Abstract
Cold-inducible RNA-binding protein (Cirp) was the first cold-shock protein identified in mammals. It is structurally quite different from bacterial cold-shock proteins and is induced in response to mild, but not severe, hypothermia. To clarify the physiological function of Cirp in vivo, we produced cirp-knockout mice. They showed neither gross abnormality nor defect in fertility, but the number of undifferentiated spermatogonia was significantly reduced and the recovery of spermatogenesis was delayed after treatment with a cytotoxic agent, busulfan. Cirp accelerated cell-cycle progression from G0 to G1 as well as from G1 to S phase in cultured mouse embryonic fibroblasts. Cirp directly bound to dual-specificity tyrosine-phosphorylation–regulated kinase 1B (Dyrk1b, also called Mirk) and inhibited its binding to p27, resulting in decreased phosphorylation and destabilization of p27. Cirp did not affect binding of Dyrk1b to cyclin D1 but inhibited phosphorylation of cyclin D1 by Dyrk1b, resulting in cyclin D1 stabilization. In the spermatogonial cell line GC-1spg, suppression of Cirp expression increased the protein level of p27, decreased that of cyclin D1, and decreased the growth rate, which depended on Dyrk1b. Consistent changes in the protein levels of p27 and cyclin D1 as well as the percentage of cells in G0 phase were observed in undifferentiated spermatogonia of cirp-knockout mice. In undifferentiated spermatogonia of wild-type mice, Cirp and Dyrk1b colocalized in the nucleus. Thus, our study demonstrates that Cirp functions to fine-tune the proliferation of undifferentiated spermatogonia by interacting with Dyrk1b.
In most mammals, the testes are located in the scrotum, wherein the temperature is below that of the body cavity by 2 °C to 7 °C (1). Scrotal testes become aspermatogenic if their temperature is raised to body temperature or above, either by artificial heat or by retaining them in the abdomen (2, 3). We previously identified the first mammalian cold-shock protein in the testis and named it cold-inducible RNA-binding protein (Cirp, also called Cirbp or hnRNP A18) (4). It is constitutively expressed in the male germ cells and inducibly expressed in almost all cell types by lowering the culture temperature to 32 °C but not below 20 °C. Expression of Cirp is also induced by cellular stresses such as UV irradiation and hypoxia (5–7). In response to the stress, Cirp migrates from the nucleus to cytoplasm and affects expression of its target mRNAs (8, 9). Cirp affects cell growth and protects cells from damages induced by tumor necrosis factor α, genotoxic stress, or cryptorchidism (4, 10–13).
In an adult mouse testis, spermatogenesis is sustained by germ-line stem cells (Asingle) that undergo mitosis and self-renew or differentiate into committed Apaired and Aaligned spermatogonia, which are collectively called undifferentiated spermatogonia (14). Undifferentiated spermatogonia constitute the smallest population, less than 1%, of total testicular cells, include cells with stem-cell function, and differentiate into differentiating spermatogonia (A1–4, intermediate, and B), which will undergo meiosis after the final mitosis (15, 16). Recently, methods to enrich undifferentiated spermatogonia by flow cytometry using combinations of markers such as c-Kit and α6-integrin and the side population phenotype have been developed (17, 18).
Dual-specificity tyrosine-phosphorylation–regulated kinase 1B (Dyrk1b, also called Mirk) is a member of the conserved Dyrk/minibrain family of tyrosine-regulated, arginine-directed serine/threonine protein kinases (19). The highest level of Dyrk1b mRNA is observed in the testis and skeletal muscle, and Dyrk1b protein mediates muscle differentiation, cell survival, and cell migration (19). Biochemical analysis has revealed that Dyrk1b phosphorylates and blocks degradation of the cyclin-dependent kinase (CDK) inhibitor p27 in nontransformed cells that helps to maintain a quiescent state (20). Dyrk1b blocks quiescent cells from traversing G1 phase of the cell cycle by phosphorylating and destabilizing cyclin D1 and cyclin D3 (21).
In the present study, we have generated Cirp-deficient mice to clarify the biological function of mammalian cold-shock protein in vivo. We found that Cirp interacts with Dyrk1b and that it is required to fully maintain the undifferentiated spermatogonia.
Results
Number of Undifferentiated Spermatogonia Is Decreased in Cirp-Deficient Mice.
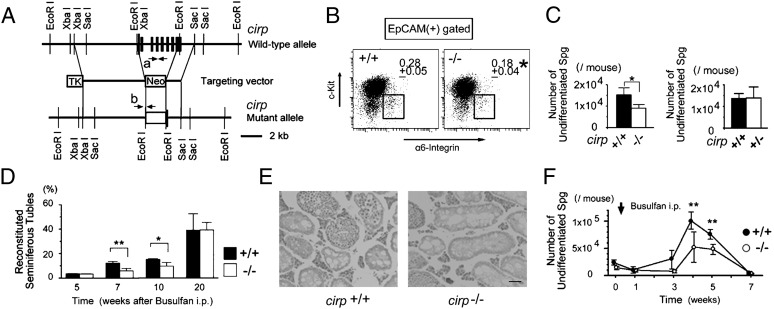
We generated Cirp-deficient mice by homologous recombination in ES cells (Fig. 1A). Intercrossing of heterozygous mutant mice generated progeny at nearly Mendelian ratios (cirp+/+:cirp+/−:cirp−/− = 58:110:47, n = 215), and cirp−/− mice were indistinguishable from wild-type littermates in appearance. When we examined fertility of cirp−/− male mice by mating them with wild-type females, the litter size (average = 5.1 mice, n = 18) was not different from that of wild-type mating (average = 4.9 mice, n = 15). We observed no reproductive defect in cirp−/− females, either. Although the testis of cirp−/− mice looked histologically normal and the numbers of total testicular cells and differentiating spermatogonia as well as the size and weight of the testis were comparable to wild type, the percentage and number of c-Kit− and highly α6-integrin+ undifferentiated spermatogonia in the testicular epithelial cell adhesion molecule (EpCAM)+ cells (18, 22) were decreased in cirp−/− mice compared with those in wild-type mice (P < 0.05) (Fig. 1 B and C). The number of undifferentiated spermatogonia in cirp+/− mice, however, was not different from that in wild-type mice (P = 0.925).
