Abstract
Progenitor cells of the first and second heart fields depend on cardiac-specific transcription factors for their differentiation. Using conditional mutagenesis of mouse embryos, we define the hierarchy of signaling events that controls the expression of cardiac-specific transcription factors during differentiation of cardiac progenitors at embryonic day 9.0. Wnt/β-catenin and Bmp act downstream of Notch/RBPJ at this developmental stage. Mutation of Axin2, the negative regulator of canonical Wnt signaling, enhances Wnt and Bmp4 signals and suffices to rescue the arrest of cardiac differentiation caused by loss of RBPJ. Using FACS enrichment of cardiac progenitors in RBPJ and RBPJ/Axin2 mutants, embryo cultures in the presence of the Bmp inhibitor Noggin, and by crossing a Bmp4 mutation into the RBPJ/Axin2 mutant background, we show that Wnt and Bmp4 signaling activate specific and nonoverlapping cardiac-specific genes in the cardiac progenitors: Nkx2-5, Isl1 and Baf60c are controlled by Wnt/β-catenin, and Gata4, SRF, and Mef2c are controlled by Bmp signaling. Our study contributes to the understanding of the regulatory hierarchies of cardiac progenitor differentiation and outflow tract development and has implications for understanding and modeling heart development.
Keywords: cardiogenesis, MesP1-cre, mesoderm patterning, canonical Wnt, progenitor differentiation in culture
Distinct cell types, such as the mesodermal progenitors of the first and second heart fields (FHF and SHF) as well as cardiac neural crest cells, contribute to the development of the vertebrate heart. The FHF lineage generates the left ventricle, whereas the SHF forms the right ventricle and contributes to outflow tract and atria. SHF and cardiac neural crest cells cooperate during the development of outflow tract vessels and valves (1–3). Mesodermal cells of the anterior postgastrulating mouse embryo and FHF and SHF progenitors express the transcription factor MesP1, which is essential for heart induction (4). FHF and SHF progenitors can be distinguished by their level of proliferation and differentiation. Cells of the FHF begin to differentiate by expressing Gata4, Baf60c, and Nkx2-5 in the cardiac crescent and cardiac tube. Cells of the SHF express Isl1, which marks undifferentiated cells (5). The development of the outflow tract involves Isl1-expressing cardiac progenitors from the anterior SHF, endocardial cells, and cardiac neural crest cells (6).
Mesodermal progenitor cells pass through several developmental stages before they become fully differentiated cardiomyocytes. During a first phase, mesodermal progenitors express a core complex of essential transcription factors and chromatin remodelers—Gata4, Brg1, and Baf60c—which induce transcription factors such as Nkx2-5, Tbx5/20, and Isl1 that define cells as cardiac progenitors (5, 7). During predifferentiation of cardiac progenitors into cardioblasts, these transcription factors induce the expression of Mef2c and Hand1/2 and also early cardiac muscle-specific genes, e.g., the genes that encode cardiac actin and myosin light chain 2a (8–10). In the differentiation phase, down-regulation of Isl1 and interaction of Mef2c/Hand1/2 with the core complex induce the expression of cardiac muscle-specific genes for structural proteins such as troponin T2 and myosin heavy chains (11, 12).
How the Notch, Wnt, and Bmp developmental signaling pathways control heart development and the heart-specific transcription factors has been studied. Notch signals repress differentiation by inhibiting Mef2c expression during early cardiogenesis in Xenopus and in embryonic stem cells and promote differentiation in both myocardium and endocardium during later developmental stages (6). The Notch intracellular domain translocates to the nucleus to form a transcriptional complex with the DNA-binding protein RBPJ and the coactivator Mastermind-like to activate target genes (6). Studies in mouse, chick, frog, and fish demonstrate that high levels of Bmp activity are necessary for the expression of Nkx2-5 and Gata4 and for myocardial differentiation (5, 9, 13, 14). Canonical Wnt signals are essential for proliferation of Isl1-expressing SHF progenitors and also promote Nkx2-5 expression and subsequent cardiac differentiation by down-regulating HDAC1 (5, 13, 15). In the presence of canonical Wnt ligands, β-catenin is stabilized and translocates to the nucleus, where it interacts with Lef/Tcf transcription factors to activate target genes (16–18). Axin proteins are negative regulators and control β-catenin degradation. Axin2 is a target gene of canonical Wnt signals, and its expression is a useful marker to define cells exposed to Wnt (19–22). Axin2-null mice are viable and have no obvious defects in cardiac development. However, increased canonical Wnt signaling is found in homozygous Axin2−/− cells after transplantation into wild-type tissues and embryos (21, 22). Deficits in skull formation in Axin2-mutant mice appear to be caused by tissue-specific up-regulation of canonical Wnt signals (20).
Results
Wnt Gain-of-Function Mutation Axin2−/− Rescues Defects of SHF Morphogenesis in Conditional RBPJ/Notch Mice.
