Abstract
Members of the RAS small GTPase family regulate cellular responses to extracellular stimuli by mediating the flux through downstream signal transduction cascades. RAS activity is strongly dependent on its subcellular localization and its nucleotide-binding status, both of which are modulated by posttranslational modification. We have determined that RAS is posttranslationally acetylated on lysine 104. Molecular dynamics simulations suggested that this modification affects the conformational stability of the Switch II domain, which is critical for the ability of RAS to interact with guanine nucleotide exchange factors. Consistent with this model, an acetylation-mimetic mutation in K-RAS4B suppressed guanine nucleotide exchange factor-induced nucleotide exchange and inhibited in vitro transforming activity. These data suggest that lysine acetylation is a negative regulatory modification on RAS. Because mutations in RAS family members are extremely common in cancer, modulation of RAS acetylation may constitute a therapeutic approach.
Members of the rat sarcoma (RAS) family of small monomeric GTPases function as molecular binary switches, with their biological activities determined by their nucleotide-binding state. When bound to GTP, RAS proteins engage a variety of downstream “effector” pathways to influence cellular behavior (1). As such, the nucleotide-binding state of RAS must be highly regulated in a cell, and this regulation is accomplished through the activity of positive and negative cofactors. Wild-type RAS has low intrinsic GTPase activity and thus relies on GTPase-activating proteins (GAPs) to hydrolyze GTP efficiently. Guanine nucleotide exchange factors (GEFs) facilitate the reloading of GDP-bound RAS with GTP. Activating-point mutations in RAS proteins are common in cancer, with missense mutations at codons 12 and 13 being the most prevalent (2). These particular mutations affect the endogenous enzymatic activity of RAS, but have a much greater effect on GAP-induced GTP hydrolysis, effectively shifting the nucleotide-binding equilibrium of RAS toward its constitutively active (i.e., GTP-bound) state (3).
The nucleotide-binding state affects RAS activity by influencing its 3D structure. When RAS binds to GTP, it undergoes a conformational change that primarily affects two regions of the protein: Switch I, which binds to effectors and GAPs, and Switch II, which is critical for GEF and GAP activity and for interaction with PI3K (4–7). Mutations that impinge on the nucleotide-dependent conformation change affect the ability of RAS to release nucleotide in the presence of GEF and to activate downstream effectors (8). In essence, proper RAS function requires the ability to cycle between its active and inactive conformations.
Within a cell, RAS proteins must associate with cellular membranes to transmit signals to downstream effector proteins. Because RAS itself is not a transmembrane protein, its proper localization is accomplished through posttranslational lipidation, primarily by irreversible farnesylation of a C-terminal cysteine (9). Mutation of the farnesylated cysteine restricts RAS to the cytoplasm, inhibiting its normal functions and its oncogenic properties. With the exception of farnesylation, the four RAS family members (H-RAS, N-RAS, K-RAS4A, and K-RAS4B) undergo distinct posttranslational modifications, both within their hypervariable regions and throughout the rest of the protein (10). These secondary modifications fine-tune RAS localization, providing unique subcellular localizations for each of the RAS isoforms. Here, we have identified a posttranslational modification of RAS—lysine acetylation—and elucidate its effect on the biochemical characteristics of RAS. Acetylation of lysine 104 affects the efficiency of nucleotide exchange and also suppresses the oncogenic activity of mutationally activated RAS.
Results
RAS Is Acetylated on Lysine 104.
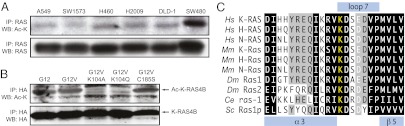
Because of the demonstrated importance of lysine acetylation in modifying protein function, we sought to determine whether RAS is acetylated and whether this modification could modulate its function. We found that endogenous mutant RAS immunoprecipitated from human cancer cell lines could be detected by Western blotting with an antibody specific for acetylated lysine (Fig. 1A). To confirm this observation and to identify the lysines that are acetylated, we performed mass spectrometry. We found that lysine 104 (K104) was the only amino acid on which we could detect acetylation (Fig. S1). To explore in more detail whether K104 is the likely site of RAS acetylation, we expressed different forms of HA-tagged K-RAS4B in 293T cells and then assessed acetylation via Western blotting. This experiment allowed us to make several key observations related to RAS acetylation. First, we found that both wild-type (G12) and mutationally activated (G12V) K-RAS4B are acetylated (Fig. 1B), suggesting that RAS acetylation is not dependent on the GTP-binding state. Next, mutation of lysine 104 to either alanine (K104A) or glutamine (K104Q) eliminated K-RAS4B acetylation, indicating that lysine 104 is likely to be the only lysine that is acetylated on K-RAS4B (Fig. 1B). Finally, inhibition of K-RAS farnesylation via mutation of cysteine 185 to serine (C185S) did not affect acetylation, indicating that K-RAS4B does not need to associate with a membrane to be acetylated (Fig. 1B).
Fig. 1.