Fig. 1.
Reduced number of undifferentiated spermatogonia in cirp-knockout mice. (A) Targeted disruption of the cirp gene. Structures of the wild-type allele, targeting vector, and mutant allele are shown together with the relevant restriction sites. The filled rectangles indicate the exons of the cirp gene. Neo, TK, and arrows (a and b) indicate a pgk-neo selection cassette, a thymidine kinase cassette, and the locations of primers for genomic PCR, respectively. (B) Flow cytometric analysis of undifferentiated spermatogonia. The fractions of undifferentiated spermatogonia (boxed) were compared between wild-type (+/+) and cirp-knockout (−/−) mice. *Significantly different from +/+ mice (P < 0.05). Data are from four +/+ and three −/− 12-wk-old mice. (C) Numbers of undifferentiated spermatogonia in +/+, −/−, and heterozygous knockout (+/−) mice. Significant decrease (*P < 0.05) was seen in −/− mice but not in +/− mice (P = 0.93). Values represent mean ± SD. Data are from eight +/+, three −/−, and four +/− mice. (D) Percentage of reconstituted seminiferous tubules after busulfan treatment. *Significantly different (P < 0.05). **Significantly different (P < 0.01). Values are mean ± SD (n = 3 each). (E) Histological sections of the testes at 7 wk after busulfan treatment. Sections are stained with hematoxylin and eosin. (Scale bar: 50 μm.) (F) Numbers of undifferentiated spermatogonia after busulfan treatment. ● and ○ indicate the numbers in +/+ and −/− mice, respectively. Values represent mean ± SD (n = 3–7). **Significantly different between +/+ and −/− mice (P < 0.01).
To determine whether the observed decrease in the number of undifferentiated spermatogonia has biological meaning, we analyzed the recovery process of spermatogenesis after i.p. injection of the alkylating agent busulfan (30 mg/kg body weight). The percentage of seminiferous tubules showing spermatogenesis was less than 3% and not different between wild-type and cirp−/− mice at 5 wk after the injection (Fig. 1D). At 7 and 10 wk, however, significantly fewer tubules were reconstituted in cirp−/− mice compared with wild-type mice (Fig. 1E). Consistently, the number of undifferentiated spermatogonia was significantly less in cirp−/− mice at 4 and 5 wk after the busulfan treatment (Fig. 1F).
To assess whether stem cells of other cell lineages are affected by deficiency of Cirp, we analyzed purified bone marrow cells enriched with hematopoietic stem cells (23, 24). Their numbers were comparable between cirp−/− and wild-type mice (Fig. S1 A and B). The competitive reconstitution assay did not reveal any significant difference between them even with an additional irradiation of recipient mice (Fig. S1 C and D). In contrast to spermatogonia, cirp mRNA expression was barely detectable in the analyzed hematopoietic cells (Fig. S1E).
Cirp Accelerates Cell-Cycle Progression in Cultured Mouse Embryonic Fibroblasts (MEFs).
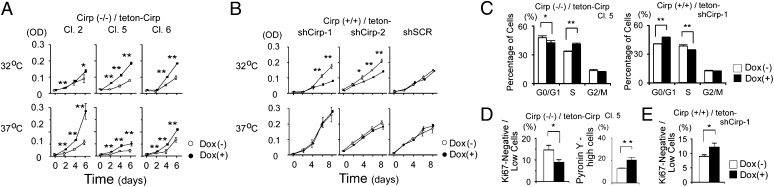
To analyze the effect of Cirp in vitro, we made several MEF cell lines that inducibly change the protein level of Cirp (Fig. S2 A and B). As shown in Fig. 2A, cirp−/− MEFs proliferated faster when Cirp was expressed both at 32 °C and 37 °C. When expression of Cirp was suppressed by shRNAs at 32 °C, proliferation of cirp+/+ MEFs was suppressed (Fig. 2B). The suppressive effect was reversed by expression of shRNA-insensitive Cirp construct (Fig. S2 C and D), suggesting that the observed effect was not attributable to off-target effects of shRNAs.
Fig. 2.
