Abstract
We determined if soy isoflavones have dose-related estrogenic and methylation effects. Thirty-four healthy premenopausal women were randomized to 40 mg or 140 mg isoflavones daily through one menstrual cycle. Breast specific and systemic estrogenic effects were assessed measuring the estrogenic marker complement (C)3 and changes in cytology, whereas methylation assessment of 5 cancer related genes (p16, RASSF1A, RARβ2, ER, and CCND2) was performed on intraductal specimens. Serum genistein significantly increased after consuming both isoflavone doses. Cytology did not significantly change at either isoflavone dose. Serum C3 levels posttreatment were inversely related to change in serum genistein (r = −0.76, P = 0.0045) in women consuming low but not high dose isoflavones. The RARβ2 hypermethylation increase posttreatment correlated with the posttreatment genistein level considering the entire group (r 0.67, P = 0.0017) and those receiving high-dose isoflavones (r = 0.68, P = 0.021). At the low but not the high isoflavone dose, CCND2 hypermethylation increase correlated with posttreatment genistein levels (r = 0.79, P = 0.011). In summary, the inverse correlation between C3 and genistein suggests an antiestrogenic effect. Isoflavones induced dose-specific changes in RARβ2 and CCND2 gene methylation, which correlated with genistein levels. This work provides novel insights into estrogenic and methylation effects of dietary isoflavones.
INTRODUCTION
Isoflavones are compounds found in plants that act like estrogens. The importance of these compounds relates to their action on the estrogen receptor, which may influence cancer risk. One of the most commonly consumed plant products is soy, which contains isoflavones. The two isoflavones in soy that have gained the most research attention are genistein and daidzein. Genistein (5,7,4′-trihydroxyisoflavone) binds to the active site of the estrogen receptor (1) and increases luciferase in cells with an estrogen response element–luciferase reporter gene construct that coexpresses ERα and ERβ (2,3). Genistein and other soy isoflavones inhibit ER negative (−) breast cancer cell growth, although some studies have indicated that they may increase ER positive(+) breast cancer cell growth and may interfere with the antitumor activity of tamoxifen (4).
Treatment with dietary levels of genistein during puberty leads to a lower incidence of mammary tumors in rats challenged with carcinogens as adults (5), whereas genistein administered during adult life but not during puberty had no effect on mammary tumors (6). Asian women who consumed tofu during adolescence but not in adult life still had a lowered incidence of breast cancer compared with those who never consumed tofu or only consumed tofu as an adult (7). The effect of early exposure to isoflavones on future breast cancer risk may be due to epigenetic changes such as alterations in DNA methylation.
Cytosine residues methylated by DNA-cytosine methyltransferase-1 (DNMT1) are associated with loss of transcription of the target gene (8). DNMT1 activity is elevated in malignant cells, and this is associated with increased cell proliferation, tumorigenesis, and tumor progression (8). Genistein (2–20 μmol/l) was found to inhibit DNMT, reverse DNA hypermethylation and reactivate RARβ, p16, and O6-methylguanine methyltransferase (MGMT) in esophageal squamous carcinoma and prostate and mammary cancer cells in vitro (9,10). Genistein also inhibited cell growth at these concentrations. In theory, prevention or reversal of hypermethylation-induced inactivation of key tumor suppressor or receptor genes by DNMT inhibitors such as genistein and daidzein could be an effective approach for cancer prevention, but little is known about the methylation effects of these compounds in humans.
Asian populations that have low rates of breast cancer consume 20 to 80 mg/day of genistein, whereas dietary intake of genistein in the United States is 1 to 3 mg/day (7). In a case-control study, a significant reduction in breast cancer risk was found in both premenopausal and postmenopausal women who consumed supplemental isoflavones (11). Circulating ovarian hormone levels were decreased (estradiol, 25%; progesterone, 45%) in premenopausal women after they received 36 oz of soymilk (113–207 mg/day isoflavones) through one menstrual cycle (12). Six-month ingestion of 37 mg/day genistein led to increased plasma estradiol levels, increased nipple aspirate fluid (NAF) volume, and epithelial hyperplasia in NAF in a subset of premenopausal women (13). In general, clinical studies of isoflavones have observed lesser effects in postmenopausal compared to premenopausal women (14).