Interference with Notch signaling arrests cardiac development (6, 23–25). In accordance, MesP1-cre–induced mutation of RBPJlox/lox (hereafter, “RBPJ mutant”) reduced the size and compactness of the heart, as revealed by analyses of H&E-stained sections at embryonic day (E) 9.75 (Fig. 1 A and B and Table S1). The right ventricles were strongly affected, and the expression of the predifferentiation factor Hand2 was lost (Fig. 1 C and D). In situ hybridizations showed a pronounced down-regulation of Gata4 in the heart and of Isl1 in the splanchnic mesoderm and outflow tract myocardium at E9.25 (Fig. 1 E and F and Fig. S1 A and B). Ablation of RBPJ (Fig. S1 C–D′) strongly attenuated canonical Wnt signaling, as seen by the reduced expression of the Wnt target genes Axin2 and Lef1, particularly in the rudimentary anterior SHF and SHF derivatives at E8.5–9.25 (Fig. S1 E–L and Table S2). Moreover, a major reduction in Lef1 and Isl1 coexpression occurred in the splanchnic mesoderm (Fig. S1 M–P). Axin2 expression also was visualized in mice that carried a Wnt reporter, the heterozygous Axin2LacZ allele (19, 21). LacZ was expressed in splanchnic mesoderm and SHF derivatives in control mice (13) but was reduced at these sites in the RBPJ mutants (Fig. 1 G and H). These data indicate cross-talk between Notch/RBPJ and canonical Wnt signaling, operating particularly in the SHF, in early heart morphogenesis.
Fig. 1.
The Axin2 mutation restores Wnt and Bmp signaling and substantially rescues the RBPJ phenotype in the heart. (A and B) H&E staining of transverse sections of hearts of control and RPPJ mutants at E9.75. Right ventricles (RV) are marked by arrows. LV, left ventricle. (C and D) Whole-mount in situ hybridization for Hand2 in controls and RBPJ mutants at E9.75. (E and F) In situ hybridization on sections for Isl1 at E9.25. (G and H) Canonical Wnt activation as determined on transverse sections of whole-mount in situ hybridizations for Axin2-LacZ in controls (Axin2LacZ/+) and RBPJ mutants (RBPJ/Axin2LacZ/+) at E9.25. Right ventricles (RV; see Inset magnifications) and outflow tract myocardium (OFT) are marked by arrows and arrowheads, respectively, and splanchnic mesoderm is marked by asterisks. (I–L) Whole-mount in situ hybridization for Nkx2-5 in controls, RBPJ single-mutant, RBPJ/Axin2 double-mutant, and Axin2 single-mutant embryos at E9.25. (M–T) Immunofluorescence analysis for Isl1 (red) and cre (green) (M–P), and TropT2 (red) and Nkx2-5 (green) (Q–T) in indicated genotypes. See also magnifications of splanchnic mesoderm (M′–P′). FG, foregut. (U) Heatmap of gene-expression arrays of RBPJ/Axin2 double mutants vs. controls and RBPJ and Axin2 single mutants. The restored expression of particular genes is shown in orange for cardiac-specific genes, green for Wnt genes, and magenta for Bmp-controlled genes. (V) qRT-PCR analyses of mRNA expression at E9.25 in the heart region of controls (gray bars), Axin2 single (blue bars), RBPJ single mutants (magenta bars), and RBPJ/Axin2 double mutants (blue/magenta-striped bars). Error bars represent SEM (n = 4). *P < 0.05, **P < 0.01, ***P < 0.005. AU, arbitrary units.
We confirmed by genetic means that Notch/RBPJ and Wnt/β-catenin interact during SHF morphogenesis, and we generated compound MesP1-cre;RBPJlox/lox;Axin2−/− mutants (hereafter “RBPJ/Axin2 double mutants”). Remarkably, the Axin2−/− mutation substantially but not completely rescued the heart phenotypes at E9.25 in RBPJ mutants (Fig. 1 I–L and Table S1). In particular, the size of the right ventricles and outflow tracts and also the expression of Nkx2-5 were normalized (Fig. 1 J and K). Elimination of Axin2 alone did not produce heart hyperplasia (Fig. 1L). Pronounced activation of canonical Wnt signaling by β-galactosidase expression occurred in splanchnic mesoderm and ventricular walls of RBPJ/Axin2LacZ/LacZ mutants, as analyzed by immunohistology of Cre+ cells (Fig. S2 A–B′′). Canonical Wnt signals previously had been shown to be essential for proliferation of Isl1-expressing SHF progenitors (5, 13). However, proliferation in Isl1-expressing cells was little affected in RBPJ single mutants and RBPJ/Axin2+/− mutants (Fig. S2 C and D). However, in RBPJ/Axin2+/− mutants, a pronounced rescue of the number of Isl1-expressing cells in the splanchnic mesoderm and of ventricular cardiomyocyte differentiation was observed, evidenced by the restored expression of troponin T2 (Fig. 1 M–T and Fig. S2E). Thus, coexpression of Nkx2-5 and troponin T2 were not observed in RBPJ mutant hearts, whereas robust coexpression was seen in RBPJ/Axin2 double mutants (Fig. 1 Q–T).
