Abstract
By virtue of their ability to induce apoptosis and regulate growth, differentiation, and cytokine responses, the tumor necrosis factor receptor (TNFR) superfamily members have emerged as attractive targets for anticancer therapeutics. Agonistic antibodies to apoptosis-inducing TNFRs, such as death receptor 5 (DR5), although displaying impressive activities against a variety of tumors in preclinical models, appear to be less active in clinical trials. We report that the in vivo apoptotic and antitumor activities of these antibodies have an absolute requirement for the coengagement of an inhibitory Fcγ receptor, FcγRIIB. Anti-DR5 antibodies of the type currently in clinical trials have weak FcγRIIB binding and thus are compromised in their proapoptotic and antitumor activities in both colon and breast carcinoma models. Enhancing FcγRIIB engagement increases apoptotic and antitumor potency. Our results demonstrate that Fc domain interactions are critical to the therapeutic activity of anti-DR5 antibodies and, together with previous reports on agonistic anti-CD40 antibodies, establish a common requirement for FcγRIIB coengagement for optimal biological effects of agonistic anti-TNFR antibodies.
Keywords: cancer immunotherapy, Fc engineering, human FCGR2B, antibody-dependent cell-mediated cytotoxicity
The tumor necrosis factor receptor (TNFR) superfamily members are widely expressed on normal and malignant tissues (1, 2) Based on the signaling pathway used by their cytoplasmic domains, TNFRs can be broadly divided into those receptors that signal through TNFR-associated factors (TRAFs) and those known as “death receptors,” which signal through Fas-associated protein with death domain (FADD) adaptor molecules and result in apoptosis. Signaling transmitted by the former group regulates many biological processes, including the growth, differentiation, and response to cytokines, in a wide variety of immune cells. Death receptor-induced apoptosis plays an important role in the regulation of homeostasis for a variety of tissues, including the liver, as well as in the regulation of innate immune cells (3). These pathways are also responsible for the intrinsic antitumor response (3, 4), thereby preventing the expansion of transformed cells. The central role of TNFRs in regulating both immune responses and apoptosis, along with their expression by many malignant tissues, have made TNFR molecules attractive therapeutic targets for cancer immunotherapy (2). Several agonistic anti-TNFR antibodies have been developed for the treatment of solid tumors, including anti-CD40, a member of the TRAF family of TNFRs, for its ability to activate cytotoxic T cells, and anti-DR5, a member of the FADD family of TNFRs, for its direct apoptotic activity against tumor cells (5, 6). DR5, (also known as TRAIL-R2) and its ligand TRAIL are of particular interest, because DR5 is expressed on a variety of tumor cells including colon and breast carcinomas, and because studies in mouse models have shown that DR5 can be targeted to promote strong tumor eradication without damaging normal tissues (7, 8). Like other death receptors, after triggering by TRAIL or agonistic antibodies, DR5 induces apoptosis by recruiting the FADD adaptor protein, which activates caspase-8 and the downstream caspase-3 to execute apoptosis (1).
Preclinical testing of agonistic anti-TNFR antibodies, including anti-CD40 and anti-DR5, demonstrated significant activity in eradicating established tumors and limiting metastatic disease (8–10). However, results from early clinical trials, although promising, did not match the preclinical activities observed (5, 6, 11–15). We reasoned that these differences may result in part from species-specific interactions between the Fc domains of these antibodies and their human Fc receptors, which were not addressed in the preclinical studies that led to the development of these antibodies. We and others recently reported that agonistic anti-CD40 antibodies indeed require specific Fc receptor engagement to activate cytotoxic T cells and thereby target tumor cells (16, 17). In contrast to the Fc receptor requirement for cytotoxic antibodies, such as anti-CD20 and anti-Her2neu, anti-CD40 antibodies display an absolute requirement for coengagement of the inhibitory Fcγ receptor FcγRIIB, and consequently, its in vivo activity can be enhanced through FcγRIIB-targeted Fc engineering (16–18).
The IgG Fc receptors (FcγRs) have emerged as central determinants of the in vivo function of IgG antibodies for such diverse activities as cytotoxicity, virus and toxin neutralization, immunomodulation, and anti-inflammatory responses (19, 20). In both mouse and human, the FcγR system comprises several immunoreceptor tyrosine-based activation motif-containing activating FcγRs (FcγRI, FcγRIII, and FcγRIV in mouse; huFcγRI, huFcγRIIA, and huFcγRIIIA in human) and one immunoreceptor tyrosine-based inhibitory motif-containing inhibitory FcγR (FcγRIIB in mouse and huFcγRIIB in human). Activating FcγRs are absolutely required for antibody-dependent cell-mediated cytotoxicity (ADCC), which is vital during cancer immunotherapy (18, 21). The Fc interaction with activating FcγRs is also critical for IgG antibody-mediated neutralization of bacterial toxins and viral pathogens (22–24). In contrast, FcγRIIB is a negative regulator of immune cell activation and antibody–antigen immune complex-triggered inflammation, thereby contributing to the regulation of effector cell activation (25, 26). In addition, FcγRIIB’s expression on follicular B cells and plasma cells is critical to the maintenance of peripheral tolerance (27–30). Each FcγR interacts differently with Fcs of the different IgG subclasses within a species and displays unpredictable cross-species interactions, precluding extrapolation from one species to another. These interactions are critical determinants of the different biological activities of each IgG subclass. Increased understanding of Fc–FcγR interactions has promoted Fc engineering that generates Fcs with enhanced or reduced binding affinities to individual FcγRs, thereby optimizing the development of therapeutic antibodies (31–33). Here we report on the FcγR requirements for agonistic antibodies to apoptosis-inducing members of the TNFR superfamily, using DR5 as an example.
