Abstract
Recurrent apneas are characterized by transient repetitive cessations of breathing (two breaths duration or longer) resulting in periodic decreases in arterial blood PO2 or chronic intermittent hypoxia (IH). Patients with recurrent apneas and experimental animals exposed to chronic IH exhibit cardio-respiratory morbidities. The purpose of this article is to highlight the current information on the transcriptional mechanisms associated with chronic IH. Studies on rodents and cell cultures have shown that IH activates a variety of transcription factors including the hypoxia-inducible factor-1 (HIF-1), c-fos (immediate early gene), nuclear factor of activated T-Cells (NFAT), and nuclear factor kB (NF-kB). The signaling pathways associated with transcriptional activation associated with IH differ from continuous hypoxia (CH). Compared to same duration and intensity of CH, IH is more potent in activating HIF-1 and c-fos and also results in long-lasting accumulation of HIF-1α and c-fos mRNA, a phenomenon that was not seen with CH. IH-evoked transcriptional activation by HIF-1, c-fos as well as the resulting activator protein-1 (AP-1) requires reactive oxygen species (ROS)-mediated signaling and involves complex feed-forward interactions between HIF-1 and ROS. Chronic IH evoked cardio-respiratory responses are absent in Hif-1a+/− mice, and hypertension elicited by chronic IH is absent in mice lacking NFAT3c. These studies indicate that cardio-respiratory responses to chronic IH depend on complex interactions between various transcription factors resulting in alterations in several down stream genes and their protein products.
Keywords: Hypoxia-inducible factor-1, NFAT, Activator protein-1, Nuclear factor kB, intermittent hypoxia, reactive oxygen species, NADPH oxidase
1. Introduction
Recurrent apneas are characterized by transient repetitive cessations of breathing (two breaths duration or longer) resulting in cyclical decreases in arterial blood PO2 or chronic intermittent hypoxia (IH). An estimated 4–5% of adult males, 2–4% of females after menopause, and 50–70% of premature infants experience chronic IH as a consequence of recurrent apneas (Nieto et al., 2000; Poets et al., 1994). Patients with recurrent apneas exhibit cardio-respiratory co-morbidities including pulmonary as well as systemic hypertension, myocardial infarction, stroke, ventilatory abnormalities, and sudden death (Shahar et al., 2001). Similar cardio-respiratory changes were also reported in rodents exposed to chronic IH (reviewed in Prabhakar et al., 2007). Studies on rodents and cell cultures have shown that IH activates several transcription factors. The purpose of this article is to summarize what is currently known on the effects chronic IH on activation of transcriptional factors, underlying mechanisms and the potential contribution of transcriptional activators on chronic IH- evoked cardio-respiratory responses. In contrast to IH associated with recurrent apneas, wherein each hypoxic episode lasts no more than couple of breaths, exposing individuals to few hours of hypoxia per day for a few weeks, which is also intermittent in nature, improves cardio-respiratory functions (Serebrovskaya et al., 1999). Due to constraints of space, this article focuses on transcriptional responses to IH simulating recurrent apneas only.
2. Hypoxia and transcription factors
Hypoxia activates several genes via recruiting specific transcription factors. The resulting protein products maintain homeostasis by enhancing tissue perfusion, ATP generation, glycolysis etc. Transcriptional activators that are affected by continuous hypoxia (CH) include: hypoxia inducible factors (HIF-1 and HIF-2); nuclear factor kappa B (NF-kB), cyclic AMP response element binding protein (CREB), activating protein-1 (AP-1), p53, early growth response-1 (Egr-1), nuclear factor for interleukin 6 (NF-IL6) (Cummins and Taylor, 2005). With the exception of HIF-1, the effects of hypoxia on other transcription factors can be cell-type and cell-state specific. The following section summarizes the effects of IH on some of these transcription factors.
3. IH and Hypoxia Inducible Factor-1 (HIF-1)
HIF-1 is a heterodimeric protein that is composed of a constitutively expressed HIF-1β subunit and an O2-regulated HIF-1α subunit (Wang et al., 1995). HIF-1 mediated transcriptional activation requires increased HIF-1α expression, dimerization with HIF-1β and interaction with co-activators p300 (adenovirus EIA-associated 300-kDa protein) and CBP (cyclic AMP-responsive element-binding protein).