RAS is acetylated on lysine 104. (A) Detection of acetylated RAS in human cancer cell lines. Endogenous RAS was immunoprecipitated from a panel of colorectal and lung cancer cell lines and detected by Western blotting for acetylated lysine (Ac-K) or for RAS itself. Acetylated RAS is detectable in all cell lines that were examined. (B) Effects of mutations on K-RAS4B acetylation. Ectopic HA-tagged forms of K-RAS4B were expressed in 293T cells and then immunoprecipitated with anti-HA antibody. Western blotting for Ac-K revealed that mutation of K104 to either alanine (A) or glutamine (Q) abolishes acetylation. Mutational activation, via mutation of codon 12 to valine, on its own does not affect acetylation. Likewise, inhibition of K-RAS4B farnesylation, via mutation of C185 to serine, does not affect acetylation. (C) Alignment of RAS homologs. K104, which lies at the border between the α3 helix and loop 7, is conserved in all RAS homologs from humans to budding yeast. Hs, Homo sapiens; Mm, Mus musculus; Dm, Drosophila melanogaster; Ce, Caenorhabditis elegans; Sc, Saccharomyces cerevisiae.
K104 is in loop 7, between the α3 and β5 regions of RAS (5, 11), which lies outside the Switch I and II domains that are primarily responsible for RAS function. Interestingly, lysine at this position is conserved in RAS orthologs from humans to yeast, suggesting that this residue may be important for an evolutionarily conserved function of RAS (Fig. 1C). Based on this observation, we sought to determine how modulation of RAS acetylation affects its function.
K104 Acetylation Affects K-RAS Transforming Activity but Not Localization.
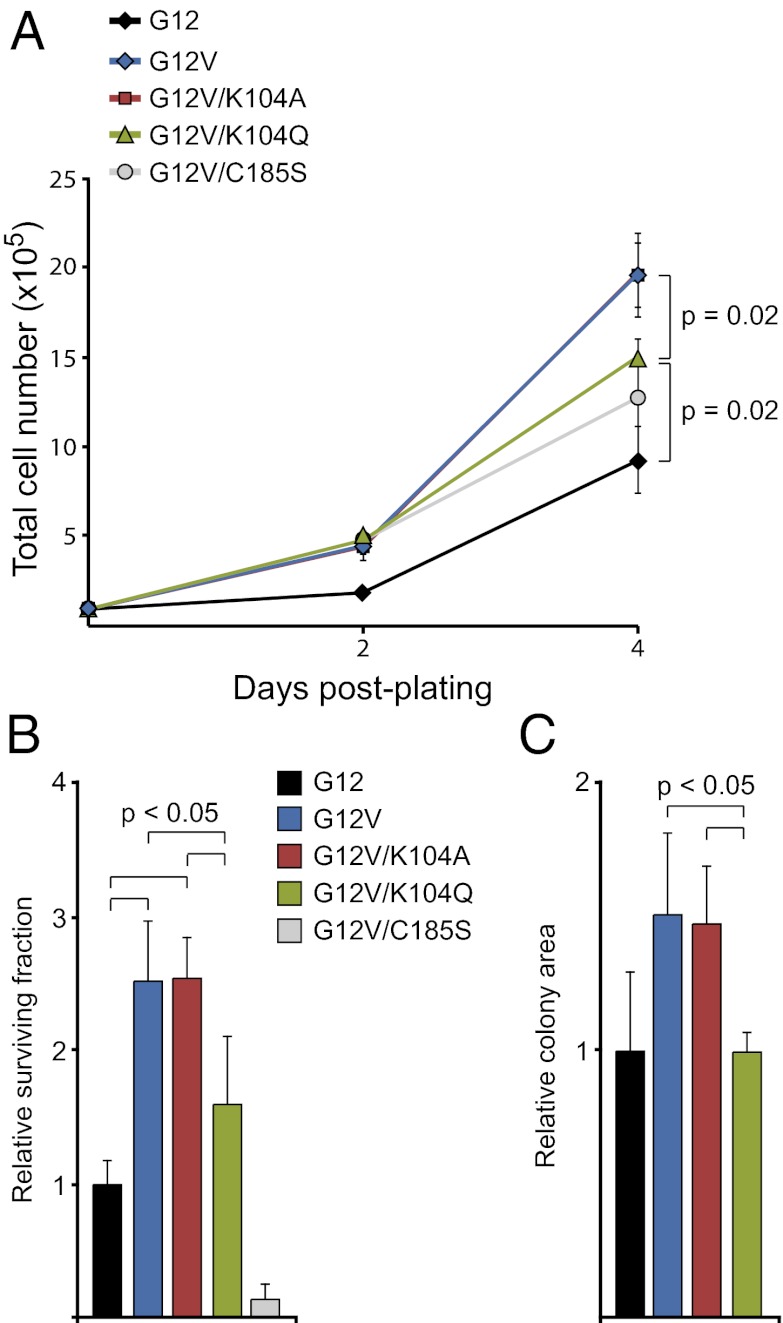
To determine whether there are functional consequences of acetylating RAS on K104, we examined the in vitro transforming activity of mutationally activated (G12V) K-RAS4B that also was mutated at codon 104 (Fig. S2). Mutation of K104 to alanine (K104A), which essentially locks K-RAS4B into a constitutively deacetylated state, did not affect the proliferation of NIH3t3 cells relative to those expressing G12V (Fig. 2A). Alternately, mutation of K104 to glutamine (K104Q), which mimics constitutive acetylation, led to an attenuated proliferation phenotype similar to G12V/C185S, which is restricted to the cytoplasm (Fig. 2A). Next, we assessed the ability of these same mutants to affect the clonogenic survival of NIH3t3 cells in a focus-formation assay. In this assay, the G12V and G12V/K104A forms of K-RAS enhanced cell survival, but G12V/K104Q did not (Fig. 2B). Consistent with the effect on proliferation, colonies that developed from cells expressing G12V/K104Q were significantly smaller than those expressing G12V or G12V/K104A (Fig. 2C). Together, these data suggest that K104 acetylation is a negative regulatory modification on K-RAS.
