Abstract
Tight junctions (TJs) are dynamic cellular structures that are critical for compartmentalizing environments within tissues and regulating transport of small molecules, ions, and fluids. Phosphorylation-dependent binding of the transmembrane protein occludin to the structural organizing protein ZO-1 contributes to the regulation of barrier properties; however, the details of their interaction are controversial. Using small angle X-ray scattering (SAXS), NMR chemical shift perturbation, cross-saturation, in vitro binding, and site-directed mutagenesis experiments. we define the interface between the ZO-1 PDZ3-SH3-U5-GuK (PSG) and occludin coiled-coil (CC) domains. The interface is comprised of basic residues in PSG and an acidic region in CC. Complex formation is blocked by a peptide (REESEEYM) that corresponds to CC residues 468–475 and includes a previously uncharacterized phosphosite, with the phosphorylated version having a larger effect. Furthermore, mutation of E470 and E472 reduces cell border localization of occludin. Together, these results localize the interaction to an acidic region in CC and a predominantly basic helix V within the ZO-1 GuK domain. This model has important implications for the phosphorylation-dependent regulation of the occludin∶ZO-1 complex.
Keywords: membrane-associated guanylate kinase, tricellulin, calmodulin
Tight junctions (TJs) are highly polarized gates that control the flux of fluids, proteins, and even ions across sheets of endothelial or epithelial cells (1, 2). These barriers function in a range of tissues, including the vasculature of the central nervous system, kidney, and gut epithelium. Dysregulation of barrier properties is associated with a host of disease states including cancer, stroke, diabetic retinopathy, and inflammatory bowel syndrome (3–5). Furthermore, viral and bacterial pathogens exploit specific TJ proteins to gain access to host cells. Thus, understanding the contribution of TJ components in barrier formation and regulation may aid the development of therapies to restore barrier properties, control barrier properties to promote drug delivery to regions of the CNS, and for the development of novel antibacterial and antiviral compounds.
Occludin is an integral membrane, vesicle-trafficking, MAL and related proteins for vesicle trafficking and membrane link (MARVEL) protein (6), that has a role in regulating TJ properties (7). For example, in endothelial cells, it regulates TJ barriers in response to cytokines, such as IFN-γ (8, 9), and growth factors, such as VEGF (10). Removal of the cytoplasmic C-terminal coiled coil domain (CC; residues 413–522) of occludin leads to cytoplasmic localization with increased tracer flux through the junctions and an inability to maintain the apical localization of marker proteins (11, 12), identifying the CC as a key element in regulation (13). The CC directly associates with the SH3-U5-GuK domains of the membrane-associated guanylate kinase homolog (MAGUK) protein, ZO-1 (14). ZO proteins are characterized by their core PDZ3-SH3-GuK (PSG) domains, preceded by two additional PDZ domains and separated by unique regions (U1–6), each with distinct functions (15).
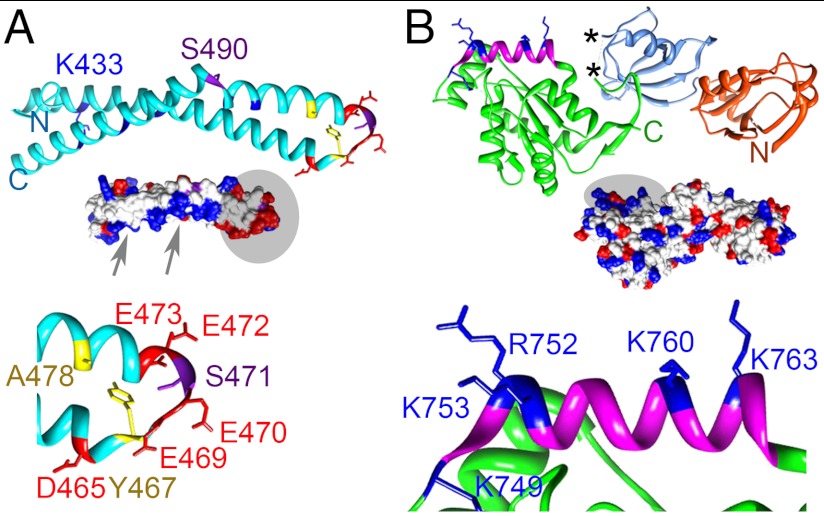
The interaction of ZO-1 and occludin is modulated by phosphorylation of residues in CC that affects the properties of TJs (7). Biochemical and biophysical experiments have led to two distinct models for the complex (Fig. 1). In the first, (Model 1) a cluster of basic residues on CC (K433, K444, K485, K488, K504, and K511) form the interface with ZO-1 (16). In the second (Model 2), the interface was formed between the acidic surface of CC and regions of the ZO-1 U5 and GuK domain (17–19). The crystal structures of ZO-1 PSG and SH3-GuK domains (20–22) seemed to support the Model 2, since the surface of the GuK domain is largely basic (Fig. 1B). The potential contributions of the ZO-1 U6 (not part of the minimal CC binding domain) or U5 motifs to the interface are unknown as they are absent from the structures. Thus, no clear consensus exists on how occludin and ZO-1 interact or how phosphorylation modulates this interaction.
Fig. 1.
Ribbon diagrams showing location of key residues in CC and PSG. Residues characterized in detail are labeled and shown in stick representation. (A, Top) Ribbon diagram of CC. K433, K444, K485, K488, K504, and K511 (basic face) (16) are blue. (Middle) Surface representation with arrows and a circle highlighting the basic (blue) and acidic (red) surfaces, respectively. (Bottom) Residues in the loop between the first two helices are drawn as sticks (acidic, red; hydrophobic, yellow; phosphorylatable Ser, purple). All other residues are shown as a cyan ribbon. (B, Top) Ribbon diagram of PSG (PDZ3, orange; SH3, mid-blue; GuK, green) showing basic residues in helix V (blue). The asterisk (*) denotes the ends of the U5 motif. (Middle) Surface representation colored as in A. A circle highlights the region around helix V. (Bottom) Residues within helix V involved in CaM binding (20).