3.1. IH and HIF-1α protein expression
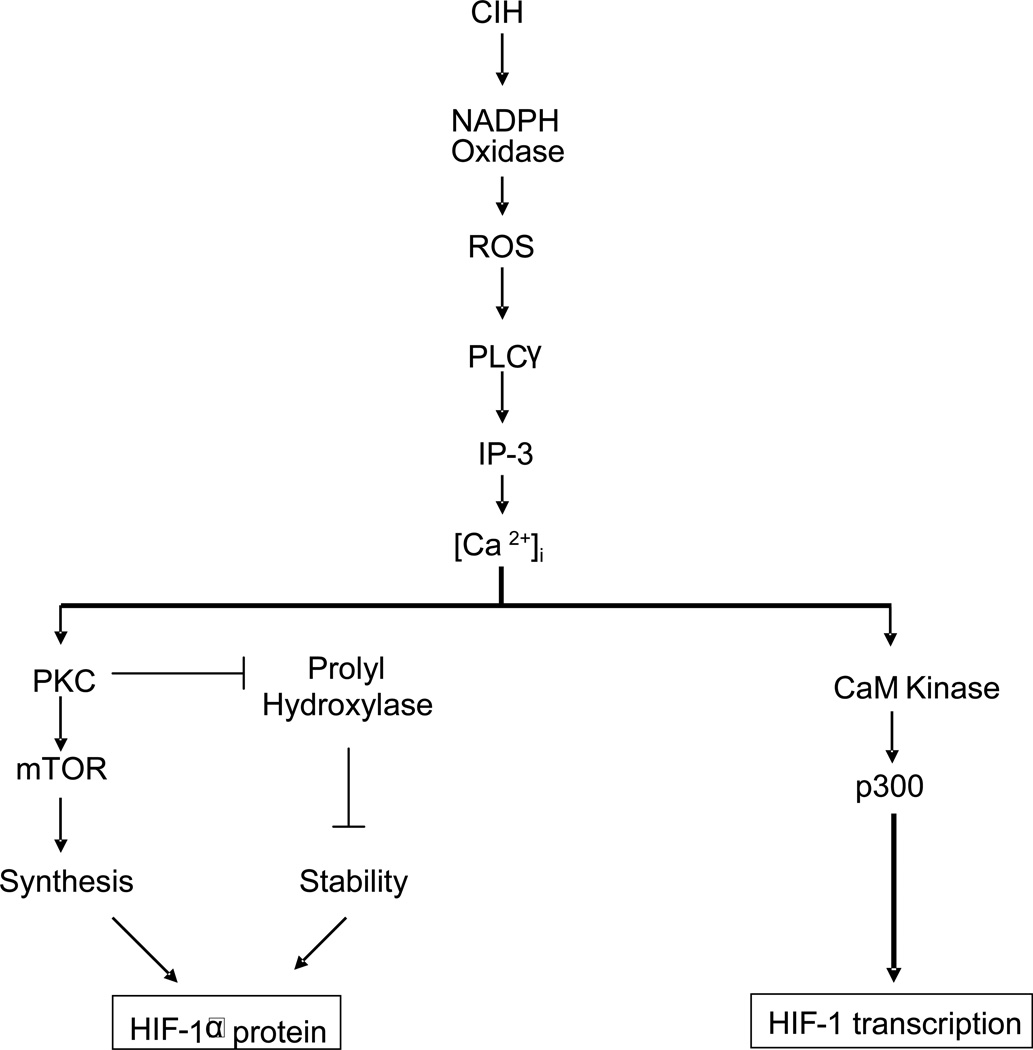
IH up-regulates HIF-1α protein in the central nervous system of mice (Peng et al 2006), and in PC12 cell cultures (Yuan et al., 2005). Lam et al. 2008 reported an increase in the HIF-1α transcript but not the protein during IH. HIF-1α accumulation by CH requires decreased O2-dependent proline hydroxylation, ubiquitination, and proteasomal degradation of the HIF-1α subunit (Coleman and Ratcliffe, 2007). The mechanism associated with IH-evoked HIF-1α accumulation is complex and requires not only decreased proline hydroxylation but also increased protein synthesis via activation of mTOR (mammalian target of rapamycin) as summarized in Figure 1. Recent study by Yuan et al (2008) reported that ROS generated by NADPH oxidase and the resulting changes in intracellular Ca2+ are the primary signaling events that trigger HIF-1α accumulation by IH.
Figure 1.
