Abstract
Although there is a great deal of knowledge regarding the phylo- and ontogenetic plasticity of the neocortex, the precise nature of environmental impact on the newborn human brain is still one of the most controversial issues of neuroscience. The leading model–system of experience-dependent brain development is binocular vision, also called stereopsis. Here, we show that extra postnatal visual experience in preterm human neonates leads to a change in the developmental timing of binocular vision. The onset age of binocular function, as measured by the visual evoked response to dynamic random dot correlograms (DRDC-VEP), appears to be at around the same time after birth in preterm (4.07 mo) and full-term (3.78 mo) infants. To assess the integrity of the visual pathway in the studied infants, we also measured the latency of the visual-evoked response to pattern reversal stimuli (PR-VEP). PR-VEP latency is not affected by premature birth, demonstrating that the maturation of the visual pathway follows a preprogrammed developmental course. Despite the immaturity of the visual pathway, clearly demonstrated by the PR-VEP latencies, our DRCD-VEP data show that the visual cortex is remarkably ready to accept environmental stimulation right after birth. This early plasticity makes full use of the available extra stimulation time in preterm human infants and results in an early onset of cortical binocularity. According to our data, the developmental processes preceding the onset of binocular function are not preprogrammed, and the mechanisms turning on stereopsis are extremely experience-dependent in humans.
Keywords: experience-dependent development, evoked potential
Stereopsis provides accurate depth perception by aligning the views of the two eyes in some of the rodents and in most carnivores, primates, and humans. The binocular system is unique among other cognitive capacities because it is alike across a large number of species; therefore, a remarkable collection of molecular, cellular, network, and functional data (1–6) is available to advance the understanding of human development. This system is also unique in its relatively abrupt onset during ontogeny. The onset of binocular function follows the emergence of eye-specific organization of the visual cortex into ocular dominance columns, which seem to develop with the initial guidance of intrinsic molecular and electrical signals (1–3). Later on, in a distinct phase of development called the “critical period,” the ocular dominance columns become particularly open to alteration by extrinsic environmental signals (4, 5). The well-defined timeline of developmental events is another valuable characteristic of binocular vision (6), persistently bringing it into the limelight of studies on cortical plasticity.
To address the origin of early plasticity of the binocular system in humans, we studied preterm human neonates compared with full-term infants. We asked whether early additional postnatal experience, during which preterm infants have an ∼2 mo of extra environmental stimulation and self-generated movement, leads to a change in the developmental timing of binocular function. Here, we introduce a developmental model that directly compares preterm and full-term infants with respect to the onset of a particular function. Studying preterm infants helps to clarify the nature of developmental processes that ignite the onset of a function, and it can conclusively be determined whether early binocular development is a result of preprogrammed or experience-dependent processes. Assuming that the individual onset ages of a particular visual function approximate normal distribution, the time of onset for a population is best localized by the steepest part of the cumulative onset-distribution function (Fig. 1). As is explained in Fig. 1, the onset age of a particular function can be expressed either in postnatal age (PNA) or in adjusted age. Preprogrammed processes are indicated by an equivalent time of onset in preterm and in full-term infants, as expressed in adjusted age, whereas a delayed onset time is expected in preterm compared with full-term infants on a PNA scale (Fig. 1A). Experience-dependent processes are indicated by a shorter onset time in preterm vs. full-term infants, as expressed in adjusted age, whereas an equivalent time of onset in preterm and in full-term infants is expected as expressed in PNA (Fig. 1B). A diplomatic balance between preprogrammed and experience-dependent processes would naturally fall in between these options. This paradigm allowed us to obtain surprisingly clear-cut results: we found that binocular function is experience-dependent at the extreme, and human cortical development, as probed by our experiment, does not follow a preprogrammed course.