We used gene-expression profiling of dissected cardiac tissue (heart, including SHF) to study the mechanism of the rescue of the cardiac differentiation program in RBPJ/Axin2 mutants (Fig. 1U). Compared with RBPJ single-mutant tissues, the expression of many down-regulated genes was largely restored in RBPJ/Axin2 double mutants (compare the third and second columns in Fig. 1U and see Tables S1 and S3). Among these genes were those required for cardiac differentiation, e.g., cardiac actin (Actc1), SRF, and Mef2c (identified in orange in Fig. 1U). Moreover, the expression of >20 genes that encode components and targets of canonical Wnt signaling, e.g., Wnt2, Dkk1, and Lef1 (identified in green in Fig. 1U; see also Table S3, and for Wnt target genes see www.stanford.edu/group/nusselab/cgi-bin/wnt/) were up-regulated also. Surprisingly, the expression of many genes encoding members of the Bmp pathway (Bmp4, BmpR1a) and Bmp target genes (Smad6, Msx2) (indentified in magenta in Fig. 1U; see also Table S3) was rescued also. We confirmed restoration of expression of selected cardiac differentiation genes (SMA, SRF, and troponin T2) and of Wnt and Bmp target genes by quantitative RT-PCR (qRT-PCR) (Fig. 1V). Furthermore, Bmp4 but not Bmp7 expression was restored (Fig. 1V; see also below). The majority of these genes were not or were only slightly affected in Axin2 single mutants [Fig. 1U (compare first and fourth columns) and V]. Thus, our genetic data indicate that Wnt/β-catenin acts downstream of RBPJ/Notch and controls Bmp signals in morphogenesis and differentiation of cardiac cells.
Wnt/β-Catenin and Bmp Signals Downstream of Notch/RBPJ Are Crucial for Different Stages of Cardiac Progenitor Differentiation.
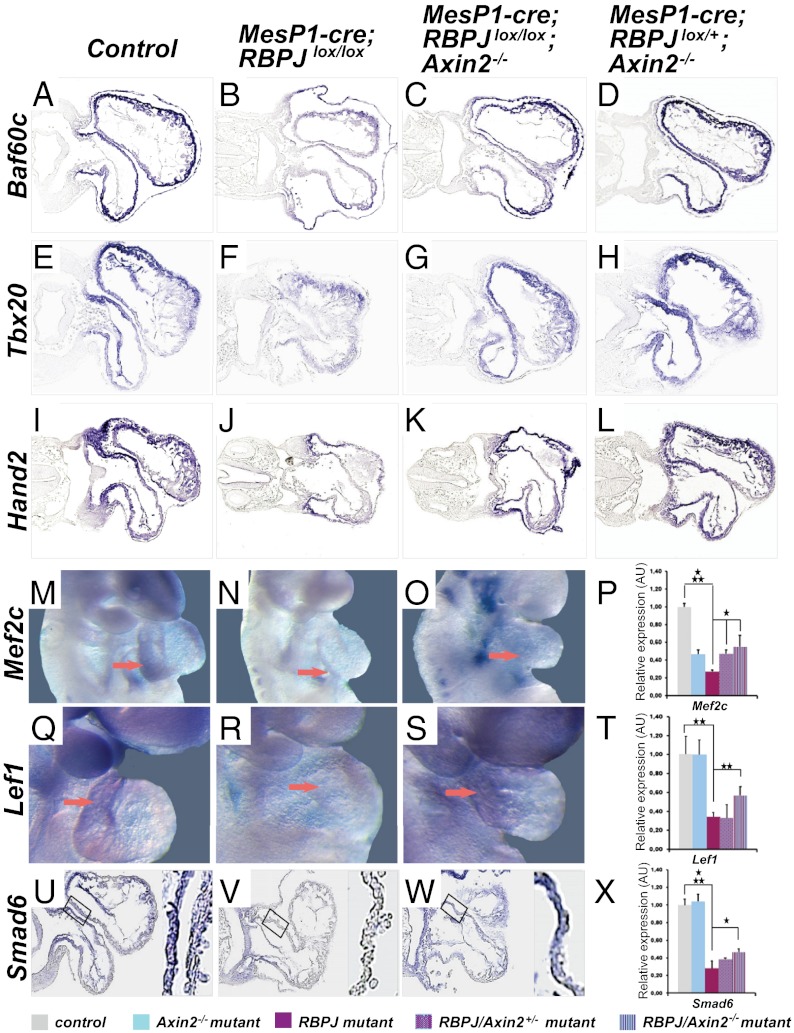
The minimal core complex, Gata4 and Baf60c, that induces cardiac progenitor specification was down-regulated in the RBPJ mutants and was rescued by Axin2 ablation, as confirmed by in situ hybridization (Fig. 2 A–D; see also below). The factors produced during progenitor specification, Nkx2-5, Isl1, SRF, and Tbx20 were similarly down- and up-regulated (Figs. 1 I–L and V and Figs. 2 E–H and Table S2). The expression of factors that mark predifferentiation, Hand2 and Mef2c, also was down-regulated in RBPJ mutants and was rescued by Axin2 ablation (Fig. 2 I–P). Elimination of one allele of Axin2 sufficed to rescue, in part, the expression of Mef2c (quantifications are given in Fig. 2P). In situ hybridization also confirmed the reduced expression of Lef1, Wnt2, and the Bmp target gene Smad6 in SHF derivatives of RBPJ mutants and their up-regulation in RBPJ/Axin2 double mutants (Fig. 2 Q–X and Fig. S2 F–I). The expression of the Notch/RBPJ target gene Hey3 was not restored, but Hey1 and Hey2 were normalized by Axin2 ablation (Fig. S2 J–P and Table S3) (6). The expression of Tenascin C (Fig. S2 Q–T) and of other genes not yet described as being involved in SHF morphogenesis [e.g., protocadherin (PCDH7) (Fig. S2 U–X) and G protein-coupled receptor Gpr83, Nkd2, and others (identified in Table S3)] was normalized. Thus, Wnt/β-catenin and further signals regulated by Wnt are required for the expression of transcription factors that are essential for specification of cardiac progenitors as well as for differentiation, i.e., the induction of heart-specific structural proteins.
