Abstract
Giant insects, with wingspans as large as 70 cm, ruled the Carboniferous and Permian skies. Gigantism has been linked to hyperoxic conditions because oxygen concentration is a key physiological control on body size, particularly in groups like flying insects that have high metabolic oxygen demands. Here we show, using a dataset of more than 10,500 fossil insect wing lengths, that size tracked atmospheric oxygen concentrations only for the first 150 Myr of insect evolution. The data are best explained by a model relating maximum size to atmospheric environmental oxygen concentration (pO2) until the end of the Jurassic, and then at constant sizes, independent of oxygen fluctuations, during the Cretaceous and, at a smaller size, the Cenozoic. Maximum insect size decreased even as atmospheric pO2 rose in the Early Cretaceous following the evolution and radiation of early birds, particularly as birds acquired adaptations that allowed more agile flight. A further decrease in maximum size during the Cenozoic may relate to the evolution of bats, the Cretaceous mass extinction, or further specialization of flying birds. The decoupling of insect size and atmospheric pO2 coincident with the radiation of birds suggests that biotic interactions, such as predation and competition, superseded oxygen as the most important constraint on maximum body size of the largest insects.
Keywords: paleoecology, temperature, maximum likelihood estimation
Because metabolic oxygen demand increases with increasing body size, environmental oxygen concentration (pO2) is frequently invoked as an important constraint on the size of animals (1–6). Giant late Paleozoic insects, with wingspans as large as 70 cm, are the iconic example of the oxygen-body size link; hyperoxic conditions during the Carboniferous and Permian are thought to have permitted the spectacular sizes of the largest insects ever (2, 4). The physiological basis linking insect body size and pO2 has been elucidated in numerous experimental tests (5, 7, 8). Body size and metabolic rate respond to pO2 when insects are reared in hypoxic or hyperoxic atmospheres (7, 8), although the effects are not uniform in all taxa (9). Flying insects should be particularly susceptible to variations in atmospheric pO2 because their flight musculature has high energy demands (10), particularly during periods of active flight (11, 12). The volume occupied by tracheae, tubes that transport oxygen throughout the body, scales hypermetrically with body volume, imposing further surface area-to-volume constraints on maximum size (13, 14).
Although these responses underscore the physiological importance of oxygen, developmental plasticity exhibited by different insect groups may not be indicative of evolutionary changes (15), especially in natural settings where other abiotic influences, biotic interactions, and selective pressure from allometric scaling of life-history traits are also important (16–20). For example, temperature can also be an important influence on insect body size via physiological effects on metabolic oxygen demand and ecological effects on food supply, growing season, and foraging time (20). Furthermore, the relationship between atmospheric pO2 and maximum insect size is more complicated than implied by coincidence of late Paleozoic hyperoxia and insect gigantism, raising the possibility that other factors, such as competitive or predatory interactions with flying vertebrates (birds, bats, and pterosaurs), may have contributed to or even been the dominant control on evolutionary size trends (18, 19). The rich fossil history of insects (21) contains the only record of evolutionary body-size trends in response to changing atmospheric pO2 (22), temperature, and evolution of flying predators and competitors (23). Our quantitative analysis of evolutionary size trends from more than 10,500 measured fossil insect specimens confirms the importance of atmospheric pO2 in enabling the evolution of large body size, but suggests that the pO2-size relationship was superseded by biological factors following the evolution and aerial specialization of flying vertebrates, particularly birds.