Fig. 2.
Effect of K104 mutation on transforming activity. (A) Effect of K104 mutations on proliferation. The proliferation of NIH3t3 cells stably expressing different forms of K-RAS4B was measured over 4 d. G12V K-RAS4B enhanced proliferation relative to wild-type (G12) K-RAS4B. Mutation of K104 to alanine (K104A), which mimics the nonacetylated form of K-RAS4B, did not affect the ability of G12V K-RAS4B to enhance proliferation. Mutation of K104 to glutamine (K104Q), which mimics acetylation, or of C185 to serine (C185S), which prevents farnesylation, attenuates the hyperproliferative effect of G12V K-RAS4B. (B) Effect of K104 mutations on clonogenic survival. The ability of different forms of K-RAS4B to promote focus formation was assessed in stably expressing NIH3t3 cells. G12V K-RAS4B enhanced clonogenic survival. Mutation of K104 to alanine (K104A) did not affect the ability of K-RAS4B to enhance survival. As with proliferation, mutation of K104 to glutamine (K104Q) attenuates the prosurvival effect of K-RAS4B. The C185S mutant functions as a dominant negative in this assay. All data are normalized to the surviving fraction of cells ectopically expressing wild-type K-RAS4B. (C) Effect of K104 mutations on colony size. Similar to its effect in the proliferation assay, the K104Q mutation had a significant effect on the size of colonies in the focus-formation assay. All data are normalized to the average colony size of cells expressing wild-type K-RAS4B.
We next investigated the mechanism by which acetylation of K104 could negatively affect the function of RAS. Because other forms of posttranslational modification (e.g., lipidation and ubiquitination) affect RAS trafficking to cellular membranes, we first examined whether K104 mutations that affect acetylation also impinge on subcellular localization. To track K-RAS4B localization in live cells, we fused it to the C terminus of mCherry and followed the fusion protein by confocal microscopy. As expected, mCherry-K-RAS4B localized to the plasma membrane in both COS-1 and HeLa cells (Fig. S3A). Mutation of K104 to either arginine or glutamine did not affect K-RAS4B localization, suggesting that K-RAS4B acetylation, unlike other types of posttranslational modification, is not important for its ability to traffic to the plasma membrane (Fig. S3A). This observation suggests that K104 acetylation influences K-RAS function via a unique molecular mechanism.
K104 Acetylation Is Predicted to Affect Switch I and II Stability.
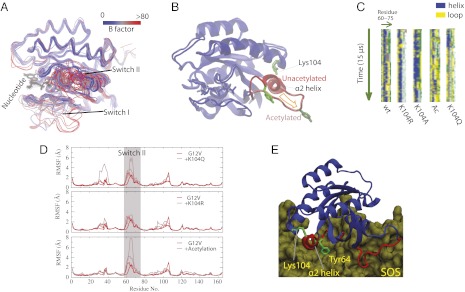
To gain insight into potential mechanisms by which acetylation might affect RAS transforming activity, we used molecular dynamics (MD) simulations to determine how acetylation of lysine 104 influences the structure of K-RAS. The compilation of crystal structures of K-RAS indicates that the protein is conformationally flexible at the Switch I and Switch II regions (Fig. 3A). These crystal structures suggest two conformational states for the protein. In the “ordered” state, the Switch I domain maintains contact with the phosphate groups of the bound nucleotide and the helix of the Switch II (α2 helix) remains intact, whereas in the “disordered” state the Switch I domain is flipped away from the nucleotide and the α2 helix is largely dissolved. Previous studies have indicated a dynamic equilibrium between the two states (12, 13). Our simulations suggest that K104 acetylation destabilizes the ordered conformation—in particular the α2 helix of Switch II—in favor of the disordered conformation (Fig. 3B and Movies S1 and S2).
Fig. 3.