Results
Impaired Antitumor Effects of MD5-1 in Fcgr2b−/− Mice.
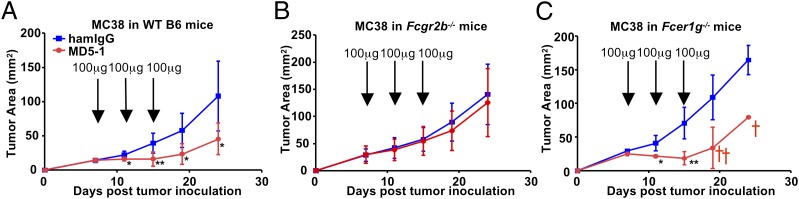
To study whether FcγRs contribute to the antitumor activities of agonistic anti-DR5 antibodies in vivo, we studied the antitumor activity of MD5-1, an agonistic Armenian hamster IgG2 anti-mouse DR5 antibody (8), in mice carrying FcγR mutations using the MC38 colon carcinoma model. As reported previously (10) and shown in Fig. 1A, MD5-1 treatment significantly inhibited the growth of established MC38 tumors in B6 WT mice. This antitumor effect was abolished in B6 mice deficient for FcγRIIB (Fcgr2b−/−; Fig. 1B), but was unaffected in B6 mice lacking the FcR common γ chain (Fcer1g−/−; Fig. 1C), which is required for all activating FcγRs. These results demonstrate that the antitumor effect of MD5-1 requires FcγRIIB, but not activating FcγRs. Interestingly, although MD5-1 significantly slowed MC38 growth in Fcer1g−/− mice, it also caused significant mortality.
Fig. 1.
MD5-1 uniquely depends on FcγRIIB for its tumoricidal activity in the MC38 model. MC38 cells were inoculated s.c. into WT (A), Fcgr2b−/− (B), and Fcer1g−/− (C) mice on the B6 background, then treated with MD5-1 antibodies at the indicated doses at the time points indicated by arrows. Representative tumor growth curves of four to six mice are shown. The cross represents mortality. Error bars represent SD. *P < 0.05; **P < 0.01.
MD5-1–Induced Hepatotoxicity Requires FcγRIIB.
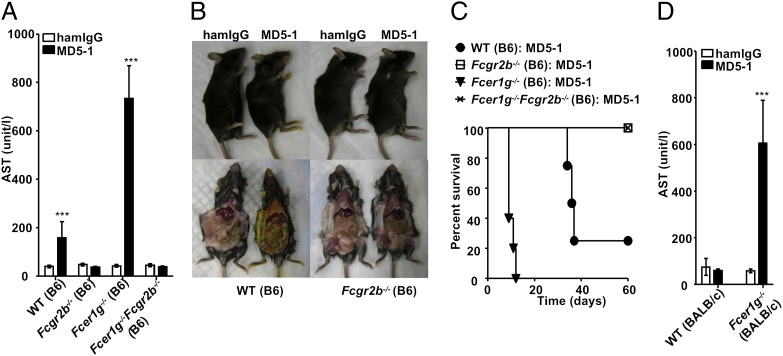
We investigated the accelerated mortality of Fcer1g−/− mice treated with MD5-1 to determine the cause of this toxicity. Previous studies demonstrated that MD5-1 induced hepatotoxicity in a strain-dependent manner, resulting in cholestatic liver injury in B6 mice, but not in BALB/c mice (34). In susceptible strains like B6, MD5-1 treatment triggers apoptosis in cholangiocytes and causes cholestatic liver disease. To study the effect of inhibitory and activating FcγRs on MD5-1–induced hepatotoxicity, we treated B6 and BALB/c mice with selective FcγR mutations with MD5-1 and analyzed them for hepatotoxicity. MD5-1 treatment resulted in elevated serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels and jaundice, all of which are indications of liver failure, in WT B6 mice, but not in BALB/c mice, resulting in death within 2 mo after treatment (Fig. 2 A–D and Figs. S1 and S2), as reported previously (34). These hepatotoxic phenotypes were accelerated in Fcer1g−/− B6 mice (Fig. 2 A and C and Fig. S1A) and required a lower dose of MD5-1 to result in this phenotype compared with WT B6 mice (Fig. S2). In contrast, none of these hepatotoxicity phenotypes was observed in B6 mice deficient for FcγRIIB (Fig. 2 A–C and Fig. S1A).
Fig. 2.
