Abstract
OBJECTIVE
The purpose of this study was to investigate the impact of prenatal exposure to chlorpyrifos on 3-year neurodevelopment and behavior in a sample of inner-city minority children.
METHODS
As part of an ongoing prospective cohort study in an inner-city minority population, neurotoxicant effects of prenatal exposure to chlorpyrifos were evaluated in 254 children through the first 3 years of life. This report examined cognitive and motor development at 12, 24, and 36 months (measured with the Bayley Scales of Infant Development II) and child behavior at 36 months (measured with the Child Behavior Checklist) as a function of chlorpyrifos levels in umbilical cord plasma.
RESULTS
Highly exposed children (chlorpyrifos levels of >6.17 pg/g plasma) scored, on average, 6.5 points lower on the Bayley Psychomotor Development Index and 3.3 points lower on the Bayley Mental Development Index at 3 years of age compared with those with lower levels of exposure. Children exposed to higher, compared with lower, chlorpyrifos levels were also significantly more likely to experience Psychomotor Development Index and Mental Development Index delays, attention problems, attention-deficit/hyperactivity disorder problems, and pervasive developmental disorder problems at 3 years of age.
CONCLUSIONS
The adjusted mean 36-month Psychomotor Development Index and Mental Development Index scores of the highly and lower exposed groups differed by only 7.1 and 3.0 points, respectively, but the proportion of delayed children in the high-exposure group, compared with the low-exposure group, was 5 times greater for the Psychomotor Development Index and 2.4 times greater for the Mental Development Index, increasing the number of children possibly needing early intervention services.
Keywords: pesticides, chlorpyrifos, neurodevelopment, behavior problems
In June 2000, the US Environmental Protection Agency (EPA) announced a ban on the sale of the insecticide chlorpyrifos for residential use, effective December 31, 2001.1 This regulatory action was intended to phase out nearly all indoor and residential uses of this organophosphate,2 one of the most commonly used pest control substances in the United States. Before the ban, residential use of chlorpyrifos was particularly heavy in New York City. For example, during 1997, the amount of the insecticide applied by licensed applicators in New York City exceeded the amount applied in any other county in New York State, including agricultural communities. Other commonly detected insecticides included the organophosphate diazinon and propoxur.3
As a result of heavy indoor use, largely for cockroach control, pesticide exposure is widespread among pregnant women living in New York City.3–8 Specifically, 72% to 85% of women participating in 2 different cohort studies reported using some form of pest control during pregnancy,3,7–9 and 46% reported using ≥1 of the higher-toxicity methods, such as exterminator sprays, can sprays, and pest bombs.3 A survey of pest control measures conducted in 2000 and 2001 by the office of the New York State Attorney General found that 93% of New York City public housing residents applied pesticides at home and more than one half did so approximately once per week.6
In a previous report from the present cohort, detectable levels of chlorpyrifos were found in 99.7% of personal air samples and 100% of indoor air samples from stationary residential monitors for a sample of pregnant women.8 Chlorpyrifos was also detected in 64% to 70% of blood samples collected from mothers and newborns at delivery. Maternal levels and newborn levels were correlated strongly, which indicates that this pesticide crosses the placenta readily.3 Since the ban, levels of chlorpyrifos in personal and indoor air samples have decreased at least threefold and plasma blood levels have decreased more than fivefold among women and infants participating in the present longitudinal study.3
Experimental work showed links between chlorpyrifos exposure during pregnancy and deficits in fetal growth and neurocognitive development in rats.10 Prenatal chlorpyrifos exposure was shown experimentally to inhibit acetylcholinesterase, to downregulate muscarinic receptors, to inhibit the adenylate cyclase signaling cascade, to decrease brain DNA and RNA synthesis, and to suppress neurite outgrowth.10–14 Many organophosphate compounds are lipophilic and cross the placenta.15 Prenatal exposure is a source of concern because acetylcholinesterase seems to act as a neurotropic factor during brain development.16 Organophosphates may also disrupt brain development through noncholinergic mechanisms, at doses that cause only minimal acetylcholinesterase inhibition.16–18 Unlike classic teratologic cases, in which the greatest sensitivity is seen during the first trimester, the window of vulnerability for organophosphates is likely to extend from the embryonic period into postnatal life.13 Exposures during the spurt in brain growth, which in human pregnancies begins during the third trimester, may be particularly deleterious.11,12,16,19–21 The defects seen experimentally are in the same neurotransmitter pathways as those that are characteristic of nicotine-related changes, and there is evidence that some of these effects are delayed, which suggests that the programming of synaptic development has been altered.13,22 Such changes may emerge or reemerge later in development, accompanied by behavioral anomalies.23