Fig. 2.
Canonical Wnt and Bmp signaling downstream of Notch/RPBJ are crucial for the different stages of cardiac differentiation at E9.25. (A–L) In situ hybridization on transverse sections of hearts for Baf60c, Tbx20, and Hand2 in indicated genotypes at E9.25. (M–X) Whole-mount in situ hybridizations of embryos or on transverse sections of hearts for Mef2c, (M, N, O), Lef1 (Q, R, S), and Smad6 (U, V, W) (outflow tract/right ventricles are marked by arrows) and corresponding qRT-PCR analyses (P, T, X) in controls, RBPJ single mutants, RBPJ/Axin2+/−, RBPJ/Axin2 double mutants, and Axin2 single mutants. Error bars represent SEM (n = 4). *P < 0.05, **P < 0.01, ***P < 0.005. AU, arbitrary units.
Axin2 Mutation Restores Expression of Crucial Transcription Factors in FACS-Sorted Cardiac Progenitors of RBPJ Mutants.
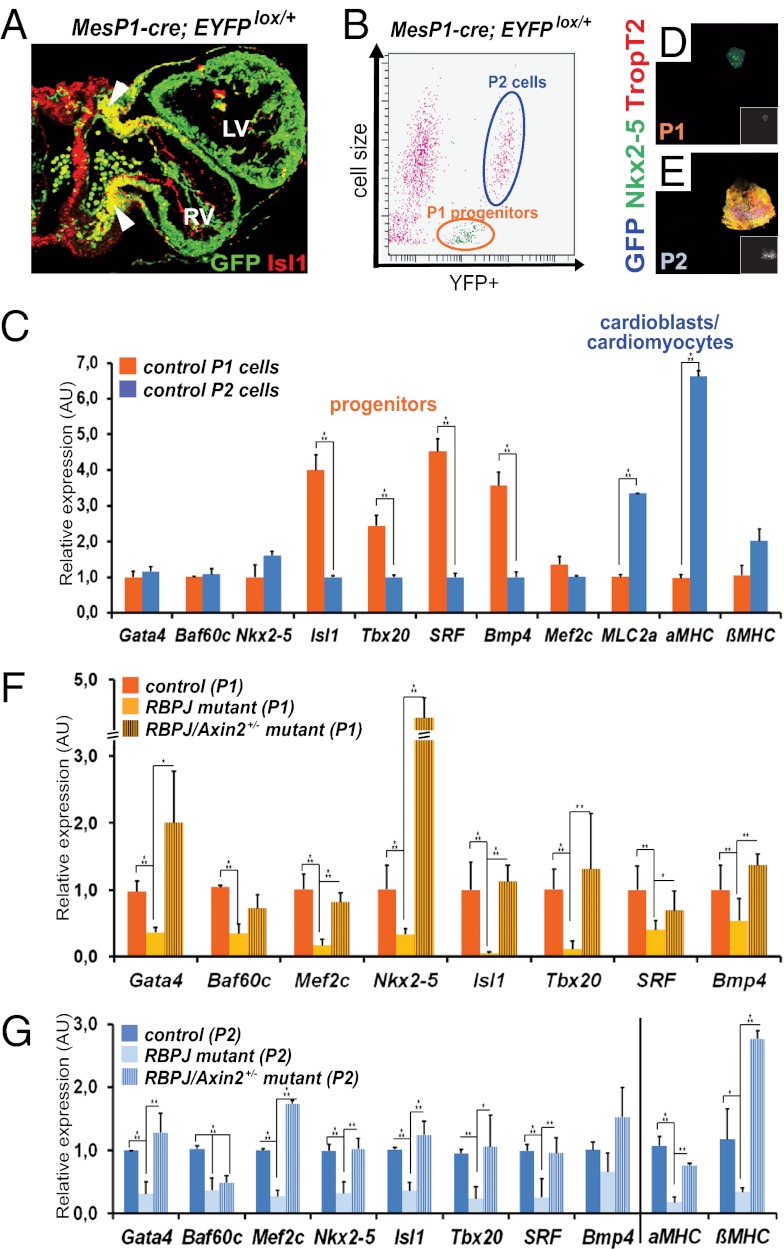
Our analyses show that Axin2 ablation rescues cardiac development of RBPJ mutants during the formation of cardiac progenitor cells. To confirm the stage and cell type precisely, we used FACS to isolate cardiac progenitors. A reporter line that expresses EYFP in a cre-inducible manner was crossed into controls and mutants that carry MesP1-cre (26). Reporter expression was observed in MesP1-derived mesodermal cardiac progenitor cells that express Isl1, in particular in the splanchnic mesoderm and distal outflow tracts, and in cells of the left and right ventricles (arrowheads in Fig. 3A; merged yellow fluorescence marks coexpression of Isl1 and GFP). Two distinct EYFP+ cell populations from the cardiac tissue (heart, including SHF) were isolated: small-diameter P1 cells (outlined in orange in Fig. 3B) and large-diameter P2 cells (outlined in blue in Fig. 3B). The identity of the sorted cells obtained from control hearts was verified by analyses of Isl1 and αMHC expression. RT-PCR showed that P1 cells strongly expressed the progenitor-specific factors Isl1, Tbx20, and SRF, suggesting that they represent cardiac progenitors (orange bars in Fig. 3C). In contrast, P2 cells expressed reduced levels of Isl1, Tbx20, and SRF but high levels of MLC2a and αMHC typical for differentiated cardiac cells (blue bars in Fig. 3C), suggesting that P2 cells are cardioblasts and/or cardiomyocytes. Immunofluorescence showed that isolated P1 progenitors expressed Nkx2-5, whereas P2 cells had high levels of cardiac troponin (Fig. 3 D and E). When isolated P1 and P2 cells were cultured for 7 d, P1 cells underwent cardiac differentiation in the presence of Activin A and Bmp4 (27), as analyzed by immunofluorescence for Nkx2-5 and troponinT2 (Fig. S3 A–B′). At the end of the culture period, cardiac troponin in P1 and P2 cells was equal. Thus, P1 cells are indeed cardiac progenitors that retain the capacity to differentiate. P1 progenitor cells strongly expressed Notch and Wnt pathway components and target genes, in particular Jag2 and Notch4 and Wnt2, Axin2, and Lef1, but these genes were seen to a lesser extent in P2 cells (Fig. S3C). These genes were expressed in cardiac tissue of RBPJ/Axin2 double mutants but not in RBPJ mutants (Table S3). Other Notch pathway components, i.e., Dll4, Jag1, and Notch1/2, were highly expressed in the differentiated P2 cardiac cells (Fig. S3C). We used P1 and P2 cell populations of RBPJ single-mutant and