In this study, we present a structural model for the minimal complex between occludin and ZO-1 (CC∶PSG) where residues 468–475 (REESEEYM) in the CC acidic head directly interact with helix V of ZO-1 GuK. This model is consistent with our data from SAXS, NMR, in vitro, and ex vivo binding studies and in transiently transfected Madin–Darby canine kidney (MDCK) cells. Furthermore, we found that a phosphopeptide (YREEpS471EEYM) bound approximately 20-fold tighter than its nonphosphorylated equivalent to PSG, implicating S471 phosphorylation in regulating binding. In light of these results, we discuss roles for CC phosphorylation in complex stability and TJ regulation.
Results
CC and PSG Form an Extended Complex in Solution.
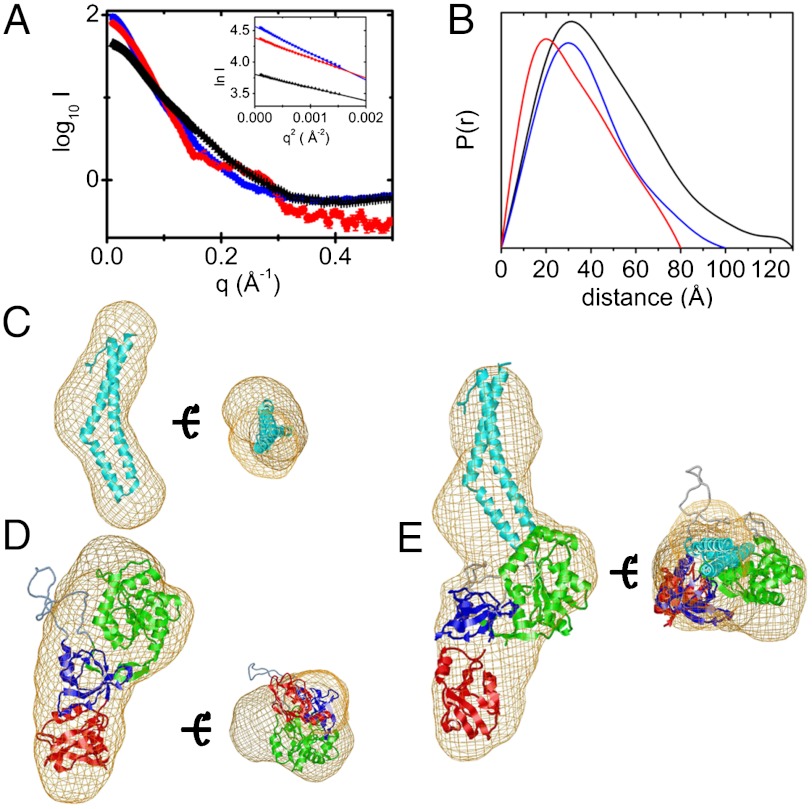
To discriminate between the opposing models for the ZO-1∶occludin complex (16, 19), we measured the SAXS curves of the minimal binding domains (CC and PSG) alone and in their binary complex (Fig. 2A). From the Guinier plot, all samples were monodisperse (Fig. 2A and SI Appendix, Fig. S1A) and monomeric, based on the relative scattering intensities at zero angle and Porod volumes of CC and PSG. The molecular weight of the CC∶PSG complex was consistent with a 1∶1 stoichiometry, and this was confirmed in a titration experiment (SI Appendix, Fig. S1B). The radius of gyration (Rg) extrapolated to zero concentration of CC and PSG were 23.2 ± 0.4 Å (approximately 24 Å, 1WPA.PDB, 3G7C.PDB) (7, 16) and 28.9 ± 0.3 Å (approximately 29 Å, 3SHW.PDB) (22), respectively, and 35.6 ± 0.6 Å for the complex (Fig. 2A). These values correspond to a centers-of-mass separation of 51 ± 2 Å from the parallel axis theorem (23), suggesting an extended complex. Further, the distance distribution functions, P(r), and maximum chord length, Dmax, for the CC and PSG alone and in complex support this conclusion (Fig. 2B). The shape of the P(r) curve for CC is indicative of a rod (Dmax = 80 Å), while PSG is more compact (Dmax = 95 Å), consistent with their crystal structures (CC Dmax approximately 75 Å, 1XAW.PDB; PSG Dmax approximately 93 Å, 3SHW.PDB). The complex is significantly longer (Dmax = 140 Å).
Fig. 2.
Analysis of the SAXS data for CC, PSG, and their binary complex. (A) Plot of log of scattered intensity versus scattering angle, q, for CC (black), PSG (red), and their binary complex (blue); (Inset) Guinier plot for each. (B) P(r) curves. (C) Fit of CC (cyan ribbon), (D) PSG (PDZ3, red; SH3, blue; GuK, green), and (E) the binary complex, into their respective ab initio envelopes (beige net).
The SAXS-derived molecular envelope of CC was rod-like (Fig. 2C), while PSG was boot-shaped in high or low NaCl (Fig. 2D and SI Appendix, Fig. S1 C and D) with the SH3 at the heel, GuK forming the ankle, and PDZ3 the toes. The envelope of the complex was extended at the ankle, presumably due to binding of the CC (Fig. 2E). Crystal structures of CC and PSG were fit as rigid molecules to the SAXS data (SI Appendix, Fig. S2) (24), either unconstrained or constraining the relative placement of CC and PSG (SI Appendix, Fig. S2). Constraining either E470 or 472 (CC, acidic head) to be < 7 Å from K760 (PSG, GuK, helix V) resulted in the best fit to the data (SI Appendix, Fig. S2) and gave marginally better χ2 values than without constraints. This arrangement is consistent Model 2 (17–19). Constraints involving K433 (CC), to test Model 1, gave much poorer fits to the scattering data (SI Appendix, Fig. S2). Moreover, constraining the model to be a homodimer of PSG, rather than a CC∶PSG complex, fit the data poorly (χ2 = 3.1), consistent with the observation that dimerization occurs through PDZ2, which was not in our construct (25).
