Abstract
Four serological tests were evaluated for their ability to detect passively acquired maternal antibodies to Bovine herpesvirus 1. A blocking enzyme-linked immunosorbent assay demonstrated superior sensitivity in the detection of such antibodies in calves up to 9–11 months old, versus calves up to 7 months old for other tests.
Transplacental transfer of antibodies from dam to fetus does not occur in ruminants, horses, and swine. Maternal antibodies are transferred to the neonate via immunoglobulin (Ig) G-rich colostrum. The ability of the neonate to absorb these colostral antibodies into its systemic circulation is highest in the first 24 h following birth (1,2). In cattle, maternally derived serum antibodies to Bovine herpesvirus 1 (BHV-1, also known as infectious bovine rhinotracheitis virus) have been shown to decay in a predictable fashion, with a biological half-life of about 3 wk (3,4,5). In most cases, BHV-1 maternal antibodies are undetectable in neonatal calves of 6 mo of age in a standard serum neutralization (SN) test, although they may occasionally be detected after this age (3,4).
Serum neutralization tests and various enzyme-linked immunosorbent assays (ELISAs) are routinely used for BHV-1 antibody detection (3). Bovine herpesvirus 1 is distributed throughout the world but has been eradicated in Denmark and Switzerland (3). For Canadian cattle to be qualified for export to Denmark, their health certificates require that they have been shown to be seronegative to BHV-1 in a blocking ELISA. In contrast to other serological tests (3,4), use of this blocking ELISA at the Animal Diseases Research Institute (ADRI) revealed that calves older than 6 mo frequently had detectable levels of BHV-1 antibodies.
The study described herein was designed to evaluate the sensitivity of 4 serological methods (indirect ELISA, blocking ELISA, standard SN, modified SN) for the detection of maternally derived antibodies to BHV-1, with a particular interest in investigating the duration that antibodies were detectable after birth.
Sixty-two red Angus heifer calves from an Alberta ranch were used in this study. The calves were born to dams that had been vaccinated with an inactivated infectious bovine rhinotracheitis, bovine viral diarrhea, and parainfluenza type 3 vaccine (Triangle 3; Ayerst Veterinary Laboratories, Guelph, Ontario). The calves were born between January 26 and April 4 in year 1. Serum samples were collected on March 14, July 5, September 9 or 20, November 14, and December 19 in year 1 and on January 29 in year 2. In a routine management step, the calves were also vaccinated with clostridial vaccine. Although specific records are not available, the routine procedure of the ranch is to vaccinate calves in June with a combined bacterin-toxoid product containing immunizing antigens derived from Clostridium chauvoei; C. septicum; C. novyi type B; C. haemolyticum; C. perfringens types B, C, and D; and C. tetani (Tasvax 8; Schering-Plough Animal Health, Pointe-Claire, Quebec).
The standard serological tests in use at the ADRI were employed in this study. Details of the SN test (standard and modified) procedures have been described previously (6). Briefly, 2-fold serial dilutions of heat inactivated serum (56°C, 30 min) were mixed with equal volumes (0.025 mL) of the Colorado strain of BHV-1 containing 102 ± 0.5 TCID50. The serum with virus mixtures were incubated for 1 h or 24 h at 37°C in a CO2 (5%) incubator, respectively, for the standard SN or modified SN tests, after which Madin-Darby bovine kidney cells were added to the mixture and the plates incubated at 37°C in a CO2 (5%) incubator for 3 d prior to reading.
The indirect ELISA was a modification of a previously described method (7) that used 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) as the chromogen and Triton X-100 extracted BHV-1 (108 strain) as the ELISA antigen (8).