To date, relatively few studies of pesticide effects on human neurodevelopment have been published. Young et al24 found associations between prenatal maternal organophosphate exposure and deviant neonatal reflexes, which are very early functional indicators of neurologic damage. Guillette et al25 found that 4- and 5-year-old children in a Mexican agricultural community with high levels of organophosphate and organochlorine pesticide use showed decreased stamina, hand-eye coordination, drawing ability, and short-term recall, compared with children from a nearby community with low levels of use. However, there was no biomarker of exposure, there was no control for confounding, interviewers were not blinded to group status, and the assessment tools were not validated. A more-recent study investigated long-term neurobehavioral development in young children after illegal residential applications of the organophosphate pesticide methyl-parathion.26 Urinary and environmental measures were used to document exposure levels, and adverse effects were seen for tasks that involved short-term memory and attention. Parents of exposed children also reported that their children had more behavioral and motor skill problems.26 Because the experimental literature has shown adverse neurodevelopmental consequences of exposure to chlorpyrifos at subclinical exposure levels, there is concern that risks to pregnant women and their offspring may go undetected.22,27,28
In the present cohort, previous work demonstrated significant inverse associations between umbilical cord chlorpyrifos levels and birth weight and length.4,29 Among infants born before the EPA ban, birth weight decreased by 67.3 g (95% confidence interval [CI]: −116.6 to −17.8 g; P = .008) and birth length decreased by 0.43 cm (95% CI: −0.73 to −0.14 cm; P = .004) for each 1-unit increase in logarithmically transformed cord plasma chlorpyrifos level,4 which showed that exposures were sufficiently high to produce fetal growth deficits. The purpose of the present study was to evaluate the longer-term effects of prenatal chlorpyrifos exposure on preschool behavior and child neurodevelopment during the first 3 years of life.
METHODS
Study Subjects
The subjects for this report were participants in an ongoing prospective cohort study of inner-city mothers and their newborn infants who were born between February 1998 and May 2002.29 The study was initiated in 1997, to evaluate the effects of prenatal exposures to ambient and indoor pollutants on birth outcomes, neurocognitive development, asthma, and procarcinogenic damage in a cohort of mothers and newborns from minority communities in New York City. The study gathered information on prenatal pesticide use in response to growing concerns regarding the extent of residential insecticide use in New York City30 and the levels of pesticide concentrations in air and biospecimens.31
Nonsmoking women (classified by self-report and validated with blood cotinine levels of <15 ng/mL), 18 to 35 years of age, who self-identified as black or Dominican and who registered at the obstetrics/gynecology clinics at New York Presbyterian Medical Center and Harlem Hospital by the 20th week of pregnancy were approached for consent to participate. Eligible women were free of diabetes mellitus, hypertension, known HIV infection, and documented or reported drug abuse and had resided in the area for ≥1 year. The retention rate for the full cohort was 82.72% at the 3-year follow-up assessment. There were no significant differences between women retained in the study and those lost to follow-up monitoring with respect to maternal age, ethnicity, marital status, education, and income and gestational age and birth weight of the newborn. The study was approved by the institutional review board of Columbia University, and informed consent was obtained from all participants.
Of 648 consenting women, 536 were active participants in the ongoing study at the time of this report, and 254 of their children had reached the age of 3 years with (1) prenatal maternal interview data, (2) biomarkers of chlorpyrifos exposure levels from maternal and/or cord blood samples obtained at delivery, (3) postnatal observational data on the quality of the home caretaking environment, and (4) a neurobehavioral outcome assessment at ≥1 yearly evaluation (12, 24, and 36 months). Of the 254 children in the research sample, all of the requisite data were available for 228 children at 12 months, for 227 at 24 months, and for 228 at 36 months. There were 189 children with outcome assessments and other required data at all 3 time points.
Maternal Interview
A 45-minute questionnaire was administered to each woman by a trained bilingual interviewer during the third trimester of pregnancy. Information included demographic characteristics, home characteristics, lifetime residential history, history of active and passive smoking, occupational history, maternal education and income level, alcohol and drug use during pregnancy, and history of residential pesticide use. The questionnaire was based on one used in a previous study and was adapted for the New York City population.31 Information on residential exposure to environmental tobacco smoke (ETS) was obtained from a set of questions about timing, frequency, and amount of exposure to cigarette smoke in the home. Residential exposure to ETS has the greatest potential impact on mother and child, because of the stability, duration, and intensity of exposure in urban apartments such as those of our study participants. There was a significant difference in mean cotinine levels between residentially exposed and nonexposed mothers (P < .05), but there was no significant difference between mothers reporting work exposure only and nonexposed mothers. Therefore, classification of self-reported ETS exposure was based on residential history only.