Genistein and daidzein belong to a larger class of compounds called polyphenols, which have documented effects on DNA methylation (15). Genistein, and to a lesser extent daidzein, reversed DNA hypermethylation and reactivated RARβ, p16, and MGMT in mammary cancer cells in vitro (6), whereas two animal studies have observed increased methylation effects with treatment (16,17). Thus, the demethylation effects of genistein and daidzein on tumor suppressor genes in vitro appears to be at odds with the methylating effects of genistein in the two animal studies. Furthermore, no studies to date have examined the effect of dietary isoflavones on gene methylation in humans. In theory, prevention or reversal of hypermethylation-induced inactivation of key tumor suppressor genes by genistein and daidzein could be an effective approach for cancer prevention.
We examined the response of genes frequently methylated in breast cancer to orally administered genistein and daidzein in the breast tissue of healthy premenopausal women using mammary ductoscopy (MD). Our attempts to use methylation arrays were unsuccessful; however we observed treatment related methylation effects using quantitative methylation specific PCR (qMS-PCR) in our assessment of 5 genes (p16, RASSF1A, RARβ2, ER, and CCND2) known to be methylated in breast cancer (18–20). Estrogenic effects of isoflavones were determined by measuring circulating levels of the estrogenic marker complement (C)3 (21) and evaluating the cytologic profile of breast ductal epithelial cells. We correlated the estrogenic and methylation changes with isoflavone dose and with circulating levels of genistein.
MATERIALS AND METHODS
Subjects
Premenopausal subjects (19–54 yr) with no history of atypia, in situ, or invasive breast cancer were recruited after Internal Review Board (IRB) approval. All procedures were conducted in accordance with the ethical standards of the University of Missouri IRB. Women who were pregnant, lactating, or had nursed within 20 mo of study enrollment were excluded. Subjects consuming supplements in the past month containing alfalfa, black cohosh, flax meal, flax seed, ginseng, hops, licorice, red clover, thyme, tumeric, verbana, vitex agnus castus, or using Chinese, Ayurvedic, or Tibetan medicines were excluded. Subjects were administered a food frequency questionnaire to determine their daily isoflavone consumption. Subjects ingesting >3 mg isoflavones/day from foods were required to eliminate these foods for at least one month prior to starting the trial and were counseled regarding soy-containing foods to avoid.
A prospective, double-blind, randomized trial was conducted in 34 subjects with two doses of isoflavones. Fifteen women were randomly assigned to consume one low dose capsule (18.6 mg isoflavones: 13.2 mg genistein, 5.2 mg daidzein, and 0.2 mg glycitein) twice daily for a total daily phytoestrogen dose of 37.2 mg, and 19 were assigned to consume one high dose capsule (64.4 mg isoflavones: 45.3 mg genistein, 18.2 mg daidzein, and 0.9 mg glycitein) twice daily for a total daily phytoestrogen dose of 128.8 mg. Both treatments were given through one menstrual cycle. The doses selected were based on the typical Asian consumption of isoflavones (low dose) and studies of documented effects of isoflavones on mammary glands (high dose) (12,13). Randomization was conducted via the method of sealed envelopes, prepared in advance by the study's biostatistician, Dr. John Hewett. The clinical research pharmacists were responsible for opening the envelopes and dispensing the appropriate capsules to each subject. All other study personnel were blinded as to group assignment. Subjects began and ended supplement treatment during the first 10 days of their menstrual cycle. Isoflavone preparations were provided as a single lot by the Solae Company (St. Louis, MO). Each subject served as their own control by collecting samples before and after treatment. Compliance was assessed by a capsule calendar and collection of unused capsules.
Specimen Collection
NAF, blood, and MD samples were collected before and one menstrual cycle after isoflavone intervention and prepared for cytologic and biologic analysis as previously described (22,23). Both pre and posttreatment samples from all women were collected during the first 10 days of their menstrual cycle. Whereas NAF samples from the left and right breast were kept separate, MD samples from each breast were combined to increase the total sample available for methylation and cytologic analyses. For MD collection, a nipple grid was used to indicate the location of the duct cannulated at baseline. Attempts were made to cannulate the same duct before and after treatment.
C3 Studies
Pre and posttreatment samples of serum and NAF from the same breast were analyzed for C3 using an enzyme immunoassay kit (Assay Designs, Ann Arbor, MI).