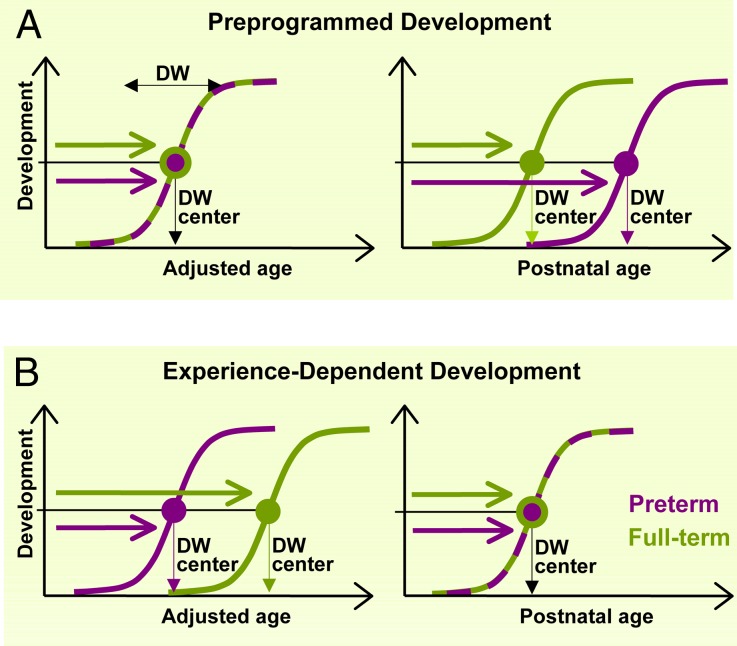
Fig. 1.
Theoretical predictions for the early development of a particular brain function in the extreme cases of preprogrammed development (A) vs. experience-dependent development (B). The interaction between brain maturation and experience can be studied by comparing preterm (violet coloring) and full-term (green coloring) populations at an early age. Development of a particular function begins with a fully immature state and ends with a fully mature state, defining a developmental window (DW), within which developmental processes occur. To compare developmental timing of different functions in preterm and full-term populations, we localize DWs by their center, which is the steepest part of the developmental function. Assuming normal distribution, onset age of an abrupt-onset function is defined by the highest slope of the cumulative onset-distribution function (DW center, as in Fig. 2). In the case of a gradually developing function, DW center refers to the developmental age where improvement is the most rapid (as in Fig. 3). Age is expressed on two complementary scales: adjusted age is the time elapsed after the first day of the last menstrual period minus the average physiological gestational period (9 mo) of the full-term population (in other words, the age the child would be if the pregnancy had actually gone to term); PNA is the time elapsed since birth. Exclusively preprogrammed development predicts identical DW centers for preterm and full-term infants on the adjusted age scale, whereas it predicts an advantage of the full-term population on the PNA scale. Experience-dependent development, on the other hand, would predict an advantage of the preterm population on the adjusted age scale, and identical DW centers on the PNA scale. These clear-cut predictions ensure that the two hypotheses (preprogrammed vs. experience-dependent development) can be contrasted with each other effectively in this paradigm.
To avoid confounding factors from retinal or neurologic injury of the premature visual system, we only included “low-risk” premature infants, who were not affected by the consequences of long-term respiratory treatment or reanimation and did not have major disabilities resulting from, e.g., cerebral lesions or retinopathy of prematurity, as detailed in SI Text. At birth, the mean adjusted age of the preterm group (n = 15) was 31.27 ± 3.03 wk and that of the full-term group (n = 15) was 39.07 ± 1.33 wk.