The binding of PSG to CC was characterized in a quantitative capture assay (SI Appendix, Fig. S3). PSG (2 μM) was soluble and did not bind to GST beads alone or in the presence of GST, even in 50 mM NaCl. It was efficiently captured on these beads by GST-CC (SI Appendix, Fig. S3A, 2 μM). The amount captured was saturable and the maximum relative intensity for the captured PSG and CC was consistent with a 1∶1 stoichiometry at 50 mM NaCl (SI Appendix, Fig. S3 B and C). Under these conditions, the apparent dissociation constant, Kd, was 1.7 ± 0.2 μM and increased with NaCl concentrations (SI Appendix, Fig. S3C, 6 ± 1 μM at 100 mM NaCl; 12 ± 2 μM at 150 mM NaCl,). These data indicate a net displacement, Δn, of 1.7 Na+ plus Cl- in forming the GST-CC∶PSG complex (SI Appendix, Fig. S3C), consistent with an ionic interface.
CC Binds to Basic Residues in Helix V of the ZO-1 GuK Domain.
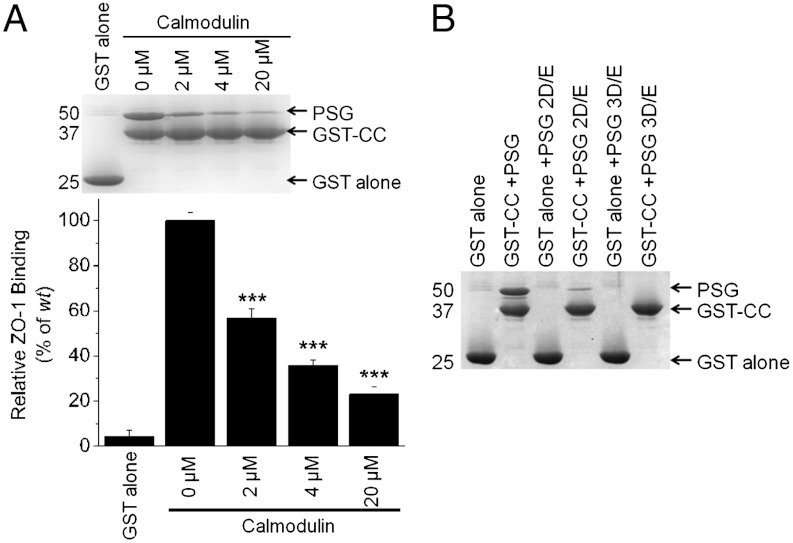
To define the CC binding surface on PSG, we focused on the region in and around residues 749–768 (helix V, GuK), a conserved, predominantly basic region that contributes to calmodulin (CaM) binding (20). The CaM∶PSG complex by SAXS was elongated (Rg 35.4 ± 0.4 Å) with a 1∶1 stoichiometry. The SAXS-derived envelope suggested that CaM bound to PSG in a similar position to CC (SI Appendix, Fig. S4). Further, CaM inhibited, in a dose-dependent manner, the capture of PSG by GST-CC (Fig. 3A), suggesting that they share a common binding site on ZO-1. The basic residues in helix V GuK are a primary determinant of CaM binding (20). Therefore, we tested the effect on CC binding of the variants used in the CaM studies [(20); K749D/R752D/K753E (PSG 3D/E) and K760E/K763E (PSG 2D/E) in helix V of ZO-1 GuK)]. Both PSG variants were captured in significantly reduced amounts by GST-CC (Fig. 3B), demonstrating that CC and CaM are direct competitors for PSG and share a binding site that includes basic residues within helix V GuK.
Fig. 3.
CaM competes with CC for binding to the ZO-1 GuK domain. (A) Addition of increasing amounts of purified CaM inhibits the capture of PSG by GST-CC. A representative Coomassie-stained gel of the GST-CC and PSG bound to glutathione resin as a function of added CaM with a summary of the amount of captured PSG relative to GST-CC relative (n = 3). For statistical significance: * p < 0.05, ** p < 0.01, and *** p < 0.001. (B) The charge swaps in helix V of PSG 2D/E and 3D/E cannot bind CaM (20) and are not captured by GST-CC on glutathione beads.
The Acidic Head of CC Forms the Interface.