Results
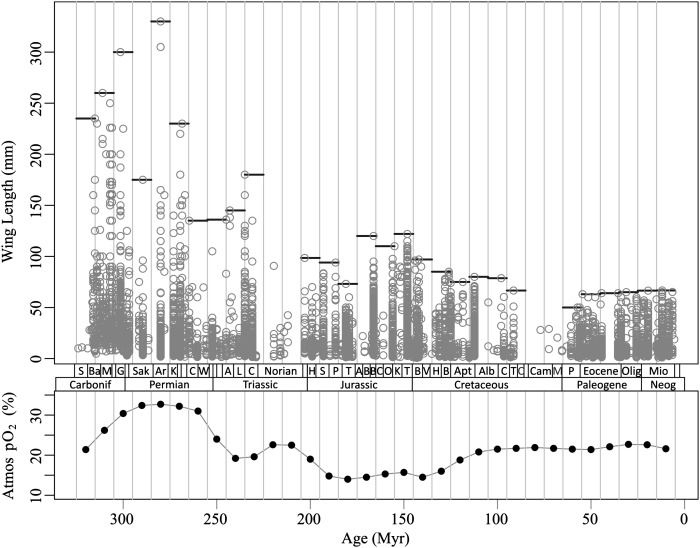
Trends in maximum body size broadly parallel the atmospheric pO2 pattern, including an increase to a Carboniferous-Early Permian maximum, decrease in the Late Permian and Early Triassic, secondary peak in the Middle Triassic, and further decrease in the Late Triassic-Early Jurassic (Fig. 1). Although the largest insects are rare, observed trends cannot be explained by sampling bias (SI Text) and the overall size decrease from the Permian to Recent occurs despite a significant increase in the richness and abundance of fossil insects in the Cenozoic (21). The overall correlation between pO2 and maximum wing length in each 10-Myr bin is highly significant (r = 0.54, P = 0.002), although the strength of the size-oxygen relationship is greatly diminished (P = 0.42) after controlling for collection paleolatitude (a proxy for temperature) via multiple linear regression or for autocorrelation in body-size data using generalized least-squared regression (P = 0.62, P = 0.80 including paleolatitude). These results, as well as instances of decoupling, such as an Early Cretaceous decrease in insect size during a substantial increase in atmospheric oxygen, imply that atmospheric oxygen did not control maximum body size over the entire evolutionary history of insects, or that it was not the only control.
Fig. 1.
Phanerozoic trends in insect wing lengths and atmospheric pO2 (GEOCARBSULF model). Maximum size in each 10-Myr bin containing more than 50 measurements is indicated by black lines.
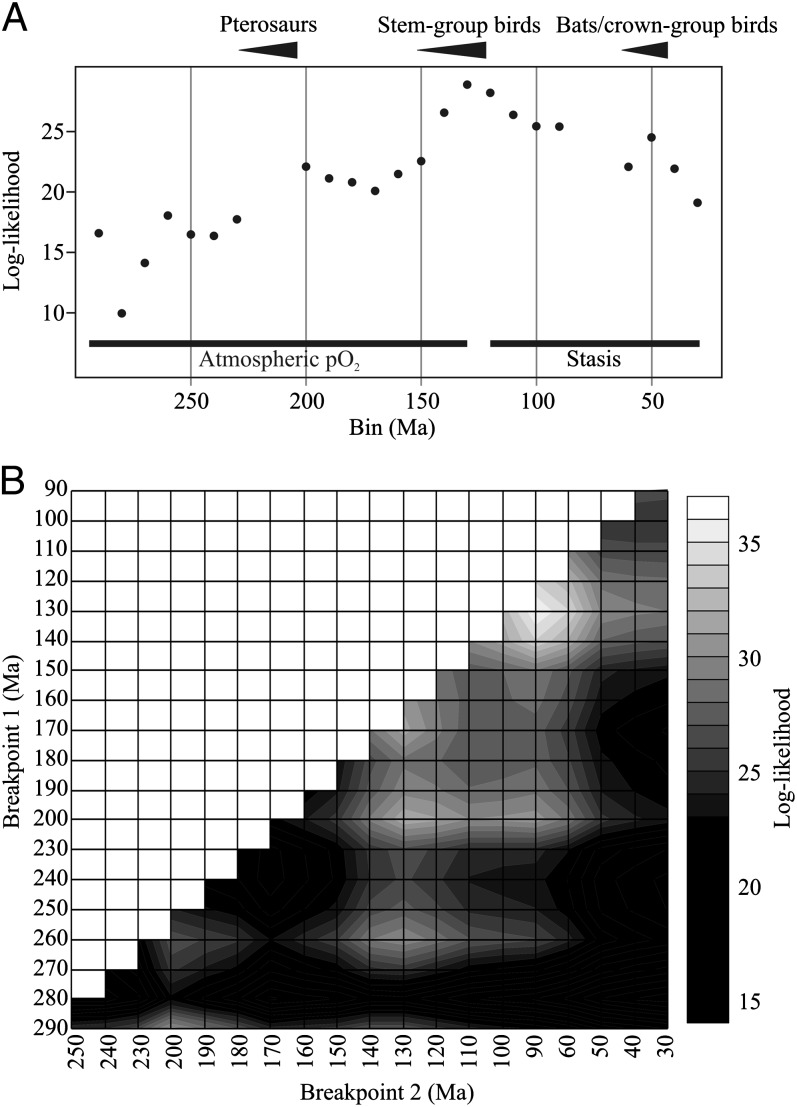
To test the apparent decoupling of pO2 and size records, we used maximum-likelihood estimation to compare models with a shift in evolutionary dynamics of maximum body size, from oxygen control to stasis. We generated two-phase models and three-phase models (oxygen control, followed by body-size stasis, followed by an independent interval of stasis) and adjusted the timing of the evolutionary shift to find the best-supported breakpoint. In the models, the intercept (reflecting the maximum size) was allowed to vary between independent intervals of stasis, but the variance (of the residuals around the regression line) was assumed to be the same and was held constant. The best-supported two-phase model had a breakpoint between 140 and 130 Ma (a breakpoint between 130 and 120 Ma performed nearly as well) (Fig. 2A). The best-supported three-phase model indicated breakpoints at 140 Ma (as in the two-phase model) and between 90 and 60 Ma (Fig. 2B).
Fig. 2.