Impact of K104 acetylation on RAS structure. (A) Superposition of 96 resolved RAS structures in the PDB. The structures collectively show that Switch I and Switch II are conformationally highly variable. Consistently, the B factors (color-coded) of these crystal structures also indicate that the two Switch regions are the most flexible in RAS proteins. (B) Representative conformation of acetylated RAS G12V. The conformation was generated by 15-μs MD simulation. For comparison, a snapshot from a simulation of nonacetylated RAS G12V was superimposed. As shown, the acetylation leads to significant rearrangement at the α2 helix and Tyr64. Note that the two simulations represented here were initiated from one identical RAS structure (PDB ID 121P). (C) The helicity of the α2-helix region in simulations of GTP-bound G12V RAS. In the stripes each pixel represents the secondary structure (color-coded) of a residue (60–75, x-dimension) at a given time in simulation (y-dimension). The bars are blue to a large extent, indicative of an intact α2-helix, for the wild-type (wt), K104R, and K104A, but not for acetylated RAS (Ac) and K104Q, in which the helix was dissolved in the simulations. (D) Residue-specific root mean square fluctuations (RMSF) in simulations. Both the acetylation and the acetylation-mimetic K104Q mutation lead to higher instability of the Switch II region. By contrast, K104R, which preserves the positive charge of the residue, does not destabilize the Switch II. (E) Lys104 and the Switch II α2 helix in an RAS protein interacting with SOS1 (PDB ID 1XD2). Here the α2 helix and Tyr64 of Switch II are highlighted because of their importance in RAS-SOS binding. As shown, the α2 helix interacts with Lys104 electrostatically at the C terminus of the helix.
The effect of K104 acetylation on RAS structure likely results from perturbation of electrostatic interactions within the Switch II region. K104, which is positively charged before acetylation, is located adjacent to the C terminus of the α2 helix and interacts favorably with the helix dipole. This interaction is a common mechanism for helix stabilization in proteins. K104 acetylation removes the charge of the residue and consequently disrupts the favorable interaction with the α2 helix, leading to dissolution of the α2 helix and higher conformational fluctuations within the region, as was observed in our simulations (Fig. 3 C and D). Similarly, the K104Q mutation, which mimics acetylation by neutralizing the residue, is predicted also to destabilize the α2 helix. Indeed, the destabilizing effect was observed in our simulations where the K104Q mutation was introduced (Fig. 3 C and D). Together, these observations suggest that the K104Q mutation does mimic K104 acetylation at the molecular level.
The normal GTP hydrolysis cycle for RAS requires GAP and GEF. The conformational dynamics of the Switch I domain plays a central role in GAP activity, whereas GEF activity relies upon the protein–protein interface between the α2 helix of RAS and GEF. Within this interface, Tyr64, Met67, and Tyr71 form a hydrophobic core that is critical for proper interaction (Fig. 3E) (14). As shown in Fig. 3B, the destabilization of the α2 helix of Switch II by K104 acetylation leads to displacement of Tyr64, and therefore we predicted that the RAS–GEF interaction would be hindered. Thus, based on our simulation findings, we inferred a mechanism by which acetylation, and by extension the K104Q mutation, could disrupt K-RAS transforming activity by obstructing nucleotide exchange by hindering the RAS–GEF interaction.
K104 Is Critical for GEF-Induced Nucleotide Exchange.
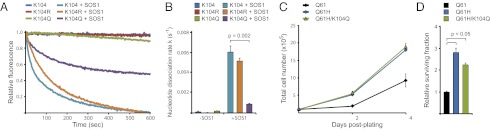
Based on our MD simulations, we tested the hypothesis that K104 acetylation affects GEF-induced nucleotide exchange on RAS. To this end, we performed in vitro assays in which the capacity of the catalytic domain of the RAS GEF SOS1 to stimulate nucleotide exchange was analyzed by monitoring the dissociation of methylanthraniloyl (mant)-GDP from purified K-RAS4B. SOS1 was able to promote efficiently the nucleotide exchange of G12V K-RAS and G12V/K104R K-RAS (which, like K104A, mimics the deacetylated state), but G12V/K104Q K-RAS was relatively resistant to the effects of SOS1 (Fig. 4 A and B). Moreover, we found that the K104Q mutation altered the GTP-binding state of K-RAS4B in NIH3t3 cells (Fig. S3B). Consistent with our simulation data, these data indicate that mutation of K104 to glutamine, and, by extension, acetylation of K104, affects the catalysis of RAS nucleotide exchange by SOS1. If acetylation of K104 exerts a general effect on RAS function, we would expect to see a broad effect on downstream signaling through RAS effector pathways. Indeed, cells expressing G12V/K104Q K-RAS4B exhibited attenuated signaling through the MAPK and PI3K pathways relative to cells expressing G12V K-RAS4B (Fig. S3 C and D).
Fig. 4.
K104 acetylation affects GEF activity. (A) In vitro nucleotide exchange assay. In the absence of GEF (SOS1), all the forms of K-RAS4B assayed retained nucleotide over the course of the experiment. SOS1 accelerated nucleotide release on wild-type (K104) and K104R K-RAS4B, but K104Q K-RAS4B was largely resistant. All GEF assays were done on G12V K-RAS4B ± mutation of K104. (B) Quantification of nucleotide exchange. Nucleotide dissociation rates for each condition were calculated from three independent experiments. SOS1-induced nucleotide exchange was decreased significantly in K104Q K-RAS4B relative to wild-type. (C) Effect of K104Q mutation on proliferation in a Q61H background. Q61H K-RAS4B enhanced proliferation of NIH3t3 cells relative to wild-type (Q61) K-RAS4B. Mutation of K104 to glutamine (K104Q) did not affect the hyperproliferative effect of activated K-RAS4B. (D) Effect of K104Q mutation on clonogenic survival in a Q61H background. Q61H K-RAS4B enhanced clonogenic survival of NIH3t3 cells. As with proliferation, the K104Q mutation did not significantly affect the prosurvival function of Q61H K-RAS4B. All data are normalized to the surviving fraction of cells ectopically expressing wild-type K-RAS4B.