Accelerated proliferation of MEFs expressing Cirp. (A and B) Effects of Cirp on cell growth. Cells from Cirp(−/−)/teton-Cirp clones 2, 5, and 6 (Cl. 2, Cl. 5, and Cl. 6, respectively) were derived from cirp−/− MEFs that express Cirp in the presence of Dox. Cirp(+/+)/teton-shCirp cells were derived from cirp+/+ MEFs that Dox-inducibly express shRNAs that knockdown Cirp expression (shCirp-1 and shCirp-2) or scrambled RNAs (shSCR). Cells were cultured in the presence (+) or absence (−) of Dox at the indicated temperatures. Cell numbers were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. Values are mean ± SD (n = 3 or 4). *P < 0.05. **P < 0.01. (C) Analysis of the cell cycle by flow cytometry. BrdU incorporation and DNA content were analyzed in indicated cells cultured at 32 °C in the presence (+) or absence (−) of Dox. Percentages of cells in indicated phases of cell cycle are shown. Values are mean ± SD (n = 3). *P < 0.05. **P < 0.01. (D and E) Percentages of cells in G0 phase in the presence (+) or absence (−) of Dox. Ki67 expression, stainability with pyronin Y, and DNA content were analyzed in indicated cells by flow cytometry. Values are mean ± SD (n = 3). *P < 0.05.
We next analyzed the effect of Cirp on the cell cycle by BrdU incorporation and propidium iodide staining. When exogenous Cirp was expressed in cirp−/− MEFs at 32 °C, the percentage of cells in G0/G1 phase was reduced and that in S phase increased significantly (Fig. 2C). Conversely, when expression of Cirp was suppressed in cirp+/+ MEFs at 32 °C, the percentage of cells in G0/G1 phase was increased and that in S phase reduced significantly (Fig. 2C). The changes in the Cirp protein levels did not affect the percentage of the sub-G1 population (Fig. S3A). These results indicate that expression of Cirp accelerates the G0/G1-to-S phase transition without affecting apoptosis.
The cells with 2N DNA content and that are not positively stained with anti-Ki67 antibody are considered to be in the G0 phase of the cell cycle (25). The G0 state is characterized by significantly decreased ribosomal RNA synthesis and lowered total cellular protein content. When Cirp was expressed in cirp−/− MEFs, the percentage of cells in the fraction of Ki67low or Ki67− was significantly decreased, and the percentage of cells highly positive for pyronin Y staining, which stains polysomal RNA, was increased (Fig. 2D). Conversely, the percentage of G0 cells was significantly increased when Cirp expression was suppressed in cirp+/+ MEFs at 32 °C (Fig. 2E). These results demonstrate that Cirp accelerates cell-cycle progression from G0 to G1 as well as from G1 to S phase in cultured MEFs.
Cirp Binds to Dyrk1b and Inhibits Its Kinase Activity.
Yeast two-hybrid screening of a mouse testis cDNA library using Cirp as bait suggested that the C-terminal glycine-rich domain of Cirp binds to Dyrk1b (Fig. S4A).
When exogenously expressed as GFP-fusion proteins in HEK293T cells, full-length Cirp and its glycine-rich domain, but not its N-terminal RNA-binding domain, were coimmunoprecipitated with Dyrk1b as expected (Fig. S4 B and C). The region of Dyrk1b that interacted with Cirp was the kinase domain (Fig. S4D). Binding of endogenous Cirp and Dyrk1b was detected in MEFs of the cirp+/+ genotype cultured at 32 °C (Fig. S4E). The recombinant Cirp protein bound to recombinant Dyrk1b protein in vitro, indicating a direct interaction (Fig. S4F). Immunocytochemistry demonstrated that Cirp was induced and colocalized with Dyrk1b in the nucleus at 32 °C in cirp+/+ MEFs (Fig. S4G). In undifferentiated and differentiating spermatogonia, Cirp and Dyrk1b mRNAs were expressed, and their proteins were colocalized in the nucleus (Fig. S4 H and I).
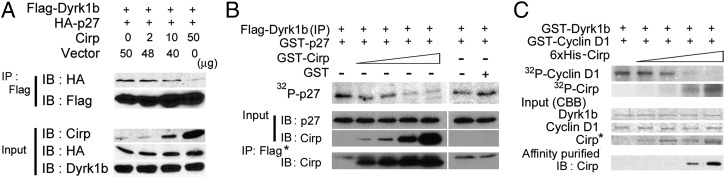
Dyrk1b phosphorylates p27 and cyclin D1 to affect cell-cycle progression (19). When increasing amounts of Cirp were expressed in HEK293T cells, decreasing amounts of p27 were coimmunoprecipitated with Dyrk1b (Fig. 3A). In contrast, the amount of cyclin D1 coimmunoprecipitated with Dyrk1b was not decreased in the presence of Cirp and its binding to Dyrk1b (Fig. S5A). The presence of cyclin D1 did not affect the binding of Cirp to Dyrk1b either, and Cirp was coimmunoprecipitated with cyclin D1 as well (Fig. S5 B and C). These results indicate that Cirp forms a complex together with Dyrk1b and cyclin D1.
Fig. 3.
Inhibition of Dyrk1b kinase activity by Cirp. (A) Effects of Cirp on binding of p27 to Dyrk1b. Plasmids were cotransfected into HEK293T cells as indicated. Proteins immunoprecipitated (IP) with anti-FLAG antibody and inputs were analyzed by Western blotting (IB) using the indicated antibodies. +, Present; −, absent. (B) Effects of Cirp on in vitro kinase activity of Dyrk1b toward p27. Immunoprecipitants (IP) were prepared from HEK293T cells expressing FLAG-Dyrk1b by using anti-FLAG antibody. Their kinase activity was assayed in the presence of [γ-32P]ATP, GST-p27, and increasing amounts of GST-Cirp or GST. Reaction mixtures were separated by SDS/PAGE and analyzed by autoradiography. Proteins coprecipitated with protein G Sepharose beads (*) from the reaction mixtures without 32P and inputs (2%) were analyzed by IB using the indicated antibodies. (C) Effects of Cirp on in vitro kinase activity of Dyrk1b toward cyclin D1. Recombinant GST-Dyrk1b was incubated with [γ-32P]ATP, GST-cyclin D1, and increasing amounts of His-tagged Cirp and analyzed as in B. The lower images show Coomassie blue staining (CBB) of the indicated proteins used in the assay and IB of proteins coprecipitated with glutathione Sepharose beads from the reaction mixtures without 32P (affinity-purified). *1/4 of input. Experiments were repeated three (A) and four (B and C) times, with similar results.
