Abstract
Human pluripotent stem cells (hPSCs) offer the potential to generate large numbers of functional cardiomyocytes from clonal and patient-specific cell sources. Here we show that temporal modulation of Wnt signaling is both essential and sufficient for efficient cardiac induction in hPSCs under defined, growth factor-free conditions. shRNA knockdown of β-catenin during the initial stage of hPSC differentiation fully blocked cardiomyocyte specification, whereas glycogen synthase kinase 3 inhibition at this point enhanced cardiomyocyte generation. Furthermore, sequential treatment of hPSCs with glycogen synthase kinase 3 inhibitors followed by inducible expression of β-catenin shRNA or chemical inhibitors of Wnt signaling produced a high yield of virtually (up to 98%) pure functional human cardiomyocytes from multiple hPSC lines. The robust ability to generate functional cardiomyocytes under defined, growth factor-free conditions solely by genetic or chemically mediated manipulation of a single developmental pathway should facilitate scalable production of cardiac cells suitable for research and regenerative applications.
Keywords: directed differentiation, chemically defined medium
Because human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) (1) and human induced pluripotent stem cells (iPSCs) (2, 3), can be propagated indefinitely while still retaining the capacity to differentiate into all somatic cell types, they are a potentially inexhaustible supply of human cells, including cardiomyocytes. Over the past decade, substantial advances have been made in generating cardiomyocytes from hPSCs by providing developmental cues during differentiation. Mouse visceral endoderm-like cell-conditioned medium has been shown to enhance cardiac differentiation in embryoid bodies (EBs) (4). The addition of defined growth factors, including activin A, bone morphogenic factor (BMP4), FGF2, VEGF, and Dickkopf-1 (DKK-1), can further enhance cardiomyocyte differentiation in EBs (5). However, this protocol requires monitoring of kinase domain receptor/c-kit (5) or Flk1/PDGF receptor α (6) expression to present growth factors temporally at optimal concentrations to induce efficient cardiac development and thus must be optimized individually for each cell line. This directed differentiation protocol also requires a difficult-to-scale enrichment step, such as cell sorting with an antibody against signal-regulatory protein α (SIRPA) (7), to generate a relatively (up to 98%) pure population of cardiomyocytes.
Identification of defined factors that promote cardiomyocyte differentiation also has enabled development of monolayer-based directed differentiation protocols. Sequential addition of activin A and BMP4 to defined RPMI/B27 medium has been reported to be more efficient than EB-based methods, generating more than 30% cardiomyocytes in the H7 hESC line (8). However, the efficiency of the activin A and BMP4 monolayer-directed differentiation protocol can be highly variable between cell lines and experimental repeats (9).
The Wnt signaling pathway has emerged as one of the key regulators of cardiogenesis in vivo and in vitro. Canonical Wnt ligands direct cell proliferation and cell fate determination during embryonic development through inhibition of glycogen synthase kinase 3 (Gsk3), leading to nuclear accumulation of β-catenin, which associates with T-cell factor/lymphoid enhancer-binding factor (Tcf/Lef) and activates gene transcription. In chick and frog embryos canonical Wnt signaling was shown to repress early cardiac specification (10). Wnt signaling also has been shown to have a biphasic effect on cardiac development in zebrafish, mouse embryos, and mouse ES cells (11, 12), with early Wnt signaling enhancing and later signaling repressing heart development. Endogenous Wnt signaling also is required for hESC differentiation to cardiomyocytes in the monolayer-based directed differentiation protocol (9). However, because of the complex nature of Wnt signaling on cardiac differentiation, previous reports have not identified the temporal requirements or sufficiency of canonical Wnt signaling in hPSC differentiation to cardiomyocytes. Here, we systematically optimize Wnt signaling activity during hPSC differentiation to cardiomyocytes and then illustrate that appropriate temporal modulation of regulatory elements of Wnt signaling alone via genetic approaches or small molecule inhibitors is sufficient to drive multiple hPSC lines to differentiate to cardiomyocytes efficiently, in part by regulating signaling through other pathways critical for cardiomyocyte differentiation. We demonstrated that it is possible to generate populations consisting of up to 98% cardiomyocytes with an extremely high yield (15 cardiomyocytes per hPSC input) from hPSC without any enrichment and/or purification step solely via temporal modulation of regulatory elements of Wnt signaling. We then used this method to develop a robust, inexpensive, completely defined, growth factor-free, scalable method of producing cardiomyocytes from hPSCs.
Results
Differentiation Induced by Gsk3 Inhibitors in hPSCs is β-Catenin Dependent.
To probe the activation of canonical Wnt/β-catenin signaling during hPSC specification to cardiomyocytes, we generated a series of promoter–reporter cell lines in H9 hESC and 19-9-11 human iPSC lines. These reporter lines, integrated with a lentiviral 7TGP vector, express GFP under control of a consensus TCF/LEF binding sequence promoter that reports canonical Wnt/β-catenin signaling activation (Fig. S1A) (13). Although Wnt/β-catenin activation has been reported in undifferentiated mouse embryonic stem cells and hESCs (14–16), we and others (17) failed to observe significant TCF/LEF-mediated transcriptional activity in self-renewing undifferentiated H9-7TGP hESCs in several different culture conditions, including mouse embryonic fibroblast (MEF) coculture, in MEF-conditioned medium on Matrigel, and in mTeSR1 medium on Matrigel (Fig. S1B). However, treatment of the H9-7TGP reporter line with the Gsk3 inhibitor CHIR99021 (CH) activated TCF/LEF promoter activity in mTeSR1 on Matrigel. Immunofluorescent analysis revealed that the CH-induced H9-7TGP GFP+ cells did not express Oct4 but did express Isl1 and Nkx2.5 (Fig. 1A). Heart Isl1+ cells have been shown to be capable of self-renewal and expansion before differentiation into the three major cell types: cardiomyocytes, endothelial cells, and smooth muscle cells (18, 19), and Nkx2.5 (20) is expressed in committed cardiomyocytes and cardiac progenitor cells. Similar results were obtained in the 19-9-11 7TGP iPSC line (Fig. S1C). These results indicate that CH induces differentiation of hPSCs cultured in mTeSR1. Interestingly, we observed heterogeneous activation of Wnt/β-catenin signaling in 7TGP-hPSCs upon CH treatment in mTeSR1; this heterogeneous activation may result from conflicting self-renewal signals in the mTeSR1 medium and differentiation signals from the Gsk3 inhibitor.
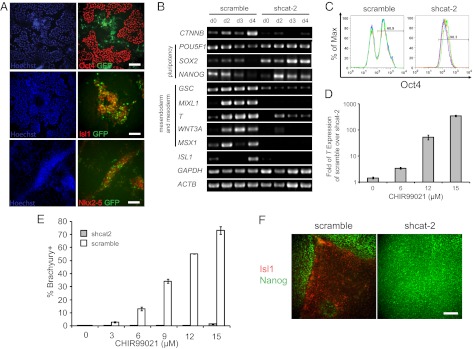
Fig. 1.
Differentiation induced by treatment with the Gsk3 inhibitor in hPSCs is β-catenin dependent. (A) H9-7TGP cells were treated with 12 μM CH in mTeSR1 for 4 d. Immunofluorescent staining for Oct4, Isl1, and Nkx2.5 was compared with GFP expression. (Scale bars, 50 μm.) (B and C) 19-9-11 shcat-2 and scramble cells were cultured on Matrigel with mTeSR1 medium containing 12 μM CH for 4 d. (B) RT-PCR analysis of pluripotent, mesendoderm, early mesoderm, and early cardiac gene expression was performed. (C) Oct4 expression on day 4 was analyzed by flow cytometry. Each colored line represents an independent replicate. n = 3. (D) 19-9-11 shcat-2 and scramble lines were cultured on Matrigel in mTeSR1 containing CH. After 2 d, the expression of T in the scramble relative to its expression in the shcat-2 line was quantified by quantitative PCR. (E) Flow cytometry analysis of brachyury expression in 19-9-11 shcat-2 and scramble cells exposed to CH for 4 d. Error bars represent SEM of three independent replicates. (F) 19-9-11 shcat-2 and scramble lines were cultured on Matrigel in mTeSR1 containing 12 μM CH. After 4 d, cells were immunostained for Nanog and Isl1. (Scale bar, 50 μm.)