RBPJ/Axin2+/− mutant hearts to define potential differences in their response to the mutations. Remarkably, loss of RBPJ in P1 and P2 cells abolished Nkx2-5, Isl1, Tbx20, and Mef2c expression (yellow bars in Fig. 3F and light blue bars in Fig. 3G). However, loss of Axin2 (one allele sufficed) substantially normalized the expression of Nkx2-5, Isl1, Tbx20, and Mef2c in P1 and P2 cells of RBPJ mutants (striped bars in Fig. 3 F and G). Furthermore, Bmp4 expression was restored in RBPJ/Axin2+/− mutant P1 cells (Fig. 3 F and G Right; see also below and Fig. S4 B–D). Thus, Axin2 ablation restored the expression of the crucial transcription factors Nkx2-5, Isl1, Tbx20, and Mef2c and the expression of Bmp4 in RBPJ mutant cardiac cells, indicating that the sequential regulation of cardiac-specific transcription factors mediated by RBPJ, Wnt, and Bmp signals occurs in mesoderm-derived cardiac progenitors as well as in the early differentiated cardiac cells.
Fig. 3.
Wnt/β-catenin activation by Axin2 ablation restores cardiac commitment and Bmp signaling in SHF progenitor cells at E9.25. (A) Immunofluorescence analysis of GFP (green) and Isl1 (red) on a transverse section of control embryos (arrowheads mark merged yellow fluorescence in MesP1-derived SHF cells). (B) FACS of YFP+ cells of MesP1-cre; EYFPlox/+ embryos at E9.25 showing two populations of YFP+ cells: P1 progenitors (outlined in orange) and P2 cells (outlined in blue). (C) Relative mRNA expression levels in the YFP+ populations (P1, orange; P2, blue) of controls show that P1 cells express higher levels of cardiac progenitor genes, and P2 cells show a marked increase in muscle-specific genes. (D and E) Immunofluorescence analysis of GFP (blue), Nkx2-5 (green), and troponin T2 (red) in P1 cells (D) and P2 cells (E). (F and G) qRT-PCR analysis of mRNA in the cardiac progenitor population (P1 cells) (F) and in P2 cells (G) of controls (MesP1-cre;EYFPlox/+; RBPJlox/+;Axin2+/−), RBPJ single mutants (MesP1-cre;EYFPlox/+; RBPJlox/lox), and double mutants (MesP1-cre;EYFPlox/+; RBPJlox/lox;Axin2+/−). Error bars represent SEM (n = 3–6). *P < 0.05, **P < 0.01, ***P < 0.005. AU, arbitrary units.
Nkx2-5, Isl1, and Baf60c are Wnt/β-Catenin Target Genes, and Gata4 and SRF Are Bmp4 Targets in Cardiac Progenitors.
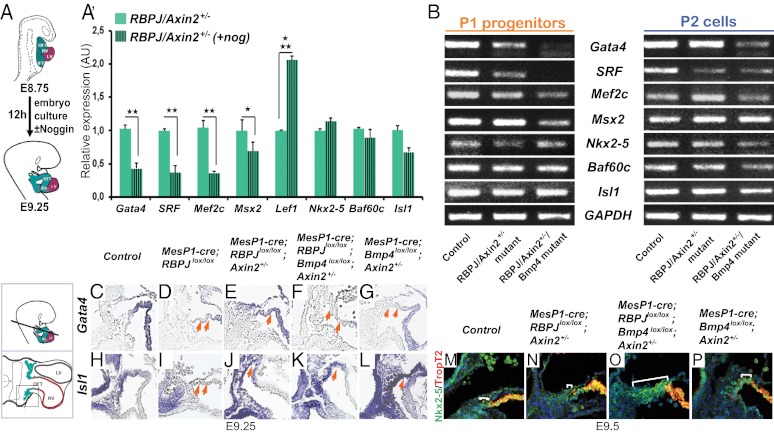
We next defined the heart-specific transcription factors controlled by canonical Wnt and/or Bmp signaling at this developmental stage. Whole E8.75 embryos were cultured in the presence or absence of Noggin, a soluble Bmp inhibitor (Fig. 4A) (14). Analysis of mRNA from dissected cardiac tissue (heart, including SHF) of control embryos treated with Noggin revealed decreased expression of all cardiac-specific transcription factors and also reduced Lef1, indicating that Noggin also affected Wnt signaling (Fig. S4A). However, analysis of RBPJ/Axin2+/− mutants, in which Wnt signaling is up-regulated, showed that Noggin treatment strongly reduced the expression of Gata4, SRF, Mef2c, and Msx2 (Fig. 4A′), indicating that Gata4, SRF, and Mef2c are controlled by Bmp signaling in the presence of Wnt signaling. In contrast, Nkx2-5, Baf60c, and Isl1 expression was not affected significantly by Noggin (Fig. 4 A′), showing that Nkx2-5, Baf60c, and Isl1 are controlled by Wnt but not by Bmp signals.