Maximum-likelihood model fit relative to timing of shift from atmospheric pO2 control to size stasis. (A) Log-likelihood plotted against all possible breakpoints in a two-phase size evolution model. Greater log-likelihoods indicate greater support for a breakpoint, implying that the best breakpoint is at 140–130 Ma. (B) Log-likelihood contours on all possible two-breakpoint combinations in a three-phase size evolution model (breakpoints must be separated by three intervals to fit the models). The best-supported pair of breakpoints is at 140–130 Ma and 90–60 Ma. The support for a 270–260 Ma breakpoint is an artifact of the shift from extremely insect-rich bins to insect-poor Late Permian bins.
We compared the best two-phase and three-phase size models with a model explaining maximum size solely as a function of pO2 and with a four-phase size model (oxygen, stasis, independent stasis, and independent stasis) also including a breakpoint at 230–200 Ma. Although a 230 Ma breakpoint was not the best supported, there was some suggestion of a transition at that time in the two-phase model selection procedure (Fig. 2B), and we included it to test for potential effects of the Late Triassic evolution of pterosaurs. Of these four models, the three-phase size model, with shifts in size dynamics at 140–130 Ma and 90–60 Ma, received the most support (Akaike weight 0.74) (Table 1). The full four-phase model, incorporating an additional shift between 230 and 200 Ma, is weakly supported (Akaike weight 0.24), and the two-phase model (Akaike weight 0.02) and the oxygen-only model receive negligible model support (Akaike weight <0.001). Models using estimated body width or body volume, and models correlating size to alternative oxygen proxies, also do not support oxygen as the primary control on body size throughout the entire history of insect evolution (SI Text). Adding the effects of paleolatitude significantly improves the model fit; the three-phase model incorporating paleolatitude is much more strongly supported than the model without paleolatitude (Akaike weight 0.92 vs. 0.08) (Table 2). Maximum body size is not well explained by paleolatitude alone, but multiple regression indicates that maximum body size was greater at low paleolatitudes, independent of changes in oxygen.
Table 1.
Model support for models comparing wing length to oxygen ordered from best supported to least supported by bias-corrected Akaike information criterion (ΔAICc) and Akaike weight
| Model | logL | ΔAICc | df | Weight |
| 3: pO2/140 Ma/90 Ma breaks | 34.03 | 0.0 | 6 | 0.743 |
| 4: pO2/200 Ma/140 Ma/90 Ma breaks | 34.69 | 2.3 | 7 | 0.236 |
| 2: pO2/140 Ma break | 28.81 | 7.2 | 5 | 0.021 |
| 1: pO2 | −280.46 | 620.0 | 3 | <0.001 |
Goodness-of-fit (log-likelihood, logL) and number of fitted parameters (df) are shown for each model.