To evaluate the role of β-catenin in Gsk3 inhibitor-induced hPSC differentiation, we generated H9 hESC and 19-9-11 iPSC lines carrying lentiviral integrated β-catenin shRNA. This shRNA efficiently down-regulated β-catenin expression compared with control scrambled sequences (Fig. S2 A–C). The β-catenin knockdown cells still maintained high Oct4 and SSEA4 expression (Fig. S2 D and E).
We then treated the β-catenin knockdown (shcat-2) and scramble 19-9-11 lines with CH in mTeSR1 medium to test whether differentiation induced by Gsk3 inhibitors requires β-catenin. Although the shcat-2 line maintained an undifferentiated morphology, the scramble line appeared to undergo differentiation (Fig. S2F), similar to unmodified cell lines treated with CH. Induction of differentiation following CH treatment in the scramble control was indicated further by the disappearance of SOX2 and NANOG and decreased OCT4 expression at day 4 (Fig. 1B). Flow cytometry analysis revealed that the percentage of cells expressing Oct4 decreased to 61% in the scramble line, whereas 98% of the shcat-2 cells expressed Oct4 (Fig. 1C). In addition, expression of genes found in mesendoderm and early mesoderm tissues (MIXL1, GSC, T, WNT3A, and MSX1) emerged in CH-treated scramble cells, but less expression of these genes was observed in CH-treated shcat-2 cells. To understand better the quantitative nature of early mesoderm induction via Gsk3 inhibition, we analyzed expression of the early mesoderm gene T in scramble and shcat-2 19-9-11 lines. As CH concentration increased, the ratio of T expression in scramble to the shcat-2 line increased (Fig. 1D). Flow cytometry analysis revealed that less than 2% of shcat-2 cells expressed brachyury upon exposure to different concentrations of CH for 4 d. In contrast, the scramble line exhibited a CH concentration-dependent increase in the fraction of cells expressing brachyury, containing 76% brachyury-positive cells following treatment with 15 μM CH for 4 d (Fig. 1E). In addition, immunostaining of the scramble cell line after treatment with 12 μM CH in mTeSR1 for 4 d showed substantial numbers of Nanog−/Isl1+ cells, whereas the shcat-2 cells treated with CH contained only Nanog+/Isl1− cells (Fig. 1F). Together these results demonstrate that treatment of undifferentiated hPSCs in mTeSR1 with Gsk3 inhibitors induces differentiation in a β-catenin–dependent manner.
Temporal Key Roles of β-Catenin for Efficient Cardiac Differentiation.
Because Gsk3 inhibition induced differentiation toward early mesoderm cells expressing Isl1 and Nkx2.5, we quantitatively assessed the effect of incorporating Gsk3 inhibitors during previously reported EB- and monolayer-directed differentiation protocols (8). For EB differentiation, undifferentiated H9 hESCs were cultured in the presence of 0–8 μM CH for 3 d before EB formation. Visual analysis of spontaneously contracting outgrowths indicated that the efficiency of cardiomyocyte differentiation peaked at 2–4 μM CH (Fig. 2A). During monolayer-based directed differentiation, sequential addition of activin A and BMP4 generated very few contracting cardiomyocytes from H9 hESCs. However, application of the Gsk3 inhibitors CH or 6-bromoindirubin-3′-oxime (BIO) 3 d before the addition of growth factors greatly enhanced cardiomyocyte generation, producing an average of 50% spontaneously contracting cardiac troponin T (cTnT)-labeled cells (Fig. 2B and Movie S1). BIO pretreatment for 3 d before addition of activin A and BMP4 also enhanced generation of cTnT-expressing cells in the IMR90C4 iPSC line in a dose-dependent manner (Fig. S3A). Together these results demonstrate that treatment of hPSCs with Gsk3 inhibitors before differentiation, using either an EB- or monolayer-directed strategy, dramatically enhanced cardiomyocyte differentiation.
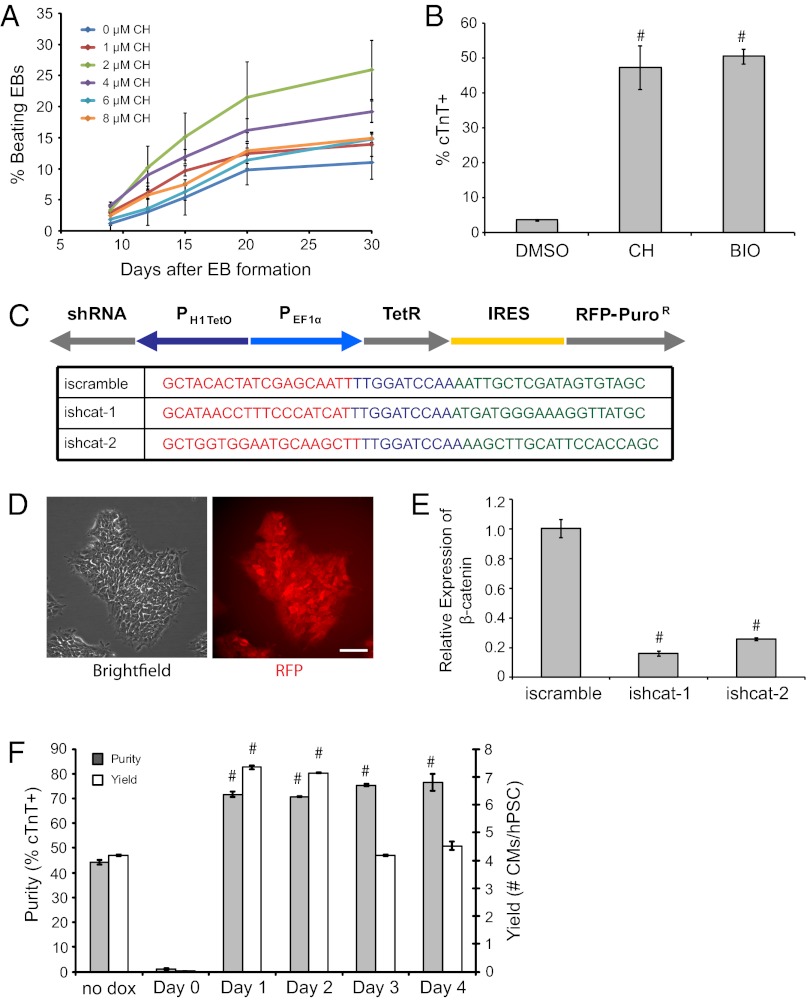
Fig. 2.
