Size is one of the most significant and fascinating characteristics of all organisms. Its significance derives from the strong relationships, especially in animals, between body size and many physiological traits and ecological characteristics (1, 2). What makes size fascinating is its extremes. They not only compel wonder at deviation from the average for a given group (3), but also provide insight into the mechanisms underlying size variation (4). Among the insects, the giant forms of the late Paleozoic have perhaps captured most attention. Many species from this period were large by modern standards; an extreme case is a member of the aerial predator group, the Protodonata, with a wingspan of ca. 71 cm (5). Modern dragonflies have wingspans typically one-tenth of the size (6). How such gigantism could have arisen and why these large species subsequently disappeared have long been staples of biology (5, 7). Although several explanations for the rise and fall of insect giants have been proposed, the physiological limits to performance or fitness at a given size and the improvements (constraints) brought by higher (lower) atmospheric oxygen concentration (partial pressures) have garnered most attention (7, 8). In PNAS, the work by Clapham and Karr (9) uses a massive compilation of fossil data to show that biotic interactions, especially predation by birds, are plausibly responsible for a decoupling of size from oxygen concentration from the Early Cretaceous onward. Their findings bring a robust and fresh ecological perspective to the discussion of insect size variation.
Size, Fitness, and Oxygen Concentration
Unlike many other ectotherms, in insects, gas exchange takes place through a system of tubes, the tracheae, that lead directly to the cells if not the mitochondria, where oxygen acts as an electron receptor during respiration (10). The exceptionally high power demands of insect flight, coupled with this gas exchange system, led early physiologists to conclude that large modern flying species may be at their performance limits (11, 12). At any larger size, the high power demands coupled with the requirements for diffusion through the smallest tubes could simply not be met. In particular, they could not be met at the ca. 21% current oxygen concentration (or ca. 21 kPa partial pressure) typically found at sea level. These conclusions led to speculation that higher oxygen partial pressures, as were becoming known for the Late Paleozoic, could have enabled species to achieve body sizes much larger than those sizes currently known (11, 12). By similar reasoning, it was later argued that declines in oxygen concentration might have led to the disappearance of these giants (7, 13).
Subsequent work has presented and to a lesser extent, explored a wide range of mechanisms that might improve the fitness or performance of larger-sized individuals under hyperoxic conditions relative to their smaller counterparts and vice versa (8). Most promising among these mechanisms is hypermetry of the tracheal system, such that the larger tracheae required for adequate performance of larger insects, under a 21% oxygen concentration, could simply not be accommodated by peripheral structures of the insect body (8). As is discussed in the work by Clapham and Karr (9), much of this recent discussion has focused on plastic responses and short-term evolution of size under laboratory conditions. These aspects do not reflect longer-term changes under more natural conditions, where organisms simultaneously experience changes in a variety of conditions, such as the presence of competitors and predators. By contrast, investigations of changes in the size distributions and maximum body sizes of naturally occurring insect assemblages certainly do, and this approach is precisely the approach adopted in the work by Clapham and Karr (9).
Assemblages Reflect Interacting Effects
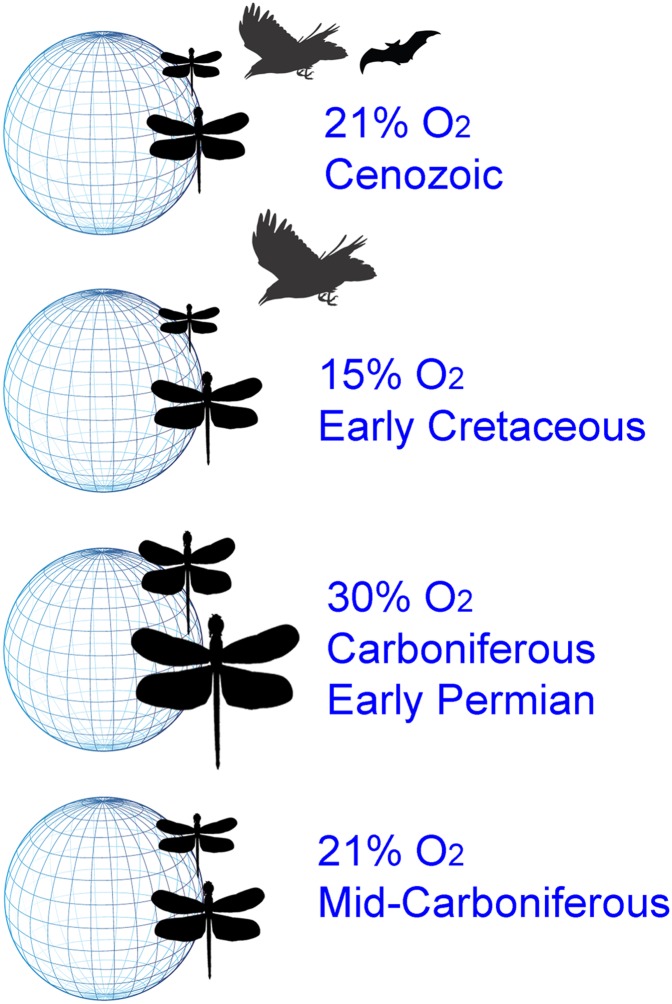
In their study, Clapham and Karr (9) investigate temporal trends in maximum insect size based on a newly compiled dataset of more than 10,500 fossil insect wing lengths. They then apply statistical models to determine the contributions of oxygen partial pressure, temperature, and stasis during different time periods, to size variation among 10-Myr time intervals (9). They include temperature (using paleolatitude as a proxy) because of the known positive relationship between the maximum size of insects in an assemblage and ambient temperature (9, 14). They find that, between the mid-Carboniferous (the start of their data series) and about 140 Mya, maximum size tracks atmospheric oxygen concentration. However, after about 130 Mya, variation is better explained by a decoupling of body size from oxygen concentration (Fig. 1) and a model of stasis, with additional changes in stasis at 90–60 Mya. The work by Clapham and Karr (9) argues that the first shift coincides with the diversification of birds and later, increases in their maneuverability. Aerially adept predators would have placed substantial pressure on large flying, presumably palatable insects. The later shift in stasis is ascribed to additional radiation of the birds and possibly, the bats, likely meaning enhanced predation, although competition for food among the aerial insect predators and highly maneuverable flying vertebrates cannot be ruled out.