NADPH oxidase signaling in intermittent hypoxia-induced HIF-1α protein expression and HIF-1 transcription
Key: CIH, chronic intermittent hypoxia; ROS, reactive oxygen species; PLCγ, phospholipase C gamma; IP-3, inositol triphosphate; PKC, protein kinase C; mTOR, mammalian target of rapamycin; HIF-1, hypoxia-inducible factor-1
The effects of IH and CH on HIF-1α accumulation differ in the following aspects: a) for a given intensity and duration, IH is more potent in increasing HIF-1α protein than CH (Yuan et al. 2005), and b) following IH, HIF-1α levels remain elevated during re-oxygenation, whereas they return to control levels within 10 min of re-oxygenation following CH (Yuan et al., 2008). The persistent accumulation of HIF-1α protein during re-oxygenation following IH requires increased protein synthesis via activation of mTOR signaling (Yuan et al., 2008).
3.2. IH and HIF-1 mediated transcriptional activation
The effects of IH on HIF-1 mediated transcriptional activation were examined in PC12 cells (Yuan et al., 2005). IH activated HIF-1-dependent transcriptional activity in a stimulus-dependent manner. Like HIF-1α protein expression, CH of comparable, cumulative duration of IH was ineffective in activating HIF-1-dependent transcription. Previous studies showed that mitogen-activated protein kinases (MAPKs) and phospho-ionositol-3 (PI-3) kinases are critical for continuous hypoxia evoked activation of HIF-1 mediated transcription (Sang et al., 2003; Seta et al., 2003). Although MAPKs (ERK-1 &2; Jun Kinase) are activated by IH, inhibitors of MAPKs and PI-3 kinase were ineffective in blocking IH-elicited activation of HIF-1 mediated transcriptional activation (Yuan et al., 2005).
HIF-1 activation by IH was inhibited by BAPTA-AM, an intracellular Ca2+ chelator (Yuan et al., 2005), suggesting the involvement of Ca2+ signaling pathways. Calcium-calmodulin-dependent kinases (CaM kinases) are one of the important Ca2+ signaling molecules. PC12 cells express CaMK-II and CH causes a transient and modest increase in CaM kinase activity (Premkumar et al., 2000). In striking contrast, IH resulted in robust and persistent activation of CaM kinase (~ 5-fold activation) and more importantly, CaM kinase inhibitor KN93 prevented HIF-1 transcriptional activation, but not HIF-1α accumulation by IH (Yuan et al., 2005).
Transcriptional activation by HIF-1 requires N- and C- terminal transactivation domains (N-TAD and C-TAD), which are separated by intervening inhibitory domain. FIH-1 (factor inhibiting HIF-1) binds to the inhibitory domain (Mahon et al., 2001) and mediates the O2-dependent hydroxylation of asparagine (Asn-803), which prevents binding of the co-activators. CaMK II stimulates C-TAD domain function of HIF-1 via a mechanism that is independent of asparaginyl hydroxylation (Yuan et al., 2005). Several lines of evidence suggest that phosphoproteins p300 and CBP (Yaciuk and Moran, 1991) are the major co-activators for HIF-1 activation (Sang et al., 2003, Ruas et al., 2002; Dames et al., 2002). Hypoxia leads to hyperphosphorylation of p300 in PC12 cells via Ca2+ signaling by IP-3 receptors (Zakrzewska et al., 2005). CaMK II phosphorylated p300 in vitro (Yuan et al., 2005) and CaM kinase inhibitor, KN-93 prevented activation of p300 by IH, These observations suggest that IH induced HIF-1 transcriptional activity is mediated by a novel signaling pathway involving phosphorylation of p300 by CaM kinase (Figure 1).
3.3. Physiological significance of IH-induced HIF-1 activation
3.3.1. Cardio-respiratory responses to chronic IH
Chronic IH has profound effects on cardio-respiratory physiology. Rodents exposed to chronic IH exhibit elevated blood pressures (Fletcher, 2001; Kumar et al., 2006, Peng et al., 2006, Kanagy et al., 2001), increased plasma catecholamine (Bao et al., 1997; Kumar et al., 2006, Peng et al., 2006), and endothelin (a peptide vasoconstrictor) levels (Kanagy et al., 2001). Basal sympathetic nerve activity was elevated in chronic IH exposed rats (Sica et al., 2000) and in recurrent apnea patients (Somers et al., 1995). Furthermore, acute hypoxia as well as hypoxic-hypercapnia -evoked sympathetic excitation was more pronounced in chronic IH-exposed rats (Sica et al., 2000).