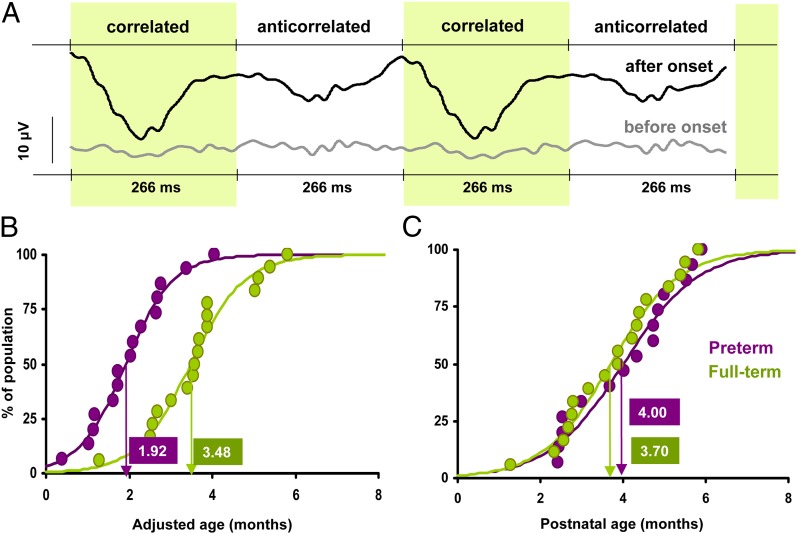
To determine the onset age of binocular function, we used a visual evoked potential (VEP) protocol with dynamic random dot correlograms (DRDC) as stimuli (7–11). The VEP protocol is useful to avoid difficulties and artifacts arising from behavioral estimation of onset times in a preverbal population, such as infants. DRDC stimuli are faultless probes of binocular function because the alternation of the stimulus can only be detected by subjects with intact cortical binocular function (8, 9). DRDCs alternated at 1.875 Hz between binocularly correlated and anticorrelated phases, resulting in a pulsating percept. VEPs were recorded from the scalp of the infants (12). Typical evoked cortical responses of preterm and full-term subjects of different ages are shown in Fig. 2A. The recorded VEPs were analyzed in the time and frequency domain, as we have described it previously (11). Although it has been debated whether VEP responses to the alternation of correlated/anticorrelated dynamic random dots are a sign of stereopsis in monkeys (13), recordings in adult human subjects and older children indicated that this response only occurs in subjects with intact binocular function (8, 9, 11). Previous studies have shown that DRDC-VEP response has an abrupt onset between 3 and 6 mo of age (8–10), and it is followed by the rapid development of stereopsis to “near-adult” level by 6–7 mo in humans (10).
Fig. 2.
Results of the DRDC-VEP experiment. (A) Representative averaged DRDC-evoked VEP responses. VEPs could not be evoked before the onset of binocular function; there is no specific response for the repeating correlated and anticorrelated phases of the stimulus. After the onset, however, VEP responses are phase locked to the DRDC stimulation frequency, which can be detected by T2circ statistic mostly in the second harmonic component (see SI Text). (B) Cumulative distribution of the onset times of DRDC evoked VEP responses in preterm (violet coloring) and full-term (green coloring) infants on an adjusted age scale. Dots represent the percentage of the population presenting DRDC response at a particular age, with a logistic curve fitting (see SI Text for curve-fitting details). Onset age of each population is estimated by the age at which 50% of the infants are responding to DRDCs. The preterm population has an earlier onset on this scale. (C) Data represented on a PNA scale. Estimated onset age is alike for preterm and full-term infants. This pattern of results clearly indicates experience-dependent development of cortical binocularity.