Dyrk1b immunoprecipitated from HEK293T cells could phosphorylate recombinant p27 in vitro (Fig. 3B). The amount of phosphorylated p27 was dose-dependently decreased in the presence of Cirp. When we incubated recombinant cyclin D1 with recombinant Dyrk1b, phosphorylation of cyclin D1 was observed (Fig. 3C). The phosphorylation was dose-dependently suppressed by Cirp. Interestingly, Cirp was also phosphorylated by Dyrk1b.
These data demonstrate that Cirp binds to and inhibits the kinase activity of Dyrk1b toward both p27 and cyclin D1, although Cirp competitively blocks the binding of Dyrk1b to p27 but not to cyclin D1.
Effects of Cirp on Cell-Cycle Progression Are Mediated by p27 and Cyclin D1 via Dyrk1b.
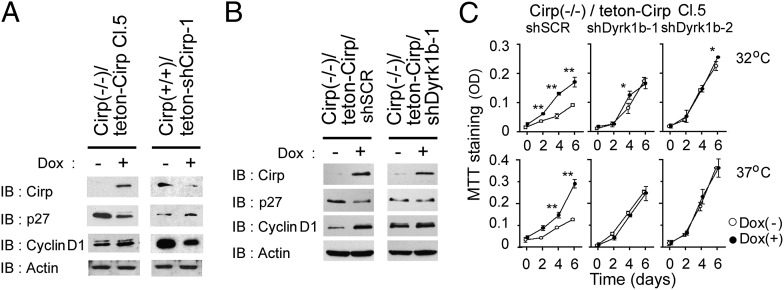
Rapid removal of p27 at the G0/G1 transition and expression of cyclin D1 at the G1/S transition are required for effective progression of the cell cycle to S phase (26, 27). When Cirp was inducibly expressed with doxycycline (Dox) in cirp−/− MEFs, expression of p27 was decreased and that of cyclin D1 was increased (Fig. 4A) because of the increased and decreased degradation rates of p27 and cyclin D1, respectively (Fig. S4 J and K). Conversely, when expression of Cirp was suppressed in cirp+/+ MEFs, expression of p27 was increased and that of cyclin D1 was decreased (Fig. 4A).
Fig. 4.
Effects of Cirp on p27 and cyclin D1 via Dyrk1b in cultured MEFs. (A) Effects of Cirp on p27 and cyclin D1 protein levels. Cirp(−/−)/teton-Cirp Cl. 5 and Cirp(+/+)/teton-shCirp-1 cells were cultured at 32 °C in the presence (+) or absence (−) of Dox. Cell lysates were analyzed by IB using the indicated antibodies. (B) Dyrk1b dependency of protein levels. Indicated cells, both derived from Cirp(−/−)/teton-Cirp Cl. 5 cells, were cultured at 37 °C and analyzed as in A. (C) Dyrk1b and cell growth. Indicated cells were cultured at 32 °C and 37 °C in the presence (+) or absence (−) of Dox. Cell numbers were assessed by MTT assays. Values are mean ± SD (n = 3). *P < 0.05. **P < 0.01. Experiments were repeated four (B) and three (C) times, with similar results.
Because p27 and cyclin D1 are known to be phosphorylated by kinases besides Dyrk1b (26, 27), we examined whether the effects of Cirp on p27 and cyclin D1 depended on Dyrk1b. We stably expressed shRNAs targeted to Dyrk1b mRNAs or scrambled shRNAs in cirp−/− MEFs that inducibly express Cirp (Fig. S5D). Induction of Cirp decreased the protein level of p27 and increased that of cyclin D1 in cirp−/− MEFs expressing scrambled shRNAs (Fig. 4B and Fig. S5E). When expression of Dyrk1b was suppressed, however, Cirp did not show these effects in cirp−/− MEFs. The growth rate of these cells was not affected by Cirp either (Fig. 4C). These data demonstrate that expression of Cirp accelerates cell-cycle progression by inhibiting the kinase activity of Dyrk1b toward p27 and cyclin D1 in MEFs.
Down-Regulation of Cirp Suppresses Cell-Cycle Progression in a Spermatogonial Cell Line in Vitro and Undifferentiated Spermatogonia in Vivo.