Table 2.
Model support for the best-fit model incorporating paleolatitude and without paleolatitude, ordered from best supported to least supported by bias-corrected AIC (ΔAICc) and Akaike weight
| Model (wing length) | logL | ΔAICc | df | Weight |
| 3: Three-phase including paleolatitude | 38.3 | 0.0 | 7 | 0.922 |
| 3a: Three-phase excluding paleolatitude | 34.03 | 4.9 | 6 | 0.078 |
| Paleolatitude only | 13.36 | 37.3 | 3 | <0.001 |
Goodness-of-fit (log-likelihood, logL) and number of fitted parameters (df) are shown for each model.
Alternative Controls on Maximum Body Size.
The maximum-likelihood model selection procedure identifies the earliest Cretaceous as an important transition in insect body-size evolution. Maximum wing length closely tracked atmospheric pO2 before 140 Ma, but became decoupled from pO2 trends and is better explained by a model of stasis after 130 Ma. The timing of the oxygen-size decoupling coincides with the Early Cretaceous diversification of birds, between their first appearance in the latest Jurassic (Archaeopteryx, ca. 150 Ma) and the presence of diverse assemblages 25 Myr later (23). Maneuverability plays a key role in aerial predation and predator evasion, and scales inversely with body size in flying insects (11), suggesting that size-selective predation pressure by flying birds is a plausible explanation for the weakening and ultimate decoupling of the size-oxygen relationship. This trend is primarily the result of body-size changes in large flying insects, such as dragonflies (but also in grasshoppers), and ground-dwelling groups, such as many beetles or cockroaches, may not follow the same pattern because the history of terrestrial predation differs from that of aerial predation. The gradual reduction in maximum insect size and extinction of large-bodied groups adapted for gliding flight (24) also coincided with the gradual acquisition of key flight characteristics, such as an alula and fused pygostyle, important for low-speed flight performance and maneuverability, in early birds (23).
Although shifting paleolatitude of insect collections cannot alone explain the observed temporal trends in body size, the larger size of fossil insects from tropical paleolatitudes, after accounting for oxygen concentration, is consistent with observed interspecific body-size clines among modern insect clades (20, 25). Our data suggest that a trend of increasing body size with temperature has been a persistent feature throughout insect evolution, but we cannot directly test models explaining these size clines by metabolic or dietary limitations on body-size (20, 25).
The model-selection procedure also supports a shift in dynamics of maximum insect size between the mid-Cretaceous and Paleocene (between 90 and 60 Ma) (Fig. 2B), although its timing and significance are obscured by the extremely sparse Late Cretaceous insect compression fossil record. The shift could reflect gradual reduction in maximum size during the Cretaceous, possibly a result of continuing flight specialization in stem-group birds (23), or a more abrupt reduction following the early Cenozoic radiation of crown-group birds (23) and bats (26). Although not expressed at the family level, there is other evidence for an end-Cretaceous extinction of insects (27) that may have affected body size, either directly through the extinction of large-bodied taxa or indirectly through food web collapse and reduction in prey availability for the largest insects, which are usually carnivorous. These hypotheses are not necessarily mutually exclusive, but cannot be tested without more data from Late Cretaceous fossil sites.
Conclusions.
Our results suggest that physiological limitations related to atmospheric pO2 were dominant only in the early history of insects, whereas maximum size became noticeably decoupled from atmospheric pO2 in the Early Cretaceous. The timing of the shifts in evolutionary size dynamics coincided with the radiation of stem-group birds; combined with the expected size-selective consequences of predation by flying vertebrates, this implies that predation pressure is a plausible explanation for the decoupling. Decreasing insect size in the Early Cretaceous, even as atmospheric pO2 increased, further supports the predation hypothesis because it coincides with the acquisition of anatomical characters in early birds that enabled greater maneuverability, and likely enhanced their prey capture abilities. The importance of multiple controls on insect body size is consistent with theory that predicts a complex suite of abiotic and biotic factors influencing evolutionary trends in natural settings. Thus, atmospheric oxygen acted effectively as a physiological constraint that limited the size of the largest insects from the Carboniferous to Triassic, but its impacts were superseded by increasingly selective size limitation, likely by flying vertebrate predators following the evolution of birds in the latest Jurassic.