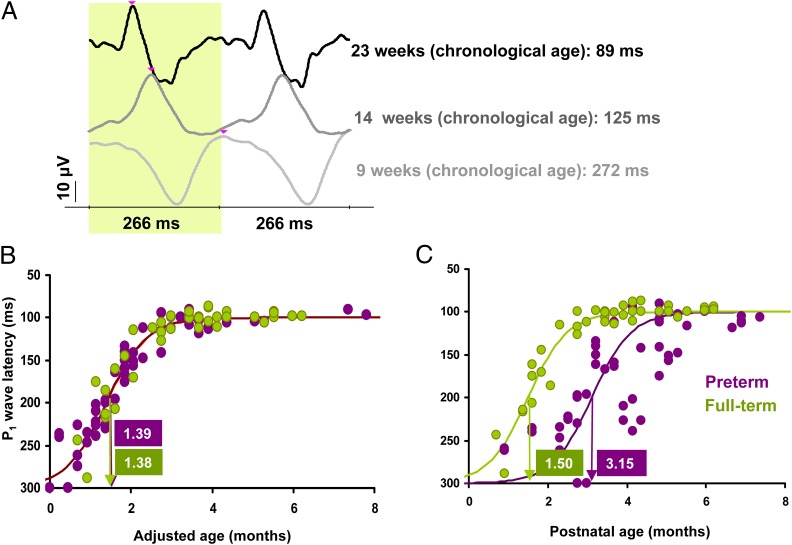
To assess the integrity of the visual pathways, and as a control condition for the DRDC stimuli, we used standard checkerboard pattern reversal (PR) stimuli in the VEP protocol (8, 10, 11). The PR-VEP response has a major positive peak (P1) at 100 ms in adults and at around 300 ms in newborns. The maturation of PR-VEP latency is most probably driven by several ontogenetic factors affecting the visual system. The underlying changes mainly include myelination of the retinocortical pathways (14), and the development of the retina, the optic system, the retinocortical, and intracortical synaptic connections are also in progress (15). Fig. 3A presents typical PR-VEP responses of our preterm and full-term subjects of different ages. It is known that for large check sizes, such as used in our study (120 min of arc), maturation of P1 is most rapid at around 6 wk of age and reaches maturity at around 5 mo (14).
Fig. 3.
Results of the PR-VEP experiment. (A) Representative averaged PR evoked VEPs. The latency of the P1 wave (marked with pink arrows) is substantially longer in younger infants. (B) Distribution of P1 wave latencies as a function of adjusted age. Dots represent individuals measured at different ages, with a logistic curve fitting (see SI Text for curve-fitting details). The greatest change in development (steepest part of the curve) occurs at the same age in preterm (violet coloring) and full-term (green coloring) infants. (C) Data represented on a PNA scale: the maturation of preterm infants is delayed. This pattern of results clearly indicates preprogrammed development of the PR-VEP response.
Results
Onset ages of binocular function based on DRDC-VEP responses are shown in Fig. 2 B and C. On the adjusted age scale, the two developmental curves are nonoverlapping. Onset time is 1.99 mo for preterm and 3.50 for full-term infants [Student t(28) = 4.46, P < 0.001; Kolmogorov–Smirnov (KS) test: P = 0.0011; see SI Text for modeling the data, statistical analysis, and detailed statistics]. As expressed in PNA, preterm and full-term groups have overlapping developmental functions, and onset times are 4.07 mo for preterm and 3.78 mo for full-term infants [Student t(28) = 0.578, P = 0.57; KS test: P = 0.5886, not significant]. This pattern of results clearly indicates that the developmental timing of the onset of the DRDC-VEP response is experience-dependent and not preprogrammed in the tested age ranges. Preterm infants make almost full use of the extra stimulation time, and the evoked response to binocular correlation appears at around the same time after birth as in full-term infants.
Maturational curves for P1 latency in the PR-VEP response are shown in Fig. 3 B and C. The results are in agreement with the literature, and clearly demonstrate that VEP latencies are independent of visual experience. On the adjusted age scale, the two curves fully overlap, and the most rapid change occurs at 1.52 mo for preterm and at 1.50 mo for full-term infants [F(80)=0.0159; P = 0.9, not significant; see SI Text for detailed statistics]. On the PNA scale, preterm and full-term groups have nonoverlapping curves, with the most rapid change at 3.40 mo for preterm and at 1.62 mo for full-term infants [F(80)=56.1; P < 0.0001; see SI Text for detailed statistics], with a 1.78-mo difference between groups, which corresponds to the mean gestational age (GA) difference (1.79 mo) between our preterm and full-term groups. This pattern of results is exactly the opposite of the one for DRDC-VEP and indicates that P1 latency in the PR-VEP response is not determined by experience; it is fully preprogrammed. The timing of cell maturation and myelination of the visual pathways, as indicated by this response latency, is not advanced by the extra stimulation time in preterm infants.