MD5-1 uniquely depends on FcγRIIB for its hepatotoxic effect. (A–C) WT, Fcgr2b−/−, Fcer1g−/−, and Fcer1g−/−Fcgr2b−/− mice on the B6 background (five mice per group) were treated with high-dose MD5-1 or hamster control IgG (300 μg/mouse repeated at 3-d intervals for a total of 1.2 mg/mouse), and analyzed for serum AST level (A), jaundice (B), and survival (C). (D) Serum AST levels of WT and Fcer1g−/− mice on the BALB/c background (four mice per group) treated with 300 μg of MD5-1 7 d earlier. ***P < 0.001. Error bars represent SD.
These results demonstrate that FcγRIIB is both necessary and sufficient for MD5-1–induced hepatotoxicity, whereas activating FcγRs are dispensable, and their deficiency appears to exacerbate the hepatotoxic effect. The hepatotoxicity induced by MD5-1 in B6 mice is not affected by the presence or absence of T cells; disease was similar in T-cell–deficient mice and WT mice (Fig. S3). To determine whether FcR common γ-chain deficiency can sensitize BALB/c mice to MD5-1–induced hepatotoxicity, we challenged BALB/c mice deficient in FcR common γ-chain with MD5-1. These mice demonstrated elevated AST and ALT levels along with premature mortality (Fig. 2D and Figs. S1B and S2). The protection of FcγRIIB-deficient mice from MD5-1–induced hepatotoxicity is not due to developmental defects in these mice; blockade of FcγRIIB by the mAb 2.4G2 attenuated the MD5-1–induced hepatotoxicity phenotypes in WT and Fcer1g−/− mice on both the B6 and BALB/c backgrounds (Figs. S1B and S2).
MD5-1–Induced Tumor Apoptosis Requires FcγRIIB.
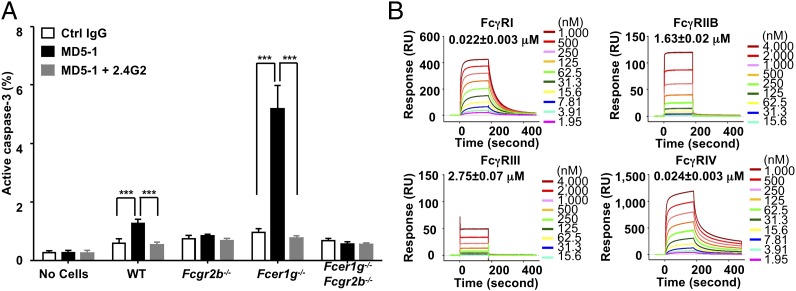
Whereas agonistic anti-DR5 antibodies may kill tumor cells by mediating ADCC or triggering DR5 receptor-mediated apoptosis, the proapoptotic activity of MD5-1 has been proposed to be responsible for both the hepatotoxicity and tumoricidal activity of MD5-1 in the MC38 model (10, 34). To test whether FcγRIIB has a direct effect on MD5-1–induced apoptosis, activation of caspase-3 was quantified in MD5-1–treated MC38 cells cultured in vitro. By itself, MD5-1 treatment did not induce significant caspase-3 activation in MC38 cells (Fig. 3A); however, in the presence of WT splenocytes, MD5-1 treatment consistently induced caspase-3 activation in a small but significant fraction of MC38 cells. This apoptosis-supporting effect of splenotyes depends on their expression of FcγRIIB; neither splenocytes deficient in all FcγRs (Fcer1g−/−Fcgr2b−/−) or deficient solely in Fcgr2b−/− supported MD5-1 to induce apoptosis in MC38 cells. In contrast, Fcer1g−/− splenocytes were superior to WT splenocytes in this assay. Blockade of FcγRIIB by 2.4G2 antibodies abolished MD5-1–induced apoptosis supported by both WT and Fcer1g−/− splenocytes. Thus, FcγRIIB plays a unique role in supporting MD5-1–induced caspase-3 activation, whereas coengagement of activating FcγRs diminishes the ability of DR5 to induce apoptosis, consistent with the observed FcγRIIB requirement in vivo for both the antitumor and hepatotoxic effects of MD5-1, as well as the negative effect of activating FcγRs on the in vivo activities of MD5-1.
Fig. 3.
FcγRIIB is necessary and sufficient for the in vitro proapoptotic activity of MD5-1. (A) Percentage of MC38 cells with active caspase-3. MC38 cells were treated with hamster control IgG, MD5-1, or a combination of MD5-1 and the FcγRIIB/III blocking antibody 2.4G2 in the absence or presence of splenocytes isolated from the indicated mice, and then analyzed for caspase-3 activation. ***P < 0.001. Error bars represent SD. Data shown are representative of three experiments. (B) SPR analysis of the binding of MD5-1 to mouse FcγRs. Real-time sensorgrams with affinity constants (KD = mean ± SD) are shown.
Mouse FcγR-Binding Property of MD5-1.