Fig. 4.
Wnt/β-catenin and Bmp signaling target different sets of cardiac-specific transcription factors to maintain differentiation of cardiac progenitors within the distal outflow tract. (A) Scheme of mouse embryo culture. Culture for 12 h in the absence or presence of Noggin was from E8.75–9.25. (A′) qRT-PCR analysis of mRNA levels of the indicated genes of RBPJ/Axin2+/− double mutants in the absence (plain green bars) and presence of Noggin (+Noggin; striped green bars). A 2.5- to threefold decrease of Gata4, SRF, and Mef2c expression was observed by treatment with Noggin. Error bars represent SEM (n = 4). *P < 0.05, **P < 0.01, ***P < 0.005. AU, arbitrary units. (B) Relative expression of indicated mRNAs in FACS P1 progenitor cells and P2 cells of controls, RBPJ/Axin2+/− double mutants, and RBPJ/Bmp4/Axin2+/− triple mutants. (C–L) In situ hybridization on transverse sections of hearts for Gata4 (C–G) and Isl1 (H–L) in indicated genotypes at E9.25 (shown are magnifications of the SHF as indicated in the scheme at left). (M–P) Immunofluorescence analysis in control (M), RBPJ/Axin2+/−(Ν), RBPJ/Bmp4/Axin2+/− triple-mutant (O), and Bmp4/Axin2+/− mutant (P) embryos for TropT2 (red) and Nkx2-5 (green) at E9.5. Bars mark the area of undifferentiated cells in the distal outflow tracts.
We also investigated the expression of various Bmp ligands in the mutant hearts. Bmp2, Bmp4, and Bmp7 expression was lost in RBPJ single mutants. Bmp4 but not Bmp2 and Bmp7 expression was restored in the heart of RBPJ/Axin2 double mutants (Fig. S4 B–O; see also Fig. 1V). It should be noted that in Bmp4 mutants Bmp7 was expressed in the entire heart, suggesting that Bmp7 might substitute for Bmp4 loss. To assess the role of the restored Bmp4 expression in the RBPJ/Axin2 double mutants and to corroborate the above assignment of signal-dependent expression of cardiac transcription factors, we introduced a conditional Bmp4 mutation (28) into the RBPJ/Axin2 mutant background (MesP1-cre;RBPJlox/lox;Bmp4lox/lox;Axin2+/−; hereafter “triple mutants”). Bmp signaling indeed was lost in the mutants, and Bmp4 expression as well as pSmad1/5/8-Isl1 costaining were abrogated in the SHF of the triple mutants at E9.25 (Fig. S4 P–T′ and Table S1). However, the quantity of Isl1-expressing cells was unchanged, demonstrating that the low pSmad1/5/8 expression was not caused by a loss of SHF cells (Fig. S4U). It should be noted that Smad1/5/8 phosphorylation was detectable in the ventricular walls of the triple mutants, indicating that these differentiated cells receive signals provided by a Bmp ligand other than Bmp4. In accordance with the embryo culture in the presence of Noggin, FACS-sorted P1 progenitors of the triple-mutant embryos displayed strongly reduced Gata4, SRF, and Mef2c expression (Fig. 4B Upper Left; the Bmp target gene Msx2 served as a control). In contrast, the additional ablation of Bmp4 had no significant effect on the expression of Nkx2-5, Isl1, and Baf60c, as shown by comparison of triple-mutant and double-mutant P1 cells (Fig. 4B Left and Fig. S5A). In differentiated P2 cells, the expression of Gata4, SRF, and Mef2c was unchanged (Fig. 4B Right and Fig. S5B). The ventricular wall contained differentiated pSmad1/5/8+ cardiac cells, which are a probable source of P2 cells (Fig. S4 P–T). In situ hybridization confirmed that the expression of Gata4, Mef2c, and Hand2 was down-regulated in the SHF at E9.25, but the expression of Isl1, Nkx2-5, and Tbx20 was not affected (Fig. 4 C–L, Fig. S5 C–R, and Table S2). The change in the expression of the cardiac transcription factors was accompanied at E9.5 by a marked arrest of differentiation in the distal outflow tract of the triple mutants, a possible consequence of the loss of Hand2 and Mef2c in the anterior SHF (Fig. 4 M–P; bars mark the area of cells lacking troponin T2 in the distal outflow tract).
In summary, we used genetics and embryo culture to show that Wnt signaling controls the expression of Nkx2-5, Baf60c, and Isl1, whereas Bmp signaling regulates Gata4, SRF, and Mef2c. Wnt and Bmp signaling act downstream of Notch in cardiac progenitors. We also unraveled a regulatory hierarchy in outflow tract development and showed that the Bmp- and Wnt-dependent expression of Hand2 and Mef2c in the anterior SHF resulted in arrested differentiation.