Biological Sample Collection
Samples of umbilical cord blood (30–60 mL) were collected at delivery by drawing blood into a heparin-containing syringe, to avoid clotting. Samples of maternal blood (30–35 mL) were collected within 2 days after delivery, in heparin-containing Vacutainer tubes (Fisher Scientific, Morres Plains, NJ), and were transported to the Columbia Center for Children’s Environmental Health laboratory for processing. Portions were sent to the Centers for Disease Control and Prevention for analysis of chlorpyrifos, cotinine, and metal levels, as described in detail elsewhere.8,32 Methods for the laboratory assay for chlorpyrifos, including quality control, reproducibility, and limits of detection (limits of detection: 0.5–1 pg/g), also were published previously.33 The parent compound for chlorpyrifos was measured in blood plasma. Lead in umbilical cord blood was analyzed through Zeeman graphite furnace atomic absorption spectrometry, with a phosphate/Triton X-100/nitric acid matrix modifier.
Measures of Child Behavior and Neurodevelopment
The Bayley Scales of Infant Development II (BSID-II) were used to assess cognitive and psychomotor development at 12, 24, and 36 months of age.34 The BSID-II is a widely used, normative value-referenced, developmental test for young children that is used frequently to diagnose developmental delay and is known to be sensitive to the effects of toxic exposures such as low-level, intrauterine, lead exposure.35,36 Each scale provides a developmental quotient (raw score/chronological age), which generates a continuous Mental Development Index (MDI) and a corresponding Psychomotor Development Index (PDI). When administered at 3 years of age, the BSID-II demonstrates only moderate predictive power for subsequent intelligence and school performance but is clinically useful for children performing in the subnormal range.34,37–39 Children’s status can be classified as normal or delayed (scores of ≤85) on the basis of a standardized cutoff point of 1 SD. In the present study, each child was tested under controlled conditions in the study office by a trained bilingual research assistant, checked for reliability. Five different testers conducted a total of 1101 BSID-II assessments over the course of the 3-year study period. Every effort was made to maximize reliability in scoring by using standardized training procedures and regular quality control. Inter-rater reliability for the 24-month BSID-II assessments was r = 0.92, on the basis of double-scoring of a random 5% of the sample.
Behavior problems were measured through maternal report on the 99-item Child Behavior Checklist (CBCL) for ages 1.5 to 5 years, which collects information on child behaviors occurring in the past 2 months.40 Syndrome scale scores are obtained by summing individual items on the subscales, and the total problems score is the sum of all items. The CBCL also yields internalizing and externalizing scores and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)-oriented scales that approximate clinical diagnoses.41 All subscales are scored continuously and categorically by using a borderline or clinical cutoff point corresponding to the 98th percentile for each domain. This measure is well validated, is easy to administer, and screens problem areas that have been linked to pesticide and ETS exposure, specifically, attention problems and attention problems with hyperactivity.
Measures of maternal intelligence and quality of the home environment were included as covariates because they are known predictors of child neurodevelopment and potential confounders of the association between exposure and outcomes. Maternal nonverbal intelligence was measured when the child was ~3 years of age, with the Test of Nonverbal Intelligence, Second Edition,42 a 15-minute, language-free measure of general intelligence that is relatively stable and free of cultural bias. The quality of the care-taking environment, measured with the Home Observation for Measurement of the Environment (HOME), was also assessed at 3 years.43 This instrument assesses physical and interactive home characteristics44 and has been used widely in studies of neurotoxicity.45
Many nonpersistent pesticides are soluble in water and therefore may partition to the water fraction of breast milk, although available data are extremely limited for chlorpyrifos. In the present study, breastfeeding was considered as a dichotomous variable (present or absent) in preliminary analyses and was found to have no relationship to any of the developmental outcomes considered in this study and no relationship to chlorpyrifos exposure. It was therefore not included in the final analyses. Nevertheless, because exposure to chlorpyrifos in breast milk may be complex, this area deserves additional study.