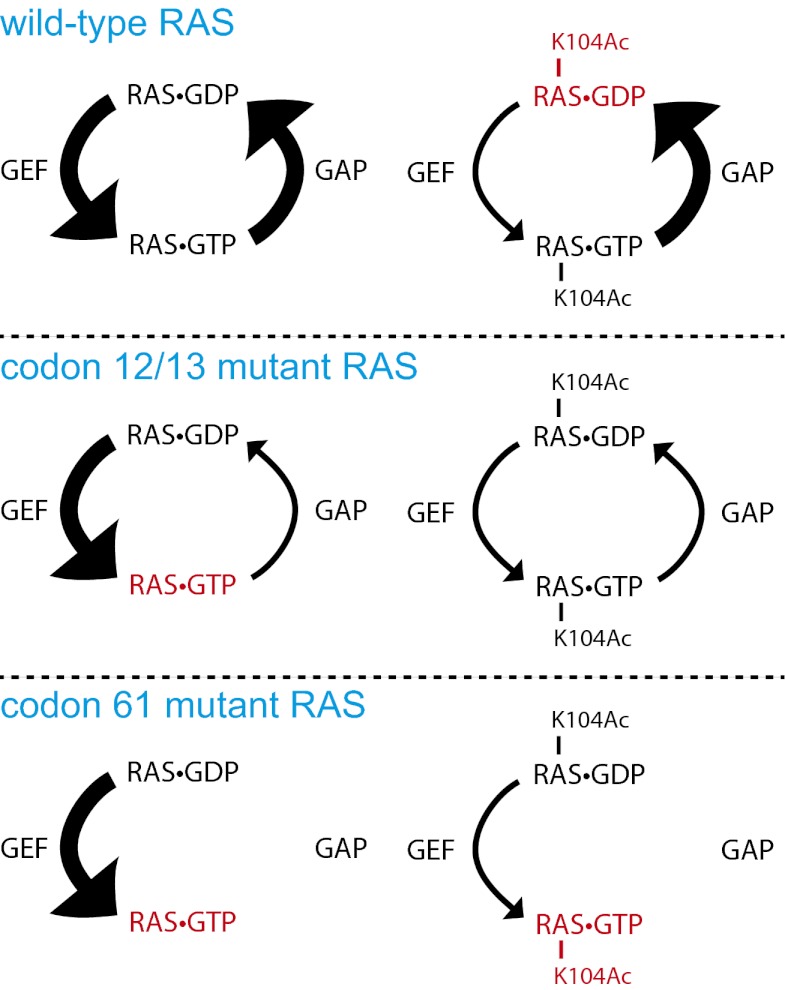
How might loss of GEF-induced nucleotide exchange affect the transforming activity of mutationally activated RAS? Mutation of RAS at codon 12 or 13 affects the association between RAS and GAP, leading to RAS activation secondary to loss of GAP-induced GTPase activity. Nevertheless, RAS that is mutated at codon 12 or 13 still retains some intrinsic GTPase activity and therefore still requires GEF to reload GTP after hydrolysis. By contrast, codon 61 mutations in RAS destroy the intrinsic GTPase activity; and so the nucleotide-binding state of these mutants presumably is not regulated by GEF (15, 16). Thus, although the transforming activity of G12V K-RAS4B was affected by an acetylation-mimetic mutation at K104 (Fig. 2), we hypothesized that the K104Q mutation would not affect the transforming activity of Q61H K-RAS4B. We tested this hypothesis formally by measuring the transforming activity Q61H K-RAS4B in NIH3t3 cells. Unlike its effect in the background of codon 12 mutation, we found that the K104Q mutation failed to inhibit the transforming activity of K-RAS mutated at codon 61 (Fig. 4 B and C). This observation is consistent with our hypothesis that defective nucleotide exchange plays a role in the impairment of transforming activity by K104 acetylation. A model for linking K104 acetylation to RAS activation state is shown in Fig. 5.
Fig. 5.
Model for the effect of K104 acetylation on wild-type and mutant RAS. When RAS is wild-type and unacetylated, its nucleotide-binding state is largely controlled by GAP and GEF, creating an equilibrium between RAS⋅GTP and RAS⋅GDP. Acetylation of K104 causes a shift in the equilibrium toward RAS⋅GDP because of insensitivity to GEF. When RAS is mutationally activated at codon 12 or 13, the equilibrium is shifted toward RAS⋅GTP because of insensitivity to GAP. This equilibrium shift can be counteracted by K104 acetylation, which suppresses GEF-induced nucleotide exchange. When RAS is mutationally activated at codon 61, the equilibrium is shifted toward RAS⋅GTP because of the loss of intrinsic GTPase activity. This equilibrium shift cannot be affected by K104 acetylation. Red lettering indicates the nucleotide state of RAS that is favored under each circumstance.