The blocking ELISA was based on a Danish protocol (9, courtesy of Dr. Lief Rønsholt, State Veterinary Institute for Virus Research, Lindholm, Denmark) with minor modifications. Microplates (Nunc Maxisorp Immunoplates; Canadian Life Technologies, Burlington, Ontario) were coated with BHV-1 ELISA antigen (identical to that used for the indirect ELISA, but at a different dilution) in 0.06 M carbonate buffer (pH 9.6) and incubated overnight at 25°C. Sera were tested in quadruplicate wells and incubated overnight at 25°C. Antigenic sites not blocked by BHV-1 antibodies in the test sera were reacted with biotinylated bovine anti-BHV-1 IgG (DAKO A/S, E382; DAKO Diagnostics Canada, Mississauga, Ontario) diluted 1:2000 in 0.5 M NaCl/0.1% Tween 20/0.03 M phosphate, pH 7.2 (STP) buffer for 1 h at 25°C. Peroxidase-conjugated avidin (DAKO A/S, P347; DAKO Diagnostics Canada), at a dilution of 1:10 000 in STP buffer, was added and incubated for 30 min at 25°C. Reactions were detected by the addition of a substrate solution consisting of 0.01% tetramethylbenzidine dihydrochloride (Sigma, T-3405; Sigma-Aldrich Canada, Oakville, Ontario) and 0.012% hydrogen peroxide (3% stock, H324; Fisher Scientific, Nepean, Ontario) in 0.1 M citrate buffer, pH 5.0. After 15 min incubation at room temperature, reactions were stopped by the addition of 1 M sulphuric acid. All volumes were 0.100 mL per well, except for the sera, which were added at 0.120 mL per well. Negative and positive control bovine sera were run on each microplate. The plates were read photometrically at 450 nm (Titertek Multiskan MCC/340 ELISA reader; Titertek Instruments, Huntsville, Alabama, USA). The working dilution of the BHV-1 ELISA antigen gave an average optical density (OD) reading of approximately 0.800 when tested with the reference negative serum. The antibody-blocking reaction was considered positive for sera if the OD value was ≤ 50% of the OD value of the reference negative serum, suspect for a reaction > 50% and ≤ 70%, and negative if the reaction was > 70%.
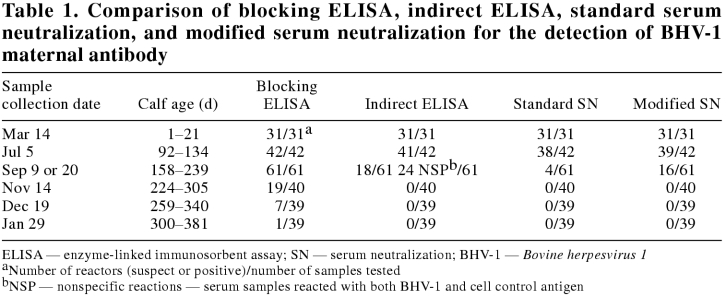
Table 1 shows the results of the 4 serological methods that were used for the detection of BHV-1 maternal antibodies in serially collected serum samples. The blocking ELISA detected anti-BHV-1 maternal antibodies for longer than did the 3 other tests. One calf still contained antibodies detectable by blocking ELISA at 345 d of age, and 7 calves harbored blocking ELISA antibodies at 259 to 310 (average 294) d of age. The standard SN test was the least sensitive: only 4 calves were seropositive with undiluted serum samples (final titer of 1/2, after the addition of virus) at the September collection. In this investigation, with the standard SN test, BHV-1 maternal antibodies were in most cases undetectable by 6 mo of age, though some animals had detectable antibody up to 222 d of age. This confirmed the observations of previous authors (3,4,5). The modified SN test detected more seroreactors than did the standard SN, and yielded higher titers than did the standard SN (data not shown).
Table 1.
The indirect ELISA was slightly more sensitive than the modified SN. However, it is important to note that, in the indirect ELISA, 24 of the 61 calves produced nonspecific reactions on samples from the September collection. These animals reacted with both cell control antigen and BHV-1 antigen coated plates. It is probable that these nonspecific reactions were due to vaccination with the clostridial vaccine, which may have contained bovine proteins.