Temporal regulation of Wnt/β-catenin signaling promotes cardiac differentiation induced by serum or growth factors. (A) H9 cells on MEFs were treated with CH in hESC medium for 3 d before forming EBs. EBs were cultured in suspension using serum containing medium for 4 d before being transferred to 0.1% (wt/vol) gelatin-coated plates. The percentage of contracting EBs was determined visually. (B) H9 cells were cultured on Matrigel and treated with DMSO, 1 μM CH, or 1 μM BIO for 3 d before exposure to 100 ng/mL activin A at day 0 and 5 ng/mL BMP4 at day 1 in RPMI/B27-insulin medium using monolayer-directed differentiation. At day 15, the percentage of cTnT+ cells in culture was assessed by flow cytometry. #P < 0.005, CH versus DMSO or BIO versus DMSO; Student’s t test. (C) Schematic of the inducible shRNA construct for β-catenin knockdown and shRNA sequences targeting β-catenin. PH1TetO represents the human H1 promoter with Tet operator sequences. Red and green sequences are forward and reverse shRNA sequences of β-catenin, respectively; the loop sequence is shown in blue. (D) Representative phase-contrast and mCherry epifluorescence images of 19-9-11 cells transduced with lentiviral vectors containing the constructs described in C and selected by puromycin treatment. (E) 19-9-11 ishcat-1 and ishcat-2 cells were cultured in mTeSR1 containing 2 μg/mL dox. After 3 d, mRNA was collected, and β-catenin expression was evaluated by quantitative PCR. Error bars represent SEM of three samples. #P < 0.005, ishcat-1 versus iscramble or ishcat-2 versus iscramble; Student’s t test. (F) 19-9-11 ishcat-1 cells were cultured in mTeSR1 medium and were treated with BIO before exposure to 100 ng/mL activin A at day 0 and 5 ng/mL BMP4 at day 1, with 2 μg/mL dox added at the indicated times. Cells were analyzed for cTnT expression by flow cytometry 15 d after initiation of differentiation. Error bars represent SEM. of three independent experiments. #P < 0.005, for each time point versus no dox; Student’s t test.
To assess the temporal requirement of β-catenin for cardiomyocyte generation, we then created 19-9-11 iPSC lines (ishcat-1 and ishcat-2) expressing two different β-catenin shRNA sequences under control of a Tet-regulated inducible promoter (Fig. 2C). Integration of the lentiviral construct was visualized by mCherry expression, and clones were selected based on resistance to puromycin (Fig. 2D). Upon the addition of doxycycline (dox), both shRNAs efficiently down-regulated β-catenin expression (Fig. 2E). We used these cell lines to examine the stage-specific roles of β-catenin during monolayer-directed differentiation induced by activin A and BMP4. Canonical Wnt signaling is essential for cardiac induction, because β-catenin knockdown upon the addition of activin A did not generate cTnT-expressing cells in the 19-9-11 ishcat-1 line (Fig. 2F). Importantly, knockdown of β-catenin expression at later stages of differentiation enhanced cardiogenesis (Fig. 2F). Similar results were observed in the 19-9-11 ishcat-2 line (Fig. S3B).
Highly Efficient Generation of Human Cardiomyocytes Solely by Modulating Regulatory Elements of Wnt Signaling.
Our results and prior studies (21–24) indicate that early induction of canonical Wnt signaling and suppression of canonical Wnt signaling at later stages of differentiation synergistically enhance yield of cardiomyocytes with other growth factors and/or serum. We next sought to determine whether modulating regulatory elements of Wnt signaling alone, in the absence of serum and exogenous growth factors, was sufficient to induce cardiogenesis. Undifferentiated inducible β-catenin knockdown hPSCs lines were treated with CH for 24 h followed by the addition of dox at various time points between day 0 and day 4 (Fig. 3A). Cardiomyocyte differentiation was assessed at day 15 by the percentage and yield of cTnT-expressing cells. In the 19-9-11 ishcat-1 line, 12 μM CH produced the most cTnT+ cells at day 15 without additional dox-induced β-catenin knockdown (Fig. S4A). Addition of dox 36 h after the addition of 12 μM CH generated 98% cTnT+ cells with yields of ∼15 cTnT+ cells per input iPSC without substantial effects on the total cell number (Fig. 3 B and C and Fig. S4B). A high purity of cTnT+ cells also was obtained using 19-9-11 ishcat-2 (Fig. 3D) and three additional (IMR90C4, 6-9-9, and H9) hPSC lines transduced with inducible β-catenin shRNA ishcat-1 (Fig. S4C).
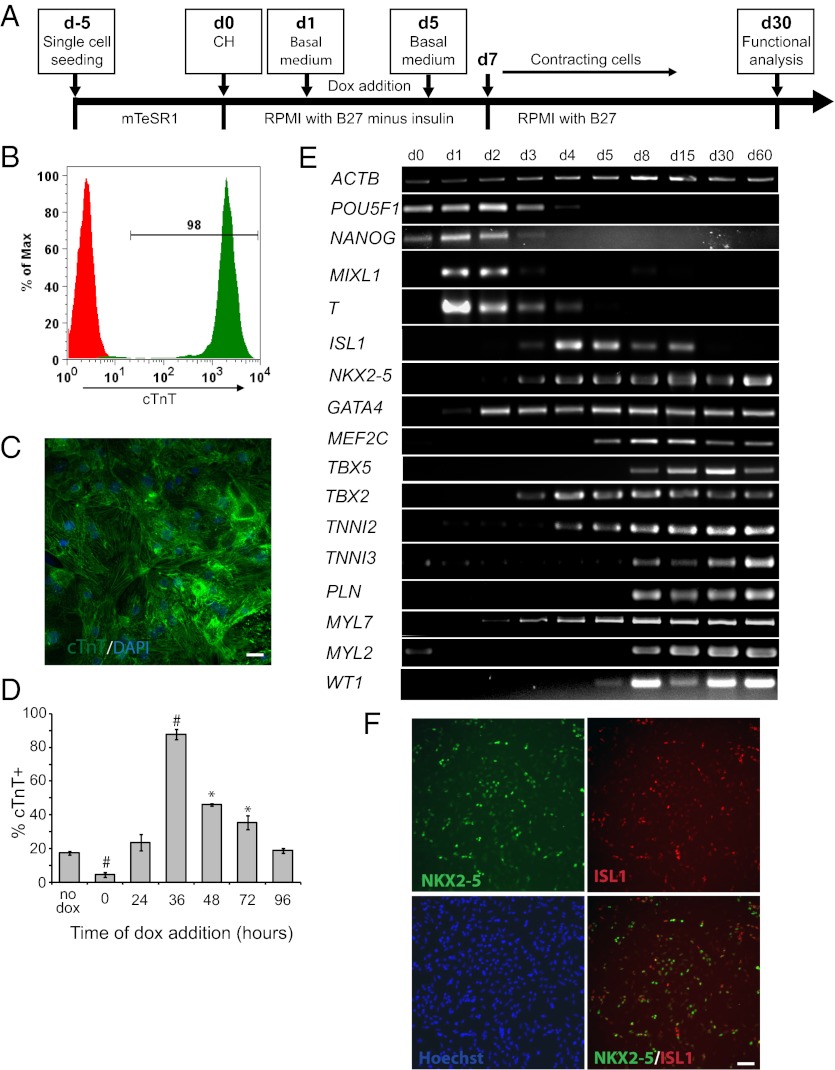
Fig. 3.
Modulating regulatory elements of Wnt signaling is sufficient for efficient and reproducible generation of human cardiomyocytes in the absence of growth factors. (A) Schematic of protocol for defined, growth factor-free differentiation of hPSCs expressing dox-inducible β-catenin shRNA to cardiomyocytes via treatment with small molecules. (B and C) 19-9-11 ishcat-1 cells were cultured as indicated in A with dox added 36 h after treatment with 12 μM CH. At day 15, cells were analyzed for cTnT expression by flow cytometry (B) or immunofluorescence (C). In B, the green histogram represents cTnT expression, and the red histogram is an isotype control. (Scale bar in C, 50 μm.) (D) 19-9-11 ishcat-2 cells were cultured as indicated in A, with dox added at different time points after treatment with 12 μM CH. At day 15, cells were analyzed for cTnT expression by flow cytometry. Error bars represent SEM of three independent experiments. *P < 0.05 and #P < 0.005, each time point versus no dox; Student’s t test. (E and F) 19-9-11 ishcat-1 cells were differentiated as described in A, with dox added 36 h after treatment with 12 μM CH. (E) At different time points, mRNA was collected, and RT-PCR analysis of pluripotent, mesendoderm, mesoderm, and cardiac gene expression was performed. (F) Day 7 cells were analyzed for Isl1 and Nkx2.5 expression by immunofluorescence. (Scale bar, 100 μm.)