The binding properties of Armenian hamster IgG2 Fc to mouse FcγRs are poorly understood, but the requirement of mouse FcγRIIB for the proapoptotic, tumoricidal, and hepatotoxic effects of MD5-1 strongly suggest that MD5-1 is able to bind mouse FcγRIIB. To determine the precise affinities between Armenian hamster IgG2 Fc and mouse FcγRs, we analyzed the binding properties of MD5-1 to mouse FcγRs by surface plasmon resonance (SPR) analysis. We found that MD5-1 binds to all mouse FcγRs, displaying low affinity to mouse FcγRIIB and FcγRIII and intermediate affinity to mouse FcγRIV and FcγRI (Fig. 3B). Thus, in contrast to mouse IgG subclasses, which display preferential binding to activating or inhibitory FcγRs (35), Armenian hamster IgG2 is promiscuous in its binding to mouse FcγRs, providing a likely explanation for the enhanced MD5-1 activity in mice deficient in activating FcγRs. Deletion of these receptors eliminates their ability to compete with FcγRIIB to engage MD5-1 and results in an effective higher local concentration of antibody to engage FcγRIIB to induce apoptosis in the target cells. Because MD5-1 is also able to engage mouse FcγRIII and IV and trigger ADCC through NK cells and macrophages, the absolute dependence on FcγRIIB for the in vivo tumoricidal activity of MD5-1 against MC38 cells indicates that the proapoptotic activity dominates in vivo over the cytotoxic activity for MC38 tumor eradication.
Fc-Engineered Anti-DR5 Antibody with Enhanced FcγRIIB Binding Is More Potent than Its Native Form in Vivo.
To test whether Fc–FcγRIIB interactions can be exploited to modulate the activity of agonistic anti-DR5 antibodies, we produced recombinant chimeric anti-DR5 antibodies containing the variable regions of MD5-1 and constant regions with different human Fcs. Among these, the WT human IgG1 Fc (hIgG1) binds to huFcγRIIB with low affinity and to huFcγRIIIA (or mouse FcγRIV) with intermediate affinity (32, 36), the N297A variant does not bind to FcγRs (37), and the S267E variant has enhanced binding to huFcγRIIB (32) and reduced binding to mouse FcγRIV (Fig. S4A). All of these antibodies retain the specificity and affinity of MD5-1 antibody to DR5, competing with MD5-1 with the same efficiency for the binding of DR5 on MC38 cells (Fig. S4B). To test the in vivo potency of these engineered chimeric antibodies, we used a novel B6 mouse strain in which the mouse Fcgr2b gene was deleted and the human FCGR2B (huFCGR2B) gene was inserted as a transgene. The huFCGR2B gene, driven by its human promoter elements, retains the specific cellular expression pattern seen in the human and is functional for FcγRIIB inhibitory and immunomodulatory activities (38).
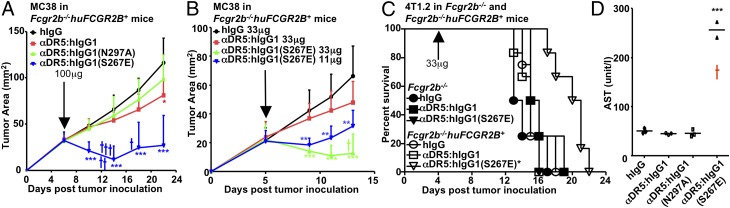
MC38 tumors were implanted in Fcgr2b−/−huFCGR2B+ mice on the B6 background and treated with the chimeric anti-DR5 antibodies. At a dose of 100 μg/mouse, αDR5:hIgG1(N297A) exhibited no antitumor activity, and αDR5:hIgG1 had very weak antitumor activity (Fig. 4A). In contrast, αDR5:hIgG1(S267E) significantly inhibited the growth of MC38 tumors in a huFCGR2B-dependent manner (Fig. 4A and Fig. S5) but also caused significant mortality (Fig. 4A). However, at a dosage of 33 μg/mouse or 11 μg/mouse, the toxic effect of αDR5:hIgG1(S267E) was attenuated while still providing significant tumoricidal activity, and in some cases MC38 tumors were completely eradicated after αDR5:hIgG1(S267E) treatment (Fig. 4B and Fig. S6).
Fig. 4.
Tumoricidal and hepatotoxic effects of agonistic anti-DR5 antibodies can be enhanced by FcγRIIB-targeted Fc engineering. (A and B) Fcgr2b−/−huFCGR2B+ mice on the B6 background (five to nine mice per group) were implanted with MC38 cells s.c. and treated with 100 μg (A) or 33 μg or 11 μg/mouse (B) of the indicated control or anti-DR5 antibodies at the indicated times. Tumor growth curves are presented. (C) Fcgr2b−/− and Fcgr2b−/−huFCGR2B+ mice on the BALB/c background (four to six mice per group) were inoculated with 4T1.2 cells i.v. and treated with 33 μg/mouse of the indicated control or anti-DR5 antibodies at the indicated times. Survival curves are shown. (D) Fcgr2b−/−huFCGR2B+ on the B6 background (three mice per group) were treated with 300 μg of the indicated control or anti-DR5 antibodies and analyzed for serum AST level 7 d later. *P < 0.05; **P < 0.01; ***P < 0.001. The cross represents mortality. Error bars represent SD.
