Abstract
Early acquisition of mate preferences or mate-preference learning is associated with signal diversity and speciation in a wide variety of animal species. However, the diversity of mechanisms of mate-preference learning across taxa remains poorly understood. Using the butterfly Bicyclus anynana we uncover a mechanism that can lead to directional sexual selection via mate-preference learning: a bias in learning enhanced ornamentation, which is independent of preexisting mating biases. Naïve females mated preferentially with wild-type males over males with enhanced wing ornamentation, but females briefly exposed to enhanced males mated significantly more often with enhanced males. In contrast, females exposed to males with reduced wing ornamentation did not learn to prefer drab males. Thus, we observe both a learned change of a preexisting mating bias, and a bias in ability to learn enhanced male ornaments over reduced ornaments. Our findings demonstrate that females are able to change their preferences in response to a single social event, and suggest a role for biased learning in the evolution of visual sexual ornamentation.
Keywords: reproductive isolation, female choice, sexual imprinting, oblique imprinting, nymphalid
The evolution of elaborate and diverse secondary sexual traits in males is often attributed to female preferences for enhanced ornamentation (1). However, evidence that sexual ornaments can be lost as well as gained demonstrates that females do not always prefer enhanced ornaments (2–4). The development of mate preferences based on social experience in juveniles (i.e., learned preferences), either through exposure to a parental phenotype (parental sexual imprinting) or by exposure to a nonparental adult phenotype [oblique imprinting (5)] may facilitate evolution of female preference for both ornament gains and losses (6–9). However, learned preferences have not been well studied as a driver for sexual ornamentation and speciation in insects, one of the most diverse animal taxa (10). This lack of studies is partially because in oblique imprinting, the form of imprinting that would apply to most insects, there is no genetic relationship between personal and learned phenotype (6, 10). However, studies of insect and spider mating systems over the last 10 y have shown that both males and females will change their mate selectivity in response to both positive and negative intersexual encounters (11–15). These preference shifts suggest that premating experience may play a role in reproductive isolation and ornament evolution in species without parental care. Here we demonstrate a mechanism by which mate-choice learning could drive the evolution of visual sexual ornaments in males: a directional bias in learning, which is independent of preexisting mate preferences, in the butterfly Bicyclus anynana (Lepidoptera: Nymphalidae, Satyrinae).
Butterfly species in the genus Bicyclus differ in the number of eyespots on the dorsal surface of their wings. This variation in patterning is hypothesized to have evolved under sexual selection based on existing male and female preferences for spot presence in the opposite sex in B. anynana (16, 17) and genus-wide sex-specific differences in rates of spot number evolution (18). Although it is currently unclear how interspecific wing-pattern variation relates to variation in female preferences within or between species, our findings suggest a possible role of mate-preference learning, including biases in learning, in the evolution of wing-pattern variation in this genus.
Results and Discussion
Premating Experience Alters Butterfly Mating Preferences.
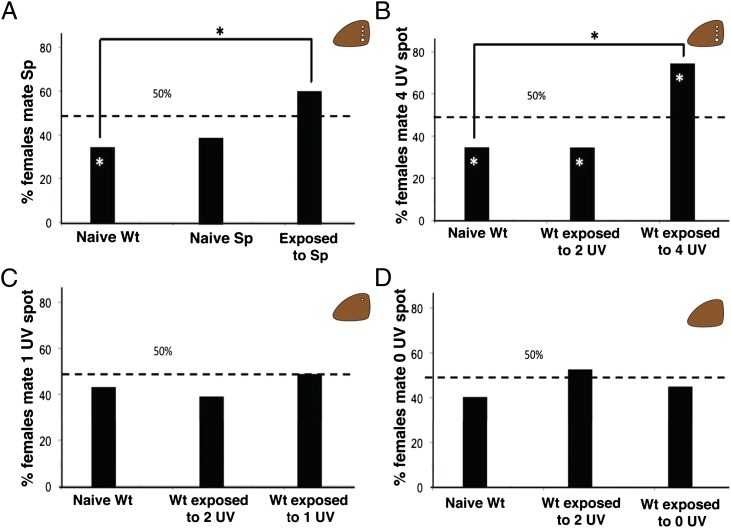
Female butterflies that are newly emerged and who have no prior adult social experience may have a preference for particular wing patterns in males. We will name this preference a preexisting mating bias. To determine whether learned mate preferences can co-occur with preexisting mating biases for male ornamentation (i.e., forewing dorsal eyespot number) in B. anynana, we first documented preexisting mating bias in females for male eyespot number and then tested whether premating exposure to a single mature conspecific male with different wing ornamentation affected mating outcome in triad mating trials. We conducted mate-choice trials using two phenotypically variant laboratory lines: (i) Wild type (Wt), a line possessing two eyespots on each forewing dorsal surface (Fig. 1A) like most wild-caught B. anynana, and (ii) Spotty (Sp), a single locus mutant that appeared spontaneously in laboratory populations, possessing four eyespots on each forewing dorsal surface (19) (Fig. 1B). Naïve Wt females mated preferentially with Wt over Sp males (χ2= 6.13, P = 0.013), demonstrating that females did not have a preexisting mating bias for signal enhancement (i.e., more spots). To assess whether females had preexisting mating bias for their own phenotype, we repeated the mating trials using naïve Sp females. Naïve Sp females did not exhibit a significant preference for either Wt or Sp males, suggesting that the preexisting mating bias of naïve Wt females for two- over four-spotted males was likely to be the result of a bias against increased number of spots instead of a bias for their own phenotype (Fig. 2A).