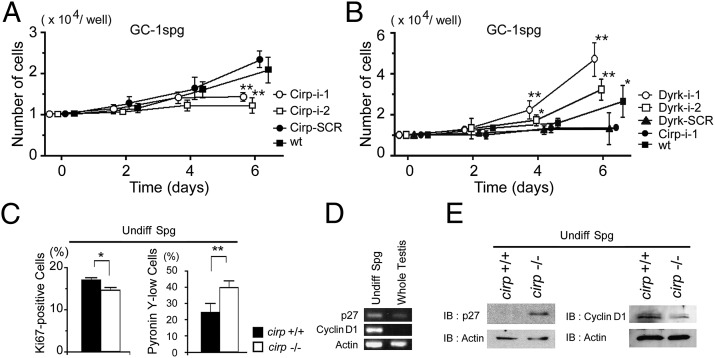
To analyze the effect of Cirp on male germ cells, we first used the mouse spermatogonial cell line GC-1spg (28). When GC-1spg cells were transduced with control retrovirus expressing scrambled shRNAs, expression of Cirp was induced at 32 °C like parental cells (Fig. S6A). In GC-1spg cells transduced with retrovirus expressing shRNAs to cirp mRNAs, the induction of Cirp was partly suppressed, the level of p27 protein was increased, and the level of cyclin D1 protein was decreased (Fig. S6B). These cells proliferated more slowly than the control cells (Fig. 5A). To confirm that growth-suppressive effect of Cirp down-regulation depends on Dyrk1b, we further down-regulated Dyrk1b expression (Fig. S6C). As shown in Fig. 5B, the impaired growth was rescued by suppressing the expression of Dyrk1b.
Fig. 5.
Impaired cell-cycle progression in Cirp-deficient spermatogonia. (A) Effects of Cirp on growth of cultured GC-1spg cells. Cells were transduced with retrovirus [murine stem cell virus-LTRmiR30-PIG (MSCV-LMP)] expressing RNAi sequences targeted to Cirp (Cirp-i-1 and Cirp-i-2) or scrambled sequence (Cirp-SCR). Cells were cultured at 32 °C, and cell numbers were assessed by MTT assays. wt, untransduced cells. Values are mean ± SD (n = 3). **P < 0.01. (B) Dyrk1b-dependent effects of Cirp on growth of GC-1spg cells. Cirp-i-1 cells were further transduced with retrovirus [Friend spleen focus-forming virus-based hybrid vector 2 (SF2)] expressing RNAi sequences targeted to Dyrk1b (Dyrk-i-1 and Dyrk-i-2) or scrambled sequence (Dyrk-SCR) and analyzed as in A. (C–E) Undifferentiated spermatogonia of cirp−/− and cirp+/+ genotypes were sorted and analyzed at 4 wk after busulfan treatment. (C) The percentages of Ki67+ (Left) and pyronin Y− or pyronin Ylow (Right) cells were assessed by flow cytometry. Values are mean ± SD [n = 3 (Left) or 4 (Right)]. *P < 0.05 and **P < 0.01, respectively, different between wild-type (+/+) and cirp-knockout (−/−) mice. (D) Detection of p27 and cyclin D1 transcripts in spermatogonia isolated from cirp−/− mice. cDNAs were analyzed by RT-PCR. (E) Protein levels of p27 and cyclin D1. Cell lysates were analyzed by IB using the indicated antibodies. Experiments were repeated three times (D and E), with similar results.
Next, we examined the effects of Cirp deficiency on the cell cycle in undifferentiated spermatogonia in vivo. At 4 wk after injection of busulfan, the percentage of Ki67+ cells was significantly smaller and the percentage of pyronin Ylow cells was significantly larger in undifferentiated spermatogonia isolated from cirp−/− mice than those from cirp+/+ wild-type mice (Fig. 5C). RT-PCR analysis demonstrated that undifferentiated spermatogonia expressed mRNAs for p27 and cyclin D1 (Fig. 5D) as well as for Cirp and Dyrk1b. Furthermore, the protein level of p27 was higher and that of cyclin D1 was lower in undifferentiated spermatogonia of cirp−/− mice compared with those of wild-type mice, although the mRNA level for p27 was not different and that for cyclin D1 was higher in cirp−/− mice (Fig. 5E and Fig. S7 A and B). Altogether, these data indicate that Cirp destabilizes p27 and stabilizes cyclin D1 at the protein level in undifferentiated spermatogonia, resulting in an accelerated cell-cycle progression in wild-type mice and decreased proliferation in Cirp-deficient mice.
Discussion
Numbers and Proliferative Activity of Undifferentiated Spermatogonia Are Decreased in cirp-Knockout Mice.
In mammals, there have been only two established cold-shock proteins reported to date, Cirp and RNA-binding motif protein 3 (Rbm3), which are structurally quite similar (5). In the mouse testis, Cirp is constitutively expressed in the germ cells and the level varies depending on the stage of differentiation, whereas Rbm3 is suggested to be expressed mainly in the Sertoli cells (3, 29). When mouse testis is exposed to heat stress, expression of Cirp and Rbm3 is decreased, suggesting that the decreased expression might be related to the heat-induced testicular damage. Indeed, overexpression of Cirp has recently been reported to reduce the damage by cryptorchidism (13). In the present study, we found that the Cirp-deficient mice have reduced numbers of undifferentiated spermatogonia, although the total numbers of testicular germ cells and fertility were not significantly decreased. When they were exposed to the cytotoxic agent busulfan, the recovery of spermatogenesis was affected slightly but significantly. In addition to Cirp, Rbm3 was expressed in spermatogonia (Fig. S7 C and D). Because its expression level was not increased in spermatogonia of the cirp−/− genotype compared with those of wild type, it seems unlikely that the relatively mild defects observed in the knockout mice are a consequence of redundancy/compensation by Rbm3. Analysis of the cirp and rbm3 double-knockout mice would clarify this possibility.