Ventilatory response to acute hypoxia is biphasic with an initial augmentation of breathing followed by a decline. The excitatory phase of the hypoxic ventilatory response (HVR) was augmented in chronic IH exposed cats (Rey et al., 2004), rats (Peng et al., 2003; Reeves and Gozal, 2006) and mice (Peng et al 2006). In addition, chronic IH also affects the ventilatory decline phase of the HVR (Reeves and Gozal, 2006) and augments ventilatory response to hypercapnia (Peng et al., 2006). Repetitive hypoxia leads to long-lasting activation of breathing, a phenomenon termed as "long-term facilitation" (LTF; Mitchell and Johnson, 2003). Prior conditioning with chronic IH augments LTF of breathing (McGuire et al., 2003; Peng et al., 2003; Reeves and Gozal, 2006).
Much of the cardio-respiratory changes for a given stimulus are reflex in nature and are regulated by sensory information from sensory receptors as well as processing of afferent inputs at the central nervous system. Carotid bodies are the primary sensory organs for detecting changes in arterial blood oxygen. It has been proposed that carotid bodies constitute the "frontline" defense system for detecting systemic hypoxia associated with apneas (Cistulli and Sullivan, 1994; Kara et al., 2003). Recent studies have shown that chronic IH leads to enhanced sensory response to acute hypoxia (Peng and Prabhakar, 2003, 2004; Rey et al. 2004; Peng et al., 2006) and long-lasting activation of baseline discharge, a phenomenon termed as sensory LTF (Peng et al., 2003, 2006). Stimulation of carotid body leads to increases in blood pressure, sympathetic excitation and breathing. Consequently, it was proposed that chronic IH-evoked sensory LTF of the carotid bodies contributes to persistent sympathetic activation and hypertension, whereas sensitization of the hypoxic sensory response may lead to instability of the respiratory control system, perpetuating apneas (Prabhakar et al., 2007).
The following section summarizes the potential contribution of HIF-1 activation to the above described alterations in cardio-respiratory responses and carotid body function elicited by chronic IH.
3.3.2. Heterozygous deficiency of HIF-1α impairs cardio-respiratory and carotid body responses to chronic IH
Complete HIF-1α deficiency results in embryonic lethality at mid-gestation, whereas Hif1α+/− heterozygous (HET) mice, which are partially deficient in HIF-1α expression, develop normally and are indistinguishable from wild type (WT) littermates under normoxic conditions (Iyer et al., 1998; Yu et al., 1999). Wild type mice exposed to chronic IH exhibited: augmented hypoxic ventilatory response; LTF of breathing; enhanced carotid body response to graded hypoxia and sensory LTF; increased blood pressures; and elevated plasma norepinephrine levels. In striking contrast, in HET mice exposed to chronic IH, carotid body responses to hypoxia were absent and all measured cardio-respiratory responses were either absent or markedly attenuated (Peng et al., 2006). HIF-1α protein expression increased in WT mice, but not in HET littermates exposed to chronic IH. Thus, the virtually complete absence of ventilatory and cardiovascular responses to chronic IH in HET mice could be attributed to lack of induction of HIF-1α protein expression in these mice.
What are the HIF-1 regulated genes that might contribute to chronic IH-evoked cardio-respiratory changes? Earlier studies have shown that hypoxia-evoked up-regulation of pre-pro ET-1 requires HIF-1 (Hu et al., 1998, Yamashita et al., 2001). Recent studies showed that ET-1 contributes to chronic IH-evoked increases in blood pressure (Kanagy et al., 2001) as well as to the sensitization of the carotid body response to acute hypoxia (Rey et al., 2004). It is likely that HIF-1 contributes to chronic IH-induced changes in blood pressure and carotid body in part by up-regulating the gene encoding pre-pro ET-1. While this remains an attractive notion, further studies are needed to test this possibility and identify other HIF-1 regulated genes that may contribute to cardio-respiratory changes elicited by chronic IH.
