Abstract
Staphylococcus aureus mastitis is an important cause of economic loss for the dairy industry. Control programs rely on the timely and accurate identification of positive quarters. The effects of sampling time and sample handling were examined in an attempt to improve the accuracy of detection of S. aureus. Premilking and postmilking milk samples were collected from 55 lactating quarters with subclinical S. aureus infection. Each sample was divided into 2 aliquots; one of which was cultured fresh, the other was frozen at -20°C for 14 days before being cultured. Analysis of variance was used to determine the effect of sampling time (premilking vs postmilking) and sample handling (fresh vs frozen) on the detection of S. aureus, as measured by the mean category for colony-forming units per millilitre (cfu/mL). A stratified analysis was required, due to interaction between sampling time and sample handling. Only a fresh postmilking sample was inferior, yielding a lower mean category for cfu/mL (P < 0.05). The ability to detect S. aureus in quarters with subclinical intramammary infection, as measured by the mean category of cfu/mL, was maximized in fresh or frozen premilking samples and in frozen postmilking samples.
Introduction
Staphylococcus aureus mastitis continues to be a costly disease in many dairy herds, despite vigorous research and extension of current udder health management programs (1,2,3). The focus of S. aureus control programs is on preventing the spread of the organism from cow to cow (4,5). Monitoring the infection status of the herd on an ongoing basis is another important aspect of control. Elimination of existing infections has proven to be difficult using standard treatment regimes, unless extensive culling is performed. For such control programs to be successful, it is essential that the producer be able to identify S. aureus-infected cows and quarters reliably.
Bacteriological culture remains the “gold standard” for the diagnosis of S. aureus-infected quarters, despite limitations in the detection of S. aureus infection. The sensitivity for detecting S. aureus from culture of a single composite milk sample in subclinically infected cows is only 58% to 63% (6,7). The low sensitivity is thought to be due to the dilution effect of milk from uninfected quarters. The sensitivity for detecting S. aureus from a single quarter sample increases to only 75% (8). The low sensitivity, regardless of the sampling method (quarter or composite), is because S. aureus is often shed in an intermittent or cyclical pattern and in numbers too low to be detected by conventional culturing methods (8). The sensitivity of detecting infected quarters can be increased to 94% to 99% if 2 or 3 consecutive samples are collected over a period of time (8,9). However, given the additional cost and labor involved, consecutive sampling may not be utilized in many investigations of herd-level mastitis.
Given the limitations of bacteriological culture, researchers have tried to identify practical methods of sample collection and handling, in an attempt to increase the sensitivity of detecting S. aureus in milk samples routinely submitted to veterinary or bacteriological laboratories. Several studies have examined the impact of time of sample collection (premilking vs postmilking) or sample handling (fresh vs frozen samples) on the ability to detect S. aureus infections, often with conflicting results (10,11,12,13,14,15).
The objective of this study was to examine the effects of both sampling time and sample handling on the detection of S. aureus in quarters with subclinical intramammary infection.
Materials and methods
Sample collection and handling
In 1997, 4 commercial Holstein herds were selected purposively for sampling, based on their history of an ongoing herd problem due to S. aureus infections. Sample collection occurred at the time of regular milking and was carried out according to current National Mastitis Council (NMC) recommendations (16). Quarters from all lactating cows in each herd were screened, prior to sample collection, by using the California Mastitis Test (CMT). A premilking and postmilking quarter sample was then collected from each quarter with a CMT score of 2 or 3. California Mastitis Test scores of 2 and 3 are reported to correlate with somatic cell counts of 800 000 to 5 000 000 and > 5 000 000, respectively (17). All premilking and postmilking samples were placed on ice and delivered directly to the Animal Health Laboratory, University of Guelph. Upon arrival at the laboratory, each premilking and postmilking milk sample was divided into 2 aliquots: the first was plated fresh for bacteriological culture, and the second was frozen at -20°C for a period of 14 d before being thawed and plated for bacteriological culture. Thus, 4 different sample types were plated for bacteriological culture: a premilking fresh, a premilking frozen, a postmilking fresh, and a postmilking frozen.