Discussion
We demonstrated that two indicators of normal visual development present widely different patterns in response to extra stimulation time in human infants born approximately 2 mo before term. VEP response latency to checkerboard PRs is not affected by the extra time, demonstrating that the maturation of the visual pathway follows a preprogrammed course. Despite the immaturity of the visual pathway, binocular function, involving cortical processing, seems to be open for experience-dependent changes right after birth even in premature infants.
Past work on the visual development of preterm infants (15–18) has not demonstrated experience-dependent cortical development so clearly. The lack of conclusiveness in earlier studies is mainly attributable to the less abrupt onset of the previously studied mechanisms, which makes conclusions more difficult to be drawn, although indications that development is affected by variations in visual experience exist (19). With respect to the development of binocular function in other vertebrates, experience dependency is rarely tested under normal stimulation circumstances, such as in our study. Preterm studies are not reasonable to consider in those species that are born with closed eyes and have a relatively short gestational period (e.g., ferrets, cats). The most commonly used experimental manipulations, mimicking naturally occurring human clinical conditions, are dark rearing, monocular form deprivation (by limiting the view of one eye), or induced misalignment of the two eyes. These usually lead to reversible reorganization of cortical ocular dominance columns in the critical period (1–6). It is assumed that the numerous molecular mechanisms uncovered with the above manipulations reveal processes that normally establish (modify and stabilize) synaptic connections in the visual cortex (2, 3, 5, 6). Although ocular dominance columns may not directly be linked to binocular function (13), column formation generally precedes the onset of the critical period and stereopsis in those species where the columns exist (2, 3, 5, 6). Both classic and modern studies support the view that neural activity driven by visual experience is essential for transforming the early rudimentary cortical connectivity patterns into a mature network in all of the studied vertebrate species (6). The development of human ocular dominance column formation is not known; however, our results indicate that the mechanisms turning on the critical period and stereopsis are flexibly timed by external stimulation.
It is remarkable that the available 2 mo of extra stimulation in preterm human infants lead to a clear advantage in cortical detection of binocular correlation. Despite the immaturity of the visual pathways, which is demonstrated by the stimulation independent P1 latency in our study, the visual cortex is ready to accept environmental stimulation right after birth. The results suggest that the developmental processes preceding the onset of binocular function are not preprogrammed and that the mechanisms turning on stereopsis are experience-dependent in humans.
Methods
Subjects.
Age terminology was used according to the recommendation of the American Academy of Pediatrics (20) (SI Text). Fifteen healthy full-term (mean birth age, 39.07 ±1.33 wk; range, 37–40 wk; mean birth weight, 3,435 ± 494 g) and 15 healthy preterm (mean birth age, 31.27 ±3.03 wk; range, 27–34 wk; mean birth weight, 1,752 ± 683 g) infants were involved in our study. Preterm and full-term infants were recruited by mailings to new parents residing in the city of Pécs (Hungary) or in nearby settlements. Parents were fully informed about the nature of the study and signed a consent form before the experiments. Infants were tested at the University of Pécs Medical School. See inclusion criteria and follow-up of the subjects in SI Text.
DRDC.
DRDCs were generated on a standard personal computer and presented on the red and green channels of a 19-inch cathode ray tube computer monitor (Samsung Model 957 MB) with 320 × 240 pixels spatial and 60-Hz temporal resolution. For dichoptic viewing, R26 low-pass (red) and YG09 band-pass (green) gelatin filters (Tobias Optic) were used. Correlated images consisted of 50% dark (black) and 50% bright (yellow) random dots; these images look identical either through the red or the green filter, respectively. Anticorrelated images contained 50% red and 50% green dots; each dark dot through the red filter corresponded to a bright dot through the green filter and vice versa. Images containing different random dot patterns were updated at 30 Hz and synchronized to the monitor refresh cycle. The alternation rate of the two phases (i.e., stimulus frequency) was 1.875 Hz. One dot in the image subtended 15 min of arc, the luminance of the bright dots through the filters was 5.85 ± 0.33 cd/m2, and the contrast was about 80%. Subjects with functional binocularity perceived a 1.875 Hz pulsation; in case of monocular viewing or without binocularity, only a 30 Hz noise was visible (11). For a more detailed description of stimulus generation, filter description, and monitor calibration procedure, see Markó et al. (11).