Cytologic Review
The Pap-stained slides were examined in blinded fashion as previously described (24,25). The cytologic endpoints were normal, hyperplasia without atypia, atypical hyperplasia, and malignant cells present.
qMS-PCR
One μg salmon sperm carrier DNA was added to DNA extracted from each MD sample, which was then sodium bisulfite treated. A two-step PCR strategy was employed for p16, RASSF1A, RARβ, ER, and CCND2, all frequently hypermethylated in breast cancer (26–28) and some known to be demethylated by genistein (10,15). First-round PCR of MD DNA, 100% methylated DNA (positive), and water (negative) controls was carried out for the 5 genes using an AmpliTaq Gold PCR kit (Applied Biosystems, Foster City, CA). For secondround SYBR green-based qMSP, diluted PCR products were amplified with specific primers of the 5 genes for both methylated and unmethylated DNA. The primer sets for qMSP have previously been reported (29,30). The percent of methylated DNA in a each sample was calculated (31). DNA that was 100% methylated or 100% unmethylated was used to generate a standard curve to quantify the percent methylated DNA in each sample.
qMS-PCRvalidation
To verify the specificity of second round qMS-PCR products, selected amplicons for p16, RASSF1A, RARβ2, ER, and CCND2 were subcloned using the TOPO-TA cloning system (Invitrogen, Carlsbad, CA). Plasmid DNA of 5 to 6 insert positive clones was isolated and sequenced using an ABI 3730 DNA Analyzer (Applied Biosystems, Foster City, CA).
Quantification of Genistein in Serum
After preparing stock and working solutions of genistein, serum samples were analyzed as previously described (32). After sample centrifugation, the supernatant was added to a β-glucuronidase solution, incubated, mixed with 2 N HCl and then loaded on a Waters C18 sep-vac-pak column (Waters Corp., Milford, MA), vacuum washed, the columns eluted with methanol, and the eluants taken to dryness. High-performance liquid chromatography was carried out on reconstituted samples using an ESA Model 582 pump with ESA Model 5600-A coularray detector (ESA, Inc., Chelmsford, MA) and a Thermal Separation Product autosampler (20 μl injection).
Statistical Analysis
Both within-group and between-group analyses were conducted. Within-group analyses to detect significant pre- to postchanges in cytology were done using the sign test. The sign was employed to test for significant within-group changes in quantitative variables such as C3 and the fraction of methylated p16, RASSF1A, RARβ2, ER, and CCND2. Spearman's correlation coefficients were computed to investigate relationships between quantitative variables such as change in genistein level and changes in C3 and the fraction of methylated p16, RASSF1A, RARβ2, ER, and CCND2. The Wilcoxon rank sum test was employed to test for between-group differences for quantitative variables such as the change in genistein level from pre- to post-treatment.
RESULTS
Subjects
Of the 198 women interested in the study, 36 enrolled and 34 completed. Both women who dropped out were randomized to the low dose isoflavone group. Only results in which before and after samples from the same subject were available are included in this report (n = 34). Median age (37 vs. 36 yr) and family history of breast cancer (6 vs. 5) in the low vs. high dosage groups were similar. A median of 94% of the recommended isoflavone capsules were consumed. qMS-PCR was performed on MD samples from 26 women for which we had both pre and posttreatment samples from a given subject, the remaining samples having inadequate DNA. BMI did not significantly influence any of the markers evaluated. The only marker influenced by agewas RASSF1A in which the increase inmethylated DNA after treatment correlated directly with subject age (r = 0.49, P = 0.02; n = 22).
Isoflavone Levels in Capsules and in Serum
Two independent laboratories analyzed capsules at baseline. Capsules presumed to contain 20 mg isoflavones contained 18.5 mg and 19.1 mg isoflavones, whereas 70 mg capsules contained 70.2 and 64.4 mg isoflavones. Isoflavone content of the capsules was evaluated at 6 monthly intervals thereafter, with a cv over time of <10%. Serum levels of genistein were measured on average 3 h after the participant's last meal. Baseline serum genistein levels (Table 1) increased after treatment (P < 0.01 for both doses: median change 144 ng/ml in the low-dose and 507 ng/ml in the high-dose group). Serum genistein change was greater after high- than low-dose treatment (P < 0.001).
TABLE 1.