Bacteriological culture techniques
Identification of bacterial colonies was performed in accordance with standard laboratory milk culture methodology (16). Briefly, a calibrated loop was used to plate 0.01 mL of milk from each sample onto trypticase soy agar (Difco Laboratories, Detroit, Michigan, USA) containing 5% sheep blood. The plates were incubated aerobically at 37°C for 48 h. All plates were examined for bacterial growth at 24 and 48 h. A presumptive diagnosis of S. aureus was based on colony morphology and appearance, a positive catalase test, pattern of hemolysis, and a positive tube coagulase test (16). Up to 50 colonies of S. aureus were recorded. If more than 50 colonies of S. aureus were present, a classification of “too numerous to count” (TNTC) was recorded. The presence of other mastitis pathogens was noted. After the initial plating, the remainder of each milk sample was incubated at 37°C for 24 h. If no bacterial growth was observed after 24 h on the initial culture, the incubated milk sample was replated. The replate was incubated for a 24 h period at 37°C and the absence or presence of S. aureus was recorded.
Case definition
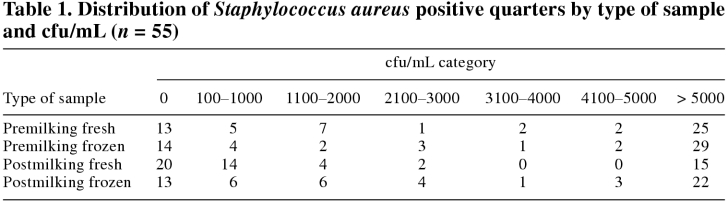
A quarter was defined as truly positive for S. aureus infection if one or more colonies were cultured from at least 1 of the 4 sample types collected or from the replated sample. Therefore, 100% specificity was assumed for the bacteriological methods. For each positive quarter, the number of colony-forming units per millilitre (cfu/mL) of milk from each of the 4 sample types was recorded in 1 of 7 categories (0, 100–1000, 1100–2000, 2100–3000, 3100–4000, 4100–5000, and > 5000 = TNTC cfu/mL).
Statistical analysis
An analysis of variance (ANOVA) (18) was performed to determine if either of the 2 treatments, sampling time or sample handling, had an effect on the ability to detect S. aureus in quarters with subclinical intramammary infection, as measured by the mean category score for cfu/mL. Because interaction was found to exist between the 2 treatments, the data was stratified by the first treatment (sampling time) and reanalyzed, using ANOVA, to look for the true effect of the second treatment (sample handling). This process was repeated by stratifying the data by the second treatment (sample handling) and then reanalyzing, using ANOVA, to look for the true effect of the first treatment (sampling time). The data was also reanalyzed excluding replated sample results. Quarter within cow and cow were considered random variables in the models.
Agreement among the various treatments (fresh premilking vs fresh postmilking, frozen premilking vs frozen postmilking, fresh premilking vs frozen premilking, fresh postmilking vs frozen postmilking) was calculated by using the kappa statistic. This was done to investigate if the various treatments were identifying the same quarters as being infected with S. aureus. The value of kappa can fall between 0 and 1, with values near 0 indicating agreement no better than would be expected by chance, and a value of 1 indicating perfect agreement (19).
Results
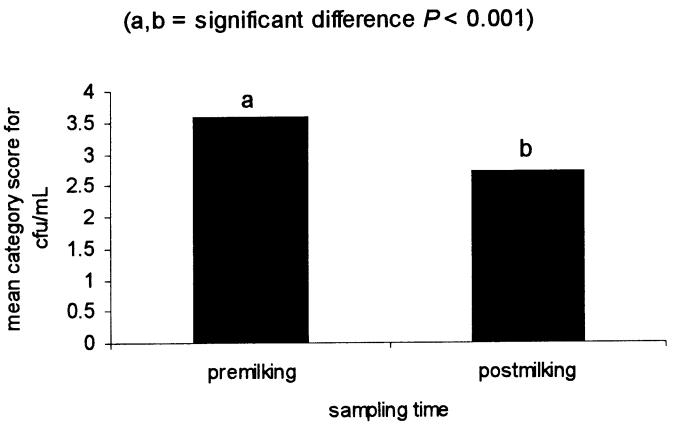
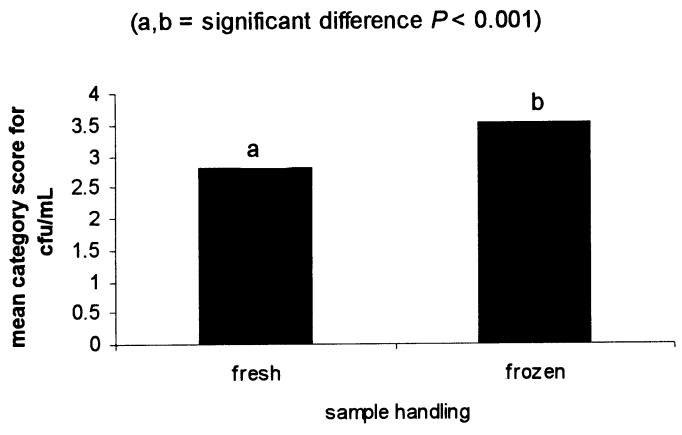
After screening 478 quarters, premilking and postmilking samples were collected from 98 individual quarters with a CMT score of 2 or 3. Of these, 55 quarters were identified as culture positive for S. aureus (see case definition). Forty-six quarters were found on routine culture and an additional 9 quarters were found with the replating technique. Replating increased the detection of S. aureus-positive quarters by 20%. The frequency distribution of S. aureus-positive quarters by type of sample and cfu/mL category is shown in Table 1. Analysis of variance, before stratification by treatment, revealed that both sampling time and sample handling had an effect on the ability to detect S. aureus in quarters with subclinical intramammary infection, as measured by the mean category score for cfu/mL. When examining the effect of sampling time, it was found that bacteriological culture of a premilking sample yielded a significantly higher mean category score for cfu/mL than did a postmilking sample (Figure 1). When examining the effect of sample handling, it was found that bacteriological culture of a frozen sample yielded a significantly higher mean category score for cfu/mL than did a fresh sample (Figure 2).