Fig. 1.
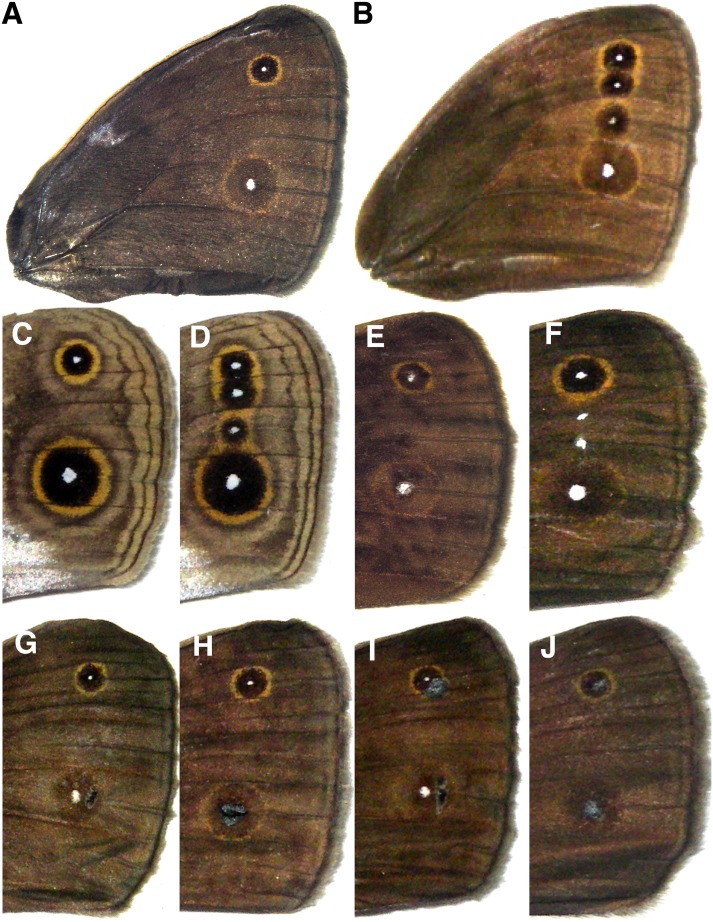
Forewing patterns of the butterflies used in mating trials. (A and C) Wt male. (B and D) Sp male. (E) Wt male with two painted UV-reflective white spots on top of the natural eyespot centers. (F) Wt male with two additional painted UV-reflective white spots. (G) Wt males with black paint next to one eyespot center. (H) Wt male with black paint over one eyespot center. (I) Wt male with black paint next to both eyespot centers. (J) Wt male with black paint over both eyespot centers. A, B, and E–J show dorsal surfaces; C and D show ventral surfaces.
Fig. 2.
Outcome of mate choice trials. (A) Females exposed to Sp males mated with Sp males more often than naïve Wt females; naïve Wt (n = 40), naïve Sp (n = 42), Wt exposed to Sp males (n = 75). (B) Female’s preexisting mating bias was reversed by exposure to males with enhanced ornamentation; naïve Wt (n = 25), Wt exposed to Wt males with two painted UV spots (n = 25), Wt exposed to Wt males with four painted UV spots (n = 25). (C) Females did not alter their preference after exposure to male with reduced ornamentation on dorsal forewing; naïve Wt (n = 25), Wt exposed to Wt males with one UV spot (n = 25), Wt exposed to Wt males with two UV spots (n = 26). (D) Females did not alter their preference after exposure to males with no ornamentation on dorsal forewing; naïve Wt (n = 25), Wt exposed to Wt males with two UV spots (n = 26), Wt exposed to Wt males with zero UV spots (n = 26). Asterisk represents statistically significant preference/significantly different mating patterns.
We then demonstrated that naïve female preexisting mating biases could be altered by early visual experience during a single, short (3 h), exposure to a male displaying the novel Sp phenotype. Wt females exposed to a single Sp male during the sexually unreceptive period immediately following emergence mated with Sp males in subsequent mate choice trials more frequently than did naïve Wt females (χ2 = 5.28, P = 0.021) (Fig. 2A). However, females did not exhibit a statistically significant preference for Sp males. This result is possibly because of confounding factors associated with the Sp mutation, such as additional spots on the ventral as well as dorsal surface of the forewing or a difference in sex pheromone chemical composition or concentration. To control for these possible pleiotropic effects, we repeated this experiment using Wt males modified by the addition of two UV-reflective spots of paint to the dorsal surface of each wing, so that they either had two UV-reflective spots per wing and resembled Wt (Fig. 1E) or four UV-reflective spots and resembled Sp (see Fig. S1 for reflectance spectra of modified UV spots). Therefore, the only difference between the males used in either the premating exposure period or the mating trial was the number of UV-reflective spots on the dorsal surface of the forewing (Fig. 1F). Females exposed to a single extraornamented (Sp-like) male mated preferentially with extraornamented males (χ2 = 7.95, P = 0.005), and females exposed to normal-ornamented (Wt-like) males mated preferentially with normal-ornamented males (χ2 = 5.01, P = 0.025) (Fig. 2B). Neither male courting nor male activity level during the premating exposure was correlated with mating outcome (Table S1), and all behaviors were rejected as significant parameters for a stepwise nominal logistic model of mating outcome (all effect test P values >0.1).