A vast majority of stem cell functions reside in the undifferentiated spermatogonia population (30). The findings that the undifferentiated spermatogonia expressed Cirp and that proliferation of the spermatogonial cell line was suppressed when Cirp expression was decreased suggest that the observed defect in cirp−/− mouse testis is mainly intrinsic to the spermatogonia rather than their environment. Consistent with this notion, no effect of Cirp deficiency was observed in hematopoietic stem cell populations that normally do not express Cirp.
Cells overexpressing Cirp are more resistant to various stresses, and down-regulation of Cirp inhibits cell growth in some cancer cells (31). We found that cell-cycle progression is inhibited at both G1-to-S and G0-to-G1 phase transitions with no change in the sub-G1 population in Cirp-deficient MEFs. Consistently, the proportion of undifferentiated spermatogonia in G0 phase was increased in cirp−/− mice compared with wild-type mice after treatment with busulfan. The numbers of apoptotic testicular cells were not apparently different between cirp−/− and wild-type mice after busulfan treatment, and the sensitivity of cultured MEFs of the cirp−/− genotype to busulfan was not affected by expression of Cirp (Fig. S3 B and C). In addition, expression of Cirp stimulated proliferation of the spermatogonial cell line. Thus, the decrease in the number of undifferentiated spermatogonia in cirp−/− mice could be considered mainly attributable to decreased cell proliferation rather than increased cell death.
The growth-stimulatory effects of Cirp on spermatogonia and MEFs seem contradictory to our original finding in BALB/3T3 cells (4) and consistent with the finding in CHO cells (32). We have also observed growth-suppressive effects of Cirp in NIH/3T3 and U-2 OS cells (Fig. S8A). Growth-stimulatory effects have been observed by others in PC3 and LNCaP human prostate cancer cells and MEFs, but not in NIH/3T3, HeLa, IMR90, or human mammary epithelial cells (10, 12). These two classes of cell lines that differently respond to Cirp expression were not distinguished by the protein levels of cyclin D1, CDK inhibitors such as p16 and p27, Dyrk1b, or Cirp (Fig. S8 B and C). Further studies are thus necessary to clarify this issue. Cirp probably fine-tunes the proliferation of cells by promoting and suppressing growth signals through distinct protein–protein and protein–RNA interactions, which depend on multiple factors, including the cell types, stresses, and conditions of cells.
Cirp Binds to and Inhibits the Kinase Activity of Dyrk1b Toward p27 and Cyclin D1.
Dyrk1b autophosphorylates the second tyrosine in the Tyr-X-Tyr motif, an event that is essential for full kinase activity (33). Once phosphorylated, Dyrk1b functions as a serine/threonine kinase. Ran-binding protein M (RanBPM) directly binds to Dyrk1b and inhibits its kinase activity toward hepatocyte nuclear factor 1α (HNF1α) (34). In contrast, direct binding to dimerization cofactor of HNF1 from muscle (DCoHm) potentiates Dyrk1b’s activity as an activator of HNF1α (35). Although phosphorylation of RanBPM or DCoHm by Dyrk1b has not been detected (34), we found that Cirp directly binds to and is phosphorylated by Dyrk1b, resulting in inhibition of its kinase activity toward p27 and cyclin D1.
p27 opposes cell-cycle progression by inhibiting cyclin E/Cdk2 (27). The expression level of p27 is high during G0 phase but decreases rapidly on reentry of the cells into G1 phase. Ser10 is the major site of p27 phosphorylation by Dyrk1b in resting cells that leads to protein stability (20). Previous studies have suggested a role of p27 in male germ-cell development. Mice deficient in S-phase kinase-associated protein 2 (Skp2), which mediates ubiquitin-dependent degradation of p27, exhibit a progressive loss in spermatogenic cells (36). Conversely, testes from p27-knockout mice are significantly larger (37) and contain increased numbers of type A spermatogonia (38), and EpCAM+ spermatogonia from the knockout mice proliferate more actively than those from wild-type mice do (39). We found that Cirp decreases the protein level of p27 and increases cell proliferation via Dyrk1b in MEFs and the spermatogonial cell line and that the p27 protein level and cells in G0 phase were increased in Cirp-deficient undifferentiated spermatogonia. These results strongly suggest that the absence of Cirp increases phosphorylation of p27 by Dyrk1b, resulting in its stabilization and reduced cell-cycle progression in undifferentiated spermatogonia of the cirp−/− genotype.
D-type cyclins are G1-specific cyclins that associate with Cdk4 or Cdk6 and promote restriction point progression during G1 phase (40). In accordance with the immunohistochemical study by Beumer et al. (41), we detected expression of cyclin D1 in undifferentiated spermatogonia. The protein level of cyclin D1 was decreased in undifferentiated spermatogonia of cirp−/− mice compared with wild-type mice. Although binding to Cirp decreases the activity of Dyrk1b to phosphorylate cyclin D1 as well as to p27, the binding inhibits the binding of Dyrk1b to p27 but not to cyclin D1. After phosphorylation by Dyrk1b, p27 remains a functional CDK inhibitor, does not translocate from the nucleus, and colocalizes with Dyrk1b in G0 (20). Mitogen stimulation then causes cells to enter G1, reduces Dyrk1b levels (42), and initiates the translocation of p27 to the cytoplasm to be degraded. Thus, induction at moderately low temperatures, colocalization in the nucleus with Dyrk1b, and inhibition of Dyrk1b’s binding to p27 would contribute to the ability of Cirp to enhance degradation of p27. Cyclin D1 is translocated into the cytoplasm during S phase, where it is destroyed by the proteasome after phosphorylation at Thr286 by glycogen synthase kinase 3. Dyrk1b phosphorylates cyclin D1 at Thr288, which also enhances its rapid turnover (26). The nuclear complex formation of Dyrk1b, Cirp, and cyclin D1 would thus inhibit the nuclear export and degradation of cyclin D1. Although Cirp itself is a substrate and seems to compete with cyclin D1 for phosphorylation (Fig. 3C), it is also possible that Cirp has an independent mechanism for inhibiting Dyrk1b activity. The precise molecular mechanism by which Cirp inhibits the kinase activity of Dyrk1b remains to be elucidated.