Molecular analysis of this differentiation process revealed dynamic changes in gene expression with the induction of the primitive streak-like genes T (25) and MIXL1 (26) shortly after CH addition and down-regulation of pluripotency markers OCT4 and NANOG within 4 d (Fig. 3E). Expression of the cardiac transcription factor NKX2.5 (27) began at day 3 and persisted throughout the 60-d experiment. ISL1, a gene that marks progenitors of the secondary heart field in the early embryo (18), also was detected at day 3, but ISL1 expression ceased by day 30. TBX5 (28), GATA4 (29), and MEF2C (30) are important regulators of cardiomyocyte development, and their expression has been used to convert fibroblasts directly into cardiomyocytes (31). These three genes were expressed at different time points following β-catenin knockdown, and expression of these genes persisted for the full 60 d of the experiment (Fig. 3E). In addition to ISL1, TBX5, and NKX2.5, the cardiac progenitor marker WT1 (32) also was expressed during cardiac differentiation. Immunostaining showed the presence of substantial numbers of Isl1+ and/or Nkx2-5+ cells during differentiation (Fig. 3F).
The optimal differentiation conditions illustrated in Fig. 3B, 12 μM CH followed by dox treatment at 36 h, produced relatively pure (up to 98%) cardiomyocytes that contracted spontaneously as coordinated sheets (Movie S2) in multiple (>50) independent experiments in the 19-9-11 ishcat-1 line, demonstrating consistency and reproducibility. These cardiomyocytes were maintained as spontaneously contracting cells in culture for more than 6 mo (Movie S3). The cardiomyocytes exhibited normal cardiac sarcomere organization, demonstrated by immunofluorescent staining of α-actinin, MLC2a, and cTnT (Fig. 4A and Fig. S4 D–G). Scanning electron microscopy also identified cells with myofibrillar bundles and transverse Z-bands (Fig. 4B and Fig. S4H) and cells enriched in mitochondria (Fig. 4B). Intercalated disks with desmosomes (Fig. S4I), typical of cardiomyocytes, were observed also.
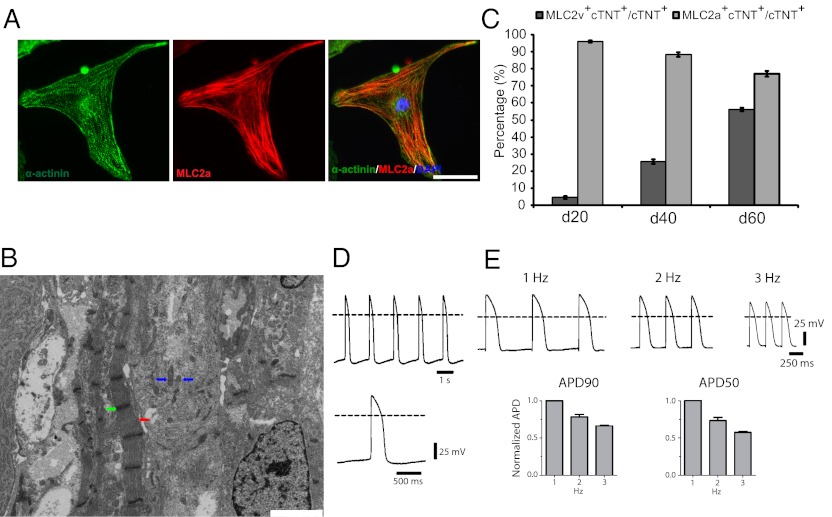
Fig. 4.
Structural and functional characterization of cardiomyocytes generated from hPSCs via modulation of Wnt signaling. (A) Cardiomyocytes were generated from 19-9-11 ishcat-1 cells using the protocol described in Fig. 3A, with treatment with 12 μM CH at day 0 and 2 μg/mL dox 36 h later. At day 30, cells were individualized and replated on 0.1% (wt/vol) gelatin-coated coverslips. Immunostaining for α-actinin and MLC2a shows sarcomere organization. (Scale bar, 50 μm.) (B) Transmission electron microscopic images of beating clusters derived from the 19-9-11 ishcat-1 line as described in A shows myofibrils (red arrow) with Z-bands (green arrow) and mitochondria (blue arrows). (Scale bar, 2 μm.) (C) 19-9-11 ishcat-1 cells were differentiated as described in Fig. 3A, with 12 μM CH added at day 0 and 2 μg/mL dox added 36 h later. Cells were assayed for cTNT, MLC2v, and MLC2a by flow cytometry at the indicated time points. Error bars represent the SEM of three independent experiments. Day 20, day 40, and day 60 are significantly different from each other (P < 0.05) when compared using one-way ANOVA and Tukey post hoc tests. (D) Microelectrode recordings of action potential activity were collected at day 29 in cardiomyocytes derived from the 19-9-11 ishcat-1 cells differentiated as described in A. Dashed lines indicate 0 mV. (E) (Upper) Representative recordings of action potentials collected during field stimulation at 1, 2, and 3 Hz as indicated. (Lower) Bar graphs showing average (± SEM) fractional changes in action potential duration at 90% (APD90) and 50% (APD50) repolarization obtained by normalizing to the values observed in response to 1-Hz stimulation. Data represent SEM of four independent experiments.
The expression pattern of the two major myosin light-chain 2 isoforms, MLC2a and MLC2v, can provide information regarding the diversity and maturity of the cardiomyocytes. To monitor quantitatively the differential expression of myofilament proteins involved in cardiomyocyte specification, we profiled MLC2a and MLC2v expression 20, 40, and 60 d after the induction of differentiation. At day 20, very few cTnT+ cells contained detectable levels of MLC2v, a marker of mature ventricular cardiomyocytes (33–35), but virtually all cTnT+ cells contained MLC2a, which is expressed in atrial and immature ventricular cardiomyocytes (Fig. 4C) (33). By day 60, greater than 50% of the cTnT+ cells expressed MLC2v, whereas the percentage of cTnT+ cells expressing MLC2a decreased to less than 80%, suggesting maturation of a population of ventricular cardiomyocytes.
To provide an initial assessment of the functional competence of cardiomyocytes generated by manipulation of canonical Wnt signaling in the absence of growth factors, we performed sharp microelectrode electrophysiological recordings at 29 d after the addition of CH. Representative recordings of ventricular-like action potentials are shown (Fig. 4D). Cardiomyocytes also exhibited rate adaptation, as evidenced by decreases in the duration of action potentials in response to stimulation at increasing frequencies (Fig. 4E). The observed decreases in duration were comparable in magnitude to those previously observed for hESC- and iPSC-derived cardiomyocytes (36, 37). These results suggest that the ion channels and regulatory proteins involved in action potential generation and regulation are expressed normally in cardiomyocytes generated by Wnt pathway manipulation alone.
Together, these results indicate that spontaneously contracting cardiomyocytes can be generated efficiently from hPSCs solely by manipulating regulatory elements of Wnt signaling in the absence of exogenous growth factors.
Induction of TGF-β Superfamily Signaling by Gsk3 Inhibitors.