3.3.3. Role of reactive oxygen species (ROS) in cardio-respiratory responses to chronic IH and evidence for feed forward interactions of HIF-1 with ROS
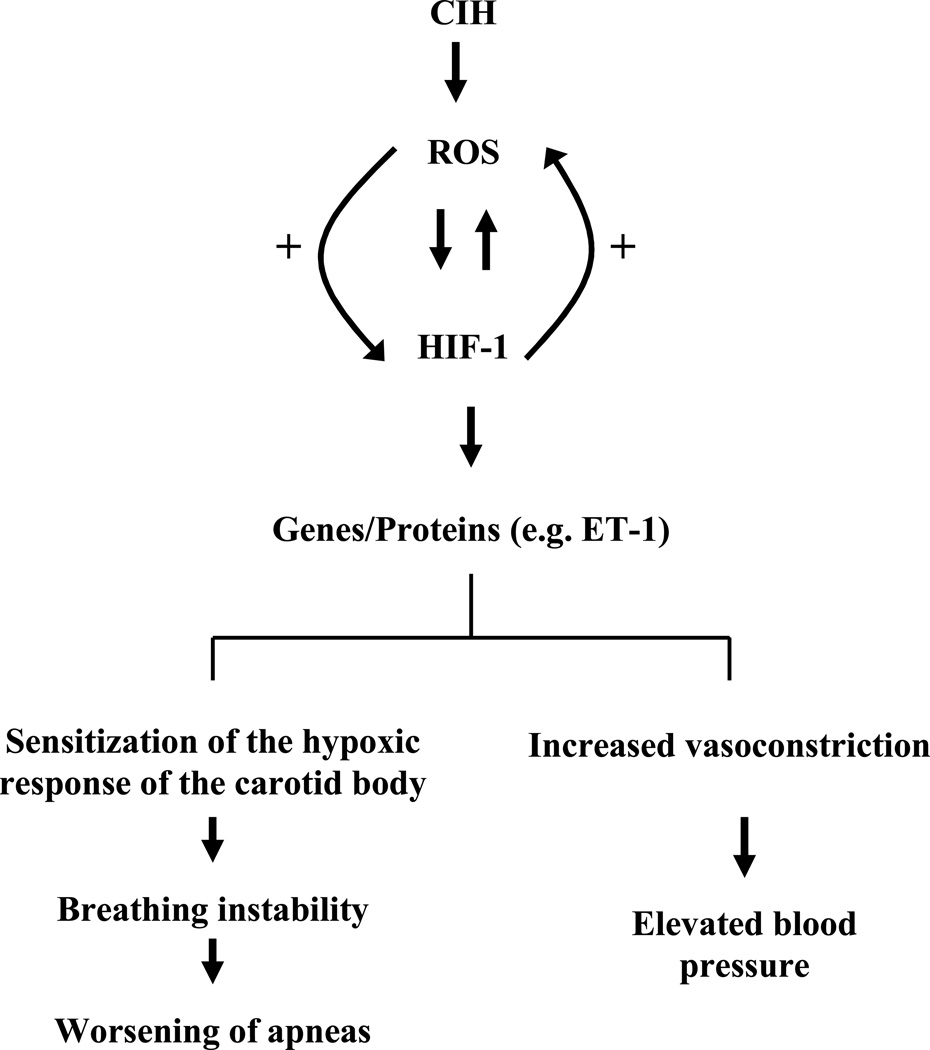
It was proposed that reactive oxygen species (ROS) are generated during the re-oxygenation phase of IH and ROS contribute to chronic IH-evoked physiological responses (Prabhakar, 2001). Indeed, chronic IH increases ROS in several tissues including the carotid body (Peng et al., 2003), adrenal medulla (Kumar et al., 2006), and brainstem (Ramanathan et al., 2005; Row et al., 2003; Veasy et al., 2004). The chronic IH-induced increase in ROS appears to arise in part from the inhibition of the mitochondrial electron transport chain (ETC) specifically at complex I (Yuan et al., 2004) as well as from activation of NADPH oxidase (Zhan et al., 2005; Yuan et al., 2008). More importantly, treating chronic IH exposed rodents with anti-oxidants MnTMPyP [manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride] or NAC [N-acetyl cysteine] prevent chronic IH-evoked cardio-respiratory responses (Peng et al., 2003, Peng and Prabhakar, 2004; Kumar et al., 2006; Troncoso Brindeiro et al., 2007) and changes in carotid body function (Peng et al., 2003 a and b). Anti-oxidants also prevent HIF-1 activation by IH in mice (Peng et al., 2006) and cell cultures (Yuan et al., 2008). Intriguingly, chronic IH elevates ROS levels in WT but not in HET mice deficient in HIF-1α (Peng et al., 2006). These observations indicate complex positive interactions between HIF-1 and oxidants. It is likely that chronic IH may initially trigger an increase in ROS levels either by inhibiting mitochondrial electron transport chain at complex I or by activating NADPH oxidase. The increased ROS in turn up-regulates HIF-1α. Once HIF-1 is activated, it may promote persistent increase in ROS either by stimulating oxidants or by inhibiting antioxidants (Figure 2), a possibility that require further studies.