Fig. 1.
Maximum body size of insect assemblages, represented here by the insect silhouettes, has varied considerably through geological time. Using a modeling approach and a compilation of more than 10,500 measurements of wing size of insect fossils, the work by Clapham and Karr (9) shows that the relationship between maximum size in an assemblage and atmospheric oxygen concentration (or partial pressure) was maintained from the Mid-Carboniferous until ∼130 Mya in the Early Cretaceous. Thereafter, the relationship was decoupled by predation, initially by birds (20) and later, by birds and bats. Moreover, the work by Clapham and Karr (9) also shows that the tendency of increasing maximum insect size to the tropics (14) is a constant feature of insect assemblages through time.
These findings provide an entirely fresh line of evidence in favor of the formerly mooted physiological mechanisms (7, 8, 13). In particular, previous investigations have tended to note the relationship between maximum size and oxygen concentration rather than model it explicitly. The work by Clapham and Karr (9) also provides evidence showing that the relationship is decoupled by biotic interactions. Predation makes good sense ecologically, even if inferred. Much research exists in favor of size-selective predation of insects (15). Moreover, in ecologically analogous situations, such as the introduction of predators to previously predator-free systems, a common outcome is extinction, whereas the introduction of competitors rarely leads to such profound impacts (16). In consequence, predation seems an excellent candidate for explaining the change in the oxygen-size relationship in insects.
By exploring explicitly and then rejecting the likely influence of sampling artifacts and showing that their results hold for body volume, which is of considerable significance from a physiological performance perspective (8, 11, 12), the work by Clapham and Karr (9) provides robust additional support for their findings. Moreover, by showing that the best models always include paleolatitude
Predation seems an excellent candidate for explaining the change in the oxygen-size relationship in insects.
(as a proxy for temperature), the work by Clapham and Karr (9) also shows that the positive maximum size–temperature relationship is not simply a recent phenomenon. Several explanations for such a relationship exist, and most encompass the improvement in performance brought by higher metabolic rates at warmer temperatures (14, 17). Indirectly, the fossil evidence presented here suggests that such mechanisms have deep historical roots in the insects if not in other groups.
Conclusions and Perspectives
To date, the discussion of insect size variation over geological time has typically been characterized by brief pause to consider biotic interactions followed by much focus on physiological mechanisms. By introducing data and modern modeling, the work by Clapham and Karr (9) provides an innovative approach that integrates these seemingly contradictory perspectives. This approach is the hallmark of good science—the development of hypotheses that seamlessly integrate previously competing ideas. Nonetheless, much remains about gigantism and its subsequent disappearance that needs to be explained. Other arthropods, including ground dwellers, and several vertebrate groups also had large-bodied forms in the late Paleozoic, which subsequently disappeared (13). Although a sampling mechanism associated with the end-Permian mass extinction may account for some of the disappearances, much room exists for exploring and integrating other mechanisms. These mechanisms include the physiological effects of declining oxygen concentrations on thermal tolerance, which may have a size bias in species relying on diffusion, including the aquatic immature forms of many insects (18), and the impacts of hypoxia on species ranges (19). Integrated approaches, such as the one developed in the work by Clapham and Karr (9), provide an important avenue for furthering such work. Moreover, they serve as powerful reminders that neglect of ecological interactions is unwise when trying to understand or perhaps, forecast how assemblages respond to abiotic environmental change.