In summary, we have demonstrated unique physiological function of the mammalian cold-shock protein Cirp that might partly explain why testis should be kept cool. Cirp fine-tunes the cell-cycle progression of undifferentiated spermatogonia as well as fibroblasts and cancer cells by interacting with and suppressing kinase activity of Dyrk1b to modulate the protein levels of p27 and cyclin D1 (Fig. S9). Because Cirp affects proliferation of immature male germ cells and could act as an oncoprotein in some tumors, these findings may pave the way for development of new diagnoses and treatments for male infertility and cancers.
Materials and Methods
Generation of the Targeting Vector.
The 6.4-kb XbaI/EcoRI fragment of the genomic DNA clones of the cirp locus (Fig. 1A) was inserted between the neomycin-resistance cassette and the HSV thymidine kinase gene, both of which are driven by the phosphoglycerate kinase 1 (PGK1) promoter, in the pPNT vector. The 1.4-kb EcoRI/SacI fragment was inserted upstream of the neomycin-resistance gene (Fig. 1A).
Isolation of Undifferentiated Spermatogonia.
Isolation of undifferentiated spermatogonia was performed as described by Takubo et al. (18) with minor modifications.
Additional materials and experimental procedures are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. C. Toniatti and Dr. H. Bujard for the Bs/IRES-M2 plasmid; Dr. T. Shinohara, Dr. M. Sugai, and Dr. R. J. Mayer for helpful comments; and Dr. K. Nonoguchi for technical assistance. This work was supported in part by the Smoking Research Foundation of Japan, the Cooperative Research Project Program at the Institute of Development, Aging, and Cancer at Tohoku University; and Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121524109/-/DCSupplemental.
References
- 1.Harrison RG, Weiner JS. Abdomino-testicular temperature gradients. J Physiol. 1948;107:48–49. [Google Scholar]
- 2.Carrick F, Setchell B. The evolution of the scrotum. In: Calaby J, Tyndale-Biscoe T, editors. Reproduction and Evolution. Canberra: Australian Academy of Science; 1977. pp. 165–170. [Google Scholar]
- 3.Nishiyama H, et al. Decreased expression of cold-inducible RNA-binding protein (CIRP) in male germ cells at elevated temperature. Am J Pathol. 1998;152:289–296. [PMC free article] [PubMed] [Google Scholar]
- 4.Nishiyama H, et al. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol. 1997;137:899–908. doi: 10.1083/jcb.137.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita J. Cold shock response in mammalian cells. J Mol Microbiol Biotechnol. 1999;1:243–255. [PubMed] [Google Scholar]
- 6.Wellmann S, et al. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. J Cell Sci. 2004;117:1785–1794. doi: 10.1242/jcs.01026. [DOI] [PubMed] [Google Scholar]
- 7.Yang C, Carrier F. The UV-inducible RNA-binding protein A18 (A18 hnRNP) plays a protective role in the genotoxic stress response. J Biol Chem. 2001;276:47277–47284. doi: 10.1074/jbc.M105396200. [DOI] [PubMed] [Google Scholar]
- 8.De Leeuw F, et al. The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor. Exp Cell Res. 2007;313:4130–4144. doi: 10.1016/j.yexcr.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Weber DJ, Carrier F. Post-transcriptional regulation of thioredoxin by the stress inducible heterogenous ribonucleoprotein A18. Nucleic Acids Res. 2006;34:1224–1236. doi: 10.1093/nar/gkj519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurai T, et al. Cirp protects against tumor necrosis factor-alpha-induced apoptosis via activation of extracellular signal-regulated kinase. Biochim Biophys Acta. 2006;1763:290–295. doi: 10.1016/j.bbamcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Artero-Castro A, et al. Cold-inducible RNA-binding protein bypasses replicative senescence in primary cells through extracellular signal-regulated kinase 1 and 2 activation. Mol Cell Biol. 2009;29:1855–1868. doi: 10.1128/MCB.01386-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng Y, Kulkarni P, Inoue T, Getzenberg RH. Down-regulating cold shock protein genes impairs cancer cell survival and enhances chemosensitivity. J Cell Biochem. 2009;107:179–188. doi: 10.1002/jcb.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou KW, Zheng XM, Yang ZW, Zhang L, Chen HD. Overexpression of CIRP may reduce testicular damage induced by cryptorchidism. Clin Invest Med. 2009;32:E103–E111. doi: 10.25011/cim.v32i2.6027. [DOI] [PubMed] [Google Scholar]
- 14.Meistrich ML, van Beek MEAB. Spermatogonial stem cells. In: Desjardins C, Ewing LL, editors. Cell and Molecular Biology of the Testis. Oxford, UK: Oxford Univ Press; 1993. pp. 266–294. [Google Scholar]
- 15.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Oatley JM, Brinster RL. Spermatogonial stem cells. Methods Enzymol. 2006;419:259–282. doi: 10.1016/S0076-6879(06)19011-4. [DOI] [PubMed] [Google Scholar]