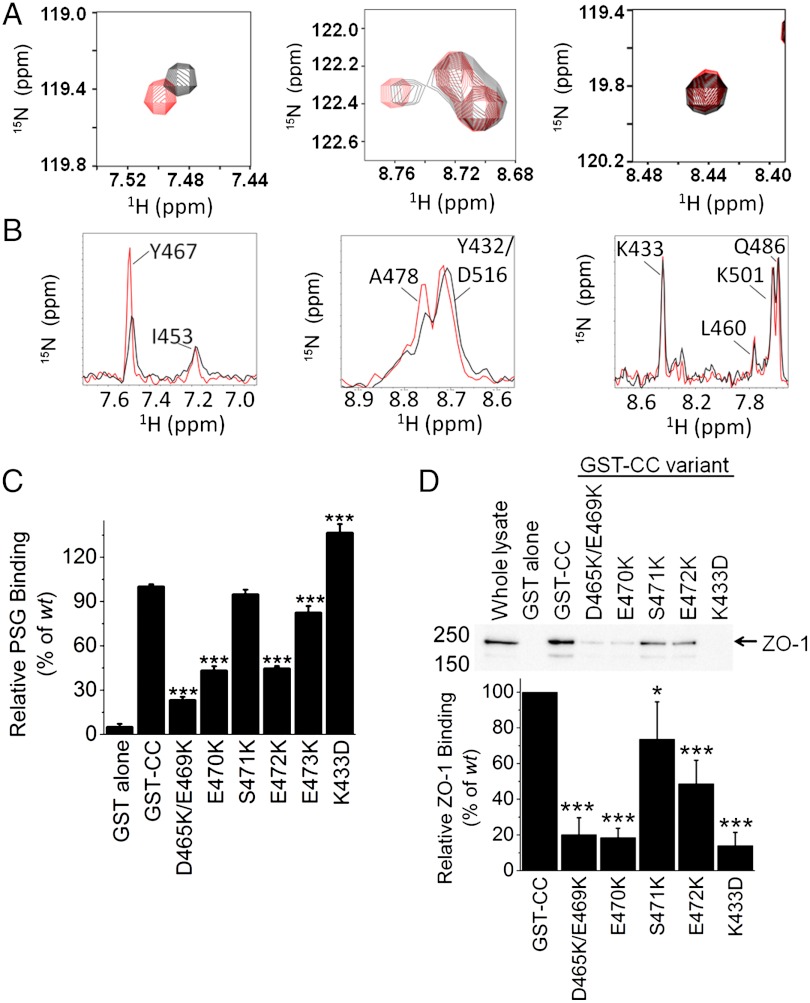
In the optimal SAXS-based model, the acidic head of CC forms part of the ZO-1 binding site; however, the data does not conclusively exclude binding via the opposite end of CC near its N and C termini (residues 414–439 and 508–522). To resolve this question, we performed NMR chemical shift perturbation and cross-saturation experiments. Backbone resonance assignment of 109 of 112 residues in CC was accomplished with the transverse relaxation optimized spectroscopy (TROSY) form of standard triple resonance experiments (SI Appendix, Fig. S5) (26, 27) and provided the basis for identification of the CC-binding surface. Chemical shift perturbation experiments comparing CC and CC∶PSG identified two clusters of residues that showed small but detectable changes in the TROSY spectrum. The first cluster was located within helix 1 (N454, E456, and R459) and the second in the acidic head (Fig. 4A and SI Appendix, Fig. S6; L464, D465, Y467, E470, S471, E473, M475, and A478). No clusters in chemical shift perturbations were observed for residues at the opposite end of the CC. The observed chemical shifts are relatively small because the CC∶PSG interaction is most likely dominated by side chains with little perturbation of the backbone environment. There was further evidence of complex formation when comparing the TROSY spectra of 15N, 2H labeled (random fractionally deuterated) PSG with and without unlabeled CC (SI Appendix, Fig. S7). Upon addition of CC numerous resonances appeared between 8.1–8.5 ppm 1H and 120–126 ppm 15N and a large peak associated with an unfolded resonance signal disappeared, suggesting some structural rearrangement in PSG upon complex formation.
Fig. 4.
The acidic head of CC mediates interaction with ZO-1. (A) Close-up of TROSY spectrum of perdeuterated [15N]-apo (red) CC overlaid with PSG-bound perdeuterated [15N]-CC (black) showing the shifts for residues affected by the addition of CC (Y467, A478) and those unaffected (K433). (B) 1D traces of the residues in A from cross-saturation relaxation experiments for saturation at 15 ppm (red) and 1 ppm (black). (C) The in vitro capture efficiency of PSG by GST-CC is reduced by charge reversals at residues 465/469, 470, 472, and 473 in the acidic head of CC. (D) The variant GST-CC, except K433D, had a similar effect upon the capture of full-length ZO-1 from MDCK lysates. Relative binding (below the gel) was the intensity ratio of the ZO-1 band in each lane and the wt condition.
To determine whether the chemical shift perturbations result from direct binding or allosteric effects, NMR cross-saturation experiments (28) were performed. Perdeuterated [15N]-CC was bound to protonated, unlabeled PSG at 2∶1, 1∶1, and 1∶2 molar ratios. Due to the tendency of free PSG to aggregate at high protein concentrations and prolonged incubation at 27 °C, total PSG was kept at 100 μM and short collection times were used (< 24 h). To overcome the sensitivity limitations, buffers containing 40% and 70% D2O were used instead of the more usual 90% D2O. To avoid potential false positives arising from the spin diffusion effect, we used a conservative criterion for identifying perturbed resonances. L464, D466, Y467, and A478 were at the interface in these experiments (Fig. 4B and SI Appendix, Fig. S8). No residues around the N and C termini showed significant cross-saturation relaxation effects. Taken together, the SAXS and NMR experiments demonstrate that CC binds with its acidic head in proximity to PSG, while the opposite end, containing its N and C termini, is furthest from this interface.
To probe the contribution of specific residues in the acidic head to complex stability, the effect of single (E470K, E472K, and E473K) and double (D465K/E469K) substituted variants of GST–CC were evaluated in PSG capture assays (Fig. 4C). The E473K variant marginally reduced capture (82% of wt, p < 0.001) while more dramatic reductions were observed for the E470K, E472K, and D465K/E469K variants [43%, 47%, and 23% of wt (all p < 0.001), respectively]. The E470K, E472K, and D465K/E469K GST-CC variants also showed reduced capture of full-length ZO-1 from MDCK cell lysates (Fig. 4D; 18%, 49%, and 20% of wt, respectively, p < 0.001) confirming the importance of the negative charges on CC in mediating its interaction with ZO-1. By contrast, the K433D substitution, located on the basic face of CC, displayed opposite effects in vitro (Fig. 4C) and in lysate (Fig. 4D). In vitro, K433D enhanced capture of PSG (136% of wt, p < 0.001) but, as reported previously (16), it abolished the interaction with full-length ZO-1 (14% of wt, p < 0.001), suggesting that K433 may be involved in a contact present in full-length ZO-1 (29) but not in PSG. A possible candidate is the acidic U6 motif (residues 803–888), that regulates complex stability but is not required for the binding of the minimal interacting domains (29). However, we cannot exclude other possibilities, such as post-translational modifications of ZO-1 or additional factors present in the extract.