Statistical Analyses
Maternal education was categorized as high school graduate or nongraduate. Dichotomization was preferred because of differences in years of education between women raised in the Dominican Republic and those raised in the United States and because a high school degree has implications for employment.
Although testing of the biomarker cotinine was available, cotinine is only a short-term marker of ETS exposure and does not indicate exposure throughout pregnancy. Therefore, cotinine testing was used only to validate maternal self-report, which was the preferred measure of ETS exposure in this study. The association between self-reported ETS exposure and cotinine assay results was highly significant (χ2 = 48.57; P < .001) and of comparable magnitude for black women (χ2 = 26.89; P < .001) and Hispanic women (χ2 = 19.16; P < .001), which supports the validity of self-report in this sample.
As described previously,8,29 chlorpyrifos levels in paired maternal and umbilical cord plasma samples were correlated strongly (r = 0.76; P < .001, Spearman’s rank). In cases in which the umbilical cord sample was not collected (12% of subjects), the mother’s values were used, on the basis of a formula derived from regression analyses described previously.4 Levels were logarithmically transformed to normalize a positively skewed distribution, and values below the limits of detection (0.5–1 pg/g) were assigned a value of one half the detection limit concentration. In preliminary analyses, we found no indication of either a linear or nonlinear dose-response relationship between chlorpyrifos levels and developmental outcomes. Chlorpyrifos levels were categorized into 4 groups, that is, undetectable levels (n = 80) and 3 tertiles in the detectable range: tertile 1 (n = 65), tertile 2 (n = 39), and tertile 3 (n = 44). As in a previous report of chlorpyrifos effects on birth weight,4,29 the only group for which mean 36-month BSID-II scores were significantly lower was the group with the highest exposure level (>6.17 pg/g). Specifically, analysis of variance showed significant PDI deficits in the highest tertile, compared with each of the other groups (undetectable: P = .02; lowest tertile: P = .02; middle tertile: P = .03). The only significant mean group difference in MDI scores was between the highest tertile and the lowest tertile (P = .03). The most highly exposed group and the undetectable group had lower mean MDI and PDI scores than did the 2 middle levels. On the basis of these preliminary analyses, and consistent with our previous reports, a dichotomized exposure variable was used, classifying subjects into high exposure (>6.17 pg/g) or lower exposure (≤6.17 pg/g).
Intrauterine lead levels in the sample ranged from 0.15 µg/dL to 2.80 µg/dL and were logarithmically transformed to approximate a normal distribution. Lead levels were available, however, for only a subset of children (n = 89). Within this subset, there was no significant relationship between prenatal lead levels and chlorpyrifos levels (r = −0.08; P = .49).
BSID-II scores have a floor of 50, which is the minimal obtainable score. A small number of children were considered untestable at each age and therefore were missing BSID-II scores, as follows: 1.4% of 24-month MDI scores, 0.7% of 24-month PDI scores, 0.4% of 36-month MDI scores, and 0.4% of 36-month PDI scores. There were no untestable children at the 12-month assessment.
Associations between prenatal chlorpyrifos exposure and developmental outcomes were estimated by using multivariate linear regression for continuous scores and logistic regression for categorical outcomes. To estimate the chlorpyrifos effect on neurodevelopment over time (from 12 to 36 months of age), general linear modeling (GLM) for repeated measures was used, including only those children with complete data at all 3 time points.46 Effect estimates, 95% CIs, and P values (α set at .05) were derived by using SPSS for Windows (release 13.01; SPSS, Chicago, IL). In addition to the prenatal indicator for high pesticide exposure, all models included terms for prenatal ETS exposure, gender, ethnicity, gestational age at birth, quality of the home care-taking environment, maternal educational level (high school degree versus no high school degree), and maternal IQ. Covariates were included in models as possible confounders if they (1) had a significant association with level of pesticide exposure and any measure of developmental outcomes in this sample, (2) altered the estimate of chlorpyrifos effect by ≥10%, or (3) had been identified as confounders in comparable studies. Maternal intelligence scores were missing for 29 women, and the sample mean was substituted for those missing values, to maximize sample size. Analyses conducted with and without the imputed values showed no differences in the magnitude of the covariate or exposure effects. Interactions of chlorpyrifos with all exposure variables and sociodemographic covariates were tested and found to be nonsignificant. Potential mediators of the exposure-development association included birth weight and birth length (growth parameters shown previously to be associated with prenatal chlorpyrifos exposure). Mediation was tested by including birth weight and birth length in separate models and determining whether the estimate of the pesticide effect was attenuated in the presence of each fetal growth parameter.