Genistein and complement 3 concentrations in serum and nipple aspirate fluid (NAF) before and after isoflavone interventiona
| 20 mg Isoflavones |
70 mg Isoflavones |
|||||
|---|---|---|---|---|---|---|
| Before | After | Δ | Before | After | Δ | |
| Serum genistein (ng/ml) | ||||||
| Mean | 17.3 (12) | 172.8 (12) | 155.5 (12) | 26.3 (15) | 620.5 (15) | 594.3 (15) |
| SD | 16.7 | 85.0 | 92.5 | 48.2 | 360.6 | 367.3 |
| Median | 15.8 | 171.1 | 143.6 | 14.8 | 528.5 | 507.3 |
| Serum C3 (ng/ml) | ||||||
| Mean | 24,138 (14) | 23,252 (14) | −886 (14) | 18,432 (17) | 16,157 (17) | −2,275 (17) |
| SD | 13,395 | 12,478 | 6,843 | 12,512 | 12,837 | 15,594 |
| Median | 27,673 | 17,828 | 446 | 14,191 | 12,040 | −1,925 |
| NAF C3 (ng/mg)b | ||||||
| Mean | 0.37 (9) | 0.15 (9) | −0.23 (9) | 0.35 (14) | 0.51 (14) | 0.16 (14) |
| SD | 0.37 | 0.15 | .43 | 0.44 | 0.95 | 0.81 |
| Median | 0.23 | 0.070 | −0.03 | 0.14 | 0.12 | −0.00 |
| NAF C3 (ng/ml) | ||||||
| Mean | 49.6 (9) | 20.5 (9) | −29.1 (9) | 48.4 (14) | 62.2 (14) | 13.8 (14) |
| SD | 65.1 | 30.4 | 77.5 | 70.7 | 152.9 | 113.5 |
| Median | 17.6 | 15.5 | −4.3 | 23.4 | 13.1 | −0.4 |
The values were obtained as follows: The change (Δ) that occurred in each individual (after treatment — before treatment) was calculated. Then the mean, SD, and median values for the changes were calculated. Change in serum genistein was significant for both the low- and high-dose isoflavones groups (P < 0.01 for both). Change in serum C3, NAF C3 (ng/ml), and NAF C3 (ng/mg) were not significant for either dosage group. Numbers in parentheses represent subjects with matched before and after treatment samples.
ng/mg: ng C3 per mg total NAF protein.
No Estrogenic Effect Observed With Isoflavones
A nonsignificant trend toward lower C3 levels was observed after isoflavone treatment (Table 1). Among women in the lowbut not the high-dose group, posttreatment serum C3 levels were inversely related to the change in serum genistein (r = −0.76, P = 0.0045). NAF C3 levels were not significantly influenced by genistein levels. Five of 15 subjects in the low-dose group and 7 of 19 subjects in the high-dose group had hyperplasia before and/or after treatment, whereas the remaining subjects had normal cytology before and after treatment. There was no significant effect of isoflavone treatment, regardless of dose or genistein level, on either NAF or MD cytology.
qMS-PCR Results
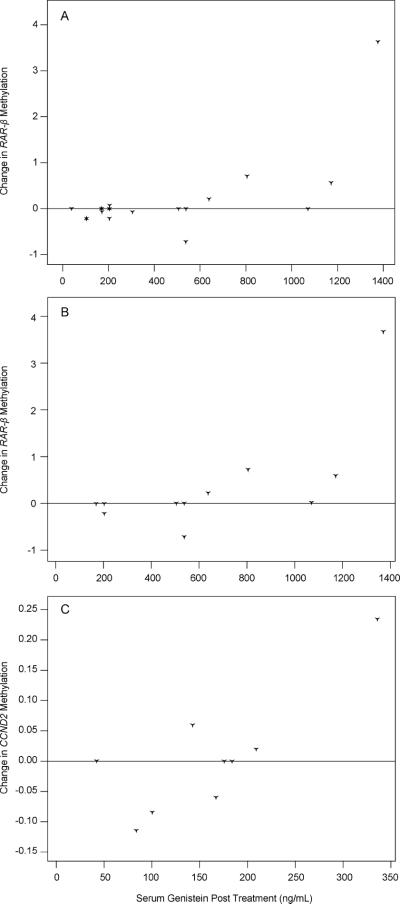
Pre and posttreatment samples from 26 subjects (13 receiving a low dose and 13 receiving a high dose of isoflavones) were analyzed by qMS-PCR for 5 genes (Table 2). There were no significant treatment-related changes for any of the genes, considering all subjects or by dose. However, considering all subjects, the change in RARβ2 was correlated (Fig. 1A) with the posttreatment level of genistein (r = 0.67, P = 0.0017). In general, RARβ2 methylation decreased posttreatment at circulating genistein levels below 600 ng/ml, with the opposite effect for circulating levels of genistein greater than 600 ng/ml.
