Abstract
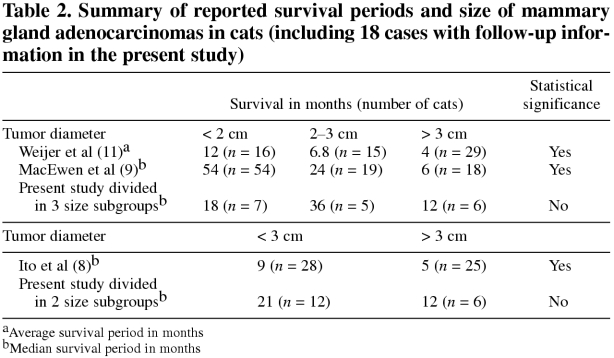
Mammary carcinomas and adenocarcinomas (MACs) are relatively common tumors in cats. The postexcisional survival period of affected cats is inversely proportional to tumor size, but the reported median survival periods for different tumor size categories is quite variable. This variability diminishes the prognostic value of reported data. In our study, cats with MACs greater than 3 cm in diameter had a 12-month median survival period, whereas those with MACs less than 3 cm in diameter had a 21-month survival period. Survival periods for cats with MACs smaller than 3 cm ranged from 3 to 54 months; therefore, tumor size alone is of limited prognostic value in cats with MACs smaller than 3 cm in diameter. In cats with MACs larger than 3 cm in diameter, tumor size appears to have much higher prognostic relevance, because this study, as well as others, have indicated that cats with MACs greater than 3 cm in diameter have a poor prognosis, with median survival periods ranging from 4 to 12 months.
Introduction
Feline mammary gland tumors rank third in frequency, following lymphoid and cutaneous neoplasms (1,2,3,4). Most feline mammary tumors have a malignant appearance on microscopic examination, and a large proportion are life-threatening even after complete excision (1,2,5,6,7). A statistically significant correlation between tumor size and postoperative survival period of cats has been a common finding (8,9,10,11). The postexcisional survival period of cats with mammary carcinomas and adenocarcinomas (MACs) greater than 3 cm in diameter has been reported to be 6 mo or less (8,9,11). Some retrospective studies found that the postexcisional survival period of cats with MACs less than 3 cm in diameter was 12 mo or less (8,11), but MacEwen et al (9) reported that cats diagnosed with MACs smaller than 2 cm survived for 54 mo and those with MACs 2 to 3 cm in diameter survived for 24 mo.
Human and veterinary pathologists are constantly faced with determining prognoses for patients with different types of tumors. Contrary to the situation in human medicine, there are limited retrospective studies in veterinary medicine that have investigated prognosis and survival period in animals with tumors. In addition, these few studies and review papers have sometimes generated inconsistent results, as exemplified by the variable lengths of survival period for cats with MACs of various sizes. Consequently, veterinary pathologists are faced with the relevance of the size of feline MACs as a prognostic factor. The purpose of this study was to determine the survival period of cats in western Canada with histologically diagnosed and surgically excised MACs and to determine if the size of feline MACs could be used as a reliable prognostic factor.
Materials and methods
Data collection
Biopsy reports of 59 cats diagnosed with proliferative disorders of mammary glands by the Department of Veterinary Pathology and Prairie Diagnostic Services between 1989 and 1999 were reviewed. The histologic diagnoses were as follows: 13 fibroadenomatous hyperplasias, 9 mammary adenomas, and 37 MACs. Survey forms were sent to veterinary clinics that submitted MACs to acquire the following information: i) tumor location, size, and gross appearance (solitary vs. multiple, well-circumscribed vs. invasive/non-movable); ii) treatment; and iii) duration before excision, postexcisional behavior, and eventual outcome. If tumor size was not indicated in the survey forms or clinical histories, the size of the formalin-fixed tumor was used, as recorded in the pathology gross descriptions.
Terminology
Survival period was defined as the time from first detection of the tumor to either the time of death or the date on which the cat was last known to be alive. Postexcisional survival period was defined as the time from excision of the tumor to either the time of death or the date on which the cat was last known to be alive. Tumor-free interval was defined as the time from excision of the tumor to the date on which the tumor first recurred, the date of death, or the date on which the absence of recurrence was confirmed, if the cat was still alive.