Checkerboard PR.
PR-VEPs were recorded as a control condition for the DRDC-VEP, mostly to see whether the DRDC stimulation frequency could be followed by the visual system of the infant. Check size was 120 min of arc, and stimulation frequency was 1.875 Hz. This frequency was identical to the one used during DRDC stimulation. The contrast was 95%, and the luminance of the white checks was 106 ± 5.04 cd/m2.
Experimental Procedure.
The PNA of infants at the first session was 10.73± 1.47 and 11.66± 1.09 wk for the full terms and preterms, respectively. Infants were tested repeatedly, normally once in every month (average repetition rate was: 4.92 ± 0.52 wk). Measurements were continued until the appearance of the DRDC-VEP response or until the PR-VEP P1 latency was near 100 ms. Infants were placed in a comfortable child seat or on the lap of their parents, at 0.5 m from a 19-inch cathode-ray tube monitor. The screen, which was the only light source in the darkened room, subtended about 30° × 40° within the visual field of the infants. To attract and maintain attention, a steady transparent monocularly visible image (e.g., a smiling sun) served as a fixation object at the center of the screen. Sound-making toys were also used to keep the attention of the infant as long as possible. Data acquisition was suspended during agitated or inattentive behavioral phases. PR-VEP recording preceded DRDC-VEP recording. Each DRDC-VEP recording block lasted at least for 70–100s, or up to the limit of the cooperation of the infants. Each combined PR- and DRDC-VEP session was usually shorter than 10–15 min. In those cases (5% of all sessions) where sleep or the refusal of wearing goggles prevented the completion of measurements, testing was repeated a few days later.
Recording and Data Analysis.
Gold-plated electrodes were placed at Oz-Fz position according to the 10–20 system, with a ground at Cz. EEG signals were amplified, band-pass filtered between 0.5–250 Hz, and sampled at 960 Hz. Signals were collected and processed with a Power1401 (Cambridge Electronic Design) data-acquisition device. Off-line analysis was started with artifact rejection. For DRDC-VEPs, records were subdivided into 2.133-s nonoverlapping epochs and then fast-Fourier-transformed, and the Fourier components of the first, second, and fourth harmonics of the stimulus frequency were used for size-dependent artifact rejection. Vectors greater than 30 μV were considered as artifacts. Signal reliability and detection of cortical binocularity was based on the T2circ statistics (20) carried out on the second harmonics, with significance defined at P < 0.01. T2circ is a statistical method designed to analyze periodic events in the recorded electrical signals. T2circ values are a useful measure of response reliability: higher values represent more reliability (i.e., clearer correlation between signal and response). Failure of T2circ to find statistical significance indicates that binocular visual stimulation is not correlated with brain activity. A more detailed description of the statistical method can be found in the report by Markó et al. (11). Onset age of cortical binocularity was defined as the mean age between the last testing day without and the first testing day with the DRDC-VEP response. We carried out a similar analysis for PR-VEPs; however, 1.066-s epochs were used and reversal frequency was considered as fundamental frequency. Signal reliability test provided by T2circ was followed by a manual determination of the P1 wave latency. Data not passing the T2circ reliability test were excluded from further analysis.
Data modeling, statistical analysis, and detailed data analysis are provided in SI Text.
Supplementary Material
Acknowledgments
The idea of testing preterm human infants in the DRDC-VEP protocol came from the late Bela Julesz, the inventor of random-dot stereograms. Julesz and colleagues (7–9) initiated the DRDC-VEP protocol in adults and infants in the 1980s; however, the preterm study remained a plan until now. This research was supported by National Science Foundation, Hungary Grant OTKA (Hungarian National Science Funds) NF60806 and Social Renewal Program, Hungary Grant TÁMOP-4.2.2/08/1/KMR.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203096109/-/DCSupplemental.