This shift in preference in response to a premating exposure to an adult male with enhanced wing ornamentation demonstrates that preexisting mating biases can be modified by premating experience in a butterfly. The brevity of the exposure period required to produce a change in mating outcome, coupled with the lack of a correlation between male courting behavior and female response to exposure, suggests that B. anynana is capable of a sexual imprinting-like behavior, in that females responded to the presence of the male and not to a specific male-female interaction that occurred during the training period (6–8, 20). Our findings differ from previous mate-preference learning studies in invertebrates, in which a stereotypic courting experience was necessary to produce an observable exposure effect (12–14, 21–23). Although the use of the triad mating assay introduces male competition as a potential confounding factor for our results, the only parameter that differed between the treatments was female experience, suggesting that the observed shift in mating outcome is the result of a shift in female behavior, not male-male competitive ability. This shift in mating outcome in response to premating social experience is a unique demonstration of a learned mate preference in Lepidoptera, and a unique demonstration of a modification of a preexisting mating bias by premating experience in an invertebrate, whereas similar modifications were also recently shown in a vertebrate species (24).
Lepidopterans are currently used as models for investigating genetic linkages between genes for trait preference and genes for trait production (25–28). The presence of a learned-mate preference in this group highlights the importance of considering rearing environment when designing experiments to test the genetic basis of behavioral traits. Previous studies examining genes associated with reproductive isolation in butterflies used either wild-caught adults or laboratory-reared animals from emergence cages containing both males and females (25–28). Therefore, although the observed mating preferences may be because of genetic linkage between genes for trait preference and genes for trait production, the alternative hypothesis of preference learning immediately following emergence cannot be ruled out.
Test of Biased Learning.
Mate-preference learning is predicted to homogenize populations and prevent trait evolution because of a high rate of exposure to (and consequent learned preferences for) the common phenotype (29). However, biased learning might counteract homogenization of preference and lead to trait evolution if individuals are biased toward learning specific categories of phenotypes, such as ornament enhancements (30, 31). We therefore tested for the presence of a bias in female mate-preference learning by exposing Wt females to Wt males manipulated to display reduced-ornamentation, mimicking the inferred ancestral B. anynana male phenotype [one dorsal UV-reflective forewing spot per wing (18)] (Fig. 1H), and assessing whether females learned preferences for this reduced-ornamentation phenotype as readily as they learned a preference for an enhanced-ornamentation phenotype.
When given a choice between males with the ancestral phenotype (one spot per forewing) and males with the extant phenotype (two spots per forewing), naïve Wt females mated randomly (χ2 = 0.360, P = 0.548) (Fig. 2C). In addition, there was no effect of exposure to either the B. anynana Wt phenotype (χ2 = 0.618, P = 0.433), or the ancestral phenotype (χ2 = 0.00, P = 1.00) on mating outcome, and male behavior during the exposure period did not influence mating outcome in either treatment (Table S1) [stepwise nominal logistic model with full factorial design rejected all behaviors as significant parameters (all effect test P values >0.1)]. A power analysis comparing effect sizes for learning increased vs. decreased visual signals is presented in Table S2.
We conclude that not all visual signals are learned equally by B. anynana (Table S2), and that females do not have a preexisting mating bias against deviations from the population average, because we did not observe a preexisting bias for two- over one-spotted males. The observed biased learning for enhanced male ornaments provides a possible mechanism for the preferential spread and fixation of enhanced phenotypes in a population without parental care, even when these phenotypes represent a minority of visual models in the population. Further study of a female’s response to premating exposure to multiple phenotypes simultaneously, or sequentially, will be necessary to determine the importance of biased learning in promoting assortative mating in a variety of social scenarios.
Possible causes for biased learning in B. anynana, where females learn to prefer four spots (over two) better than one spot (over two), could include: (i) a better perceptual ability to distinguish between four and two spots compared with one and two spots, or (ii) an easier learning of enhanced signals over reduced signals. To test these two hypotheses we conducted choice trials between normal males and modified males with zero spots (Fig. 1 I and J). By creating the same total difference in ornament number formerly permitting preference learning, we directly address the hypothesis that a difference of two spots is easier to learn than a difference of one. A failure of females to change their preference when exposed to nonornamented males would lend support to the hypothesis that signal enhancement is easier to learn than signal reduction in this species.
Naïve females mated randomly with zero- and two-spot males (χ2 = 1.007, P = 0.316). Females exposed to nonornamented (zero spot) males also mated randomly with either male type (χ2 = 0.361 P = 0.548) (Fig. 2D) (see Table S2 for power analysis). However, unlike females exposed to four- or one-spot males, the mating outcome of females exposed to zero-spot males was influenced by the training male’s behavior (females exposed to males who frequently displayed their dorsal wing surfaces during the training period learned to prefer the zero-spot males better) (Table S3). The asymmetric response of females to early exposure to males with zero, one, and four spots suggests that, although these sexual ornaments are both gained and lost in the Bicyclus lineage (18) (Fig. 3A), signal enhancement may be easier to learn than signal reduction for B. anynana.