TABLE 2.
Percent DNA methylated in 5 genes in healthy premenopausal women before and after treatment with soy isoflavonesa
| 20 mg Isoflavones |
70 mg Isoflavones |
|||||
|---|---|---|---|---|---|---|
| Gene | Before | After | Δ | Before | After | Δ |
| p16 | ||||||
| Mean | 0.37(12) | 0.30(12) | −0.06(12) | 0.72(11) | 0.84(11) | 0.11(11) |
| SD | 0.55 | 0.41 | 0.67 | 1.49 | 1.60 | 2.22 |
| Median | 0.12 | 0.16 | −0.01 | 0.17 | 0.14 | −0.02 |
| RASSF-1A | ||||||
| Mean | 1.48(13) | 1.24(13) | −0.24(13) | 1.22(9) | 1.21(9) | −0/01 (9) |
| SD | 2.32 | 1.61 | 1.17 | 0.92 | 1.73 | 1.59 |
| Median | 0.82 | 0.99 | −0.00 | 0.98 | 0.70 | −0.26 |
| RARβ2 | ||||||
| Mean | 0.18(10) | 0.12(10) | −0.06(10) | 0.18(10) | 0.57(10) | 0.39(10) |
| SD | 0.23 | 0.16 | 0.10 | 0.27 | 1.09 | 1.15 |
| Median | 0.05 | 0.05 | −0.03 | 0.05 | 0.18 | 0.00 |
| ER | ||||||
| Mean | 0.45(9) | 0.53(9) | 0.08(9) | 2.11(9) | 0.89(9) | −1.22(9) |
| SD | 0.40 | 0.79 | 0.74 | 4.76 | 2.35 | 5.30 |
| Median | 0.19 | 0.66 | 0.05 | 0.22 | 0.10 | −0.14 |
| CCND2 | ||||||
| Mean | 0.05(11) | 0.06(11) | 0.00(11) | 0.03(11) | 0.02(11) | −0.00(11) |
| SD | 0.06 | 0.09 | 0.09 | 0.03 | 0.03 | 0.03 |
| Median | 0.03 | 0.00 | 0.00 | 0.02 | 0.01 | 0.00 |
The values were obtained as follows: The change (Δ) that occurred in each individual (after treatment – before treatment) was calculated. Then the mean, SD, and median values for the changes were calculated. Change in p16, RASSF-1A, RAR/β2, ER, and CCND2 were not significant for either dosage group. Results were excluded if unreliable. Only matched results are shown. Numbers in parentheses represent subjects with matched before and after treatment samples.
FIG. 1.
Change in methylation of RARβ2 and CCND2 correlated with serum genistein level. Points above zero represent an increase in DNA methylation; points below zero represent a decrease. Methylation of RARβ2 for all subjects is shown in panel A and only subjects consuming high-dose isoflavones in panel B. When serum genistein levels were less than 600 ng/ml, a decrease in RARβ2 methylation was observed; whereas with serum genistein levels over 600 ng/ml, increased methylation was seen. Similar effects on CCND2 methylation are shown in panel C. For CCND2, a genistein level of 200 ng/ml provided the conversion point for methylation.
At the higher (but not the lower) dose, RARβ2 methylation changes were correlated (Fig. 1B) with genistein posttreatment (r = 0.68, P = 0.021) and genistein change (r = 0.68, P = 0.022). At the lower dose (Fig. 1C), change in CCND2 methylation correlated with genistein level posttreatment (r = 0.79, P = 0.011). CCND2 methylation generally decreased in subjects with posttreatment genistein levels less than 200 ng/ml and increased in subjects with higher posttreatment genistein levels. Methylation changes in p16, RASSF-1A and ER were not correlated with genistein level posttreatment.
In each of the three graphs of Fig. 1, there is a single observation that was markedly changed by isoflavone intervention. Notably, Spearman's correlation, which was used to calculate associations between genistein levels and methylation, is based on ranks and therefore not severely impacted by a single extreme observation. Nonetheless, we determined the association between genistein and methylation change after excluding the single extreme observation from each analysis. After doing this, each association (RARβ2 methylation change, all subjects, with genistein: r = 0.51, P = 0.03; RARβ2 methylation change, high-dose isoflavone group, with genistein: r = 0.50, P = 0.14; and CCND2 methylation change, low-dose isoflavone group, with genistein: r = 0.43, P = 0.29) remained strong, with significance limited by sample size.