E470 and E472 in CC Contribute to TJ Localization in Cells.
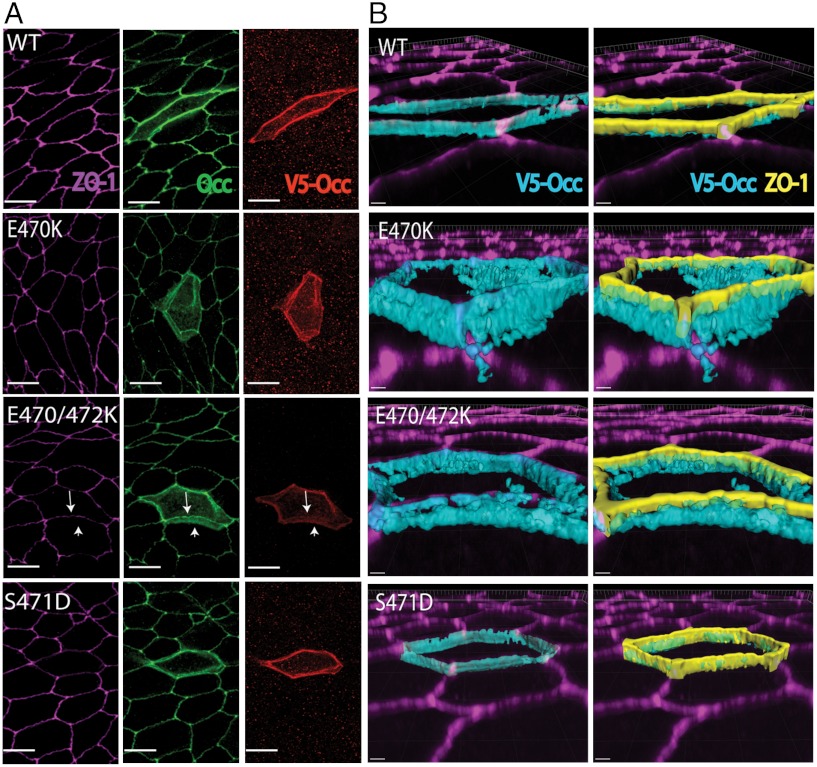
Since E470K and E472K substitutions had profound effects on ZO-1 binding, full-length V5-tagged occludin or variants containing these substitutions were transiently transfected into MDCK cells. Expression of wt occludin resulted in immunostaining at the cell membrane, with the majority occurring at cell∶cell contacts, colocalizing with ZO-1 and endogenous occludin (Fig. 5A). The 3D rendering of these confocal images revealed good colocalization of wt occludin with ZO-1 at the apical membrane border (Fig. 5B). By contrast, the E470K and E470K/E472K occludin variants showed increased localization in the cytoplasm and at the lateral border (Fig. 5B) and reduced staining at cell contacts, with loss of ZO-1 colocalization. To quantify the change in localization, the ratio of Triton X-100-soluble and insoluble expressed occludin was compared to ZO-1. As expected, the majority of wt occludin was found in the Triton X-100-insoluble fraction, along with ZO-1. However, the E470K and E470K/E472K variants were located in the soluble fraction, with a 66% (p < 0.01) decrease in the ratio of Triton X-100-insoluble∶soluble occludin for the double variant (SI Appendix, Fig. S9). These results suggest that the interaction of ZO-1 with the acidic head of CC is necessary for either the transport to, or maintenance of, occludin at TJs.
Fig. 5.
Charge reversal mutations in the acidic head disrupt occludin localization. Confocal images of immunocytochemistry for ZO-1, occludin, and V5-tagged occludin exogenously expressed in MDCK cells transiently transfected with V5 tagged wt, E470K, E470K/E472K, or S471D variants of occludin. (A) Max projected confocal image. Arrows indicate failure of E470K/E472K mutant to colocalize with ZO-1. There is good localization for S471D. Bar represents 10 μm. (B) Topical surface of V5 staining and V5 staining plus ZO-1 rendered in 3D volume. Bar represents 2 μm.
Phosphorylation of S471 May Enhance Occludin∶ZO-1 Complex Stability.
The two acidic residues, E470 and E472, with a key role in localizing occludin at TJs, flank S471, a previously identified phosphosite (7). To investigate the effect of S471 phosphorylation, occludin containing a S471D substitution (S471D) was transiently transfected into MDCK cells. Using confocal microscopy, S471D demonstrated good apical border colocalization with ZO-1 (Fig. 5).