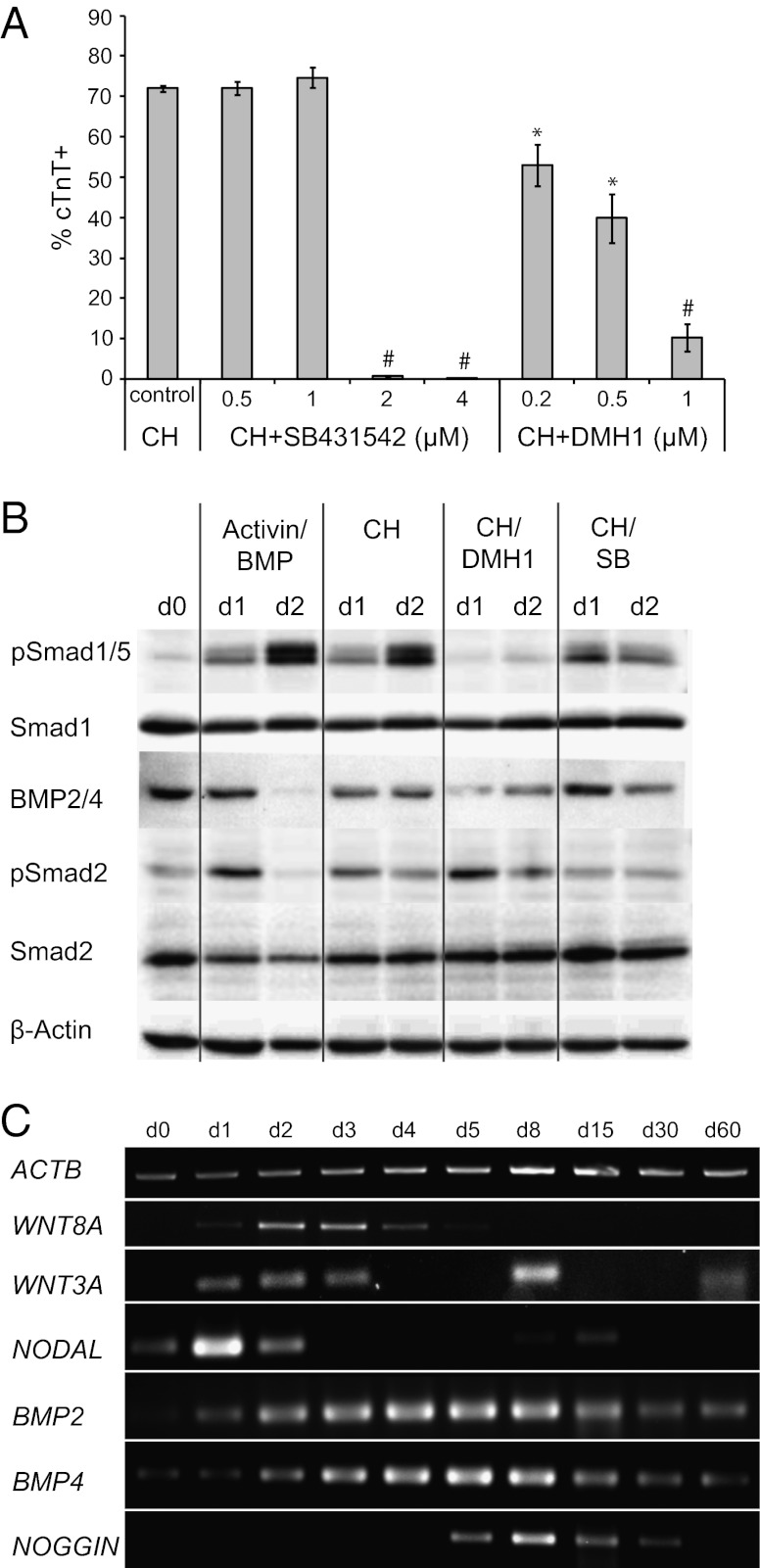
To determine whether canonical Wnt signaling requires TGF-β superfamily signaling to induce cardiogenesis, we quantified cTnT+ cell generation in 19-9-11 ishcat-2 cells when activin A and BMP4 signaling antagonists were presented during the first 24 h of cardiomyocyte induction with 12 μM CH. All samples were treated with dox at 48 h. SB431542 (SB), an inhibitor of activin A receptor-like kinase ALK5 and its relatives ALK4 and ALK7 (38) completely blocked cardiomyocyte specification at concentrations greater than 2 μM (Fig. 5A). Addition of DMH1, a dorsomorphin analogue that inhibits the BMP ALK2 receptor (39), also decreased the percentage of cTnT+ cells in a concentration-dependent manner (Fig. 5A). To investigate further the role of TGF-β superfamily signaling in modulating regulatory elements in Wnt pathway-mediated cardiogenesis, we assessed Smad1/5 and Smad2 phosphorylation downstream of BMP4 and activin A signaling, respectively. As expected, substantial Smad1/5 and Smad2 phosphorylation was detected in cells that had been treated with activin A and BMP4. CH treatment resulted in Smad1/5 and Smad2 activation at levels comparable to those induced by activin A and BMP4. Smad1/5 phosphorylation was strongly attenuated by DMH1, whereas SB reduced Smad2 phosphorylation. Interestingly, endogenous BMP2/4 was detected in undifferentiated hPSCs and cells following CH treatment (Fig. 5B and Fig. S5). Gene-expression analysis revealed that BMP2 and BMP4 were up-regulated gradually upon CH treatment and persisted throughout the differentiation process, whereas a transient up-regulation upon CH treatment was observed for NODAL expression (Fig. 5C). These results indicate that activin/Nodal and BMP signaling are necessary for cardiogenesis induced via modulating regulatory elements of the Wnt pathway and suggest that this signaling may result from endogenous Nodal and BMPs produced during differentiation.
Fig. 5.
Induction of TGF-β superfamily signaling by treatment with a Gsk3 inhibitor. (A) 19-9-11 ishcat-2 cells were treated with 12 μM CH, 12 μM CH plus 0.5–4 μM SB, or 12 μM CH plus 0.2–1 μM DMH1 for 24 h. All samples were treated with 2 μg/mL dox 48 h later. At day 15, the percentage of cTnT+ cells was assessed by flow cytometry. Error bars represent SEM of three independent experiments. *P < 0.05; #P < 0.005, each point versus control; Student’s t test. (B) Expression of BMP2/4 and expression and phosphorylation of Smad proteins were analyzed via Western blot in 19-9-11 ishcat-2 cells undergoing differentiation by 100 ng/mL activin A at day 0 and 5 ng/mL BMP4 at day 1 or treatment with 12 μM CH, 12 μM CH plus 1 μM DMH1, or 12 μM CH plus 1 μM SB for 24 h followed by the addition of 2 μg/mL dox at 36 h. (C) 19-9-11 ishcat-2 cells were differentiated to cardiomyocytes, as shown in Fig. 3A, with 12 μM CH treatment at day 0 and the addition of 2 μg/mL dox at 36 h. At different time points, Wnt and TGF-β pathway gene expression was assessed by RT-PCR.
Differentiation of hPSCs to Cardiomyocytes in Fully Defined, Growth Factor-Free Conditions via Small Molecule Modulation of Regulatory Elements of Wnt Signaling.