Figure 2.
ROS-signaling in chronic intermittent hypoxia-evoked cardio-respiratory responses
Key: CIH, chronic intermittent hypoxia; ROS, reactive oxygen species; HIF-1, hypoxia-inducible factor-1; ET-1, endothelin-1; “+” represents positive feed-forward mechanism
4. IH and Nuclear Factor of activated T-Cells (NFAT)
The role of NFAT family of transcription factors which include NFATc 1, 2, 3 and 4, and NFAT5/TonEBP (Rao et al., 1997), in genetic regulation of immune response is extensively studied. However, recent studies have shown that NFAT transcription factors also play a role in variety of other physiological systems including the nervous (Nguyen and Di Giovanni, 2008) and vascular systems (Wada et al., 2002, Amberg et al., 2004). A rise in intracellular Ca2+ and subsequent activation of calcineurin is necessary for NFAT activation (Jain et al., 1995). Calcineurin dephosphorylates several residues in the translocation domain of NFAT resulting in nuclear import. NFAT activation, although increases expression of certain genes (Hogan et al., 2003), it may also repress expression of other genes, especially those encoding K+ channels (Amberg et al., 2004; Nieves-Cintron et al., 2006). A recent study by de Frutos et al (2008) examined the effects of chronic IH on NFATc3 transcriptional activity and its significance in blood pressure changes by IH. These investigators reported increased NFATc3 transcriptional activity in aorta and mesenteric arteries and elevated blood pressures in mice exposed to chronic IH, and these responses were either absent or attenuated in NFATc3 deficient mice or by treating wild type mice with cyclosporine, an inhibitor of calcineurin. ET-1 is a potent activator of NFAT (Stevenson et al., 2001). Because chronic IH elevates ET-1 in rodents, de Frutos et al (2008) suggested that IH-induced increases in blood pressure may involve ET-1 mediated NFAT activation. Since ET-1 is a HIF-1 regulated down-stream gene, it is likely that IH-induced activation of NFATc3 involves HIF-1 mediated up-regulation of pre-pro-ET-1. In addition, NFAT is notable for its ability to bind cooperatively with other transcription factors such as the activator protein-1 (AP-1; Fos/Jun; see below) to regulate gene transcription.
5. IH and Activator Protein-1 (AP-1)
The protein products encoded by immediate early genes (e.g., c-fos, c-jun) form either homo (Jun/Jun) - or heterodimeric (Fos/Jun) protein complexes designated activator protein-1 (AP-1) that drives transcription of a variety of genes (Morgan and Curran., 1989).
5.1. IH activates c-fos transcription
Greenberg et al. (1999) reported increased levels of c-Fos protein in the central nervous system of chronic IH exposed rats. IH also increases c-fos mRNA expression in cell cultures, which was in part due to increased transcriptional activation (Yuan et al., 2004). IH-evoked c-fos transcription requires Serum Response Element (SRE), Calcium Response Element (CRE) but not the AP-1/CRE like cis-element (FAP). Interestingly, the magnitude of c-fos activation by IH was dependent on the duration of re-oxygenation between the hypoxic episodes rather than the duration of the hypoxic episodes itself. Exposure to comparable cumulative duration of CH had virtually no effect on c-fos mRNA expression and c-fos promoter activation (Yuan et al., 2004). Following IH, c-fos mRNA remained elevated for at least 3 h during the re-oxygenation, whereas it returned to control levels within 1h of re-oxygenation following CH (Yuan et al., 2004). The mechanisms by which IH leads to long lasting increase in c-fos mRNA, however, remain to be studied.
5.2. c-fos is required for AP-1 transcriptional activation by IH
IH increases AP-1 transcriptional activity in PC12 cells and antisense c-fos abolished this effect (Yuan et al., 2004), suggesting that heterodimerization of Fos/Jun is required for AP-1 activation. Anti-sense c-fos also blocked IH-induced tyrosine hydroxylase (TH) mRNA expression, an AP-1 regulated gene (Yuan et al., 2004). Since TH is the rate limiting enzyme in catecholamine biosynthesis, the elevated catecholamine levels reported in IH exposed rodents (Bao et al., 1997, Kumar et al., 2006; Peng et al., 2006) and recurrent apnea patients (Ziegler et al., 1997) might in part be due to AP-1 mediated up-regulation of the TH gene.