References
- 1.Crowley JC, Katz LC. Early development of ocular dominance columns. Science. 2000;290:1321–1324. doi: 10.1126/science.290.5495.1321. [DOI] [PubMed] [Google Scholar]
- 2.Sur M, Rubenstein JLR. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 3.Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 5.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 6.Sengpiel F, Kind PC. The role of activity in development of the visual system. Curr Biol. 2002;12:R818–R826. doi: 10.1016/s0960-9822(02)01318-0. [DOI] [PubMed] [Google Scholar]
- 7.Julesz B, Kropfl W, Petrig B. Large evoked potentials to dynamic random-dot correlograms and stereograms permit quick determination of stereopsis. Proc Natl Acad Sci USA. 1980;77:2348–2351. doi: 10.1073/pnas.77.4.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braddick O, et al. Cortical binocularity in infants. Nature. 1980;288:363–365. doi: 10.1038/288363a0. [DOI] [PubMed] [Google Scholar]
- 9.Petrig B, Julesz B, Kropfl W, Baumgartner G, Anliker M. Development of stereopsis and cortical binocularity in human infants: Electrophysiological evidence. Science. 1981;213:1402–1405. doi: 10.1126/science.7268443. [DOI] [PubMed] [Google Scholar]
- 10.Birch E, Petrig B. FPL and VEP measures of fusion, stereopsis and stereoacuity in normal infants. Vision Res. 1996;36:1321–1327. doi: 10.1016/0042-6989(95)00183-2. [DOI] [PubMed] [Google Scholar]
- 11.Markó K, et al. Contrast independence of dynamic random dot correlogram evoked VEP amplitude. J Vis. 2009;9:8.1–10. doi: 10.1167/9.4.8. [DOI] [PubMed] [Google Scholar]
- 12.Odom JV, et al. DL ISCEV standard for clinical visual evoked potentials (2009 update) Doc Ophthalmol. 2010;120:111–119. doi: 10.1007/s10633-009-9195-4. [DOI] [PubMed] [Google Scholar]
- 13.Cumming BG, Parker AJ. Responses of primary visual cortical neurons to binocular disparity without depth perception. Nature. 1997;389:280–283. doi: 10.1038/38487. [DOI] [PubMed] [Google Scholar]
- 14.McCulloch DL, Orbach H, Skarf B. Maturation of the pattern-reversal VEP in human infants: A theoretical framework. Vision Res. 1999;39:3673–3680. doi: 10.1016/s0042-6989(99)00091-7. [DOI] [PubMed] [Google Scholar]
- 15.Weinacht S, Kind C, Mönting JS, Gottlob I. Visual development in preterm and full-term infants: A prospective masked study. Invest Ophthalmol Vis Sci. 1999;40:346–353. [PubMed] [Google Scholar]
- 16.Mirabella G, Kjaer PK, Norcia AM, Good WV, Madan A. Visual development in very low birth weight infants. Pediatr Res. 2006;60:435–439. doi: 10.1203/01.pdr.0000238249.44088.2c. [DOI] [PubMed] [Google Scholar]
- 17.Ricci D, et al. Visual function at 35 and 40 weeks’ postmenstrual age in low-risk preterm infants. Pediatrics. 2008;122:e1193–e1198. doi: 10.1542/peds.2008-1888. [DOI] [PubMed] [Google Scholar]
- 18.Bosworth RG, Dobkins KR. Chromatic and luminance contrast sensitivity in fullterm and preterm infants. J Vis. 2009;9:15, 1–16. doi: 10.1167/9.13.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyler CW, Norcia AM. In: Adaptive Processes in Visual and Oculomotor Systems. Keller EL, Zee D, editors. Oxford: Pergamon Press; 1986. pp. 95–100. [Google Scholar]
- 20.Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalogr Clin Neurophysiol. 1991;78:378–388. doi: 10.1016/0013-4694(91)90099-p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.