With the blocking ELISA, the antibodies could still be detected at 9 to 11 mo of age, a time frame 3 to 4 mo longer than in previously published reports (3,4,5). In a previously published comparison of various serological tests, blocking ELISAs were found to be generally more sensitive than indirect ELISAs in the detection of antibodies to BHV-1 (3,10). In the blocking ELISA employed here, 0.120 mL of undiluted serum was reacted with antigen overnight, whereas in the indirect ELISA, 0.100 mL of 1/100 diluted serum was incubated with antigen for 2 h. Thus, the former test received 120 times more volume of sample for a longer period of time. Unfortunately, undiluted samples could not be used in the indirect ELISA due to unacceptably high background reactions. Cattle treated with non-BHV-1 biologicals, such as bacterial and viral vaccines, may produce antibodies to the bovine proteins contained in the vaccines. These antibodies may generate nonspecific reactions in the indirect ELISA, as revealed in the September serum samples.
The study size was not large, but the results indicated that the blocking ELISA is superior for detecting small amounts of passively acquired BHV-1 maternal antibodies. Also, the nature of the blocking ELISA eliminates the nonspecific reactions seen with the indirect ELISA, thus making it useful for detecting BHV-1 antibodies in calves exposed to non-BHV-1 vaccines. Such a test is important in circumstances where animals may be rejected following export to countries using a similar methodology.
Footnotes
Acknowledgments
We thank Mrs. Betty Larsen and L4 Ranches for providing the calves, the animal care and maintenance, and the use of the ranch facilities. We also thank C. Wickens, D. Patterson, W. Pickering, and S. Prins for their excellent technical assistance. CVJ
Address correspondence and reprint requests to Dr. Lorne Jordan.
References
- 1.Tizard I. An Introduction to Veterinary Immunology. 6th ed. Philadelphia: WB Saunders, 2000:210–221.
- 2.Murphy FA, Gibb EPJ, Horzinek MC, Studdert MJ. Veterinary Virology. 3rd ed. San Diego: Academic Pr, 1999:127–144.
- 3.Van Oirschot JT. Infectious bovine rhinotracheitis/infectious pustular vulvovaginitis. In: Manual of Standards for Diagnostic Tests and Vaccines. 4th ed. Paris: Office International des Epizooties, 2000:381–391.
- 4.Brar JS, Johnson DW, Muscoplat CC, Shope RE, Meiske JC. Maternal immunity to infectious bovine rhinotracheitis and bovine viral diarrhea viruses: duration and effect on vaccination in young calves. Am J Vet Res 1978;39:241–244. [PubMed]
- 5.Menanteau-Horta AM, Ames TR, Johnson DW, Meiske JC. Effect of maternal antibody upon vaccination with infectious bovine rhinotracheitis and bovine virus diarrhea vaccines. Can J Comp Med 1985;49:10–14. [PMC free article] [PubMed]
- 6.Deregt D, Cho HJ, Kozub GC. A comparative evaluation of two sensitive serum neutralization tests for bovine herpesvirus-1 antibodies. Can J Vet Res 1993;57:56–59. [PMC free article] [PubMed]
- 7.Cho HJ, Bohac JG. Sensitivity and specificity of an enzyme-linked immunosorbent assay for the detection of infectious bovine rhinotracheitis viral antibody in cattle. Can J Comp Med 1985;49: 189–194. [PMC free article] [PubMed]
- 8.Cho HJ, Jordan L, Entz S, Pickering W. An indirect ELISA for the detection of bovine antibody against IBR virus. Version 5.0. Lethbridge, Alberta: Canadian Food Inspection Agency, Animal Diseases Research Institute, 1998.
- 9.Rønsholt L. Procedure for the IBR antibody blocking ELISA at the Danish Reference Laboratory. Lindholm, Denmark: State Veterinary Institute for Virus Research, 1994.
- 10.Perrin B, Bitsch V, Cordioli P, et al. A European comparative study of serological methods for the diagnosis of infectious bovine rhinotracheitis. Rev Sci Tech Off Int Epiz 1993;12:969–984. [DOI] [PubMed]