DISCUSSION
Whether genistein and daidzein act on the ER to increase or decrease tumor growth is likely related to the dose ingested and their subsequent circulating levels. Serum isoflavone levels increased in a dose-dependent manner when consuming the supplement. Within-group levels varied, likely related to known individual differences in isoflavone metabolism and excretion (33). Because of this variation, we evaluated the influence of both isoflavone dose and circulating genistein levels on estrogenic and methylation markers.
For the assessment of methylation changes related to treatment, the entire cell sample was evaluated, similar to other published studies evaluating methylation changes in intraductal samples (31,34). Using methylation-specific PCR, as was performed in the current study, one methylated allele can be identified in 1,000 methylated alleles; so MSP is an appropriate approach for the detection of abnormal cells in a background of normal cells (35).
Both RARβ2 and CCND2 methylation decreased with low and increased with high circulating levels of genistein (Fig. 1). Although the changes in methylation prevalence in RARβ and CCND2 based on genistein level posttreatment were statistically significant, the biologic significance of these changes is at present uncertain. Aberrant hypermethylation of CpG islands is an early event in cancer development (36). Euhus et al. (37) evaluated methylation using MSP in multiple groups of women including low and high breast cancer risk subjects. The 90% threshold value of methylated DNA in high risk women for RARβ was approximately .06 higher in the high-risk than in low-risk group and for CCND2, approximately .03 higher in the high-risk than in the low-risk group. As seen in Fig. 1, the changes in methylation prevalence after isoflavone treatment exceeded these differences for both genes in a number of the treated subjects. In general, low-dose genistein decreased or had no effect on methylation, whereas the high dose increased methylation of these two genes. Our findings at higher circulating genistein levels are consistent with preclinical studies demonstrating increased methylation after isoflavone treatment (16,17) and suggest that circulating genistein level is a better predictor of methylation response than isoflavone dose.
Of the two estrogenic parameters evaluated, C3 was influenced by the intervention, but cytology was not. This is perhaps not surprising, as changes in protein expression generally precede changes in cell morphology (38). Previously an estrogenic effect, based on higher NAF pS2 levels, was observed after two weeks of low-dose isoflavones (38); however we observed a trend toward lower C3 levels after one menstrual cycle treatment. Several factors may account for the different findings. First, the earlier study was conducted for two weeks, so many women began the study in one phase of their menstrual cycle (luteal or follicular) and ended in the other; whereas in our study, all women started and ended the study in the follicular phase. Second, in the earlier study, women with an abnormality requiring biopsy to exclude cancer were enrolled, whereas we only enrolled healthy women. Finally, whereas we evaluated C3 to determine estrogenic effect of isoflavone intervention, the prior study evaluated pS2. These markers may assess estrogenic effect differently.
Hypermethylation of RARβ is a frequent event in breast cancer and is correlated with the presence of sentinel lymph node metastases, an important adverse prognostic factor in breast cancer (18,39). Promoter hypermethylation of CCND2, an important cell-cycle-regulatory gene that controls the transition from G1 to S phase, has been observed in in situ breast cancer, suggesting that gene silencing of CCND2 through hypermethylation is an early event in breast carcinogenesis (40).
A recent study detected hypermethylation of RARβ and CCND2 in 21% and 5%, respectively, of healthy BRCA gene mutation carriers (36). We observed baseline methylation levels in the breasts of healthy premenopausal women that were generally low but not zero, thereby providing useful benchmark information for future methylation studies. Our finding that the direction of changes in RARβ2 and CCND2 methylation depended on circulating levels of genistein suggests different mechanisms of action for high vs. low levels of isoflavones.
In summary, among healthy premenopausal women consuming isoflavones for one menstrual cycle, we found no estrogenic effects and preliminary evidence of an antiestrogenic effect. Isoflavones caused significant changes in the methylation levels of RARβ2 and CCND2 in the breast, the direction of which was dependent on the circulating levels of genistein. This study provides a novel, noninvasive approach to define the impact of dietary isoflavones on mammary cells and therefore the risk of breast cancer. The degree to which soy isoflavones influence breast hypermethylation deserves further study.
ACKNOWLEDGMENTS
This study was supported in part by National Institutes of Health grant CA105330.
Footnotes
Copyright of Nutrition & Cancer is the property of Lawrence Erlbaum Associates and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. Howerer, users may print, download, or email articles for individual use.
