhibition of ROS production. J Neurosci Res. 2010;88:428–437. doi: 10.1002/jnr.22213. [DOI] [PubMed] [Google Scholar]
- Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante RJ, Beal MF. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson's and Huntington's diseases. J Neurochem. 2009;109:1427–1439. doi: 10.1111/j.1471-4159.2009.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J. Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke. 2008;39:3057–3063. doi: 10.1161/STROKEAHA.108.520114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelli SA, Solenski NJ, Rosenthal RE, Fiskum G. Mechanisms of ischemic neuroprotection by acetyl-l-carnitine. Ann NY Acad Sci. 2005;1053:153–161. doi: 10.1196/annals.1344.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Signore AP, Zhou Z, Wang S, Cao G, Chen J. Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: potential signaling mechanisms. J Neurosci Res. 2006;83:1241–1251. doi: 10.1002/jnr.20816. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Cao G, Gao Y, Chen J. Signal transducers and activators of transcription 5 contributes to erythropoietin-mediated neuroprotection against hippocampal neuronal death after transient global cerebral ischemia. Neurobiol Dis. 2007;25:45–53. doi: 10.1016/j.nbd.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jia H, Liu J, Ao N, Yan B, Shen W, Wang X, Li X, Luo C, Liu J. Combined R-alpha-lipoic acid and acetyl-L-carnitine exerts efficient preventative effects in a cellular model of Parkinson's disease. J Cell Mol Med. 2010;14:215–225. doi: 10.1111/j.1582-4934.2008.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006a;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Zhao J, Moore AN, Clifton GL, Dash PK. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005;82:499–506. doi: 10.1002/jnr.20649. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, Humala N, Thiyagarajan M, Wang J, Pasinetti GM. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006b;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, Jonghe PD, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V, Schroder JM, Vance JM. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]