6. IH and Nuclear Factor kB (NF-kB)
NF-kB is an important transcriptional regulator of inflammatory mediators. Ryan et al (2006) reported increased NF-kB activity in monocytes derived from obstructive sleep apnea patients and this effect was associated with elevated serum tumor necrosis factor-α (TNF-α) and interleukin-8 (IL-8) levels (downstream gene products of NF-kB). Elevated TNF-α levels correlated with arterial O2 de-saturation, and treatment of apneas with continuous positive airway pressure (CPAP) normalized TNF-α levels. Similar increase in NF-kB activation was also reported in mice exposed to IH as well as white blood cells of apnea patients (Greenberg et al., 2006). IH also stimulates NF-kB-mediated transcriptional activation in HeLa cells (Ryan et al., 2005). However, the mechanisms by which IH activates NF-kB remain to be investigated.
6.1. IH and possible interactions between NF-kB and HIF-1
Recent studies suggest interactions between NF-kB and HIF-1. Belaiba et al (2007) reported that NF-kB plays a role in HIF-1α mRNA induction by hypoxia while Rius et al (2008) showed that NF-kB is critical for hypoxia-evoked HIF-1α accumulation as well as HIF-1 mediated transcription in the liver and brain. HIF-1α promoter contains active NF-kB binding sites at −197/188 upstream of the transcription start site (van Uden et al., 2008). Since IH activates both HIF-1 and NF-kB it is of interest to examine the mechanisms involved in the interaction of these two transcriptional activators.
Acknowledgements
We sincerely acknowledge the contributions of Dr. Y.J. Peng to the animal experiments. The research reported in this article is supported by grants from National Institutes of Health (Heart, Lung and Blood Institute) PO1HL-90554, RO1HL-76537.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem. 2004;279:47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol. 1997;83:95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- Belaiba RS, Bonello S, Zähringer C, Schmidt S, Hess J, Kietzmann T, Görlach A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007;18:4691–4697. doi: 10.1091/mbc.E07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cistulli PA, Sullivan CE. Pathophysiology of sleep apnea. In: Saunders NA, Sullivan CE, editors. Sleep and Breathing. New York, NY: Dekker; 1994. pp. 405–448. [Google Scholar]
- Coleman ML, Ratcliffe PJ. Oxygen sensing and hypoxia-induced responses. In: Peers C, editor. Oxygen Sensing and Hypoxia-Induced Responses. London: Portland Press; 2007. pp. 1–15. [DOI] [PubMed] [Google Scholar]
- Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch-European J Physiol. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, Wright PE. Structural basis for Hif-1 alpha /CBP recognition in the cellular hypoxic response. Proc Natl Acad Sci USA. 2002;99:5271–5276. doi: 10.1073/pnas.082121399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frutos S, Duling L, Alò D, Berry T, Jackson-Weaver O, Walker M, Kanagy N, González Bosc L. NFATc3 is required for intermittent hypoxia-induced hypertension. Am J Physiol Heart Circ Physiol. 2008;294:H2382–H2390. doi: 10.1152/ajpheart.00132.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EC. Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- Greenberg HE, Sica AL, Scharf SM, Ruggiero DA. Expression of c-fos in the rat brainstem after chronic intermittent hypoxia. Brain Res. 1999;816:638–645. doi: 10.1016/s0006-8993(98)01222-0. [DOI] [PubMed] [Google Scholar]
- Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006;343:591–596. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245:894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001;37:511–515. doi: 10.1161/01.hyp.37.2.511. [DOI] [PubMed] [Google Scholar]
- Kara T, Narkiewicz K, Somers VK. Chemoreflexes – physiology and clinical implications. Acta Physiol Scand. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S-Y, Tipoe GL, Liong EC, Fung ML. Differential expressions and roles of hypoxia-inducible factor-1α,-2α and 3α in the rat carotid body during chronic and intermittent hypoxia. Histol Histopathol. 2008;23:271–280. doi: 10.14670/HH-23.271. [DOI] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1 and VHL to mediate repression of HIF-1 transcriptional activity. Genes and Development. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J Appl Physiol. 2003;95:1499–1508. doi: 10.1152/japplphysiol.00044.2003. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Di Giovanni S. NFAT signaling in neural development and axon growth. Int J Dev Neurosci. 2008;26:141–145. doi: 10.1016/j.ijdevneu.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study, Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Nieves-Cintrón M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol. 2003a;94:2342–2349. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci. USA. 2003b;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poets CF, Samuels MP, Southall DP. Epidemiology and pathophysiology of apnoea of prematurity. Biol Neonate. 1994;65:211–219. doi: 10.1159/000244055. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- Premkumar DR, Mishra RR, Overholt JL, Simonson MS, Cherniack NS, Prabhakar NR. L-type Ca(2+) channel activation regulates induction of c-fos transcription by hypoxia. J Appl Physiol. 2000;88:1898–1906. doi: 10.1152/jappl.2000.88.5.1898. [DOI] [PubMed] [Google Scholar]