REFERENCES
- 1.Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, et al. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. Embo J. 1999;18:4608–4618. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4363. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 3.Legler J, van den Brink CE, Brouwer A, Murk AJ, van der Saag PT, et al. Development of a stably transfected estrogen receptor-mediated luciferase reporter gene assay in the human T47D breast cancer cell line. Toxicol Sci. 1999;48:55–66. doi: 10.1093/toxsci/48.1.55. [DOI] [PubMed] [Google Scholar]
- 4.Duffy C, Cyr M. Phytoestrogens: potential benefits and implications for breast cancer survivors. J Womens Health (Larchmont) 2003;12:617–631. doi: 10.1089/154099903322404276. [DOI] [PubMed] [Google Scholar]
- 5.Hilakivi-Clarke L, Onojafe I, Raygada M, Cho E, Skaar T, et al. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. Br J Cancer. 1999;80:1682–1688. doi: 10.1038/sj.bjc.6690584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, et al. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–558S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 7.Barnes S, Peterson TG, Coward L. Rationale for the use of genistein-containing soy matrices in chemoprevention trials for breast and prostate cancer. J Cell Biochem Suppl. 1995;22:181–187. doi: 10.1002/jcb.240590823. [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Yan L, Davidson NE. DNA methylation in breast cancer. Endocr Relat Cancer. 2001;8:115–127. doi: 10.1677/erc.0.0080115. [DOI] [PubMed] [Google Scholar]
- 9.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 10.Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, et al. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11(Pt 1):7033–7041. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- 11.Ingram D, Sanders K, Kolybaba M, Lopez D. Case-control study of phyto-oestrogens and breast cancer [see comments] Lancet. 1997;350:990–994. doi: 10.1016/S0140-6736(97)01339-1. [DOI] [PubMed] [Google Scholar]
- 12.Lu LJ, Anderson KE, Grady JJ, Kohen F, Nagamani M. Decreased ovarian hormones during a soya diet: implications for breast cancer prevention. Cancer Res. 2000;60:4112–4121. [PubMed] [Google Scholar]
- 13.Petrakis NL, Barnes S, King EB, Lowenstein J, Wiencke J, et al. Stimulatory influence of soy protein isolate on breast secretion in pre- and post-menopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785–794. [PubMed] [Google Scholar]
- 14.Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, et al. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab. 1999;84:3479–3484. doi: 10.1210/jcem.84.10.6067. [DOI] [PubMed] [Google Scholar]
- 15.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137(1 Suppl):223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 16.Day JK, Bauer AM, DesBordes C, Zhuang Y, Kim BE, et al. Genistein alters methylation patterns in mice. J Nutr. 2002;132(8 Suppl):2419S–2423S. doi: 10.1093/jn/132.8.2419S. [DOI] [PubMed] [Google Scholar]
- 17.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fackler MJ, McVeigh M, Evron E, Garrett E, Mehrotra J, et al. DNA methylation of RASSF1A, HIN-1, RAR-beta, Cyclin D2 and Twist in in situ and invasive lobular breast carcinoma. Int J Cancer. 2003;107:970–975. doi: 10.1002/ijc.11508. [DOI] [PubMed] [Google Scholar]
- 19.Giacinti L, Claudio PP, Lopez M, Giordano A. Epigenetic information and estrogen receptor alpha expression in breast cancer. Oncologist. 2006;11:1–8. doi: 10.1634/theoncologist.11-1-1. [DOI] [PubMed] [Google Scholar]
- 20.Krassenstein R, Sauter E, Al-Saleem E, Battagli C, Ehya H, et al. Detection of breast cancer in nipple aspirate fluid by hypermethylation. Clin Cancer Res. 2004;10:28–32. doi: 10.1158/1078-0432.ccr-0410-3. [DOI] [PubMed] [Google Scholar]
- 21.Wanda GJ, Starcke S, Zierau O, Njamen D, Richter T, et al. Estrogenic activity of griffonianone c, an isoflavone from the root bark of millettia griffoniana: regulation of the expression of estrogen responsive genes in uterus and liver of ovariectomized rats. Planta Med. 2007;73:512–518. doi: 10.1055/s-2007-967186. [DOI] [PubMed] [Google Scholar]