Fig. 3.
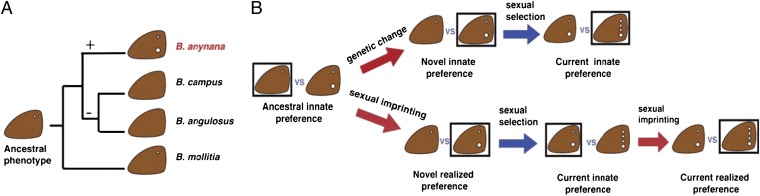
Hypothesized role of mate choice learning on the evolution of butterfly ornament diversity. (A) Ancestral wing pattern reconstruction for a clade of Bicyclus species including B. anynana showing where ornaments have been gained (+) and lost (−) in males of the species (based on ref. 25). (B) Ornament gain can be driven by a genetically determined preference for signal enhancement or a learned preference for signal enhancement (via oblique sexual imprinting). When ornament gains are driven by learned preferences, females should exhibit a genetically determined bias in learning ability toward enhanced signals instead of a genetically determined preference for signal enhancement, which is the case for B. anynana. Box signifies preference, red arrows signify change in preference, blue signify change in phenotype.
Our findings demonstrate that: (i) B. anynana females with no adult social experience have a preexisting mating bias against ornament enhancement, (ii) their preexisting mating bias can be changed by premating social experience, and (iii) they possess a bias in learning enhanced signals over reduced signals.
This biased learning in B. anynana differs from previous proposed mechanisms of mate-choice learning. B. anynana learning is not the result of associations of positive or negative experiences with particular signals, other than spot number itself, because trainer male behavior did not vary significantly between treatments (Table S4) (13, 21). This biased learning also does not reflect a simple sensory bias (32), as the preexisting mating bias and the learned preference operate in opposite directions. The observed bias in female response to premating experience also differs from that observed in Schizocosa spiders, in that B. anynana females shifted their mate choice only when exposed to enhanced males, whereas Schizocosa shifted mate choice to enhanced males regardless of the male phenotype they experienced (12). The shift in B. anynana is also independent of mate-choice copying (33), because all females were isolated from each other and unable to observe and copy the mate selection of other females at any point during the experiment. Our results, therefore, suggest that the B. anynana female response to premating experience is dependent on the male phenotype they experience, not the simple presence of a conspecific male or mate copying. Our findings therefore illustrate how mate preferences may arise from the interaction of an intrinsic capacity to learn with a range of extrinsic social stimuli.
Differences in signal conspicuousness or in signal weighting by experienced females could produce the observed bias in learning-enhanced ornamentation. Wings with white, UV spots may be more conspicuous and therefore easier to learn than wings without these spots. If this is the case, then mate-choice learning should be primarily associated with ornament enhancement in this group, and not observed in lineages that have recently undergone ornament losses. Alternatively, premating social experience could alter signal weighting. B. anynana, like many animals, uses both olfactory and visual signals to assess mate quality (16, 17, 34, 35). Sex pheromone production is variable in male B. anynana and changes with age (35, 36). Although naïve females appear to weigh presence or absence of visual and olfactory signals equally when males are matched in age (34), it is unknown whether this weighting can be changed by premating social experience. Both early exposure and shifts in relative signal conspicuousness are known to alter the relative weighting of olfactory and visual signals in mate preference in wolf spiders, damselflies, and fruitflies (13, 14, 37, 38). Exposure to a male with an enhanced visual signal could cause females to later overemphasize the visual component of male display relative to all other signals, but exposure to a male with fewer visual signals could cause females to later emphasize pheromones or other as yet unexplored signals. In this latter scenario the mating outcomes of female B. anynana would be reflecting preferences for other, nonvisual, traits instead of an inability of females to learn preferences for reduced signals.
The wing-pattern learning bias in B. anynana resembles song-learning biases in birds, where birds learn some songs (often conspecific) more readily, or instead of, other songs (often heterospecific) in their acoustic environment (39–43). Song-learning biases are suggested to be genetically determined perceptual biases that may facilitate species-specific oblique imprinting (41). Oblique imprinting has historically been undervalued in mate preference and sexual ornament evolution because of the lack of genetic connections between the phenotype of the learning individual and that of the model (5, 29), which could include individuals from other species. However, genetically determined perceptual biases that restrict the pool of suitable learning models to conspecifics or closely related individuals reduce the likelihood of an individual learning a preference for an inappropriate phenotype. Oblique imprinting of songs in birds, for example, produces very low error rates in mating with heterospecifics (10, 12–14, 21, 22, 44). Although the learning bias in B. anynana was observed using artificially modified conspecific individuals as models, the observed learning bias for additional ornaments would prevent B. anynana from forming mating preferences for close relatives that are living in sympatry, such as male Bicyclus campus and Bicyclus angulosus, with fewer dorsal forewing eyespots (18, 45) (Fig. 3A). Furthermore, oblique imprinting and subadult mate-preference learning may be particularly important in species where cross-generational changes in environment result in alternating phenotypes or seasonal polyphenisms. If individuals look different in each season, then learning a mate preference, rather than having a genetically determined mate preference, may allow successful mate recognition or mate selection in these species (46). Further research on mate-choice learning in species with and without seasonal polyphenisms is needed to determine the importance of mate-choice plasticity in maintaining this form of environmentally induced morphological plasticity.