We also analyzed the tumoricidal activity of these chimeric, Fc-engineered anti-DR5 antibodies in a 4T1.2 breast carcinoma metastasis model in BALB/c mice either deficient in mouse FcγRIIB (Fcgr2b−/−) or carrying a human transgene for human FcγRIIB on a mouse FcγRIIB-deficient background (Fcgr2b−/−huFCGR2B+). Only αDR5:hIgG1(S267E) treatment significantly prolonged mouse survival in huFCGR2B-dependent manner (Fig. 4C). Therefore, the Fc-engineered anti-DR5 antibody with enhanced FcγRIIB binding is a potent antitumor compound in multiple tumor models. We evaluated the hepatotoxicity of these chimeric, Fc-engineered anti-DR5 antibodies in B6 mice deficient in mouse FcγRIIB and transgenic for human FcγRIIB (Fcgr2b−/−huFCGR2B+). Mice were treated with 300 μg of anti-DR5 antibodies, a dose 10–30 times greater than that required to observe antitumor effects in the MC38 tumor model, and liver function was monitored by measuring serum AST and ALT levels. At these elevated levels of antibody, αDR5:hIgG1(S267E) treatment induced elevated serum AST and ALT levels or mortality (Fig. 4D and Fig. S7), demonstrating that a dosage range can be achieved at which significant antitumor effects are obtained without significant hepatotoxicity. These results suggest that tumor cells are more sensitive than cholangiocytes to the proapoptotic activity of agonistic anti-DR5 antibodies, thereby providing a therapeutic window for anti-DR5 treatment with FcγRIIB-enhanced variants.
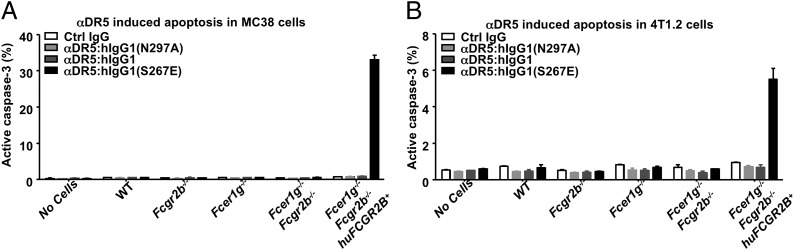
Fc-Engineered Anti-DR5 Antibody with Enhanced FcγRIIB Binding Is More Potent in Inducing Apoptosis.
To confirm that the increased in vivo antitumor and hepatotoxic activities of agonistic anti-DR5 antibodies engineered to target FcγRIIB results from increased proapoptotic activities, we analyzed anti-DR5 antibody-induced activation of caspase-3 in vitro. In the presence of splenocytes with the huFCGR2B transgene, αDR5:hIgG1(S267E) treatment induced caspase-3 activation in a large proportion of MC38 cells, in contrast to the minimal effect of αDR5:hIgG1(N297A) (Fig. 5A). A similar pattern was observed for 4T1.2 cells treated with these antibodies, albeit with approximately sixfold lower levels of caspase-3 activation (Fig. 5B), indicating that 4T1.2 cells are less sensitive than MC38 cells to agonistic anti-DR5 antibody-induced apoptosis (Fig. 3A and Fig. S8). Strikingly, αDR5:hIgG1 failed to induce significant caspase-3 activation in either MC38 or 4T1.2 cells in the presence of huFCGR2B+ splenocytes, suggesting the presence of an affinity threshold for the Fc–FcγRIIB interaction to support DR5 agonistic antibody-mediated apoptosis.
Fig. 5.
Proapoptotic activities of agonistic anti-DR5 antibodies can be enhanced by FcγRIIB-targeted Fc engineering. MC38 or 4T1.2 cells were treated with the indicated control IgG or anti-DR5 antibodies in the absence or presence of splenocytes isolated from the indicated mice and then analyzed for caspase-3 activation. The percentages of MC38 (A) and 4T1.2 (B) cells with active caspase-3 are shown. Note the different scales for each tumor line. Error bars represent SD. Results are representative of two experiments.
In Vivo Antitumor Activity of αDR5:hIgG1(S267E) Depends on Its Proapoptotic Activity.
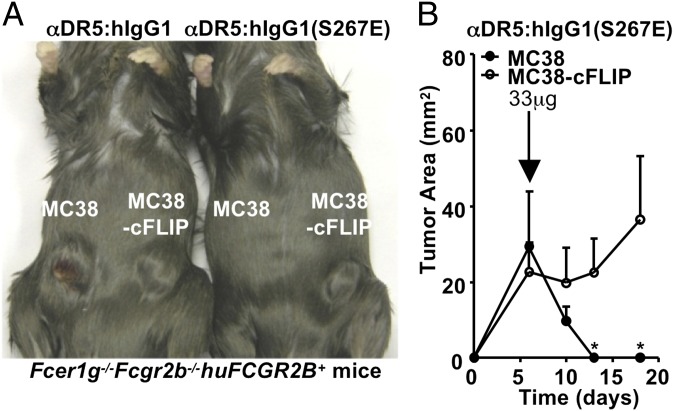
To determine whether αDR5:hIgG1(S267E) mediates its antitumor effect by inducing apoptosis in vivo, we compared the activity of this anti-DR5 antibody for its antitumor activity in B6 mice transgenic for human FCGR2B challenged with either MC38 tumors or MC38 tumors, which overexpress cFLIP, an inhibitor of caspase-8, and thus are incapable of triggering apoptosis through death domain receptor signaling (10). B6 mice transgenic for huFCGR2B challenged with MC38 cells and treated with αDR5:hIgG1(S267E) demonstrated arrest of MC38 tumor cell growth, but insignificant growth arrest of MC38-cFLIP tumors when established in the same genetic background (Fig. 6 A and B). These findings indicate that DR5-induced apoptosis is the mechanism by which αDR5:hIgG1(S267E) results in tumor arrest, which requires the coengagement of FcγRIIB to mediate this effect.