Methods
We examined trends in maximum insect size, based on a unique dataset of more than 10,500 fossil insect wing lengths (Table S1) measured from compression fossils reported in the primary literature. We used R (28) to perform multiple regression analyses, including generalized least-squared regression of wing length against oxygen, which used first-order autocorrelation. We also used maximum-likelihood estimation (bbmle package) (29) to generate and test models explaining the size of the largest insect wing length in 10-Myr bins by atmospheric pO2, temperature, and stasis during different time intervals. Bins with fewer than 50 measured specimens (220-, 210-, 80-, and 70-Ma bins) were excluded from analyses. We based atmospheric pO2 on the GEOCARBSULF reconstructions (22), although our timescale incorporated updated dates for Permian and Triassic stages. Because we lack direct paleotemperature estimates for specific insect localities, we used the paleolatitude of each collection, which on average shifted from paleoequatorial latitudes in the Paleozoic to midlatitudes in the Meso-Cenozoic, as a rough proxy. Although latitudinal temperature gradients have varied over geological time and temperature is influenced by continentality (among other factors), as well as latitude, the tropics have always been warmer than temperate regions. Paleolatitude also encompasses other climatic variables such as seasonality, which is less pronounced in the tropics. We modeled the effects of predation as stasis, assuming a constant predation-limited size. This assumption is simplistic; although there are no estimates of the intensity of predation by flying vertebrates over geological time, it is likely that predation pressure or selectivity fluctuated over time, particularly during the early evolution and aerial specialization of these groups. Although body thickness, the length-scale for oxygen transport, is the most important dimension (10), the strong correlation between wing length and body dimensions (11) allows wing length to be used as a proxy for overall body size. We also estimated body volume for three large-bodied groups (Odonata, Orthoptera, and Neuroptera) that could be approximated as cylinders, based on allometric equations calculated from complete fossil specimens (Figs. S1–S3). All analyses were performed with log-transformed size data. See Figs. S4–S6 for information regarding wing length data, Tables S2 and S3 for estimated body width data, and Table S4 for the maximum-likelihood model.
Supplementary Material
Acknowledgments
We thank the many authors responsible for the systematic descriptions of the insect taxa in our dataset; G. Bechly, R. Beckemeyer, C. Brauckmann, J. Kukalová-Peck, J. Prokop, P. Vršanský, and W. Zessin provided information and publications.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204026109/-/DCSupplemental.
See Commentary on page 10745.
References
- 1.Chapelle G, Peck L. Polar gigantism dictated by oxygen availability. Nature. 1999;399:114–115. [Google Scholar]
- 2.Dudley R. Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance. J Exp Biol. 1998;201:1043–1050. doi: 10.1242/jeb.201.8.1043. [DOI] [PubMed] [Google Scholar]
- 3.Falkowski PG, et al. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science. 2005;309:2202–2204. doi: 10.1126/science.1116047. [DOI] [PubMed] [Google Scholar]
- 4.Graham J, Dudley R, Aguilar N, Gans C. Implications of the late Palaeozoic oxygen pulse for physiology and evolution. Nature. 1995;375:117–120. [Google Scholar]
- 5.Harrison JF, Kaiser A, VandenBrooks JM. Atmospheric oxygen level and the evolution of insect body size. Proc Biol Sci. 2010;277:1937–1946. doi: 10.1098/rspb.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClain C, Rex M. The relationship between dissolved oxygen concentration and maximum size in deep-sea turrid gastropods: An application of quantile regression. Mar Biol. 2001;139:681–685. [Google Scholar]