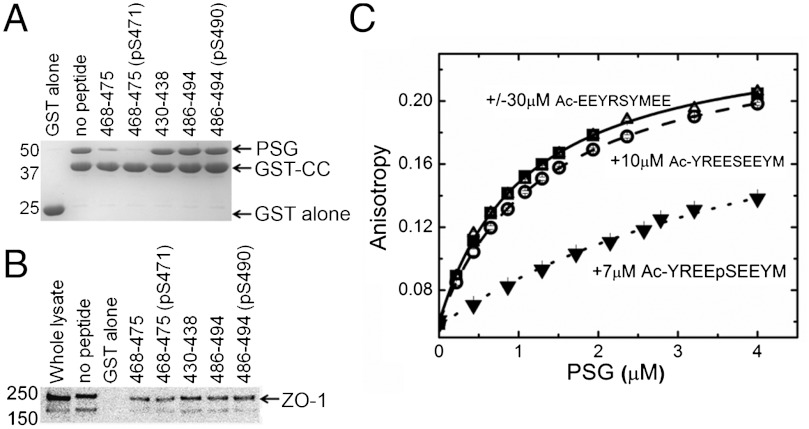
To further define the effect of S471 phosphorylation, peptides with and without a phosphogroup on S471 (residues 468–475, REESEEYM, and REEpSEEYM) were tested for their ability to inhibit PSG capture by GST-CC (Fig. 6A). Both peptides significantly inhibited the capture of PSG [19% and 33% of wt, (p < 0.001), respectively], with the phosphorylated peptide being more efficient than the nonphosphorylated peptide (p < 0.01). This observation was similar in experiments to capture endogenous ZO-1 from MDCK cell lysates (Fig. 6B; 32% and 41%, respectively, p < 0.05). By contrast, peptides containing S490 (residues 486–494, QVKGSADYK and QVKGpSADYK), a second validated phosphosite in CC (7, 10) and a control peptide encompassing K433 (residues 430–438, QLYKRNFDT) did not significantly alter capture efficiency in vitro or ex vivo.
Fig. 6.
A phosphopeptide containing S471 competes with CC binding to PSG. (A) In vitro, only inclusion of peptides (200 μM) spanning residues the acidic head, 468–475 (± pS471) inhibited capture of PSG by GST-CC on glutathione beads. (B) In MDCK lysates, only the peptides in A significantly reduced capture of full-length ZO-1 by GST-CC (n = 3). (C) Equilibrium binding of the fluorescent-labeled peptide, FITC-(miniPEG)-YREESEEYM, to PSG in the absence and presence of unlabeled peptides. In all experiments, 3 nM-labeled peptide was titrated with purified PSG. A representative titration and fit to a single binding site model is shown (▪, solid line). Inclusion of a nonfluorescently labeled version of this peptide (○, long dash, weak competition) or this peptide with S471 phosphorylated (▾, short dash, strong competition) but not a scrambled version of this sequence (Δ, no competition) reduced PSG binding compared to the unlabeled peptide alone.
To quantitate binding of the S471-containing peptides, a fluorescently labeled peptide corresponding to residues 467–475 CC [FITC—(miniPEG)—YREESEEYM] was synthesized. Based upon the changes in fluorescent anisotropy of this peptide as a function of PSG concentration, the Kd for labeled peptide binding was 1.8 ± 0.4 μM (Fig. 6C and SI Appendix), although the fluorophore and the linker appeared to contribute slightly to the interaction. In competition assays, the equivalent unlabeled peptide (Ac—YREESEEYM) bound with a Kd of 15 ± 3 μM, while the serine phosphorylated peptide (Ac—YREEpSEEYM) increased the affinity by approximately 20-fold (Kd = 0.70 ± 0.05 μM). By contrast, a scrambled peptide (Ac-EEYRSYMEE) did not affect binding of the labeled peptide (Fig. 6C). Taken as a whole, these results confirm that the acidic head of CC directly mediates its interaction with the GuK domain of PSG with high affinity and phosphorylation of S471 enhances occludin∶ZO-1 complex stability.
Discussion
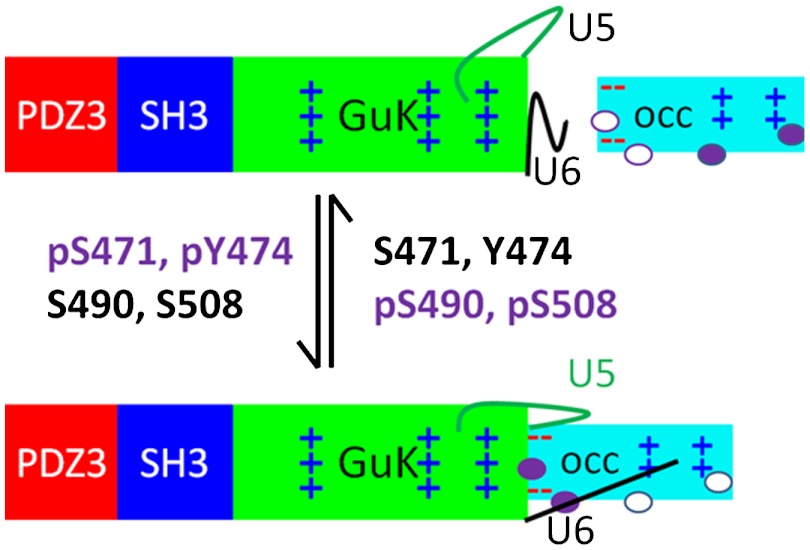
Previous studies of TJs have established a close association between the scaffolding protein, ZO-1, and the transmembrane protein, occludin, and identified multiple phosphorylation sites as potential regulators of their interaction. However, the molecular details of this interaction were unclear. The results described here suggest a model for complex formation utilizing two distinct interfaces that contain phosphorylatable residues within or around their periphery (Fig. 7). The core of the interface is electrostatic, involving D465, E469, E470, E472, and E473 in CC and residues K749, R752, K753, K760, and K763 in PSG. Consistent with this model, the stability of the complex is salt-dependent. In addition, helix V (residues 751–765) (20) is used by two other MAGUK family members for binding their phosphosites (30, 31). Finally, the coiled-coil domain of tricellulin, a homolog of occludin present at tricellular junctions, lacks the acidic residues in its head domain and does not interact strongly with PSG in SAXS and capture assays (SI Appendix, Fig. S10) (32).
Fig. 7.