Fig. 6.
αDR5:hIgG1(S267E) exert its antitumor activity through apoptosis. Fcer1g−/−Fcgr2b−/−huFCGR2B+ mice on the B6 background (three mice per group) were inoculated s.c. with MC38 cells on the left flank and MC38-cFLIP cells on the right flank, and then treated with 33 μg/mouse of the indicated anti-DR5 antibodies at the indicated time. Shown are a photo of representative mice treated with the indicated anti-DR5 antibodies (A) and tumor growth curves of MC38 and MC38-cFLIP in the αDR5:hIgG1(S267E)-treated mice (B). *P < 0.05. Error bars represent SD.
Discussion
Although both activating and inhibitory FcγRs have been reported to support the proapoptotic activities of agonistic anti-DR5 antibodies in vitro (8, 39), their in vivo contributions are less well understood. In this study, we examined the activities of agonistic anti-DR5 antibodies in vivo using their tumoricidal and hepatotoxic effects as readouts, and found that only FcγRIIB, expressed in trans, is both necessary and sufficient to support the proapoptotic activity of agonistic anti-DR5 antibodies. Both the tumoricidal and hepatotoxic effects of MD5-1 depended completely on FcγRIIB expression, and competition from activating FcγRs reduced the proapoptotic activity of anti-DR5 antibodies. Mice deficient in these activation receptors, such as Fcer1g−/− mice, displayed enhanced hepatotoxic phenotypes in both the B6 and BALB/c backgrounds compared with their WT counterparts. In addition, anti-DR5 antibodies with an Fc engineered for enhanced huFcγRIIB binding showed greatly enhanced proapoptotic activity in vitro, as well as greatly enhanced tumoricidal and hepatotoxic activities in vivo, demonstrating that the requirement for FcγRIIB engagement can be exploited to produce more potent agonistic anti-DR5 antibodies.
Wilson et al. (39) recently reported that either activating or inhibitory FcγRs were sufficient to support proapoptotic signaling triggered by drozitumab (a human DR5-specific antibody with human IgG1 Fc). However, consistent with our results reported here, their in vivo analysis found that although drozitumab or chimeric anti-human DR4 and DR5 antibodies with mouse IgG1 Fcs were able to inhibit xenograft tumor growth in Rag2−/− mice either WT or deficient in activating FcγRI and III, drozitumab failed to significantly inhibit tumor growth on an Fcgr2b−/− background (39), in agreement with our finding that FcγRIIB is not only necessary but sufficient for in vivo antitumor activity.
Another previous study reported different effects of FcγRs on the antitumor activity of MD5-1 in the 4T1 tumor model in BALB/c mice (8). In that model, activating FcγRs were required and FcγRIIB was dispensable for the antitumor effect of MD5-1 (8). Our data indicate that MD5-1, through its ability to engage the activating FcγRIII and IV, also may mediate antitumor activity through ADCC. Indeed, the proapoptotic activity of MD5-1 was substantially weaker for 4T1.2 cells (a line derived from 4T1) than for MC38 cells (Figs. 3A and 5 and Fig. S8), and in the absence of Fc optimization to enhance FcγRIIB binding, proapoptotic activity might be negligible in vivo. Thus, although it has been suggested that MD5-1 exerts its antitumor activity in the 4T1 model through its proapoptotic activity, ADCC cannot be ruled out as an alternative explanation for the requirement of activating FcγRs. The requirement for activating FcγR-bearing CD11b+ ADCC effector cells (8, 10) supports the interpretation that ADCC may be important for the antitumor activity of MD5-1 in the 4T1 model, but not in the MC38 model. The strong hepatotoxic effect of MD5-1 and its tumoricidal activity against MC38 cells in Fcer1g−/− mice excluded a significant contribution of ADCC in these effects. In addition, the resistance of MC38-cFLIP to MD5-1 and αDR5:hIgG1(S267E) suggests that proapoptotic activities of anti-DR5 antibodies play a dominant role in the MC38 model. The dominant contribution of proapoptotic activity by MD5-1 in the MC38 model and the superiority of αDR5:hIgG1(S267E) over αDR5:hIgG1 in both the MC38 and 4T1.2 models suggest that proapoptotic activity of agonistic anti-DR5 antibody is more potent than its ADCC activity as an antitumor mechanism. This conclusion is also supported by the observation that MD5-1–derived anti-DR5 antibodies with mouse IgG2a Fc, which is the most potent mouse Fc for ADCC and has much higher affinity to mouse activating FcγRs compared with human IgG1 Fc or the S267E variant of human IgG1 Fc (Fig. S4A; ref. 36), failed to show strong antitumor activities in WT B6 mice (Fig. S9). In addition, the association of increased FcγRIIB expression with tumor development (40) also may make FcγRIIB-dependent agonistic anti-DR5 antibodies more potent in antitumor responses than ADCC (and activating FcγRs)-dependent anti-DR5 antibodies.