- 22.Sauter ER, Daly M, Linahan K, Ehya H, Engstrom PF, et al. Prostate-specific antigen levels in nipple aspirate fluid correlate with breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:967–970. [PubMed] [Google Scholar]
- 23.Sauter ER, Ehya H, Klein-Szanto AJ, Wagner-Mann C, MacGibbon B. Fiberoptic ductoscopy findings in women with and without spontaneous nipple discharge. Cancer. 2005;103:914–921. doi: 10.1002/cncr.20865. [DOI] [PubMed] [Google Scholar]
- 24.Sauter ER, Ehya H, Schlatter L, MacGibbon B. Ductoscopic cytology to detect breast cancer. Cancer J. 2004;10:33–41. doi: 10.1097/00130404-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Sauter ER, Ross E, Daly M, Klein-Szanto A, Engstrom PF, et al. Nipple aspirate fluid: a promising non-invasive method to identify cellular markers of breast cancer risk. Br J Cancer. 1997;76:494–501. doi: 10.1038/bjc.1997.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoque MO, Feng Q, Toure P, Dem A, Critchlow CW, et al. Detection of aberrant methylation of four genes in plasma DNA for the detection of breast cancer. J Clin Oncol. 2006;24:4262–4269. doi: 10.1200/JCO.2005.01.3516. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Rong M, Iacopetta B. DNA hypermethylation in breast cancer and its association with clinicopathological features. Cancer Lett. 2006;237:272–280. doi: 10.1016/j.canlet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Sharma G, Mirza S, Prasad CP, Srivastava A, Gupta SD, et al. Promoter hypermethylation of p16INK4A, p14ARF, CyclinD2 and Slit2 in serum and tumor DNA from breast cancer patients. Life Sci. 2007;80:1873–1881. doi: 10.1016/j.lfs.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Gustafson KS, Furth EE, Heitjan DF, Fansler ZB, Clark DP. DNA methylation profiling of cervical squamous intraepithelial lesions using liquid-based cytology specimens: an approach that utilizes receiver-operating characteristic analysis. Cancer. 2004;102:259–268. doi: 10.1002/cncr.20425. [DOI] [PubMed] [Google Scholar]
- 30.House MG, Guo M, Iacobuzio-Donahue C, Herman JG. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis. 2003;24:193–198. doi: 10.1093/carcin/24.2.193. [DOI] [PubMed] [Google Scholar]
- 31.Fackler MJ, McVeigh M, Mehrotra J, Blum MA, Lange J, et al. Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res. 2004;64:4442–4452. doi: 10.1158/0008-5472.CAN-03-3341. [DOI] [PubMed] [Google Scholar]
- 32.Curran EM, Judy BM, Newton LG, Lubahn DB, Rottinghaus GE, et al. Dietary soy phytoestrogens and ERalpha signalling modulate interferon gamma production in response to bacterial infection. Clin Exp Immunol. 2004;135:219–225. doi: 10.1111/j.1365-2249.2003.02368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora—implications for health. Mol Nutr Food Res. 2007;51:765–781. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- 34.Evron E, Dooley WC, Umbricht CB, Rosenthal D, Sacchi N, et al. Detection of breast cancer cells in ductal lavage fluid by methylation-specific PCR. Lancet. 2001;357:1335–1336. doi: 10.1016/s0140-6736(00)04501-3. [DOI] [PubMed] [Google Scholar]
- 35.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 36.Locke I, Kote-Jarai Z, Fackler MJ, Bancroft E, Osin P, et al. Gene promoter hypermethylation in ductal lavage fluid from healthy BRCA gene mutation carriers and mutation-negative controls. Breast Cancer Res. 2007;9:R20. doi: 10.1186/bcr1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Euhus DM, Bu D, Ashfaq R, Xie XJ, Bian A, et al. Atypia and DNA methylation in nipple duct lavage in relation to predicted breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:1812–1821. doi: 10.1158/1055-9965.EPI-06-1034. [DOI] [PubMed] [Google Scholar]
- 38.Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017–4024. doi: 10.1210/jcem.84.11.6152. [DOI] [PubMed] [Google Scholar]
- 39.Fraker LD, Halter SA, Forbes JT. Growth inhibition by retinol of a human breast carcinoma cell line in vitro and in athymic mice. Cancer Res. 1984;44(Pt 1):5757–5763. [PubMed] [Google Scholar]
- 40.Evron E, Umbricht CB, Korz D, Raman V, Loeb DM, et al. Loss of cyclin D2 expression in the majority of breast cancers is associated with promoter hypermethylation. Cancer Res. 2001;61:2782–2787. [PubMed] [Google Scholar]