Statistical analysis
Statistical analysis of collected data was performed using statistical software (GraphPad Prism 3.00; GraphPad Software, San Diego, California, USA). The Kaplan-Meier product limit method was used to generate survival and tumor-free interval curves (12). Death or euthanasia due to tumor related problems (repeated local recurrence, distant and local metastasis) or progressively deteriorating health status of unknown cause was considered an endpoint. To be conservative, death or euthanasia, for reasons unknown or not indicated in the survey form, were also considered as endpoints. Only cats that were still alive and cats that were euthanized for problems unrelated to the tumor were included in this study as censored data. A Kaplan-Meier log-rank test was used to evaluate the relationship between survival period and variables tested (diameter of tumor, age, sex, and breed of affected cats). In addition, linear correlation coefficients (R) and 95% confidence intervals were calculated for the same variables and survival periods by using Pearson's correlation test.
Results
Clinical findings
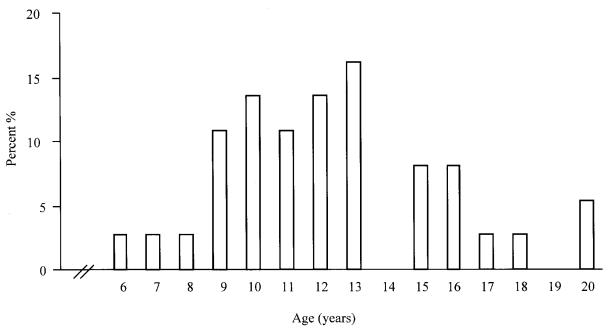
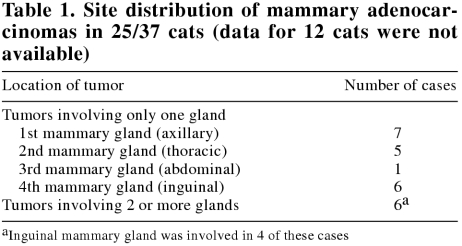
The mean age of 37 cats at first detection of MACs was 12.3, s = 3.3 y, with an increased prevalence among 9- to 13-year-old cats (Figure 1). Of the 37 cats, 22 (59%) were domestic shorthair, 4 (11%) were domestic longhair, 4 (11%) were Siamese, and 2 (5%) were mixed-breed; breeds of the 5 (14%) remaining cats were not available. At the time of the surgical excision of the MACs, 28 (76%) cats were spayed females, 7 (19%) were intact females, and 2 (5%) were castrated males. The date of ovariohysterectomy was available for only 9 (32%) cats; mean and median age at time of ovariohysterectomy was 2.3, s = 2.2 y and 1 y, respectively. Feline MACs were solitary in 18 (49%) cases, and multiple (presented simultaneously with 2 or more lumps) in 13 (35%) cases; information was unavailable for the remaining 6 (16%) cases. Information on tumor size and gross evidence of invasion were available for 29 and 12 cases, respectively. The mean diameter of submitted tumors was 2.3, s = 1.4 cm. On gross examination at the time of tumor excision, 8 were well-circumscribed, 3 were infiltrative, and 1 had metastasized to the local lymph node. Site distribution of the MACs is presented in Table 1.
Figure 1. Age distribution of 37 cats at first detection of mammary adenocarcinoma.
Table 1.