Although biases in mate-preference learning have not yet been reported in species outside of B. anynana, they may have been overlooked because of the long training periods used in other mate-preference learning studies (13, 14, 47–49). Song-learning biases are especially apparent in laboratory studies when training periods or event number are reduced (reviewed in ref. 40). If wing-pattern learning biases operate similarly to song-learning biases we would expect learning biases to become apparent in more taxa as experimental training event number and duration are reduced. The possible role of mate-preference learning biases in reinforcing species boundaries is an exciting and largely unexplored area.
One additionally intriguing question is whether biases in mate-preference learning might also play a role in establishing species boundaries in the first place. If this bias toward learning-enhanced signals arose in an early lineage of Bicyclus, predating the current wing pattern, it is possible that the bias toward learning enhancement permitted the spread and fixation of the enhanced two-spot forewing B. anynana male wing pattern (Fig. 3B, Lower) from a one-spot ancestor. Further enhancement of male ornamentation may have not yet occurred in male B. anynana because of predation pressures against conspicuous wing patterns in male butterflies (50). The apparent ease with which sexual ornaments are gained and lost in this genus (18)—and the high diversity of sexual ornamentation in lepidopterans in general (51, 52)—raise the question of whether genetic variation in learning biases are associated with both gains and losses of sexual ornaments in this group, either as secondary reinforcing mechanisms or as causal drivers of divergence.
Variation in genetic perceptual biases—coupled with drift—are hypothesized to have contributed to the high diversity in oscine bird song (53). In Bicyclus, natural selection is thought to operate primarily on ventral wing surfaces, leaving dorsal wing surfaces available for elaboration by sexual selection (50, 54). This signal partitioning paired with biased signal learning could provide the substrate for the incredible signal diversification observed in some butterflies. However, further research across species is necessary to clarify the extent to which genetic variation in learning bias drives the evolution and diversification of sexual signals.
Conclusions
These findings demonstrate that a brief social interaction can alter preexisting mating preferences and highlight the potential for biased learning to drive assortative mating and potentially reproductive isolation in species without parental care. Future research on mate-choice learning should assess the role that multiple other signals (olfactory, gustatory, tactile, and so forth) play in visual signal learning, the prevalence of preference learning in taxa with seasonal polyphenisms compared with other taxa, and the role of biased learning in driving assortative mating and speciation.
Materials and Methods
Animal Culture.
B. anynana is a subtropical African butterfly that has been maintained in the laboratory since 1988. A colony was established in New Haven, CT from hundreds of eggs collected from a laboratory colony in Leiden, The Netherlands (originally established from 80 gravid females collected in Malawi in 1988). The species currently exhibits a sexually monomorphic wing pattern, in both natural and laboratory populations, with two forewing eyespots on the ventral and dorsal surfaces (Fig. 1 A and C), seven hindwing eyespots on the ventral surface, and zero to three hindwing eyespots on the dorsal surface. The white, UV-reflective scales at the center of the dorsal wing eyespots in males, but no other eyespot trait, have previously been demonstrated to be important in female mate selection (16, 17).
Although B. anynana is seasonally polyphenic in morphology and behavior (17, 55), all butterflies used in this study were reared in wet season conditions in a walk-in climate chamber at 27 °C, 80% humidity, and 12-h:12-h light:dark photoperiod to remove any effect of seasonal phenotype on mating outcome or learning ability. Larvae were fed on young corn plants and adults were fed on mashed banana. All behavioral assays (choice trials and training events) were conducted under sunlamps and in front of East-facing windows at 25–28 °C. Behavioral assays were conducted using cylindrical hanging net cages (30 cm diameter × 40 cm height). Butterflies eclosed in strain-specific (Wt or Sp) emergence cages and were removed and isolated before noon on the day of eclosion from pupa (day 1). The Sp line has been maintained in isolation in the laboratory since 1993, and the two lines have been maintained in the laboratory using similar mating regimes; that is, we perform egg collections every generation for each line from line-specific cages containing multiple adult individuals (> 50 individuals in both Sp and Wt lines). After emergence males were put in sex- and age-specific cages where they could not see females, and females were isolated from all other butterflies (males and females) until use in a training event or mating trial.
Behavioral Assays.
All experiments followed the same basic design. Virgin females were isolated from all other butterflies the morning of emergence (day 1) and either completely isolated for 3 d before preference trials (all naïve treatments) or exposed to a single virgin male of a given phenotype for 3 h the morning of emergence and then completely isolated until the preference trial on day 3 (all exposure treatments).
In all preference trials virgin females were given a choice on day 3 between two virgin males, matched in age and wing size, with no prior female experience. A randomly selected male of each phenotype being tested was introduced to the female’s cage. Males ranged between 2 and 6 d old. Female mating outcome was determined by dusting the females’ abdomen with orange “rodent-tracking” florescent dust that is only transferred to a male abdomen during copulation (56). Males were checked every morning, and the trial was ended when one male had orange dust on his abdomen, at which point his phenotype was recorded.