Discussion
Here we used mouse genetics, FACS enrichment of cardiac progenitors, and embryo culture with pharmacological inhibitors to unravel the regulatory hierarchies of Wnt/β-catenin, Bmp, and Notch signaling during cardiac progenitor differentiation and outflow tract development. The study may have implications for understanding and modeling heart development.
We show that cardiac defects of a conditional RBPJ mutation at E9 could be rescued substantially by ablating the negative Wnt regulator Axin2, indicating that Wnt/β-catenin is a central downstream effector of Notch signaling in cardiac progenitor differentiation and outflow tract morphogenesis (Fig. S6). Moreover, the expression of Bmp4 and Bmp-controlled genes was restored in such double mutants, suggesting that Wnt/β-catenin acts upstream of Bmp signaling in this developmental context. The rescue by Axin2 was virtually complete with respect to the restoration of canonical Wnt signaling in the heart, as assessed by the expression of the endogenous Axin2 promoter (Axin2LacZ) and of Wnt target genes such as Lef1 or by the expression of cardiac-specific genes in isolated cardiac progenitors. However, cardiac morphogenesis was attenuated, indicating that, in addition to Wnt signaling, Notch signaling may control other essential target genes, e.g., Bmp2 and Bmp7 (shown in this study) or Fgf (5, 23). Outflow tract morphogenesis is known to depend on signals provided by cardiac progenitors from the anterior SHF as well as endocardial, neural crest, and endodermal cells (5, 6, 29). In RBPJ/Axin2 double mutants, diffusible morphogens (Wnt ligands and Bmp4, here shown by gene profiling and by in situ hybridization) may affect gene expression and cell death in MesP1− cells, such as neural crest or endodermal cells. Thus the up-regulated expression of such morphogens in double mutants also might contribute indirectly to the rescued development of nonmesoderm-derived cells (29). In previous analyses of mice that carried a conditional Jagged1 mutation or that expressed a dominant-negative Maml using Mef2c-cre also exhibit SHF morphogenesis affects and reduced Bmp and Fgf signaling (23). In contrast, in studies using Isl1-cre, elevated Wnt/β-catenin signaling has been observed after conditional mutation of Notch1 but not RBPJ (24, 25). The difference in phenotypes might be caused by the use of distinct cre lines to target cardiac cells or by effects that are not mediated by canonical Notch signaling.
The question arises as to how the two developmental signaling systems, Wnt/β-catenin and Bmp, control the expression of the transcription factors required for cardiac differentiation. Our results suggest a model in which Wnt/β-catenin regulates the crucial transcription factors Baf60c, Nkx2-5, and Isl1 at an early stage of progenitor formation (Fig. S6). At this stage Wnt/β-catenin also regulates Bmp4 signaling, which in turn activates Gata4 and SRF. Together, these two sets of effectors activate the transcription factors Mef2c and Hand2, thus allowing cardioblast formation. To corroborate the nature of genes that are regulated by either Wnt/β-catenin or Bmp signaling, we used embryo culture in the presence of the Bmp inhibitor Noggin and introduced a conditional Bmp4 mutation into RBPJ/Axin2 mice. Both experiments confirmed the distinct regulation of the two sets of nonoverlapping transcription factors by Wnt/β-catenin and Bmp signaling (Fig. S6). Analyses of Bmp4 mutants indicate that Bmp4 signals control the expression of Gata4 and SRF; however, residual Bmp7 expression observed in such mutants cannot take over the role of Bmp4. Apparently, Notch signaling regulates Bmp2 and Bmp7 independently of Wnt, because the expression of Bmp2 and Bmp7 is not rescued in RBPJ/Axin2 mice, whereas Bmp4 expression is rescued in a Wnt-dependent manner. In RBPJ/Axin2 double mutants, the initiation of Hand2 and Mef2c expression is rescued, and this rescue depends on Bmp4, as assessed by the analysis of RBPJ/Axin2/Bmp4 triple mutants. In Bmp4 single mutants, however, Mef2c and Hand2 are not misexpressed. These observations might reflect the fact that Bmp2 and Bmp7 are expressed in Bmp4 mutants, whereas all these Bmp ligands are silenced in RBPJ/Axin2/Bmp4 mutants. We find that Wnt signaling promotes Nkx2-5, Isl1, and Baf60c. That Wnt controls Nkx2-5 has been noted previously, and it has been suggested that this control is mediated by a down-regulation of HDAC1 (15). Several effects of Bmp signaling in cardiomyocyte differentiation have been described previously High Bmp activity is required for Gata4 expression and for the initiation of Nkx2-5 expression at E8, but our data indicate that at E9 the maintenance of Nkx2-5 no longer is Bmp dependent (9, 13, 14). Furthermore, miRNA-dependent fine-tuning of Isl1 also is regulated by Bmp signals (12).