Discussion
Posttranslational modification is a common way for a cell to fine-tune the activity of a protein. In the case of RAS, multiple posttranslational modifications (e.g., farnesylation, palmitoylation, diubiquitination) serve to regulate its subcellular localization. Other modifications (e.g., nitrosylation, monoubiquitination) appear to regulate RAS activity directly. We have identified a posttranslational modification of RAS: acetylation on lysine 104 (K104). The K104 acetylation mark shares similarities and differences with other RAS modifications. For example, acetylation is similar to diubiquitination because it does not depend on the nucleotide-binding state, but differs from diubiquitination in that it does not require prior membrane localization (Fig. 1B) (17). Our data suggest that RAS is acetylated in the cytoplasm and that acetylation may be an important mechanism for regulating RAS activity. K104 was shown previously to be monoubiquitinated on K-RAS4B and H-RAS, but it is not the primary site of monoubiquitination, and the functional consequences of this modification on this residue are not clear (18).
We set out to answer two major questions relating to RAS acetylation. The first was whether acetylation is a positive or negative regulatory modification. Although mutationally activated (G12V) K-RAS transforms NIH3t3 cells, an acetylation-mimetic mutation (K104Q) attenuates this transforming activity (Fig. 2). The observation indicates that acetylation is a negative regulatory (i.e., antioncogenic) modification and distinguishes it from other modifications that are required for RAS function and therefore are prooncogenic [e.g., farnesylation and nitrosylation (19)]. The second question we addressed was the nature of the mechanism linking acetylation to negative regulation of RAS function. Our MD simulations implicated K104 acetylation in the destabilization of Switch II, which is critical for the interactions between RAS and GEF and between RAS and PI3K and for GAP-induced GTP hydrolysis (6,7). Our biochemical analysis demonstrated that K-RAS with an acetylation-mimetic mutation at K104 is resistant to nucleotide exchange catalyzed by SOS1, an RAS GEF (Fig. 4A). Given that the K104Q mutation also suppressed transforming activity, these data suggested that resistance to GEF activity underlies the negative regulation of RAS. Based on this model, we correctly predicted that the K104Q mutation would not affect the transforming activity of K-RAS mutated at codon 61 because codon 61 mutations abolish intrinsic GTPase activity, in essence releasing RAS from the requirement for positive regulation by GEF (Fig. 4B). Taken together, our data indicate that acetylation on K104 modulates RAS activity by interfering with GEF-induced nucleotide exchange (Fig. 5). Based on pharmacologic studies that inhibit RAS binding to SOS1, a similar strategy for inhibiting RAS activity downstream of receptor tyrosine kinases has been proposed (20, 21).
RAS family members are among the most commonly mutated oncoproteins in human cancers (22). Clearly, there is great need to develop new therapies to treat cancers expressing mutant RAS. Because of its mode of activation, however, RAS has proven difficult to inhibit directly with small molecules. Two alternative approaches to generating targeted therapies against RAS have emerged. The first approach is founded on the observation that RAS is modified by farnesyltransferase to promote its association with the plasma membrane (23). In theory, inhibition of farnesyltransferase should prevent K-RAS from localizing to the cellular compartment from which it transmits its oncogenic signal. Farnesyltransferase inhibitors ultimately failed in the clinic, however, because K-RAS, the most commonly mutated family member, is prenylated efficiently by geranylgeranyltransferase in the absence of farnesyltransferase activity (24). The second approach for K-RAS–related therapy focuses on kinases that function in downstream signaling pathways. However, because RAS engages multiple downstream pathways in ways that are context dependent, it has been difficult to predict a priori which effector pathways should be targeted under specific circumstances. Without a doubt, a therapeutic strategy that targets RAS itself would be the most effective. Our data suggest the modulation of acetylation, either through activation of its acetyltransferase or through inhibition of its deacetylase, might constitute an approach to RAS-related cancer therapy.
Materials and Methods
Plasmid Construction, Cell Lines, and Mass Spectrometry.
All constructs were generated by a PCR-based strategy using a site-directed mutagenesis kit (Stratagene) according to the manufacturer’s instructions. Cells were cultured in DMEM supplemented with 10% (vol/vol) FBS. Stably overexpressing NIH 3t3 cells were made by infecting cells with pBabe containing each HA-tagged K-RAS4B construct and selecting in hygromycin (500 μg/mL). For growth curves, cells were plated at 1 × 105 cells per well in six-well dishes in medium containing 10% (vol/vol) FBS. Cell number was counted 2 and 4 d after plating using a hemocytometer. For the colony-forming assay, 100 cells were plated into each well of a six-well dish in medium containing 2% (vol/vol) FBS. After 2 wk, colonies were stained with 0.2% (wt/vol) crystal violet and analyzed using IMAGE J version 1.44p software (National Institutes of Health). All experiments were done in triplicate.
Mass spectrometry was performed at the Taplin Mass Spectrometry Facility at Harvard Medical School.
In Vivo Acetylation Assay and Immunoblotting.