Female Preexisting Mating Bias for Spot Number.
We conducted mating trials using both Wt and Sp naïve females. We tested Wt female preexisting mating bias using Wt and Sp males (n = 40 females), and Sp female preexisting mating bias using Wt and Sp males (n = 42 females).
Preexisting Mating Bias Modified by Experience.
We exposed Wt females to Sp males and conducted preference trials using Wt and Sp males (n = 75 females).
Male Modification for Mating Trials.
Males were painted with either: (i) two spots of white, UV-reflective paint (Fish Vision white) (Fig. 1 E and F); (ii) one spot of black paint that did not reflect in the UV (Testors enamel glossy black 1147) (Fig. 1 G and H); or (iii) two spots of black paint (Fig. 1 I and J).
Quantification of Natural Eyespot Center Reflectance and White, UV-Painted Spots.
Using a bifurcated optical fiber connected to a spectrophotometer (Ocean Optics USB2000+XR1/Spectrasuite Software), we shined white light through one branch and collected the reflectance spectra through the other branch, from a constant area on the wing (less than 0.5 mm in diameter). n = 5 butterflies for natural UV spot (eyespot center), painted UV spot, and painted extra spot. n = 3 butterflies for brown scales. Each butterfly was measured three times.
Effect of Exposure to Increased Dorsal Forewing Spot Number.
Naïve, exposed to two UV, and exposed to four UV, Wt virgin females were used in choice trials between two and four painted UV spotted males. n = 25 females per treatment.
Test of Learning Bias.
Naïve, exposed to two UV, and exposed to one UV, Wt virgin females were used in choice trials between two- and one-spot modified Wt males n = 25 for naïve females and females exposed to one spot males, n = 26 for females exposed to control Wt males.
Test of Learning Signal Absence.
Naïve, exposed to two UV, and exposed to zero UV, Wt virgin females were used in choice trials between two- and zero-spot modified Wt males. n = 25 for naïve females, n = 26 for females exposed to ornament absent and control Wt males. Note that our results of a nonsignificant preference for zero-spot and two-spot males using naïve females are not significantly different from a previously reported naïve preference for two-spot males in the Robertson and Monteiro (16) study (between study χ2 = 0.466, P = 0.4947). The lack of significance in our preference trials (despite us observing the same trend) may be because of the different lighting environments used in the two studies and the fact that here we also controlled for variation in number of dorsal hindwing spots, maintaining them constant in each choice trial. Irrespective of this difference between studies, the present experiments, all done under the same laboratory conditions, support our present conclusions.
Effect of Male Behavior on Female Learning.
We recorded all bouts of male behavior during the training period for every trial. Males exhibited the following behaviors: (i) a stereotypic courting behavior (35), (ii) flights, (iii) wing flutters, (iv) walking on the cage, and (v) circling of females in flight. A principle components analysis of male behavior by male phenotype was performed, and the first principle component (PC1: primarily composed of roughly equivalent loadings of flights, flutters, and walks) and second principle component (PC2: primarily composed of roughly equivalent loadings of circling and courting) were used as representatives of male behavior (Table S5). We also conducted a stepwise nominal logistic model with full factorial design to detect any combination of male behaviors that may have influenced female mating outcome.
Statistical Analyses.
Mating outcomes between treatments were compared using a Pearson’s χ2 test, and treatment preferences were deemed significant if mating outcomes differed significantly from random mating (50:50). The relationship between PC1 and PC2 (Table S5) and mating outcome was assessed using logistic regression (Table S1). All statistical analyses were conducted in JMP 9.
Supplementary Material
Acknowledgments
We thank Mark Fisher, Mayank Lahoti, Andrew Goldman, and Taid Rahimi, whose early experiments on butterfly learning and mate choice helped us design the final experimental set-up described here; Jeffrey Oliver, William Piel, Natasha Kelly, Ashley Bear, and two anonymous reviewers for comments on the manuscript; Seng Fatt Liew for help with the spectrophotometer; Robert Rak and Chris Bolick for rearing corn plants for the larvae; and Yale University for support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118378109/-/DCSupplemental.