Previous studies have reported a hepatotoxic effect of MD5-1 in B6 mice, but not in BALB/c mice (8, 34). Our observation that FcR common γ-chain deficiency can sensitize BALB/c mice to MD5-1–induced hepatotoxicity suggests that mice on different genetic backgrounds may have different thresholds for agonistic anti-DR5 antibody-induced hepatotoxic effects, but it is unlikely that WT mice are completely immune to this effect. Although the proapoptotic activity of agonistic anti-DR5 antibodies underlies the mechanism of these antibodies’ tumoricidal and hepatotoxic effects, we found that tumors such as MC38 are more sensitive to this mechanism, thereby providing a dosage range within which significant antitumor effects are obtained without significant liver toxicity. Three agonistic anti-human DR5 antibodies with human IgG1 Fcs in clinical trials—conatumumanb, tigatuzumab, and lexatumumab—have been reported to be well tolerated (11–15), but no significant antitumor effects have been identified. Our results suggest that this lack of clinical activity against tumor targets may be related to the low affinity between human IgG1 Fc and human FcγRIIB, thus failing to achieve the threshold required for inducing tumor apoptosis. Given that the therapeutic effects of agonistic anti-DR5 antibodies depend on the difference in sensitivity to apoptosis between cancer cells and cholangiocytes, it is possible that FcγRIIB-dependent agonistic anti-DR5 antibodies might not have a large therapeutic window in tumor cells that express DR5, but rather are resistant or have very high thresholds for DR5-mediated apoptosis. In these cases, the relatively low potency of activating FcγR-dependent anti-DR5 antibodies (compared with FcγRIIB-dependent agonistic anti-DR5 antibodies) does not preclude DR5 as a useful tumor antigen that can be targeted by these antibodies, which may promote the cross-presentation of antibody-coated tumor cells and the consequent activation of adaptive immune responses, including T-cell–dependent secondary responses (40). Indeed, the antitumor activity of MD5-1–treated 4T1 cells in BALB/c mice might be an example of this type of activity (8).
We and others recently reported that agonistic antibodies to a TRAF-signaling TNFR superfamily member, CD40, require FcγRIIB coengagement for their in vivo immunostimulatory and antitumor activities, and that the potency of these agonistic anti-CD40 antibodies can be modulated by FcγRIIB-targeted engineering (16, 17). The data that we present in this paper demonstrate that this is also true for a FADD-signaling TNFR superfamily member, DR5. Along with CD40 and DR5, agonistic antibodies to another TRAF-signaling molecule, CD30, and other death receptors, such as Fas and DR4, also have been reported to either require FcγRIIB or show greater in vivo activity when their Fcs are able to engage FcγRIIB (41–43). Based on these findings, FcγRIIB coengagement appears to be a general requirement for agonistic antibodies to both TRAF- and FADD-signaling TNFR members. Although results of in vitro studies have suggested that FcγRIIB might serve as a scaffolding for anti-CD40 or anti-DR5 antibodies to activate CD40 or DR5 signaling pathways, respectively (17, 39), as far as we know, no in vivo evidence has been published to support this notion. Both agonistic anti-CD40 and anti-DR5 antibodies require FcγRIIB coengagement, but the strength of the required Fc–FcγRIIB interaction may differ for different TNFRs. For example, whereas human IgG1 Fc supported weak but significant in vivo agonistic activity for agonistic anti-CD40 antibodies (16), no detectable apoptotic activity was detected in agonistic anti-DR5 antibodies with this same Fc. Also of note, MC38 and 4T1.2 cells display different sensitivities to apoptotic signaling triggered by agonistic anti-DR5 antibodies; 4T1.2 cells apparently have a higher threshold not reached by anti-DR5 antibodies with unmodified Fcs, including human IgG1 and hamster IgG2 Fcs. However, this threshold requirement can be overcome by modifying the Fc to enhance FcγRIIB binding, as demonstrated by the Fc-engineered αDR5:hIgG1(S267E). This observation has important implications for the design of therapeutic antibodies. FcγRIIB-targeted Fc engineering not only may be able to quantitatively increase the potency of agonistic TNFR antibodies, but also may be able to overcome the high threshold of less-sensitive tumor cells. Thus, the strength of Fc–FcγRIIB interactions needs to be optimized for different TNFR targets, and FcγRIIB-targeted Fc engineering will provide new possibilities for the development of agonistic antibodies to molecules of the TNFR superfamily for anticancer therapy.