Table 1.
Figure 1. Effect of sampling time on the mean category score for cfu/mL.
Figure 2. Effect of sample handling on the mean category score for cfu/mL.
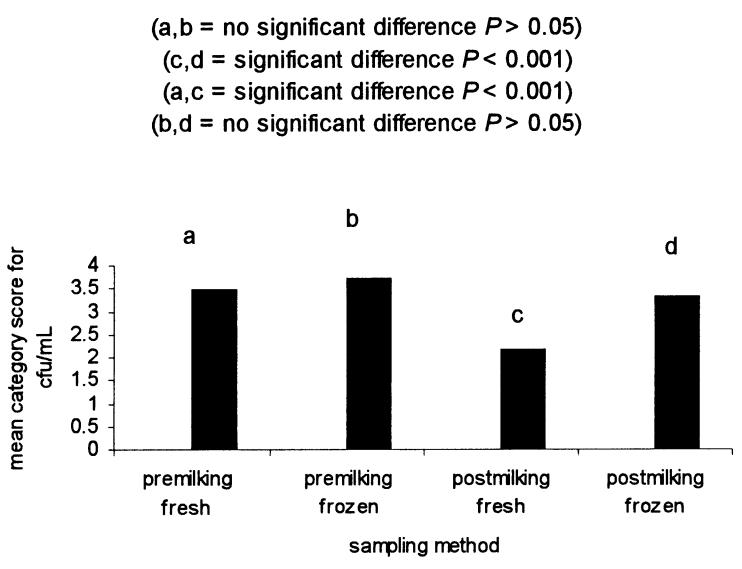
Due to the interaction between sampling time and sample handling, it was necessary to stratify by one treatment in order to determine the true effect of the other treatment (and vice versa). After stratifying by sampling time, ANOVA showed the following: if a premilking sample was cultured, there was no significant effect of sample handling on the mean category score for cfu/mL of S. aureus. However, if a postmilking sample was cultured, a frozen sample yielded a significantly higher mean category score for cfu/mL than did a fresh sample. By examining the data in a different manner, stratification by sample handling showed the following: if a fresh sample was cultured, a premilking sample yielded a significantly greater mean category score of cfu/mL of S. aureus than did a postmilking sample. However, if a frozen sample was cultured, there was no significant effect of sampling time on the mean cfu/mL of S. aureus. The data from the stratified analyses are summarized in Figure 3. Results did not change when replated data was excluded from the analysis.
Figure 3. Effect of sampling time and sample handling on the mean category score for CFU/mL.
Kappa values calculated for the various treatments were 0.62, 0.75, 0.75, and 0.70 for fresh premilking vs fresh postmilking, frozen premilking vs frozen postmilking, fresh premilking vs frozen premilking, and fresh postmilking vs frozen postmilking, respectively.
Discussion
The sensitivity of bacteriological culture for the detection of mastitis infections increases with the number of cfu/mL in the milk sampled. Thus, any method of sample collection or handling that increases the number of cfu/mL of milk should result in improved detection of S. aureus in quarters with subclinical intramammary infection. Results of this study indicate that the highest cfu/mL were counted when either a fresh or a frozen premilking sample or a frozen postmilking sample was cultured. A fresh postmilking sample yielded the lowest mean category of colonies counted. A high level of agreement among treatments indicated that the various treatments were identifying the same quarters as being infected with S. aureus.