References
- 1.Andersson M. Sexual Selection. Princeton, NJ: Princeton Univ Press; 1994. p. 559. [Google Scholar]
- 2.Morris MR. Further examination of female preference for vertical bars in swordtails: Preference for 'no bars' in a species without bars. J Fish Biol. 1998;53:(sA):56–63. [Google Scholar]
- 3.Kimball RT, Braun EL, Ligon JD, Lucchini V, Randi E. A molecular phylogeny of the peacock-pheasants (Galliformes: Polyplectron spp.) indicates loss and reduction of ornamental traits and display behaviours. Biol J Linn Soc Lond. 2001;73(2):187–198. [Google Scholar]
- 4.Wiens JJ. Widespread loss of sexually selected traits: How the peacock lost its spots. Trends Ecol Evol. 2001;16:517–523. [Google Scholar]
- 5.Verzijden MN, Lachlan RF, Servedio MR. Female mate-choice behavior and sympatric speciation. Evolution. 2005;59:2097–2108. [PubMed] [Google Scholar]
- 6.Irwin DE, Price T. Sexual imprinting, learning and speciation. Heredity (Edinb) 1999;82:347–354. doi: 10.1038/sj.hdy.6885270. [DOI] [PubMed] [Google Scholar]
- 7.Laland KN. On the evolutionary consequences of sexual imprinting. Evolution. 1994;48:477–489. doi: 10.1111/j.1558-5646.1994.tb01325.x. [DOI] [PubMed] [Google Scholar]
- 8.ten Cate C, Vos DR. Sexual imprinting and evolutionary processes in birds: A reassessment. Adv Stud Behav. 1999;28:1–31. [Google Scholar]
- 9.Grant PR, Grant BR. Hybridization, sexual imprinting, and mate choice. Am Nat. 1997;149(1):1–28. [Google Scholar]
- 10.Dukas R. Learning in the context of sexual behaviour in insects. Animal Biology. 2006;56(2):125–141. [Google Scholar]
- 11.Beckers OM, Wagner WE. Mate sampling strategy in a field cricket: Evidence for a fixed threshold strategy with last chance option. Anim Behav. 2011;81:519–527. [Google Scholar]
- 12.Hebets EA, Vink CJ. Experience leads to preference: Experienced females prefer brush-legged males in a population of syntopic wolf spiders. Behav Ecol. 2007;18(6):1010–1020. [Google Scholar]
- 13.Hebets EA. Subadult experience influences adult mate choice in an arthropod: Exposed female wolf spiders prefer males of a familiar phenotype. Proc Natl Acad Sci USA. 2003;100:13390–13395. doi: 10.1073/pnas.2333262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svensson EI, Eroukhmanoff F, Karlsson K, Runemark A, Brodin A. A role for learning in population divergence of mate preferences. Evolution. 2010;64:3101–3113. doi: 10.1111/j.1558-5646.2010.01085.x. [DOI] [PubMed] [Google Scholar]
- 15.Bailey NW, Zuk M. Field crickets change mating preferences using remembered social information. Biol Lett. 2009;5:449–451. doi: 10.1098/rsbl.2009.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson KA, Monteiro A. Female Bicyclus anynana butterflies choose males on the basis of their dorsal UV-reflective eyespot pupils. Proc Biol Sci. 2005;272:1541–1546. doi: 10.1098/rspb.2005.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prudic KL, Jeon C, Cao H, Monteiro A. Developmental plasticity in sexual roles of butterfly species drives mutual sexual ornamentation. Science. 2011;331:73–75. doi: 10.1126/science.1197114. [DOI] [PubMed] [Google Scholar]
- 18.Oliver JC, Robertson KA, Monteiro A. Accommodating natural and sexual selection in butterfly wing pattern evolution. Proc Biol Sci. 2009;276:2369–2375. doi: 10.1098/rspb.2009.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brakefield PM, French V. Butterfly wing patterns: Developmental mechanisms and evolutionary change. Acta Biotheor. 1993;41:447–468. [Google Scholar]
- 20.Ritchie MG. Sexual selection and speciation. Annu Rev Ecol Syst. 2007;38:79–102. [Google Scholar]
- 21.Dukas R. Male fruit flies learn to avoid interspecific courtship. Behav Ecol. 2004;15:695–698. [Google Scholar]
- 22.Dukas R. Learning affects mate choice in female fruit flies. Behav Ecol. 2005;16:800–804. [Google Scholar]
- 23.Miller MN, Fincke OM. Cues for mate recognition and effect of prior experience on mate recognition in Enallagma damselflies. J Insect Behav. 1999;12:801–814. [Google Scholar]
- 24.Walling CA, Royle NJ, Lindstrom J, Metcalfe NB. Experience-induced preference for short-sworded males in the green swordtail, Xiphophorus helleri. Anim Behav. 2008;76:271–276. [Google Scholar]
- 25.Melo MC, Salazar C, Jiggins CD, Linares M. Assortative mating preferences among hybrids offers a route to hybrid speciation. Evolution. 2009;63:1660–1665. doi: 10.1111/j.1558-5646.2009.00633.x. [DOI] [PubMed] [Google Scholar]
- 26.Jiggins CD, Estrada C, Rodrigues A. Mimicry and the evolution of premating isolation in Heliconius melpomene Linnaeus. J Evol Biol. 2004;17:680–691. doi: 10.1111/j.1420-9101.2004.00675.x. [DOI] [PubMed] [Google Scholar]
- 27.Kronforst MR, et al. Linkage of butterfly mate preference and wing color preference cue at the genomic location of wingless. Proc Natl Acad Sci USA. 2006;103:6575–6580. doi: 10.1073/pnas.0509685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamberlain NL, Hill RI, Kapan DD, Gilbert LE, Kronforst MR. Polymorphic butterfly reveals the missing link in ecological speciation. Science. 2009;326:847–850. doi: 10.1126/science.1179141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tramm NA, Servedio MR. Evolution of mate-choice imprinting: Competing strategies. Evolution. 2008;62:1991–2003. doi: 10.1111/j.1558-5646.2008.00419.x. [DOI] [PubMed] [Google Scholar]