Materials and Methods
Detailed information on mice, antibodies, tumor models, hepatotoxicity, in vitro proapoptotic activity analysis, SPR analysis, and statistical analysis are provided in SI Materials and Methods. All studies were performed in compliance with federal laws. All mouse experiments had been approved by the Rockefeller University Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We thank Dr. Hideo Yagita (Juntendo University School of Medicine) for kindly providing the MD5-1 hybridoma; Dr. Mark J. Smyth (University of Melbourne) for kindly providing the MC38, MC38-cFLIP, and 4T1.2 cells; P. Smith (The Rockefeller University) for expert technical assistance; and R. Anthony, S. Bournazos, and A. Pincetic (The Rockefeller University) for advice and suggestions. This work was supported in part from National Institutes of Health grants (to J.V.R.). F.L. is supported in part by Grant 2757 from the Paralyzed Veterans of America Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208698109/-/DCSupplemental.
References
- 1.Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 2.Grewal IS. Therapeutic Targets of the TNF Superfamily. New York, NY: Springer; 2009. pp. 1–220. [Google Scholar]
- 3.Daniel PT, Wieder T, Sturm I, Schulze-Osthoff K. The kiss of death: Promises and failures of death receptors and ligands in cancer therapy. Leukemia. 2001;15:1022–1032. doi: 10.1038/sj.leu.2402169. [DOI] [PubMed] [Google Scholar]
- 4.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 5.Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: The opportunities and challenges. Adv Exp Med Biol. 2009;647:8–36. doi: 10.1007/978-0-387-89520-8_2. [DOI] [PubMed] [Google Scholar]
- 6.Falschlehner C, Ganten TM, Koschny R, Schaefer U, Walczak H. TRAIL and other TRAIL receptor agonists as novel cancer therapeutics. Adv Exp Med Biol. 2009;647:195–206. doi: 10.1007/978-0-387-89520-8_14. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Stagg J, Yagita H, Okumura K, Smyth MJ. Targeting death-inducing receptors in cancer therapy. Oncogene. 2007;26:3745–3757. doi: 10.1038/sj.onc.1210374. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, et al. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199:437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 10.Haynes NM, et al. CD11c+ dendritic cells and B cells contribute to the tumoricidal activity of anti-DR5 antibody therapy in established tumors. J Immunol. 2010;185:532–541. doi: 10.4049/jimmunol.0903624. [DOI] [PubMed] [Google Scholar]
- 11.Demetri GD, et al. First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: A phase I/II open-label and double-blind study. Eur J Cancer. 2012;48:547–563. doi: 10.1016/j.ejca.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res. 2010;16:5883–5891. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- 13.Forero-Torres A, et al. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5) Cancer Biother Radiopharm. 2010;25:13–19. doi: 10.1089/cbr.2009.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plummer R, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 15.Wakelee HA, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2010;21:376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Ravetch JV. Inhibitory Fcγ receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White AL, et al. Interaction with FcγRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol. 2011;187:1754–1763. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- 18.Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239–245. doi: 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Nimmerjahn F, Ravetch JV. FcγRs in health and disease. Curr Top Microbiol Immunol. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- 20.Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Ann N Y Acad Sci. 2012;1253:170–180. doi: 10.1111/j.1749-6632.2011.06305.x. [DOI] [PubMed] [Google Scholar]
- 21.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 22.Abboud N, et al. A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J Exp Med. 2010;207:2395–2405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen ME, Xiao Y, Eisenberg RJ, Cohen GH, Isaacs SN. Antibody against extracellular vaccinia virus (EV) protects mice through complement and Fc receptors. PLoS ONE. 2011;6:e20597. doi: 10.1371/journal.pone.0020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogt MR, et al. Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fcgamma receptor and complement-dependent effector mechanisms. J Virol. 2011;85:11567–11580. doi: 10.1128/JVI.05859-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 26.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 27.Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat Immunol. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- 28.Xiang Z, et al. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007;8:419–429. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 29.Brownlie RJ, et al. Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb. J Exp Med. 2008;205:883–895. doi: 10.1084/jem.20072565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 31.Desjarlais JR, Lazar GA. Modulation of antibody effector function. Exp Cell Res. 2011;317:1278–1285. doi: 10.1016/j.yexcr.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Chu SY, et al. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcgammaRIIb with Fc-engineered antibodies. Mol Immunol. 2008;45:3926–3933. doi: 10.1016/j.molimm.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 33.Lazar GA, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda K, et al. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci USA. 2008;105:10895–10900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 36.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: A novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Shields RL, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 38.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human FcγR structural and functional diversity. Proc Natl Acad Sci USA. 2012;109:6181–6186. doi: 10.1073/pnas.1203954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson NS, et al. An Fcγ receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19:101–113. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Stefanescu RN, Olferiev M, Liu Y, Pricop L. Inhibitory Fc gamma receptors: From gene to disease. J Clin Immunol. 2004;24:315–326. doi: 10.1023/B:JOCI.0000029105.47772.04. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, et al. Effective therapy for a murine model of human anaplastic large-cell lymphoma with the anti-CD30 monoclonal antibody, HeFi-1, does not require activating Fc receptors. Blood. 2006;108:705–710. doi: 10.1182/blood-2005-11-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuntharapai A, et al. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J Immunol. 2001;166:4891–4898. doi: 10.4049/jimmunol.166.8.4891. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, et al. Fc gamma Rs modulate cytotoxicity of anti-Fas antibodies: Implications for agonistic antibody-based therapeutics. J Immunol. 2003;171:562–568. doi: 10.4049/jimmunol.171.2.562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.