- 7.Harrison J, et al. Responses of terrestrial insects to hypoxia or hyperoxia. Respir Physiol Neurobiol. 2006;154:4–17. doi: 10.1016/j.resp.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Klok CJ, Hubb AJ, Harrison JF. Single and multigenerational responses of body mass to atmospheric oxygen concentrations in Drosophila melanogaster: Evidence for roles of plasticity and evolution. J Evol Biol. 2009;22:2496–2504. doi: 10.1111/j.1420-9101.2009.01866.x. [DOI] [PubMed] [Google Scholar]
- 9.Rascón B, Harrison JF. Oxygen partial pressure effects on metabolic rate and behavior of tethered flying locusts. J Insect Physiol. 2005;51:1193–1199. doi: 10.1016/j.jinsphys.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Weis-Fogh T. Diffusion in insect wing muscle, the most active tissue known. J Exp Biol. 1964;41:229–256. doi: 10.1242/jeb.41.2.229. [DOI] [PubMed] [Google Scholar]
- 11.Dudley R. The Biomechanics of Insect Flight: Form, Function, Evolution. Princeton: Princeton Univ Press; 2000. p. 476. [Google Scholar]
- 12.Harrison JF, Woods HA, Roberts SP. Ecological and Environmental Physiology of Insects. Oxford: Oxford Univ Press; 2012. p. 372. [Google Scholar]
- 13.Kaiser A, et al. Increase in tracheal investment with beetle size supports hypothesis of oxygen limitation on insect gigantism. Proc Natl Acad Sci USA. 2007;104:13198–13203. doi: 10.1073/pnas.0611544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenlee KJ, et al. Synchrotron imaging of the grasshopper tracheal system: Morphological and physiological components of tracheal hypermetry. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1343–R1350. doi: 10.1152/ajpregu.00231.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fusco G, Minelli A. Phenotypic plasticity in development and evolution: Facts and concepts. Introduction. Philos Trans R Soc Lond B Biol Sci. 2010;365:547–556. doi: 10.1098/rstb.2009.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanckenhorn WU. The evolution of body size: What keeps organisms small? Q Rev Biol. 2000;75:385–407. doi: 10.1086/393620. [DOI] [PubMed] [Google Scholar]
- 17.Woods HA, Moran AL, Arango CP, Mullen L, Shields C. Oxygen hypothesis of polar gigantism not supported by performance of Antarctic pycnogonids in hypoxia. Proc Biol Sci. 2009;276:1069–1075. doi: 10.1098/rspb.2008.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butterfield NJ. Oxygen, animals and oceanic ventilation: An alternative view. Geobiology. 2009;7:1–7. doi: 10.1111/j.1472-4669.2009.00188.x. [DOI] [PubMed] [Google Scholar]
- 19.Okajima R. The controlling factors limiting maximum body size of insects. Lethaia. 2008;41:423–430. [Google Scholar]
- 20.Chown SL, Gaston KJ. Body size variation in insects: A macroecological perspective. Biol Rev Camb Philos Soc. 2010;85:139–169. doi: 10.1111/j.1469-185X.2009.00097.x. [DOI] [PubMed] [Google Scholar]
- 21.Labandeira CC, Sepkoski JJ., Jr Insect diversity in the fossil record. Science. 1993;261:310–315. doi: 10.1126/science.11536548. [DOI] [PubMed] [Google Scholar]
- 22.Berner R. Phanerozoic atmospheric oxygen: New results using the GEOCARBSULF model. Am J Sci. 2009;309:603–606. [Google Scholar]
- 23.Padian K, Chiappe L. The origin and early evolution of birds. Biol Rev Camb Philos Soc. 1998;73:1–42. [Google Scholar]
- 24.Bechly G. A revision of the fossil dragonfly genus Urogomphus, with description of a new species (Insecta: Odonata: Pananisoptera: Aeschnidiidae) Stuttgarter Beiträge zur Naturkunde, Serie B. 1998;270:1–47. [Google Scholar]
- 25.Makarieva AM, Gorshkov VG, Li BL. Temperature-associated upper limits to body size in terrestrial poikilotherms. Oikos. 2005;111:425–436. [Google Scholar]
- 26.Simmons NB, Seymour KL, Habersetzer J, Gunnell GF. Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature. 2008;451:818–821. doi: 10.1038/nature06549. [DOI] [PubMed] [Google Scholar]
- 27.Labandeira C. The fossil record of insect extinction: New approaches and future directions. American Entomologist. 2005;51:14–20. [Google Scholar]
- 28.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 29.Bolker B. 2010. bbmle: Tools for general maximum likelihood estimation. R package version 0.9.5.1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.