Working model of the CC∶PSG interaction and the role of the various phosphosites on CC. Domains shown as rectangles, colored as Fig. 1. Phosphosites are ovals that are shaded when phosphorylated. U5 and U6 are represented as lines.
The argument for a second binding interaction between occludin and ZO-1 is based upon differences in the effects of some amino acid substitutions in CC on their capture efficiency of PSG and longer ZO-1 constructs. Charge reversal substitutions of lysine residues in the basic face of CC did not affect capture of PSG in vitro but, consistent with earlier data, showed reduced capture of longer ZO-1 constructs (1–888 and full-length ZO-1) that contain U6 (16). Therefore, we speculate there is a second ionic interface that involves interactions between the basic face of CC, including K433, and acidic residues on the surface of the full-length ZO-1, perhaps within the U5 or U6 motifs (16, 29, 33) or involving a bridge with another cellular factor. The presence of this secondary interface may enhance the stability of the complex in the cellular environment and/or contribute to the regulation of the complex. One very attractive possibility is that binding of ZO-1 binding to the secondary interface controls access to the lysine-rich face of CC, which contains seven of the 12 conserved lysine residues in occludin, and thus its ubiquitin-dependent endocytosis observed in endothelial cells (10).
In vivo, the occludin∶ZO-1 interaction is modulated by phosphorylation events (7, 34–38). In occludin, four of these sites segregate structurally and possibly functionally—two adjacent to the primary interaction site (S471 and Y474) in the acidic head and two in the secondary interface (S490 and S508) on the basic face. Y474 phosphorylation is associated with binding p85 of phosphatidyl inositol 3-kinase at the leading edge of migrating cells (39) while S490 and S508 are associated with vascular permeability after VEGF treatment (7) or HIV encephalitis (40). Our results suggest a role for S471 phosphorylation in ZO-1 binding. The CC peptide 468–475 containing S471 and Y474, is more effective at blocking PSG and ZO-1 capture when phosphorylated at S471 (pS471), suggesting that phosphorylation here may enhance complex formation in vivo. Indeed, in MDCK cells, the S471D-occludin variant exhibited at least as good colocalization with ZO-1 as wt (Fig. 5). The results are consistent with the recent finding that the GuK domain has evolved as a phosphopeptide-binding module (31). The Kd for pS471 peptide binding to PSG (0.7 μM) is very similar to that of p-LGN binding to SAP97 SH3-GuK (0.22 μM). However, the Kd values of the equivalent nonphosphorylated peptides are different (S471 to PSG, approximately 15 μM; LGN to SPA97, > 100 μM). Interestingly, occludin possesses the requisite arginine at -3 relative to the phosphosite of GuK binding peptides (31); however, ZO-1 does not contain the sequence required for high affinity binding. These results are consistent with S471 phosphorylation strengthening or modulating binding to ZO-1 rather than providing an all or none binding motif. By contrast, phosphorylation of S490 reduces its interaction with endogenous ZO-1 (7). While the effect of S508 phosphorylation on ZO-1 binding is not known, its location adjacent to K504 and K511, which are required for complex formation with endogenous ZO-1 (16), suggests that S508 phosphorylation would reduce ZO-1 binding. Further, phosphorylation of either S490 or S508 correlates with opening of the barrier in endothelial cells (7, 40), suggesting that the basic face of CC is involved in regulating TJ barrier properties in vivo. Five additional phosphosites that lie in a conserved region just upstream of CC (Y398, Y402, T403, T404, and T408) also regulate phosphorylation-dependent complex formation. Based on geometric considerations, they cannot exert their effect directly at the primary interaction site and may modulate binding through a different site, perhaps close to the proposed secondary interaction site.
In summary, our model for the complex between CC and PSG, provides a framework for understanding the functional role of the occludin∶ZO-1 complex in TJ permeability via occludin phosphorylation and offers new insight into the regulation of barrier properties. These studies highlight the direct interaction of the CC, through its acidic head, with the basic region surrounding helix V in PSG. Undoubtedly, additional contacts exist in the larger TJ complex that contribute to complex organization; however, the relative contribution and mechanism of action of occludin phosphorylation on its interaction with ZO-1 is emerging. A complete understanding of the interface may uncover new therapeutic strategies to selectively alter TJ permeability in the treatment of disease or delivery of drugs to the central nervous system.
Methods
E. coli expressed human CC and human PSG were expressed as tobacco etch virus (TEV) protease cleavable His6-tagged fusion proteins; the TEV-cleaved form of each was used for SAXS and NMR experiments. A GST-tagged version of the CC (413–522) was expressed and purified using standard protocols (SI Appendix, Methods) and used in vitro and in cell lysate capture assays. The final concentrations of proteins used in individual experiments are given in the text and the figure legends. SAXS experiments were conducted at X9, National Synchrotron Light Source, Upton, NY, using a standard configuration and software. NMR experiments were conducted on Bruker Avance 600 MHz and 850 MHz spectrometers, equipped with cold probes using standard protocols. In-cell based assays were conducted with MDCK cells using standard protocols. Additional details are available in the SI Appendix.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. Marc Allaire and Lin Yang for discussions and technical assistance with small angle X-ray scattering. We thank the Pennsylvania Lions Sight Conservation and Eye Research Foundation (J.M.F.), the American Diabetes Association Grant 7-07-RA-34 (to J.M.F.), National Eye Institute Grant EY012021 (to D.A.), and the Pennsylvania Tobacco Funds (J.M.F., F.T., and D.A.) for financial support. The Kellog Eye Center imagine core is supported by National Institutes of Health Grants EY07003 and P60DK020572. The National Synchrotron Light Source and beamline X9 is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121390109/-/DCSupplemental.