Previous studies, which have examined the effects of either sampling time or sample handling, have produced conflicting results. Sears et al (11) recommended the collection of a postmilking sample as opposed to a premilking sample. Results from the study suggested that postmilking samples are less likely to be contaminated because teats and teat canals are washed during the milking process (11). With appropriate training of sampling personnel and careful attention to the sampling procedure used (NMC recommendations), the authors of the current study found very few contaminated samples among either the premilking or the postmilking samples.
In advance of completing the current study, the authors believed that sampling at postmilking would improve the ability to detect S. aureus intramammary infections. Evidence exists to support the theory that S. aureus may concentrate in the upper portion of the udder where they are bound to S. aureus-specific IgA, which is itself bound to the membranes of fat globules (20). Since a postmilking sample has a considerably higher fat content than that of a premilking sample (20), it seemed plausible that a greater concentration of organisms would be recovered in a postmilking sample. Thus, it was thought that the sensitivity for detection of a S. aureus-infected quarter would be increased if a postmilking sample was collected. Based on the results of this study, the hypothesis was rejected.
A number of studies have investigated the effect of freezing on the bacteriological culturing of S. aureus and other mastitis causing pathogens from milk samples. It has been postulated that the freezing process may rupture milk macrophages and neutrophils, releasing phagocytized bacteria. It has also been suggested that freezing may disrupt bacterial cell aggregates. With either scenario, the number of cfu/mL should increase, thereby improving the sensitivity of microbiological culture (14).
The results of this study suggest that the freezing of postmilking samples increases the sensitivity of detection of S. aureus, as compared with the use of fresh postmilking samples. However, this study did not observe a similar positive effect of freezing in premilking samples, when compared with fresh premilking samples. One possible explanation for this apparent discrepancy is in the way that the cfu/mL were counted and categorized. The authors were unable to count above 5000 cfu/mL. As such, this study would be unable to measure a difference in cfu/mL among treatments if both the sample types being compared yielded more than 5000 cfu/mL, which therefore diminished the power to discern a treatment effect. For any fresh samples that already had greater than 5000 cfu/mL (22 samples), no positive effect of freezing would be detectable, even if it did truly exist.
Based on the results of this trial, a second investigation into sampling time and sample handling using composite milk samples may be indicated. Composite samples are more economical and are used more frequently in whole herd screenings than are quarter samples. As mentioned earlier, the sensitivity of a composite sample is much lower than that of a quarter sample. However, a 1991 study of 3 S. aureus-positive herds reported that 86% to 90% of the cows classified positive by quarter sampling were positive by composite sampling (21). The effects of freezing on macrophages, neutrophils, and bacterial aggregates should also be the same, whether composite or quarter samples are collected.
This study examined the effects of sampling time and sample handling on the ability to detect S. aureus, as measured by the mean category score of cfu/mL. Methods such as consecutive sampling, increasing the inoculum volume plated, and replating are alternative ways to increase the sensitivity of detection of S. aureus by increasing the cfu/mL (9,16,22). A 1997 study compared the frequency of detection of S. aureus from direct culture of quarter milk samples with that of centrifugation and sedimentation of quarter milk samples (23). This procedure was found to increase the detection of S. aureus-positive quarters by 86.6% (range 5% to 145.5%), compared with the direct culture method (23). Strategic use of these methods in combination with the results found in this study could further improve the sensitivity of detection for S. aureus intramammary infections in dairy herds. Proper sampling procedures must continue to play a major role when collecting samples for bacteriological culture of mastitis causing pathogens.
Footnotes
Acknowledgments
The assistance of Christopher Church, Kathleen Day, and Jeromy Ten Hag for sample collection was greatly appreciated. Laboratory analyses carried out by Anna Bashiri and the staff of the Animal Health Laboratory are gratefully acknowledged. Mr. Mark McDougall of the Dairy Farmers of Ontario and Dr. Jim Fairles of Harriston-Mount Forest Veterinary Services provided assistance in identifying herds for this study. The scientific consultation of Drs. Don Barnum and Ann Godkin was invaluable. CVJ
Address correspondence and reprint requests to Dr. Jocelyn Jansen.
References
- 1.Blosser TH. Economic losses from and the national research program on mastitis in the United States. J Dairy Sci 1979;62: 119–127. [DOI] [PubMed]
- 2.Ravinderpal G, Howard WH, Leslie KE, Lissemore K. Economics of mastitis control. J Dairy Sci 1990;73:3340–3348. [DOI] [PubMed]
- 3.Kelton DF, Godkin MA, Alves D, Lissemore KD. Establishment of a sentinel dairy network to determine the prevalence of major mastitis pathogens in Ontario. Proc 9th Symp Int Soc Vet Epidemiol Econ 2000:1156–1159.