To detect acetylation of exogenously expressed K-RAS4B, HEK293T cells were transiently transfected with plasmids carrying HA-tagged K-RAS. Twenty hours after transfection, cells were lysed in TMN lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1% (vol/vol) Triton X-100, 1 mM DTT]. The soluble fraction of cell lysates was isolated by centrifugation at 16,110 × g for 10 min and was subjected to preincubation with protein-A agarose beads to remove proteins nonspecifically bound to the beads. Precleared cell lysates were incubated with anti-HA antibody for 1 h and then with protein-A agarose beads for 3 h at 4 °C. The immunoprecipitated proteins were washed three times with TMN washing buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 0.2% (vol/vol) Triton X-100, 1 mM DTT]. The precipitated proteins were resolved on a 4–20% gradient gel, and immunoblotting was conducted to detect acetylated K-RAS4B proteins using acetylated lysine-specific antibody (Cell Signaling Technology).
To examine the acetylation of endogenous RAS, cells were treated with 1 μM Trichostatin A (TSA) and 5 mM Nicotinamide (NAM). One hour after deacetylase inhibitor treatment, cells were lysed with 0.5% Nonidet P-40 buffer [150 mM NaCl, 0.5% (vol/vol) Nonidet P-40, 50 mM Tris⋅Cl (pH 8.0), 400 nM TSA, 16.5 mM NAM]. After preclearing with protein-A agarose beads, cell lysates were incubated with RAS antibody-conjugated beads (Calbiochem) overnight at 4 °C and then were washed three times with 0.5% Nonidet P-40 lysis buffer. The precipitated proteins were resolved on a 4–20% gradient gel, and immunoblotting was conducted to detect acetylated K-RAS4B proteins using acetylated lysine specific antibody (Cell Signaling Technology).
For analysis of signaling through RAS effector pathways, quantitative Western blots were visualized on a LiCor Odyssey Infrared Imaging System. Primary antibodies were as follows: rabbit α-Erk1/2, mouse α-phospho-Erk1/2 (Thr202/Tyr204), mouse α-Akt, rabbit α-phospho-Akt (Thr308). Antibodies were purchased from Cell Signaling Technology.
Live Cell Imaging.
HeLa and COS-1 cells were grown on 35-mm dishes that incorporate a 15-mm glass coverslip-sealed cutout in the bottom (MatTek) and were transfected with various forms of mCherry-tagged K-Ras4B. Images were acquired 24 h posttransfection with an inverted Zeiss 510 laser scanning confocal microscope using a 63×, NA 1.4 objective at room temperature.
MD Simulations.
All simulations were based on one RAS X-ray structure [Protein Data Bank (PDB) ID code 121P]. G12V mutation and bound GTP/Mg2+ were included in all simulations. Simulation systems were set up by placing the protein at the center of a cubic simulation box measuring 64 Å per side. In a simulation system of ∼25,000 atoms, explicitly represented water molecules were added to fill the system, and Na+ and Cl− ions were added to maintain physiological salinity (150 mM) and to obtain a neutral total charge for the system. The systems were parameterized using the Amber99SB force field with corrections for leucine, isoleucine, aspartic acid, and asparagine for the protein (25, 26); TIP3P for water (27); and extended Amber force field (28, 29) for the acetylated lysine and GTP molecules. We used the Anton specialized hardware to perform all simulations (30). We carried out equilibrium MD simulation on Anton in the NVT ensemble at 295 K using the Nose–Hoover thermostat (31) with a relaxation time of 1.0 ps. All bond lengths to hydrogen atoms were constrained using a recently developed implementation (32) of M-SHAKE (33). A 2.5-fs time step was used for the simulations. The Lennard–Jones and the Coulomb interactions in the simulations were calculated using a force-shifted cutoff of 12 Å (34). All simulations discussed in this paper were 15 μs long.
In Vitro Nucleotide Exchange Assay.
SOS-catalyzed nucleotide exchange assays were performed as previously described (35). Briefly, recombinantly purified RAS proteins (1 mM) were loaded with the fluorescent nucleotide analog mant-GDP in buffer containing 20 mM Tris (pH 7.5), 50 mM NaCl, and 4 mM EDTA for 15 min at room temperature. The reactions were supplemented with 14 mM MgCl2 and incubated for 2 h on ice. Reactions were initiated by the addition of 200 μM unlabeled GDP, and the decrease in fluorescence intensity (excitation: 355 nm; emission: 470 nm) was monitored via a luminescence spectrometer (model LS50; Perkin-Elmer). Where indicated, the reactions were supplemented with recombinantly purified equimolar SOS before the addition of excess cold nucleotide.
Supplementary Material
Acknowledgments
This work was supported by American Cancer Society Research Scholar Grant MGO-114877 (to K.M.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201487109/-/DCSupplemental.