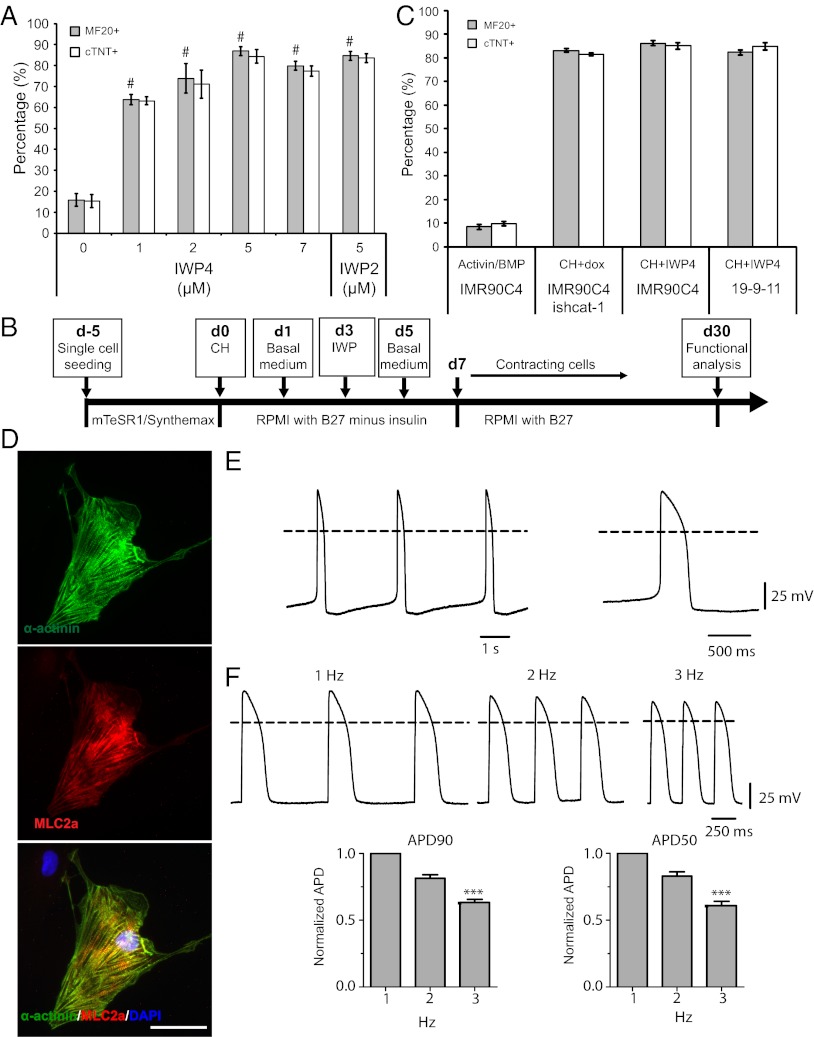
Although shRNA inhibition of β-catenin provides specific and facile temporal regulation of canonical Wnt signaling, this method requires genetic modification of the hPSC line, reducing its utility for potential clinical applications. We next used the mechanistic insight these modified lines provided regarding the sufficiency of modulating regulatory elements in canonical Wnt signaling in cardiomyogenesis to develop a completely defined, growth factor-free method of generating cardiomyocytes from unmodified hPSC lines efficiently, using only small molecule inhibitors of mediators of canonical Wnt signaling. First, 19-9-11 iPSCs were maintained in mTeSR1 on Matrigel for 5 d; then the medium was switched to RPMI/B27-insulin containing 12 μM CH. Inhibitor of Wnt production-4 (IWP4) and Inhibitor of Wnt production-2 (IWP2), which prevent palmitylation of Wnt proteins by Porcupine, thereby blocking Wnt protein secretion and activity (40), were used to inhibit Wnt signaling. Addition of 5 μM IWP4 at day 3 resulted in optimal generation of cardiomyocytes (Fig. 6A and Fig. S6A). Similar to results obtained with the 19-9-11 ishcat-1 cell line, CH treatment of 19-9-11 cells alone generated only 16% cTnT+ or MF20+ cells after 15 d, whereas adding 5 μM IWP4 or IWP2 at day 3 increased this purity to 87% cTnT+ or MF20+ cells. Similar results were obtained when CH was replaced by other Gsk3 inhibitors, including CHIR98014 and BIO-acetoxime (Fig. S6B).
Fig. 6.
Development of a protocol for differentiation of hPSCs to cardiomyocytes in fully defined conditions via small molecule modulation of regulatory elements of Wnt signaling. (A) 19-9-11 cells were cultured on Matrigel in mTeSR1 for 5 d before exposure to 12 μM CH at day 0 and 0–7 μM IWP4 or 5 μM IWP2 at day 3 in RPMI/B27-insulin. At day 15, cTnT expression and MF20 staining were assessed by flow cytometry. Error bars represent SEM of three independent experiments. #P < 0.005, each point versus no drug; Student’s t test. (B) Schematic of protocol for fully defined, growth factor-free differentiation of hPSCs to cardiomyocytes via treatment with small molecules. (C) IMR90C4 and 19-9-11 cells were cultured on Synthemax plates in mTeSR1 for 5 d before exposure to 12 μM CH at day 0 and 5 μM IWP4 at day 3 in RPMI/B27-insulin. IMR90C4 cells were differentiated with 100 ng/mL activin A at day 0 and 5 ng/mL BMP4 at day1 as a control. At day 15, cTNT and MF20 expression were assessed by flow cytometry. Error bars represent SEM of three independent experiments. (D) Cardiomyocytes were generated from 19-9-11 cells using the protocol described in C, with 12 μM CH treatment at day 0 and 5 μM IWP4 treatment 3 d later on Synthemax plates. At day 30, cells were individualized and replated on 0.1% (wt/vol) gelatin-coated coverslips. Immunostaining for α-actinin and MLC2a shows sarcomere organization. (Scale bar, 50 μm.) (E) (Left) Microelectrode recordings of action potential activity were collected at day 29 in cardiomyocytes derived from the 19-9-11 cells differentiated as described in C. (Right) Single action potential taken from the start of the recording shown at an expanded timescale. Dashed lines indicate 0 mV. (F) (Upper) Representative recording of action potentials from a cardiomyocyte derived from the 19-9-11 line during field stimulation at 1, 2, and 3 Hz as indicated. Dashed lines indicate 0 mV. (Lower) Bar graphs showing average (± SEM) fractional changes in action potential duration at 90% and 50% repolarization obtained by normalizing to the values observed in response to 1 Hz stimulation (n = 6) for cardiomyocytes exhibiting a ventricular-like action potential phenotype. A nonparametric Kruskal–Wallis test and Dunn’s posttest were used for statistical comparisons of rate adaptation. ***P < 0.001.
To achieve fully defined cardiomyocyte differentiation conditions, Matrigel was replaced with a defined peptide acrylate surface (Synthemax) during both hPSC expansion and differentiation (Fig. 6B). 19-9-11 and IMR90C4 iPSCs plated on Synthemax plates and treated with CH and IWP4 also generated ∼85% cTnT+ or MF20+ cells, comparable to the efficiency of differentiation observed after CH treatment followed by expression of β-catenin shRNA (Fig. 6C). Similar results were obtained in iPSC line 6-9-9 and hESC line H9 (Fig. S6C). These differentiated populations formed spontaneously contracting sheets of cardiomyocytes (Movies S4 and S5). The cardiomyocytes exhibited normal cardiac sarcomere organization (Fig. 6D). Representative recordings of ventricular-like action potentials are shown (Fig. 6E). The small molecule cardiac differentiation protocol predominantly generated cardiomyocytes with a ventricular-like action potential morphology (32/35, 91.5%). Atrial-like action potentials were observed less commonly (3/35, 8.5%), and nodal-like action potentials were not observed (0/35, 0%). Cardiomyocytes generated by treatment of hPSCs with small molecules also exhibited rate adaptation, as evidenced by decreases in the duration of action potentials in response to stimulation at increasing frequencies (Fig. 6F).
Discussion
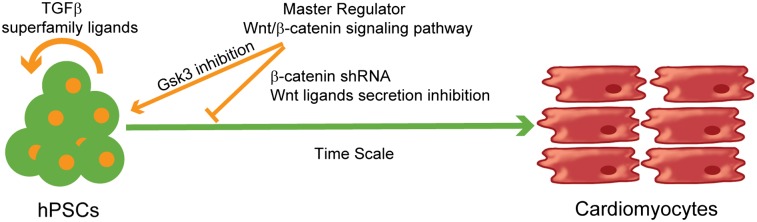
This study demonstrates efficient and robust generation of cardiomyocytes from multiple hPSC lines solely via small molecule modulation of regulatory elements of Wnt/β-catenin signaling. Using the 7TGP lines, we showed that activation of Wnt/β-catenin signaling promotes differentiation, not self-renewal, of hPSCs, a result that is consistent with a recent publication from Davidson et al. (41). Furthermore, our data demonstrate that β-catenin is essential for cardiogenesis upon hPSC treatment with activin A and BMP4. Knockdown studies conclusively demonstrated that β-catenin is required in hPSC differentiation to mesoderm and cardiac progenitors induced by Gsk3 inhibitors. In addition, β-catenin knockdown at the appropriate differentiation stage enhanced generation of cardiomyocytes during monolayer-based directed differentiation induced by ligands of the TGF-β superfamily. Recently, cardiomyocytes were isolated by cell sorting with an antibody against SIRPA, producing up to 98% purified cardiomyocytes from hPSC differentiation cultures (7). However, this technique also has disadvantages with regard to its cost, efficiency, response speed, separate resolution, and scalability. Furthermore, if cells are to be cultivated after subsequent sorting, the damage to cells caused by to the sorting process should be minimized. Instead, we demonstrated that it is possible to generate up to 98% cardiomyocytes by temporally modulating regulatory elements of Wnt signaling without subsequent enrichment or purification. Greater than 82% cardiomyocytes were obtained in six hESC and iPSC lines, including the 19-9-11 and 6-9-9 lines which exhibited low rates of cardiogenesis in EB differentiation (Table S1). Most importantly, we showed that small molecules modulating regulatory elements of a single developmental pathway, Wnt/β-catenin signaling, is sufficient for highly efficient and reproducible cardiac differentiation in multiple hPSC lines under fully defined, growth factor-free conditions. This finding suggests that canonical Wnt signaling can act as a master regulator of cardiomyocyte specification from pluripotent cells, with temporal changes in Wnt signaling regulating autocrine and paracrine signals that also are involved in cardiac development, including TGF-β superfamily pathways.