- 4.Leslie KE, Schukken YH. Herd programs for eliminating and preventing Staphylococcus aureus mastitis. Proc 32nd Annu Meet National Mastitis Council 1993;36–47.
- 5.Philpot WN. Control of mastitis by hygiene and therapy. J Dairy Sci 1979;62:168–176. [DOI] [PubMed]
- 6.Franken P, van Wuijckhuise LA, Morselt ML, Lam TJGM, Schukken YH. Evaluation of the use of composite milk samples for the diagnosis of Staphylococcus aureus and Streptococcus agalactiae intramammary infections in dairy cattle. Proc 3rd Int Dairy Fed Int Mastitis Semin 1995;2:70–71.
- 7.Lam TJGM, van Wuijckhuise LA, Franken P, Morselt ML, Hartman EG, Schukken YH. Use of composite milk samples for diagnosis of Staphylococcus aureus mastitis in dairy cattle. J Am Vet Med Assoc 1996;208:1705–1708. [PubMed]
- 8.Sears PM, Smith BS, English PB, Herer PS, Gonzales RN. Shedding pattern of Staphylococcus aureus from bovine intramammary infections. J Dairy Sci 1990;73:2785–2789. [DOI] [PubMed]
- 9.Buelow KL, Thomas CB, Goodger WJ, Nordlund KV, Collins MT. Effect of milk sample collection strategy on the sensitivity and specificity of bacteriologic culture and somatic cell count for detection of Staphylococcus aureus intramammary infection in dairy cattle. Prev Vet Med 1996;26:1–8.
- 10.Storper M, Ziv G, Saran A. Evaluation of several milk sampling methods for the diagnosis of Staphylococcus aureus and Streptococcus agalactiae mastitis. Refuah Vet 1981;38:149–153.
- 11.Sears PM, Wilson DJ, Gonzalez RN. Microbiological results from milk samples obtained premilking and postmilking for the diagnosis of bovine intramammary infections. J Dairy Sci 1991;74:4183–4188. [DOI] [PubMed]
- 12.Bashandy EY, Heider LE. The effect of freezing of milk samples on the cultural results. Zentralbl Vet Med B 1979;26:1–6. [DOI] [PubMed]
- 13.Schukken YH, Smit JAH, Grommers FJ, Vandegeer D, Brand A. Effect of freezing on bacteriologic culturing of mastitis milk samples. J Dairy Sci 1989;72:1900–1906. [DOI] [PubMed]
- 14.Villanueva MR, Tyler JW, Thurmond MC. Recovery of Streptococcus agalactiae and Staphylococcus aureus from fresh and frozen bovine milk. J Am Vet Med Assoc 1991;198: 1398–1400. [PubMed]
- 15.Murdough PA, Deitz KE, Pankey JW. Effects of freezing on the viability of nine pathogens from quarters with subclinical mastitis. J Dairy Sci 1996;79:334–336. [DOI] [PubMed]
- 16.National Mastitis Council. Laboratory Handbook on Bovine Mastitis. Rev. ed. Madison, WI: National Mastitis Council, 1999.
- 17.Fetrow J. Subclinical mastitis: biology and economics. Compend Cont Educ Pract Vet 1980;11:S223–233.
- 18.SAS Institute Inc., SAS/STAT Software: Changes and Enhancements through Release 6.12, Cary, NC: SAS Institute Inc., 1997.
- 19.Shoukri MM, Edge VL. Statistical Methods for Health Sciences. New York: CRC Press, 1996:83–91.
- 20.Sandholm M, Kaartinen I, Hyvonen P, Veijalainen K, Kuosa PL. Flotation of mastitis pathogens with cream from subclinically infected quarters. Prospects for developing a cream-rising test for detecting mastitis caused by major mastitis pathogens. Zentralbl Veterinarmed 1989;36:27–34. [DOI] [PubMed]
- 21.Hoblet K, Miller GY. Use of partial budgeting to determine the economic outcome of Staphylococcus aureus intramammary infection reduction strategies in three Ohio dairy herds. J Am Vet Med Assoc 1991;199:714–720. [PubMed]
- 22.Buelow K, Nordlund K. Factors affecting sensitivity and specificity of microbiological culture for Staphylococcus aureus. Proc 38th Annu Meet National Mastitis Council 1999;68–75.
- 23.Zecconi A, Piccinini R, Zepponi A, Ruffo G. Recovery of Staphylococcus aureus from centrifuged quarter milk samples. J Dairy Sci 1997;80:3058–3063. [DOI] [PubMed]