Footnotes
The author declares no conflict of interest.
See companion article on page 10927.
References
- 1.Peters RH. The Ecological Implications of Body Size. Cambridge, UK: Cambridge Univ Press; 1983. [Google Scholar]
- 2.Brown JH, Gillooly JF, Allen AP, Savage V, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 3.Black R. World’s Smallest Frog Discovered. 2012. Available at http://www.bbc.co.uk/news/science-environment-16491477. Accessed May 23, 2012.
- 4.Glaw F, Köhler J, Townsend TM, Vences M. Rivaling the world’s smallest reptiles: Discovery of miniaturized and microendemic new species of leaf chameleons (Brookesia) from northern Madagascar. PLoS One. 2012;7:e31314. doi: 10.1371/journal.pone.0031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shear WA, Kukalová-Peck J. The ecology of Paleozoic terrestrial arthropods: The fossil evidence. Can J Zool. 1990;68:1807–1834. [Google Scholar]
- 6.Samways MJ. Dragonflies and Damselflies of South Africa. Sofia, Bulgaria: Pensoft; 2008. [Google Scholar]
- 7.Dudley R. Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance. J Exp Biol. 1998;201:1043–1050. doi: 10.1242/jeb.201.8.1043. [DOI] [PubMed] [Google Scholar]
- 8.Harrison JF, Kaiser A, VandenBrooks JM. Atmospheric oxygen level and the evolution of insect body size. Proc Biol Sci. 2010;277:1937–1946. doi: 10.1098/rspb.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clapham ME, Karr JA. Environmental and biotic controls on the evolutionary history of insect body size. Proc Natl Acad Sci USA. 2012;109:10927–10930. doi: 10.1073/pnas.1204026109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman RF. The Insects. Structure and Function. 4th Ed. Cambridge, UK: Cambridge Univ Press; 1998. [Google Scholar]
- 11.Weis-Fogh T. Diffusion in insect wing muscle, the most active tissue known. J Exp Biol. 1964;41:229–256. doi: 10.1242/jeb.41.2.229. [DOI] [PubMed] [Google Scholar]
- 12.Miller PL. The supply of oxygen to the active flight muscles of some large beetles. J Exp Biol. 1966;45:285–304. doi: 10.1242/jeb.45.2.285. [DOI] [PubMed] [Google Scholar]
- 13.Graham JB, Dudley R, Aguilar NM, Gans C. Implications of the late Palaeozoic oxygen pulse for physiology and evolution. Nature. 1995;375:117–120. [Google Scholar]
- 14.Makarieva AM, Gorshkov VG, Li B-L. Temperature-associated upper limits to body size in terrestrial poikilotherms. Oikos. 2005;111:425–436. [Google Scholar]
- 15.Chown SL, Gaston KJ. Body size variation in insects: A macroecological perspective. Biol Rev Camb Philos Soc. 2010;85:139–169. doi: 10.1111/j.1469-185X.2009.00097.x. [DOI] [PubMed] [Google Scholar]
- 16.Davis MA. Invasion Biology. Oxford: Oxford Univ Press; 2009. [Google Scholar]
- 17.Angilletta MJ, Jr, Huey RB, Frazier MR. Thermodynamic effects on organismal performance: Is hotter better? Physiol Biochem Zool. 2010;83:197–206. doi: 10.1086/648567. [DOI] [PubMed] [Google Scholar]
- 18.Verberk WCEP, Bilton DT. Can oxygen set thermal limits in an insect and drive gigantism? PLoS One. 2011;6:e22610. doi: 10.1371/journal.pone.0022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huey RB, Ward PD. Hypoxia, global warming, and terrestrial late Permian extinctions. Science. 2005;308:398–401. doi: 10.1126/science.1108019. [DOI] [PubMed] [Google Scholar]
- 20.Brown JW, Rest JS, García-Moreno J, Sorenson MD, Mindell DP. Strong mitochondrial DNA support for a Cretaceous origin of modern avian lineages. BMC Biol. 2008;6:6. doi: 10.1186/1741-7007-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]