Cardiomyocyte differentiation was sensitive to the timing and dose of Wnt pathway modulation. During growth factor-free directed differentiation, optimization of timing of Wnt pathway regulators generated 98% cTnT+ cardiomyocytes. To achieve a high purity of cardiomyocytes by β-catenin knockdown, the addition of dox must be initiated within several hours of the 36-h postdifferentiation optimum. This result is consistent with a previous study that reported cardiac potential is restricted to a narrow window of mesoderm development (42) and an EB-based study which found that kinetics of differentiation of each cell line need to be evaluated for optimal germ-layer induction (6).
Although exogenous TGF-β superfamily growth factors are not necessary for cardiomyocyte differentiation, activin/Nodal and BMP pathway inhibitors resulted in a dramatic decrease in cTnT+ cells. Expression of Nodal and BMPs early in differentiation, when 90% of the cells are either Oct4- or brachyury-positive, suggests that endogenous Nodal/BMPs may be produced by undifferentiated cells or mesendoderm cells. Modulation of regulatory elements in Wnt pathway signaling triggers expression of a variety of developmental cues [e.g., Nodal (6), BMP2/4 (6, 8), Noggin (43), WNT3a (24), and WNT8a (9)] and transcription factors involved in cardiomyocyte differentiation [e.g., T (25), MIXL1 (26), ISL1 (18) and NKX2-5 (27), TBX5 (28), and MEF2C (30)]. The paradigm of modulating regulatory elements from a single critical developmental pathway that then results in a more complex developmental program also may simplify hPSC differentiation to other therapeutically relevant lineages.
The use of small molecules to regulate developmental programs has been described in reprogramming somatic cells to human iPSCs and directed differentiation of hPSCs to clinically relevant lineages. For example, ALK4/5/7 inhibitors have been shown to enhance reprogramming (44, 45) via overexpression of reprogramming transcription factors. LY294002 (46), a PI3K inhibitor, and IDE1 (47), an activator of the Nodal pathway, promote endodermal differentiation of hPSCs treated with serum and/or activin A. Inhibitors of Wnt production enhance serum and BMP4-based cardiac differentiation of hPSCs in EBs (23). However, these protocols require the expression of transcription factors or application of serum and/or growth factors for cell fate conversion. Here we show that small molecules alone are sufficient to convert hPSCs to cardiomyocytes efficiently when applied at the appropriate developmental stages. The use of small molecules instead of growth factors ultimately could allow inexpensive and reproducible generation of human cardiomyocytes or multipotent tissue-specific stem cells in completely chemically defined conditions, facilitating translation of these cells to high-throughput screening applications or regenerative therapies (48).
Methods
Maintenance of hPSCs.
Transgene-free human iPSCs (6-9-9 and 19-9-11) (49), lentiviral integrated human iPSC (IMR90C4) (2), and hESCs (H9, H13, H14) (1) were maintained on MEF feeders in hESC medium: DMEM/F12 culture medium supplemented with 20% (vol/vol) KnockOut serum replacer, 0.1 mM nonessential amino acids, 1 mM l-glutamine (all from Invitrogen), 0.1 mM β-mercaptoethanol (Sigma), and 10 ng/mL human bFGF (Invitrogen). Conditioned medium is hESC medium conditioned by MEFs for 24 h (50). For feeder-free culture, hPSCs were maintained on Matrigel (BD Biosciences) or Synthemax plates (Corning) in mTeSR1 medium (STEMCELL Technologies).
Cardiac Differentiation via EBs.
hPSCs were passaged onto MEFs (∼13,000 cells/cm2) and cultured in hESC medium for 2 d followed by another 3 d in hESC medium supplemented with BIO (Sigma) or CHIR99021 (Selleck). To form EBs, hPSC cell aggregates generated by dispase treatment were cultured in low-attachment plates overnight in RPMI plus 20% (vol/vol) KnockOut serum replacer. The next day, the EBs were cultured in RPMI20 (RPMI plus 20% FBS) for 4 d in suspension. EBs then were plated onto 0.1% (wt/vol) gelatin-coated six-well culture plates at 50–100 EBs per well and were cultured in RPMI20 medium. After 10 d of differentiation, the FBS concentration was reduced to 2% (vol/vol) RPMI2 (RPMI plus 2% FBS). The number of contracting EBs was assessed visually using a microscope with a 37 °C-heated stage.
Cardiac-Directed Differentiation Using Activin A and BMP4.
hPSCs maintained on Matrigel in mTeSR1 were dissociated into single cells with Accutase (Invitrogen) at 37 °C for 5 min and then were seeded onto a Matrigel-coated cell-culture dish at 100,000–200,000 cell/cm2 in mTeSR1 supplemented with 5 μM ROCK inhibitor (Y-27632; Stemgent) (day −5) for 24 h. Cells then were cultured in mTeSR1, which was changed daily. At day 0, cells were treated with 100 ng/mL activin A (R&D) in RPMI/B27-insulin. After 24 h, the medium was changed to RPMI/B27-insulin supplemented with 5 ng/mL BMP4 (R&D) for another 4 d. At day 5, the medium was changed to RPMI/B27-insulin. At day 7 the cells were transferred to RPMI/B27, and medium was changed every 3 d. When Gsk3 inhibitors were used to stimulate cardiomyocyte differentiation, cells were cultured in mTeSR1 containing BIO or CHIR99021 from day −3 to day 0.
Cardiac-Directed Differentiation via Small Molecules.
Cells were dissociated and plated as described in the activin/BMP4 protocol. When hPSCs maintained on Matrigel or Synthemax plates achieved confluence, cells were treated with CH in RPMI/B27-insulin for 24 h (day 0 to day 1). The medium was changed to RPMI/B27-insulin, followed by treatment with 2 μg/mL dox at different times between day 1 and day 5 for transgenic cell lines. For genetically unmodified lines, 5 μM IWP2 (Tocris) or IWP4 (Stemgent) was added at day 3 and removed during the medium change on day 5. Cells were maintained in the RPMI/B27 starting from day 7, with the medium changed every 3 d. Other small molecules, including SB431542 (Stemgent) and DMH1 (Sigma), were used in this study.
Lentiviral Production and Infection of Human Pluripotent Stem Cells.
The pLKO.1-based β-catenin constitutive knockdown vectors shcat-1 and shcat-2 (plasmids 19761 and 19762; Addgene) and the β-catenin–inducible knockdown vectors ishcat-1 and ishcat-2 (Biosettia) were used for lentivirus particle production. These vectors were cotransfected with the helper plasmids psPAX2 and pMD2.G (plasmids 12260 and 12259; Addgene) into HEK-293TN cells (System Biosciences) for virus production. Virus-containing media were collected at 48 and 72 h after transfection and used for human pluripotent stem cells (hPSC) infection in the presence of 6 μg/mL Polybrene (Sigma). Transduced cells were selected and clonally isolated based on resistance to 1 μg/mL puromycin.