- 30.Lynn SK. Cognition and evolution: Learning and the evolution of sex traits. Curr Biol. 2006;16:R421–R423. doi: 10.1016/j.cub.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Endler JA, Westcott DA, Madden JR, Robson T. Animal visual systems and the evolution of color patterns: Sensory processing illuminates signal evolution. Evolution. 2005;59:1795–1818. doi: 10.1554/04-669.1. [DOI] [PubMed] [Google Scholar]
- 32.ten Cate C, Rowe C. Biases in signal evolution: Learning makes a difference. Trends Ecol Evol. 2007;22:380–387. doi: 10.1016/j.tree.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Dugatkin LA. Sexual selection and imitation: Females copy the mate choice of others. Am Nat. 1992;139:1384–1389. [Google Scholar]
- 34.Costanzo K, Monteiro A. The use of chemical and visual cues in female choice in the butterfly Bicyclus anynana. Proc Biol Sci. 2007;274:845–851. doi: 10.1098/rspb.2006.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieberding CM, et al. The male sex pheromone of the butterfly Bicyclus anynana: Towards an evolutionary analysis. PLoS ONE. 2008;3:e2751. doi: 10.1371/journal.pone.0002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieberding CM, et al. Cracking the olfactory code of a butterfly: The scent of ageing. Ecol Lett. 2012;15:415–424. doi: 10.1111/j.1461-0248.2012.01748.x. [DOI] [PubMed] [Google Scholar]
- 37.Wilgers DJ, Hebets EA. Complex courtship displays facilitate male reproductive success and plasticity in signaling across variable environments. Current Zoology. 2011;57:175–186. [Google Scholar]
- 38.Griffith LC, Ejima A. Multimodal sensory integration of courtship stimulating cues in Drosophila melanogaster. Ann NY Acad Sci. 2009;1170:394–398. doi: 10.1111/j.1749-6632.2009.04367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marler P, Peters S. Selective vocal learning in a sparrow. Science. 1977;198:519–521. doi: 10.1126/science.198.4316.519. [DOI] [PubMed] [Google Scholar]
- 40.Marler P. Three models of song learning: Evidence from behavior. J Neurobiol. 1997;33:501–516. [PubMed] [Google Scholar]
- 41.Lachlan RF, Feldman MW. Evolution of cultural communication systems: The coevolution of cultural signals and genes encoding learning preferences. J Evol Biol. 2003;16:1084–1095. doi: 10.1046/j.1420-9101.2003.00624.x. [DOI] [PubMed] [Google Scholar]
- 42.Marler P, Peters S. The role of song phenology and syntax in vocal learning preferences in the song sparrow, Melospiza melodia. Ethology. 1988;77:125–149. [Google Scholar]
- 43.Marler P. Birdsong and speech development: Could there be parallels? Am Sci. 1970;58:669–673. [PubMed] [Google Scholar]
- 44.Dukas R. Learning decreases heterospecific courtship and mating in fruit flies. Biol Lett. 2008;4:645–647. doi: 10.1098/rsbl.2008.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Condamin M. Monographie du genre Bicyclus (Lepidoptera Satyridae) [Monograph of the genus Bicyclus (Lepidoptera Satyridae)] Vol 88. Senegal: Ifan-Dakar; 1973. pp. 1–324. French. [Google Scholar]
- 46.Stephens DW. Change, regularity, and value in the evolution of animal learning. Behav Ecol. 1991;2(1):77–89. [Google Scholar]
- 47.Kozak GM, Head ML, Boughman JW. Sexual imprinting on ecologically divergent traits leads to sexual isolation in sticklebacks. Proc Biol Sci. 2011;278:2604–2610. doi: 10.1098/rspb.2010.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorenson MD, Hauber ME, Derrickson SR. Sexual imprinting misguides species recognition in a facultative interspecific brood parasite. Proc Biol Sci. 2010;277:3079–3085. doi: 10.1098/rspb.2010.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verzijden MN, ten Cate C. Early learning influences species assortative mating preferences in Lake Victoria cichlid fish. Biol Lett. 2007;3:134–136. doi: 10.1098/rsbl.2006.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliver JC, Monteiro A. On the origins of sexual dimorphism in butterflies. Proc Biol Sci. 2011;278:1981–1988. doi: 10.1098/rspb.2010.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fordyce JA, Nice CC, Forister ML, Shapiro AM. The significance of wing pattern diversity in the Lycaenidae: Mate discrimination by two recently diverged species. J Evol Biol. 2002;15:871–879. [Google Scholar]
- 52.Brower AVZ. Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. Proc Natl Acad Sci USA. 1994;91:6491–6495. doi: 10.1073/pnas.91.14.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch A. The population memetics of bird song. In: Kroodsma DE, Miller EH, editors. Ecology and Evolution of Acoustic Communication in Birds. Ithaca, London: Comstock Publishing; 1996. pp. 181–197. [Google Scholar]
- 54.Brakefield PM, Larsen T. The evolutionary significance of dry and wet season forms in some tropical butterflies. Biol J Linn Soc Lond. 1984;22(1):1–12. [Google Scholar]
- 55.Brakefield PM, Reistma N. Phenotypic plasticity, seasonal climate and the population biology of Bicyclus butterflies (Satyridae) in Malawi. Ecol Entomol. 1991;16:291–303. [Google Scholar]
- 56.Joron M, Brakefield PM. Captivity masks inbreeding effects on male mating success in butterflies. Nature. 2003;424:191–194. doi: 10.1038/nature01713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.