Postexcisional behavior
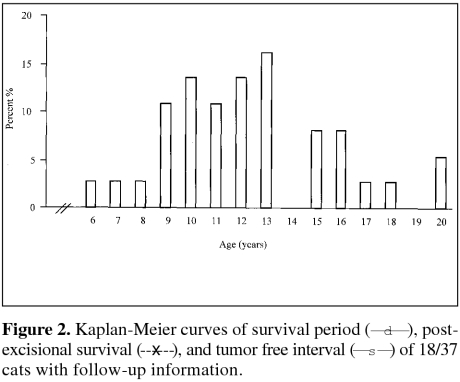
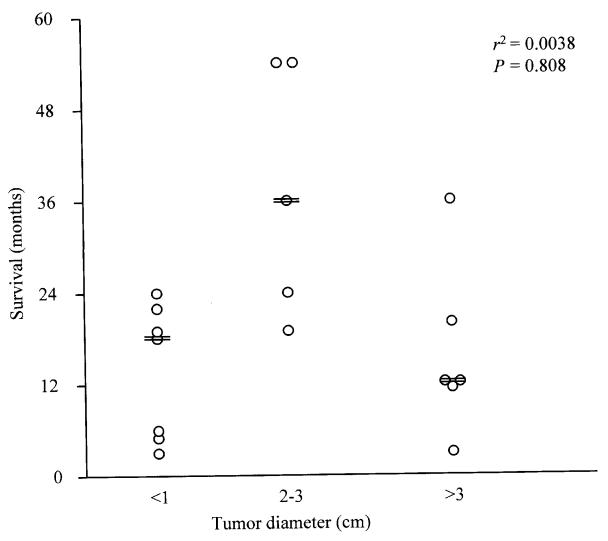
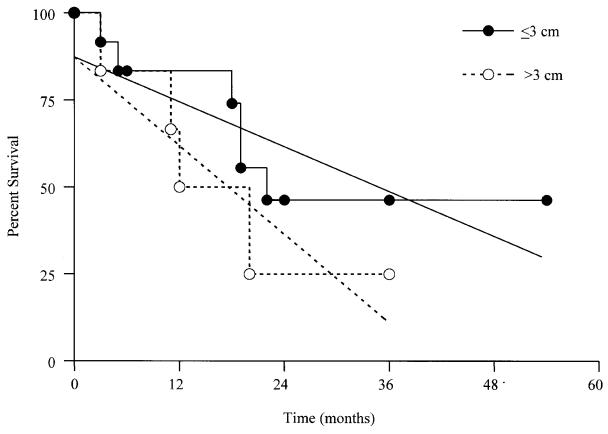
Postoperative follow-up information was available for 18 (48.6%) cats. Survival period, postexcisional survival 1time, and tumor-free interval of cats diagnosed with MACs are presented in Figure 2. The time interval between appearance of the tumor and excision ranged from 1 wk to 3 y, with 94% of the tumors being diagnosed and excised within 1 y of appearance (based on information available for 35 cases). Median survival periods of cats grouped according to tumor size are presented in Table 2 and Figure 3. A statistically significant correlation was not found between i) tumor size and survival period (Figures 3 and 4), ii) age of cat and survival period, iii) sex of cat (sexually intact vs. neutered) and survival period, and iv) breed of cat and survival period (data not shown for ii, iii and iv), analyzed by the Kaplan-Meier logrank lifetest and by Pearson's correlation test.
Figure 2.
Table 2.
Figure 3. Correlation between survival and diameter of excised mammary adenocarcinomas in 18/37 cats with follow-up information (r2 = 0.0038; P = 0.808; == median survival of size category).
Figure 4. Kaplan-Meier curves and their calculated linear slopes of survival period for 18/37 cats subgrouped by diameter of excised mammary adenocarcinomas (P = 0.0706).
Eventual outcome
Eight out of 18 (44%) cats (mean age, 13.3, s = 4.5 y) died or were euthanized due to tumor related problems (e.g. distant or local metastasis, repeated local recurrences or poor health due to unknown cause); median survival period of these cats was 15 mo (range, 3 to 22 mo). Four (22%) of 18 cats (mean age, 15.2, s = 4.1 y) were euthanized or died from indeterminate/unknown causes; these cats had a median survival period of 12.5 mo (range, 5 to 36 mo). Two (11%) of 18 cats (mean age, 18.3, s = 1.1 y) had a 4.5-year survival period and they were euthanized for tumor unrelated problems. In 1 cat, tumors recurred at 4 and 12 mo after the initial excision; contact with this patient was subsequently lost. The remaining 3 (17%) cats (mean age, 15.8, s = 3.3 y) were alive and healthy at the time of survey; their median survival period was 24 mo (range, 24 to 36 mo).
Discussion
A statistically significant correlation was not found between survival period of cats with excised MACs and any of the following variables: i) diameter of tumor (P = 0.07), ii) age, iii) breed, and iv) sex in this retrospective study. The lack of a statistically significant correlation between tumor size and survival period contrasts with previous reports (8,9,10,11) and remains unexplained. It may be due to a comparatively low number of cases with follow-up information in the present study (Table 2). A less than desirable number of cases with proper follow-up information has been one of the major obstacles to development of evidence-based medicine in the veterinary field; this problem might be overcome by publishing smaller case studies. Another possible reason for the lack of convincing statistical difference in survival between cats with “large” and “small” tumors was the wide range of survival periods for cats with MACs smaller than 3 cm in our study (Figure 3). This wide range of survival may have been affected by cultural differences influencing detection and treatment of tumors and timing of euthanasia, geographical differences in the feline population, or differences in diagnostic criteria of malignancy amongst pathologists.