RT-PCR and Quantitative RT-PCR.
Total RNA was prepared with the RNeasy mini kit (QIAGEN) and treated with DNase (QIAGEN). RNA (0.1 μg) was reverse transcribed into cDNA via Oligo (dT) with SuperScript III Reverse Transcriptase (Invitrogen). Real-time quantitative PCR was done in triplicate with iQ SYBR Green SuperMix (Bio-Rad). RT-PCR was performed with Gotaq Master Mix (Promega) and then subjected to 2% (wt/vol) agarose gel electrophoresis. ACTB was used as an endogenous control. The primer sequences are listed in Table S2.
Flow Cytometry.
Cells were dissociated into single cells and then fixed with 1% (vol/vol) paraformaldehyde for 20 min at room temperature and stained with primary and secondary antibodies in PBS plus 0.1% (vol/vol) Triton X-100 and 0.5% (wt/vol) BSA. Data were collected on a FACSCaliber flow cytometer (Beckton Dickinson) and analyzed using FlowJo. Antibodies are listed in Table S3.
Immunostaining.
Cells were fixed with 4% (vol/vol) paraformaldehyde for 15 min at room temperature and then stained with primary and secondary antibodies in PBS plus 0.4% (vol/vol) Triton X-100 and 5% (wt/vol) nonfat dry milk (Bio-Rad). Nuclei were stained with Gold Antifade Reagent with DAPI (Invitrogen). An epifluorescence microscope (DM IRB; Leica) with a QImaging Retiga 4000R camera was used for imaging analysis. Antibodies are listed in Table S3.
Electron Microscopy.
Contracting areas were microdissected and replated onto gelatin-coated glass coverslips. The clusters were fixed overnight at 4 °C in a 2.5% (vol/vol) gluteraldehyde, 2% (vol/vol) paraformaldehyde, 0.1 M cacodylate buffer solution and then were postfixed with 1% (wt/vol) osmium tetroxide. Samples were dehydrated via an ethanol gradient and embedded in Durcapan (Fluka). The glass coverslip was dissolved with hydrofluoric acid treatment. Ultrathin 60-nm sections were stained with uranyl acetate and lead citrate. Samples were visualized on a Phillips CM120 scanning transmission electron microscope.
Western Blot Analysis.
Cells were lysed in M-PER Mammalian Protein Extraction Reagent (Pierce) in the presence of Halt Protease and Phosphatase Inhibitor Mixture (Pierce). Proteins were separated by 10% (wt/vol) Tris⋅Glycine SDS/PAGE (Invitrogen) under denaturing conditions and transferred to a nitrocellulose membrane. After blocking with 5% (wt/vol) milk in Tris-buffered saline with Tween, the membrane was incubated with primary antibody overnight at 4 °C. The membrane then was washed, incubated with an anti-mouse/rabbit peroxidase-conjugated secondary antibody (1:1,000, Cell Signaling) at room temperature for 1 h, and developed by SuperSignal chemiluminescence (Pierce). Antibodies are listed in Table S3.
Electrophysiology.
Beating cardiomyocyte clusters were microdissected and replated onto glass coverslips and were maintained in RPMI2 medium before recording. Action potential activity was assessed using glass microelectrodes (50–100 MΩ; 3 M KCl) in a 37 °C bath continuously perfused with Tyrode’s solution (in mmol/L): 140 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 10 Hepes, 10 glucose (pH 7.4), NaOH. Junction potentials and capacitance were nulled, and data were acquired at 10 kHz with an AxoClamp2A amplifier and pClamp 9.2 software (Molecular Devices). Electrical field stimulation was performed using two platinum electrodes coupled to a Grass SD9 stimulator (Grass Technologies). For analysis, data were filtered offline using a low-pass Gaussian filter with a cutoff frequency of 2 kHz.
Statistics.
Data are presented as mean ± SEM. The statistical significance of differences between two groups was determined by two-tailed Student’s t test. A Kruskal–Wallis test and Dunn’s posttest were used for statistical comparisons of electrophysiology data. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Yue Wu, David Johnson, and Matt Tomai for additional experimental assistance. This work was supported by National Institutes of Health Grants R01 EB007534 and U01 HL099773 and National Science Foundation Emerging Frontiers in Research and Innovation Grant 0735903.
Footnotes
Conflict of interest statement: T.J.K. is a founder and consultant for Cellular Dynamics International, a company that uses human stem cells for drug testing.
This article is a PNAS Direct Submission.
See Author Summary on page 10759 (volume 109, number 27).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200250109/-/DCSupplemental.
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Graichen R, et al. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357–370. doi: 10.1111/j.1432-0436.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 6.Kattman SJ, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Dubois NC, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 9.Paige SL, et al. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueno S, et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naito AT, et al. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuerer C, Nusse R. Lentiviral vectors to probe and manipulate the Wnt signaling pathway. PLoS ONE. 2010;5:e9370. doi: 10.1371/journal.pone.0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anton R, Kestler HA, Kühl M. Beta-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 2007;581:5247–5254. doi: 10.1016/j.febslet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 16.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 17.Dravid G, et al. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- 18.Bu L, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 19.Qyang Y, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Elliott DA, et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods. 2011;8:1037–1040. doi: 10.1038/nmeth.1740. [DOI] [PubMed] [Google Scholar]
- 21.Willems E, et al. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res. 2011;109:360–364. doi: 10.1161/CIRCRESAHA.111.249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Hao J, Hong CC. Cardiac induction of embryonic stem cells by a small molecule inhibitor of Wnt/β-catenin signaling. ACS Chem Biol. 2011;6:192–197. doi: 10.1021/cb100323z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Y, et al. Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J Mol Cell Cardiol. 2011;51:280–287. doi: 10.1016/j.yjmcc.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran TH, et al. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells. 2009;27:1869–1878. doi: 10.1002/stem.95. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi M, et al. Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. FASEB J. 2009;23:114–122. doi: 10.1096/fj.08-111203. [DOI] [PubMed] [Google Scholar]
- 26.Davis RP, et al. Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood. 2008;111:1876–1884. doi: 10.1182/blood-2007-06-093609. [DOI] [PubMed] [Google Scholar]
- 27.Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: A novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:969. doi: 10.1242/dev.119.3.969. [DOI] [PubMed] [Google Scholar]
- 28.Bruneau BG, et al. Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev Biol. 1999;211:100–108. doi: 10.1006/dbio.1999.9298. [DOI] [PubMed] [Google Scholar]
- 29.Kuo CT, et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 30.Edmondson DG, Lyons GE, Martin JF, Olson EN. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120:1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- 31.Ieda M, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smart N, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubalak SW, Miller-Hance WC, O’Brien TX, Dyson E, Chien KR. Chamber specification of atrial myosin light chain-2 expression precedes septation during murine cardiogenesis. J Biol Chem. 1994;269:16961–16970. [PubMed] [Google Scholar]
- 34.Segev H, et al. Molecular analysis of cardiomyocytes derived from human embryonic stem cells. Dev Growth Differ. 2005;47:295–306. doi: 10.1111/j.1440-169X.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 35.Franco D, et al. Myosin light chain 2a and 2v identifies the embryonic outflow tract myocardium in the developing rodent heart. Anat Rec. 1999;254:135–146. doi: 10.1002/(SICI)1097-0185(19990101)254:1<135::AID-AR17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 36.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: Action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inman GJ, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 39.Hao J, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson KC, et al. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci USA. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kouskoff V, Lacaud G, Schwantz S, Fehling HJ, Keller G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc Natl Acad Sci USA. 2005;102:13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Q, et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–587. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin T, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ichida JK, et al. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLean AB, et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 47.Borowiak M, et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Yu J, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu C, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]