References
- 1.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004;19:331–338. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- 2.Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edelblum KL, Turner JR. The tight junction in inflammatory disease: Communication breakdown. Curr Opin Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- 5.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: Lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Pulido L, Martin-Belmonte F, Valencia A, Alonso MA. MARVEL: A conserved domain involved in membrane apposition events. Trends Biochem Sci. 2002;27:599–601. doi: 10.1016/s0968-0004(02)02229-6. [DOI] [PubMed] [Google Scholar]
- 7.Sundstrom JM, et al. Identification and analysis of occludin phosphosites: A combined mass spectrometry and bioinformatics approach. J Proteome Res. 2009;8:808–817. doi: 10.1021/pr7007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchiando AM, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010;123:2844–2852. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem. 2009;284:21036–21046. doi: 10.1074/jbc.M109.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balda MS, et al. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuse M, et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusrat A, et al. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J Biol Chem. 2000;275:29816–29822. doi: 10.1074/jbc.M002450200. [DOI] [PubMed] [Google Scholar]
- 14.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann NY Acad Sci. 2009;1165:113–120. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC. The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol. 2010;2010:402593. doi: 10.1155/2010/402593. 10.1155/2010/402593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Fanning AS, Anderson JM, Lavie A. Structure of the conserved cytoplasmic C-terminal domain of occludin: Identification of the ZO-1 binding surface. J Mol Biol. 2005;352:151–164. doi: 10.1016/j.jmb.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Muller SL, et al. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280:3747–3756. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt A, Utepbergenov DI, Krause G, Blasig IE. Use of surface plasmon resonance for real-time analysis of the interaction of ZO-1 and occludin. Biochem Biophys Res Commun. 2001;288:1194–1199. doi: 10.1006/bbrc.2001.5914. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt A, et al. Occludin binds to the SH3-hinge-GuK unit of zonula occludens protein 1: Potential mechanism of tight junction regulation. Cell Mol Life Sci. 2004;61:1354–1365. doi: 10.1007/s00018-004-4010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lye MF, Fanning AS, Su Y, Anderson JM, Lavie A. Insights into regulated ligand binding sites from the structure of ZO-1 Src homology 3-guanylate kinase module. J Biol Chem. 2010;285:13907–13917. doi: 10.1074/jbc.M109.093674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomme J, et al. The Src homology 3 domain is required for junctional adhesion molecule binding to the third PDZ domain of the scaffolding protein ZO-1. J Biol Chem. 2011;286:43352–43360. doi: 10.1074/jbc.M111.304089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan L, Chen J, Yu J, Yu H, Zhang M. The structure of the PDZ3-SH3-GuK tandem of ZO-1 suggests a supramodular organization of the MAGUK family scaffold protein core. J Biol Chem. 2011;286:40069–40074. doi: 10.1074/jbc.C111.293084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelman DM, Moore PB. Determination of quaternary structure by small angle neutron scattering. Annu Rev Biophys Bioeng. 1975;4:219–241. doi: 10.1146/annurev.bb.04.060175.001251. [DOI] [PubMed] [Google Scholar]
- 24.Petoukhov MV, Svergun DI. Global rigid body modeling of macromolecular complexes against small-angle scattering data. Biophys J. 2005;89:1237–1250. doi: 10.1529/biophysj.105.064154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanning AS, Lye MF, Anderson JM, Lavie A. Domain swapping within PDZ2 is responsible for dimerization of ZO proteins. J Biol Chem. 2007;282:37710–37716. doi: 10.1074/jbc.M707255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salzmann M, Wider G, Pervushin K, Senn H, Wuthrich K. TROSY-type triple-resonance experiments for sequential NMR assignments of large proteins. J Am Chem Soc. 1999;121:844–848. [Google Scholar]
- 27.Salzmann M, Pervushin K, Wider G, Senn H, Wuthrich K. TROSY in triple-resonance experiments: New perspectives for sequential NMR assignment of large proteins. Proc Natl Acad Sci USA. 1998;95:13585–13590. doi: 10.1073/pnas.95.23.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi H, Nakanishi T, Kami K, Arata Y, Shimada I. A novel NMR method for determining the interfaces of large protein-protein complexes. Nat Struct Biol. 2000;7:220–223. doi: 10.1038/73331. [DOI] [PubMed] [Google Scholar]
- 29.Fanning AS, et al. The unique-5 and -6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol Biol Cell. 2007;18:721–731. doi: 10.1091/mbc.E06-08-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reese ML, Dakoji S, Bredt DS, Dotsch V. The guanylate kinase domain of the MAGUK PSD-95 binds dynamically to a conserved motif in MAP1a. Nat Struct Mol Biol. 2007;14:155–163. doi: 10.1038/nsmb1195. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, et al. Guanylate kinase domains of the MAGUK family scaffold proteins as specific phospho-protein-binding modules. EMBO J. 2011;30:4986–4997. doi: 10.1038/emboj.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raleigh DR, et al. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200–1213. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riazuddin S, et al. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet. 2006;79:1040–1051. doi: 10.1086/510022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balda MS, Anderson JM, Matter K. The SH3 domain of the tight junction protein ZO-1 binds to a serine protein kinase that phosphorylates a region C-terminal to this domain. FEBS Lett. 1996;399:326–332. doi: 10.1016/s0014-5793(96)01352-x. [DOI] [PubMed] [Google Scholar]
- 35.Elias BC, et al. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem. 2009;284:1559–1569. doi: 10.1074/jbc.M804783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kale G, Naren AP, Sheth P, Rao RK. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem Biophys Res Commun. 2003;302:324–329. doi: 10.1016/s0006-291x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 37.Raleigh DR, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du D, et al. The tight junction protein, occludin, regulates the directional migration of epithelial cells. Dev Cell. 2010;18:52–63. doi: 10.1016/j.devcel.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto M, et al. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.