Several laboratories have identified cardiac progenitor and stem cells by using various protocols for sorting and culture (27). We isolated MesP1-derived EYFP+ cardiac progenitor cells of the dissected tissue (heart, including SHF) by FACS to investigate gene expression in cells that carried the cre-mediated RBPJ mutation. Cardiac progenitors (P1 cells) from control mice expressed Nkx2-5 and produced troponin T2 after 1 wk of culture in differentiation medium (27). This result demonstrates that P1 cells indeed represent cardiac progenitors that retain the capacity to differentiate into cardiomyocytes. P1 cells exhibited low Wnt and Bmp4 signaling in the absence of RBPJ, as assessed by the analysis of the respective target genes, whereas Wnt and Bmp4 signaling levels were high in controls and in RBPJ/Axin2 double mutants. The expression of Wnt- and Bmp4-dependent heart-specific transcription factors also was restored in P1 cells. The restoration did depend on Bmp4, because the expression of Mef2c and Hand2 was down-regulated in RBPJ/Axin2/Bmp4 triple-mutant P1 cells. Thus, the sequential activation of Notch, Wnt, and Bmp signaling is required during specification and differentiation of cardiac progenitor cells. In the future, the culture of isolated cardiac progenitors will be useful for biochemical investigations to define the mechanisms by which Notch, Bmp, and Wnt cooperate to control the production of the essential core transcription complexes of the embryonic heart.
Materials and Methods
A detailed description of materials and methods used in this study is given in SI Materials and Methods. Methods are briefly explained below.
The different mouse strains, RBPJlox, Axin2LacZ, Bmp4lox, MesP1-cre, and cre-inducible EYFPlox [Gt(ROSA)loxP-STOP-loxP-EYFP] and the conditions for breeding mice and genotyping primers have been described previously (4, 19, 26, 28, 30). Mutant embryos were identified by PCR using amnion or yolk sac tissue.
Whole E8.75–9.25 embryos were cultured in RPMI1640 (Lonza), 6.7% (vol/vol) FBS (Invitrogen), 10% (vol/vol) rat serum (Sigma), 0.3% D-glucose-monohydrate (Merck), 1.5% (vol/vol) penicillin-streptomycin (Invitrogen), and 16mM HEPES (Sigma) in the absence or presence of recombinant Noggin (350 ng/μL) (R&D Systems), as adapted from methods described previously (31).
Embryos were fixed and used for whole-mount in situ hybridizations or fixed, embedded, and sectioned for immunofluorescence, in situ hybridization, or H&E staining.
For microarray analyses, total RNA of E9.25 hearts, including SHF from three or four independent controls, RBPJ single-mutant embryos, and RBPJ/Axin2 double-mutant embryos were isolated using TRIzol (Invitrogen) and the RNeasy Mini cleanup kit (Qiagen). Total RNA (500 ng) was labeled and hybridized on the MouseRef-8 v2 array (Illumina) as specified by the manufacturer and data were analyzed (accession number GSE36804).
For FACS, cardiac tissue (including SHF) was collected at E9.25, trypsinized for 5 min at 37 °C, and centrifuged at 150 × g for 3 min at 4 °C. Pellets were suspended in 500 μL DMEM (Invitrogen), washed once with PBS, and passed through a cell-strainer cap (35μm) (BD Biosciences). Yellow fluorescent cells were sorted using FACS Aria (BD Biosciences), and apoptotic cells were excluded by eliminating 7-AAD+ cells (BD Biosciences).
Cells isolated by FACS were used for RNA isolation and subsequent qRT-PCR analysis or were cultured for immunofluorescence analysis.
Supplementary Material
Acknowledgments
We thank Dr. C. Birchmeier (Max Delbrueck Center for Molecular Medicine) for helpful discussions and critical reading of the manuscript, Dr. T. Honjo (Kyoto University) for providing RBPJ floxed mice, and Dr. H. P. Rahn’s FACS facility at the Max Delbrueck Center for Molecular Medicine for advice.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE36804).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121236109/-/DCSupplemental.
References
- 1.Cai CL, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 3.Waldo KL, et al. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 4.Saga Y, et al. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- 5.Vincent SD, Buckingham ME. How to make a heart: The origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- 6.MacGrogan D, Nus M, de la Pompa JL. Notch signaling in cardiac development and disease. Curr Top Dev Biol. 2010;92:333–365. doi: 10.1016/S0070-2153(10)92011-5. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- 9.Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circ Res. 2010;107:1428–1444. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang FS, Crabtree GR. Developmental biology: The early heart remodelled. Nature. 2009;459:654–655. doi: 10.1038/459654a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, et al. Bmp signaling regulates myocardial differentiation from cardiac progenitors through a MicroRNA-mediated mechanism. Dev Cell. 2010;19:903–912. doi: 10.1016/j.devcel.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci USA. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, et al. WNT signaling promotes Nkx2.5 expression and early cardiomyogenesis via downregulation of Hdac1. Biochim Biophys Acta. 2009;1793:300–311. doi: 10.1016/j.bbamcr.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Behrens J, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 17.Molenaar M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 18.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 19.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu HM, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng YA, Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6:568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian L, Mahaffey JP, Alcorn HL, Anderson KV. Tissue-specific roles of Axin2 in the inhibition and activation of Wnt signaling in the mouse embryo. Proc Natl Acad Sci USA. 2011;108:8692–8697. doi: 10.1073/pnas.1100328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.High FA, et al. Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest. 2009;119:1986–1996. doi: 10.1172/JCI38922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon C, et al. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon C, et al. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat Cell Biol. 2011;13:1244–1251. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kattman SJ, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, et al. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci USA. 2004;101:4489–4494. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prall OW, et al. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanigaki K, et al. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 31.Jones EA, et al. Dynamic in vivo imaging of postimplantation mammalian embryos using whole embryo culture. Genesis. 2002;34:228–235. doi: 10.1002/gene.10162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.