Despite these shortcomings, this study suggests that tumor size for “small” MACs (less than 3 cm in diameter) cannot be used as a reliable prognostic factor due to the wide range in survival periods (3 to 54 mo). Although this conclusion is based on a comparatively low number of cases, this wide range of survival periods is supported by variable survival periods in previously reported studies; specifically, median and mean survival periods for cats with MACs smaller than 3 cm varied from 6.8 to 54 mo (8,9,10,11) (Table 2). Accordingly, tumor size has limited prognostic value for MACs smaller than 3 cm in diameter and additional prognostic factors are needed to differentiate behaviorally “good” tumors from “bad” tumors. Clinical stage of feline MACs (8) and some of their anaplastic features (10,11) have been reported to be useful prognostic indicators. Detection of dysfunction of “gatekeeper” and “caretaker” genes regulating growth and genetic stability of tumors might prove to be valuable prognostic indicators for feline mammary tumors. In addition, demonstration of loss or attenuation of intercellular attachment (13), as well as an increased expression of laminin-binding integrins (14), proteinases (15), proteinase activators and inhibitors (16), as well as estrogen and progesterone receptors (17), might be useful in predicting the life-threatening potential of “small” feline MACs.
Tumor size appears to have a much higher prognostic relevance in MACs greater than 3 cm in diameter. Survival periods of cats with MACs greater than 3 cm in diameter was shorter than the survival period of cats with MAC smaller than 3 cm in all studies (8,9,10,11), including the present study (Table 2), even though this difference was not statistically significant (P = 0.07) in the present study (Figure 4). Median and mean survivals of cats with “large” MACs had a relatively narrow range (from 4 to 12 mo) in all studies (8,9,10,11), including the present study. Since cumulative mutations are required for a malignancy to develop from a single transformed neoplastic cell (18,19), this multistep tumor progression is directly related to the number of proliferating cells with random mutational potential and their genetic instability. Therefore, the larger the proliferating mass, the greater the life-threatening risk. Accordingly, prognosis for MACs with a diameter greater than 3 cm appears to be consistently poor.
This study supports the previously reported lack of correlation between survival period of cats with their age, breed, sex, or type of surgical excision of the tumor (8,9). The age of affected cats, gross appearance of the tumors, as well as prevalence of benign versus malignant mammary tumors in this study were similar to those in previous reports (1,5,6,7,8,9,11,20,21,22,23). The present study supports the previously reported higher prevalence of adenocarcinomas in cranial (axillary) and caudal (inguinal) mammary glands (5,11,24). However, some authors have reported that cranial glands (22) are more commonly affected by tumors, whereas others have found caudal glands to be involved more often (10).
In conclusion, this and previously published studies indicate that cats with MACs greater than 3 cm in diameter have a poor prognosis with median survival periods ranging from 4 to 12 mo. Tumor size alone is of limited prognostic value in cats with MACs smaller than 3 cm in diameter, due to the wide range of median survival periods (6.8–54 mo), so additional prognostic factors are needed to reliably prognosticate survival of cats with MACs less than 3 cm in diameter.
Footnotes
Acknowledgments
We thank Dr. Moira E. Kerr and Brenda Trask for their constructive criticism and help. In addition, the authors thank all owners of cats and veterinary clinics for their help with data collection, namely: All West Veterinary Clinic, Saskatchewan (SK); Aspen Veterinary Services Ltd., SK; Broadway Veterinary Clinic, SK; Central Animal Hospital, SK; Crestwood Veterinary Clinic, Alberta (AB); Kamsack Veterinary Clinic, SK; Lakeview Veterinary Clinic, SK; Lloydminster Animal Hospital, SK; Marquis Road Veterinary Medical Center Ltd., SK; Martensville Veterinary Clinic, SK; Poplar Grove Veterinary Services, AB; Sidney Veterinary Services, British Columbia (BC); Society for the Protection Against Cruelty to Animals, Saskatoon, SK; Terwillegar Veterinary Clinic, AB; The Clinic For Cats, BC; Westward Animal Hospital, SK. CVJ
This study was partly funded by an National Science and Engineering Research Council Undergraduate Research Award, an MRC/Burroughs Welcome Funds Student Research Award, and an Interprovincial Undergraduate Student Research Award.
Address correspondence and reprint requests to Dr. Elemir Simko.
References
- 1.Misdorp W, Else RW, Hellmen E, Lipscomb TP. Histological classification of mammary tumors of the dog and the cat. vol. 7. Washington, DC: Armed Forces Institute of Pathology, American Registry of Pathology, 1999:11–15.