- 17.Barroca V, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 18.Takubo K, et al. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell. 2008;2:170–182. doi: 10.1016/j.stem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Friedman E. Mirk/Dyrk1B in cancer. J Cell Biochem. 2007;102:274–279. doi: 10.1002/jcb.21451. [DOI] [PubMed] [Google Scholar]
- 20.Deng X, Mercer SE, Shah S, Ewton DZ, Friedman E. The cyclin-dependent kinase inhibitor p27(Kip1) is stabilized in G(0) by Mirk/dyrk1B kinase. J Biol Chem. 2004;279:22498–22504. doi: 10.1074/jbc.M400479200. [DOI] [PubMed] [Google Scholar]
- 21.Deng X, Ewton DZ, Friedman E. Mirk/Dyrk1B maintains the viability of quiescent pancreatic cancer cells by reducing levels of reactive oxygen species. Cancer Res. 2009;69:3317–3324. doi: 10.1158/0008-5472.CAN-08-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson R, Schaible K, Heasman J, Wylie C. Expression of the homophilic adhesion molecule, Ep-CAM, in the mammalian germ line. J Reprod Fertil. 1999;116:379–384. doi: 10.1530/jrf.0.1160379. [DOI] [PubMed] [Google Scholar]
- 23.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 24.Harrison DE. Competitive repopulation: A new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 25.Kubbutat MH, et al. Epitope analysis of antibodies recognising the cell proliferation associated nuclear antigen previously defined by the antibody Ki-67 (Ki-67 protein) J Clin Pathol. 1994;47:524–528. doi: 10.1136/jcp.47.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alao JP. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24–39. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larrea MD, Wander SA, Slingerland JM. p27 as Jekyll and Hyde: Regulation of cell cycle and cell motility. Cell Cycle. 2009;8:3455–3461. doi: 10.4161/cc.8.21.9789. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann MC, Narisawa S, Hess RA, Millán JL. Immortalization of germ cells and somatic testicular cells using the SV40 large T antigen. Exp Cell Res. 1992;201:417–435. doi: 10.1016/0014-4827(92)90291-f. [DOI] [PubMed] [Google Scholar]
- 29.Danno S, Itoh K, Matsuda T, Fujita J. Decreased expression of mouse Rbm3, a cold-shock protein, in Sertoli cells of cryptorchid testis. Am J Pathol. 2000;156:1685–1692. doi: 10.1016/S0002-9440(10)65039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lleonart ME. A new generation of proto-oncogenes: Cold-inducible RNA binding proteins. Biochim Biophys Acta. 2010;1805:43–52. doi: 10.1016/j.bbcan.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Hong JK, Kim YG, Yoon SK, Lee GM. Down-regulation of cold-inducible RNA-binding protein does not improve hypothermic growth of Chinese hamster ovary cells producing erythropoietin. Metab Eng. 2007;9:208–216. doi: 10.1016/j.ymben.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Lochhead PA, Sibbet G, Morrice N, Cleghon V. Activation-loop autophosphorylation is mediated by a novel transitional intermediate form of DYRKs. Cell. 2005;121:925–936. doi: 10.1016/j.cell.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 34.Zou Y, Lim S, Lee K, Deng X, Friedman E. Serine/threonine kinase Mirk/Dyrk1B is an inhibitor of epithelial cell migration and is negatively regulated by the Met adaptor Ran-binding protein M. J Biol Chem. 2003;278:49573–49581. doi: 10.1074/jbc.M307556200. [DOI] [PubMed] [Google Scholar]
- 35.Lim S, Jin K, Friedman E. Mirk protein kinase is activated by MKK3 and functions as a transcriptional activator of HNF1alpha. J Biol Chem. 2002;277:25040–25046. doi: 10.1074/jbc.M203257200. [DOI] [PubMed] [Google Scholar]
- 36.Fotovati A, Nakayama K, Nakayama KI. Impaired germ cell development due to compromised cell cycle progression in Skp2-deficient mice. Cell Div. 2006;1:4–13. doi: 10.1186/1747-1028-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakayama K, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 38.Beumer TL, et al. Regulatory role of p27kip1 in the mouse and human testis. Endocrinology. 1999;140:1834–1840. doi: 10.1210/endo.140.4.6638. [DOI] [PubMed] [Google Scholar]
- 39.Kanatsu-Shinohara M, Takashima S, Shinohara T. Transmission distortion by loss of p21 or p27 cyclin-dependent kinase inhibitors following competitive spermatogonial transplantation. Proc Natl Acad Sci USA. 2010;107:6210–6215. doi: 10.1073/pnas.0914448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JK, Diehl JA. Nuclear cyclin D1: An oncogenic driver in human cancer. J Cell Physiol. 2009;220:292–296. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beumer TL, Roepers-Gajadien HL, Gademan IS, Kal HB, de Rooij DG. Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biol Reprod. 2000;63:1893–1898. doi: 10.1095/biolreprod63.6.1893. [DOI] [PubMed] [Google Scholar]
- 42.Deng X, Ewton DZ, Pawlikowski B, Maimone M, Friedman E. Mirk/dyrk1B is a Rho-induced kinase active in skeletal muscle differentiation. J Biol Chem. 2003;278:41347–41354. doi: 10.1074/jbc.M306780200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.