- Ramanathan L, Gozal D, Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem. 2005;93:47–52. doi: 10.1111/j.1471-4159.2004.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: Regulation and function. Ann Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Reeves SR, Gozal D. Changes in ventilatory adaptations associated with long-term intermittent hypoxia across the age spectrum in the rat. Respir Physiol Neurobiol. 2006;150:135–143. doi: 10.1016/j.resp.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 2003;167:1548–1553. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- Ruas JL, Poellinger L, Pereira T. Functional analysis of hypoxia-inducible factor-1 alpha-mediated transactivation. Identification of amino acid residues critical for transcriptional activation and/or interaction with CREB-binding protein. J Biol Chem. 2002;277:38723–38730. doi: 10.1074/jbc.M205051200. [DOI] [PubMed] [Google Scholar]
- Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–830. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- Sang N, Stiehl DP, Bohensky J, Leshchinsky I, Srinivas V, Caro J. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J Biol Chem. 2003;278:14013–14019. doi: 10.1074/jbc.M209702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebrovskaya TV, Karban IN, Kolesnikova EE, Mishunina TM, Kuzminskaya LA, Serbrovsky AN, Swanson RJ. Human hypoxic ventilatory performance and blood dopamine content under intermittent hypoxic training. Can J Physiol Pharmacol. 1999;77:967–973. [PubMed] [Google Scholar]
- Seta KA, Yuan Y, Spicer Z, Lu G, Millhorn DE. In: Oxygen Sensing: Responses and Adaptation to Hypoxia. Lahiri S, Semenza GL, Prabhakar NR, editors. New York: Marcel Dekker Inc; 2003. pp. 123–152. [Google Scholar]
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto J, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: a model of sympathetic activation in the rat. Respir Physiol. 200;121:173–184. doi: 10.1016/s0034-5687(00)00126-2. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson AS, Gomez MF, Hill-Eubanks DC, Nelson MT. NFAT4 movement in native smooth muscle. A role for differential Ca(2+) signaling. J Biol Chem. 2001;276:15018–15024. doi: 10.1074/jbc.M011684200. [DOI] [PubMed] [Google Scholar]
- Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol. 2007;293:H2971–H2976. doi: 10.1152/ajpheart.00219.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Davis C, Zhan G, Hsu YJ, Fenik P, Pratico D, Gow AJ. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- Wada H, Hasegawa K, Morimoto T, Kakita T, Yanazume T, Abe M, Sasayama S. Calcineurin-GATA-6 pathway is involved in smooth muscle-specific transcription. J Cell Biol. 2002;156:983–991. doi: 10.1083/jcb.200106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaciuk P, Moran E. Analysis of specific polyclonal antiserum indicates that the E1a associated 300kDs product is a stable nuclear phosphoprotein that undergoes cell cycle specific modification. Mol Cell Biol. 1991;11:5389–5397. doi: 10.1128/mcb.11.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J Biol Chem. 2001;276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK, Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol. 2004;557:773–783. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–4432. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1α Expression by Intermittent Hypoxia: Involvement of NADPH oxidase, Ca2+ signaling, Prolyl hydroxylases, and mTOR. J Cellular Physiol. 2008 doi: 10.1002/jcp.21537. (accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska A, Schnell PO, Striet JB, Hui A, Robbins JR, Petrovic M, Conforti L, Gozal D, Wathelet MG, Czyzyk-Krzeska MF. Hypoxia-activated metabolic pathway stimulates phosphorylation of p300 and CBP in oxygen-sensitive cells. J Neurochem. 2005;5:1288–1296. doi: 10.1111/j.1471-4159.2005.03293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, Veasey SC. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 2005;172:921–929. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler MG, Nelesen R, Mills P, Ancoli-Israel S, Kennedy B, Dimsdale JE. Sleep apnea, norepinephrine-release rate, and daytime hypertension. Sleep. 1997;20:224–231. doi: 10.1093/sleep/20.3.224. [DOI] [PubMed] [Google Scholar]