References
- 1.Karnoub AE, Weinberg RA. Ras oncogenes: Split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau KS, Haigis KM. Non-redundancy within the RAS oncogene family: Insights into mutational disparities in cancer. Mol Cells. 2009;28:315–320. doi: 10.1007/s10059-009-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trahey M, McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987;238:542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- 4.Pai EF, et al. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989;341:209–214. doi: 10.1038/341209a0. [DOI] [PubMed] [Google Scholar]
- 5.Milburn MV, et al. Molecular switch for signal transduction: Structural differences between active and inactive forms of protooncogenic ras proteins. Science. 1990;247:939–945. doi: 10.1126/science.2406906. [DOI] [PubMed] [Google Scholar]
- 6.Pacold ME, et al. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103:931–943. doi: 10.1016/s0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 7.Schubbert S, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–336. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- 8.Ford B, et al. Characterization of a Ras mutant with identical GDP- and GTP-bound structures. Biochemistry. 2009;48:11449–11457. doi: 10.1021/bi901479b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hancock JF, Magee AI, Childs JE, Marshall CJ. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 10.Ahearn IM, Haigis K, Bar-Sagi D, Philips MR. Regulating the regulator: Post-translational modification of RAS. Nat Rev Mol Cell Biol. 2012;13:39–51. doi: 10.1038/nrm3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittinghofer A, Pai EF. The structure of Ras protein: A model for a universal molecular switch. Trends Biochem Sci. 1991;16:382–387. doi: 10.1016/0968-0004(91)90156-p. [DOI] [PubMed] [Google Scholar]
- 12.Geyer M, et al. Conformational transitions in p21ras and in its complexes with the effector protein Raf-RBD and the GTPase activating protein GAP. Biochemistry. 1996;35:10308–10320. doi: 10.1021/bi952858k. [DOI] [PubMed] [Google Scholar]
- 13.Spoerner M, Herrmann C, Vetter IR, Kalbitzer HR, Wittinghofer A. Dynamic properties of the Ras switch I region and its importance for binding to effectors. Proc Natl Acad Sci USA. 2001;98:4944–4949. doi: 10.1073/pnas.081441398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, Kuriyan J. The structural basis of the activation of Ras by Sos. Nature. 1998;394:337–343. doi: 10.1038/28548. [DOI] [PubMed] [Google Scholar]
- 15.Der CJ, Finkel T, Cooper GM. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986;44:167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- 16.Buhrman G, Holzapfel G, Fetics S, Mattos C. Allosteric modulation of Ras positions Q61 for a direct role in catalysis. Proc Natl Acad Sci USA. 2010;107:4931–4936. doi: 10.1073/pnas.0912226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jura N, Scotto-Lavino E, Sobczyk A, Bar-Sagi D. Differential modification of Ras proteins by ubiquitination. Mol Cell. 2006;21:679–687. doi: 10.1016/j.molcel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki AT, et al. Ubiquitination of K-Ras enhances activation and facilitates binding to select downstream effectors. Sci Signal. 2011;4:ra13. doi: 10.1126/scisignal.2001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patgiri A, Yadav KK, Arora PS, Bar-Sagi D. An orthosteric inhibitor of the Ras-Sos interaction. Nat Chem Biol. 2011;7:585–587. doi: 10.1038/nchembio.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurer T, et al. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci USA. 2012;109:5299–5304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casey PJ, Solski PA, Der CJ, Buss JE. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci USA. 1989;86:8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sebti SM, Hamilton AD. Farnesyltransferase and geranylgeranyltransferase I inhibitors in cancer therapy: Important mechanistic and bench to bedside issues. Expert Opin Investig Drugs. 2000;9:2767–2782. doi: 10.1517/13543784.9.12.2767. [DOI] [PubMed] [Google Scholar]
- 25.Hornak V, et al. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindorff-Larsen K, et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins. 2010;78:1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 28.Liu H, Duan Y. Effects of posttranslational modifications on the structure and dynamics of histone H3 N-terminal Peptide. Biophys J. 2008;94:4579–4585. doi: 10.1529/biophysj.107.115824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meagher KL, Redman LT, Carlson HA. Development of polyphosphate parameters for use with the AMBER force field. J Comput Chem. 2003;24:1016–1025. doi: 10.1002/jcc.10262. [DOI] [PubMed] [Google Scholar]
- 30.Dror RO, Jensen MO, Shaw DE. Elucidating membrane protein function through long-timescale molecular dynamics simulation. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009;2009:2340–2342. doi: 10.1109/IEMBS.2009.5335057. [DOI] [PubMed] [Google Scholar]
- 31.Hoover WG. Canonical dynamics: Equilibrium phase-space distributions. Phys Rev A. 1985;31:1695–1697. doi: 10.1103/physreva.31.1695. [DOI] [PubMed] [Google Scholar]
- 32.Lippert RA, et al. A common, avoidable source of error in molecular dynamics integrators. J Chem Phys. 2007;126:046101. doi: 10.1063/1.2431176. [DOI] [PubMed] [Google Scholar]
- 33.Krautler V, Van Gunsteren WF, Hunenberger PH. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J Comput Chem. 2001;22:501–508. [Google Scholar]
- 34.Fennell CJ, Gezelter JD. Is the Ewald summation still necessary? Pairwise alternatives to the accepted standard for long-range electrostatics. J Chem Phys. 2006;124:234104. doi: 10.1063/1.2206581. [DOI] [PubMed] [Google Scholar]
- 35.Margarit SM, et al. Structural evidence for feedback activation by Ras.GTP of the Ras-specific nucleotide exchange factor SOS. Cell. 2003;112:685–695. doi: 10.1016/s0092-8674(03)00149-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.