- 2.Moulton JE. Mammary tumors of the cat. In: Moulton JE, ed. Tumors in Domestic Animals. 3rd ed. Berkeley: University of California Press, 1990:547–552.
- 3.Dorn CR, Taylor DO, Frye FL, Hibbard HH. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. I. Methodology and description of cases. J Natl Cancer Inst 1968;40: 295–305. [PubMed]
- 4.Schmidt RE, Langham RF. A survey of feline neoplasms. J Am Vet Med Assoc 1967;151:1325–1328.
- 5.Hahn KA, Adams WH. Feline mammary neoplasia: biological behavior, diagnosis, and treatment alternatives. Feline Pract 1997;25(2):5–11.
- 6.Hayes AA, Mooney S. Feline mammary tumors. Vet Clin North Am Small Anim Pract 1985;15:513–520. [DOI] [PubMed]
- 7.Engle GC. Mammary gland neoplasia in the cat: prognosis and treatment. Feline Pract 1973;3(5):9–12.
- 8.Ito T, Kadosawa T, Mochizuki M, Matsunaga S, Nishimura R, Sasaki N. Prognosis of malignant mammary tumor in 53 cats. J Vet Med Sci 1996;58:723–726. [DOI] [PubMed]
- 9.MacEwen EG, Hayes AA, Harvey HJ, Patnaik AK, Mooney S, Passe S. Prognostic factors for feline mammary tumours. J Am Vet Med Assoc 1984;185:201–204. [PubMed]
- 10.Weijer K, Hart AAM. Prognostic factors in feline mammary carcinoma. J Natl Cancer Inst 1983;70:709–716. [PubMed]
- 11.Weijer K, Head KW, Misdorp W, Hampe JF. Feline malignant mammary tumors. I. Morphology and biology: some comparisons with human and canine mammary carcinomas. J Natl Cancer Inst 1972;49:1697–1704. [DOI] [PubMed]
- 12.Kaplan EL, Meir P. Non-parametric estimation from incomplete observation. J Am Stat Assoc 1958;53:457–481.
- 13.Jiang WG. E-cadherin and its associated protein catenins, cancer invasion and metastasis. Br J Surg 1996;83:437–46. [DOI] [PubMed]
- 14.Ziober BL, Lin CS, Kramer RH. Laminin-binding integrins in tumor progression and metastasis. Semin Cancer Biol 1996;7: 119–28. [DOI] [PubMed]
- 15.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 1997;89:1260–70. [DOI] [PubMed]
- 16.Knoop A, Andreasen PA, Andersen JA, et al. Prognostic significance of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 in primary breast cancer. Br J Cancer 1998;77: 932–40. [DOI] [PMC free article] [PubMed]
- 17.Lohmann C, Gibney E, Cotsonis G, Lawson D, Cohen C. Progesterone receptor immunohistochemical quantitation compared with cytosolic assay: correlation with prognosis in breast cancer. Appl Immunohistochem Molecul Morphol 2001;9:49–53. [PubMed]
- 18.Cotran RS, Kumar V, Collins T. Neoplasia. In: Cotran RS, Kumar V, Collins T, eds. Robbins Pathologic Basis of Diseases. 6th ed. Philadelphia: WB Saunders, 1999:260–327.
- 19.Price JT, Bonovich MT, Kohn EC. The biochemistry of cancer dissemination. Crit Rev Biochem Mol Biol 1997;32:175–253. [DOI] [PubMed]
- 20.Brodey RS. Canine and feline neoplasia. Adv Vet Sci Comp Med 1970;14:309–54. [PubMed]
- 21.Hayes HM, Jr., Milne KL, Mandell CP. Epidemiological features of feline mammary carcinoma. Vet Rec 1981;108:476–479. [DOI] [PubMed]
- 22.Hayden DW, Nielsen SW. Feline mammary tumours. J Small Anim Pract 1971;12:687–98. [DOI] [PubMed]
- 23.Patnaik AK, Liu SK, Hurvitz AI, McClelland AJ. Nonhematopoietic neoplasms in cats. J Natl Cancer Inst 1975:855–860. [PubMed]
- 24.Morrison WB. Canine and feline mammary tumors. In: Morrison WB, ed. Cancer in Dogs and Cats: Medical and Surgical Management. Baltimore: Williams & Wilkins, 1998:591–598.