RESULTS
Table 1 describes the characteristics of the sample, stratified according to level of prenatal chlorpyrifos exposure. Chlorpyrifos levels ranged from undetectable to 63 pg/g, and 20.6% of the sample was classified as having high chlorpyrifos exposure. There were significant race/ethnicity differences in the distribution of chlorpyrifos exposure, in that 24.2% of black women and 14.9% of Dominican women had high exposure. Cotinine levels ranged from 0.01 ng/mL to 8.79 ng/mL, and 37% of the sample was classified as being exposed to ETS during pregnancy. Women who were highly exposed to chlorpyrifos were significantly more likely to have residential exposure to ETS during pregnancy, compared with women with lower chlorpyrifos exposure. The sample was largely at term, because of the timing of air monitoring later in pregnancy and the exclusion of high-risk pregnancies, as defined above (only 4% of the pregnancies were <37 weeks).
TABLE 1.
Characteristics of the Study Population According to Prenatal Chlorpyrifos Exposure Level (N = 254)
| Characteristic | High Exposure (n = 50) | Lower Exposure (n = 204) | P | ||
|---|---|---|---|---|---|
| Proportion, % | Mean ± SD | Proportion, % | Mean ± SD | ||
| Maternal characteristics | |||||
| Race/ethnicity | |||||
| Black | 24.2 | 75.8 | .02 | ||
| Dominican | 14.9 | 85.1 | |||
| Age, y | 24.60 ± 5.27 | 25.09 ± 5.14 | NS | ||
| Married | 12.2 | 18.2 | NS | ||
| No high school degree | 40.0 | 33.3 | NS | ||
| Maternal IQa | 84.28 ± 12.43 | 87.26 ± 13.69 | NS | ||
| HOME score | 38.46 ± 5.95 | 39.78 ± 5.72 | NS | ||
| Infant characteristics | |||||
| Birth weight, g | 3239.58 ± 558.09 | 3450.93 ± 448.30 | .05 | ||
| Birth length, cm | 50.02 ± 2.41 | 51.05 ± 3.60 | NS | ||
| Birth head circumference, cm | 34.03 ± 1.69 | 34.35 ± 1.84 | NS | ||
| Gestational age, wk | 39.24 ± 1.38 | 39.32 ± 1.49 | NS | ||
| Male | 48.0 | 46.1 | NS | ||
| Prenatal exposure to residential ETS | 56.0 | 31.9 | .002 | ||
The sample included all children who had reached 36 months of age with all required data elements. NS indicates not significant.
Measured with the Test of Nonverbal Intelligence, Second Edition.42
Table 2 shows the unadjusted means and SDs for continuous BSID-II scores and proportions delayed for the 2 exposure groups at 12, 24, and 36 months of age. The 36-month average PDI score was significantly lower among children exposed to high levels of chlorpyrifos, compared with those exposed to lower levels (P = .01). At 36 months, significantly greater proportions of children with cognitive (P = .05) and psychomotor (P = .002) delays were observed among children exposed prenatally to high levels of chlorpyrifos. Although none of the other differences reached statistical significance in these bivariate tests, children exposed to high levels of chlorpyrifos scored lower, on average, on 5 of the 6 age-specific tests than did children exposed to lower levels. Similarly, the percentage of children experiencing developmental delay was higher among children exposed to high chlorpyrifos levels than among children with low exposure for 5 of the 6 age-specific tests.
TABLE 2.
BSID-II Means and Proportion Delayed at 12, 24, and 36 Months According to Chlorpyrifos Exposure Level (N = 254)
| Domain | Total | High Exposure | Lower Exposure | P |
|---|---|---|---|---|
| MDI score, mean ± SD | ||||
| 12 mo | 94.03 ± 9.78 | 93.72 ± 9.64 | 94.11 ± 9.84 | .810 |
| 24 mo | 85.10 ± 12.41 | 83.69 ± 12.24 | 85.45 ± 12.46 | .396 |
| 36 mo | 89.58 ± 11.41 | 87.39 ± 10.08 | 90.11 ± 11.67 | .155 |
| PDI score, mean ± SD | ||||
| 12 mo | 96.22 ± 12.21 | 93.35 ± 12.94 | 96.95 ± 11.95 | .074 |
| 24 mo | 97.04 ± 11.47 | 98.70 ± 10.90 | 96.62 ± 11.60 | .274 |
| 36 mo | 100.46 ± 12.98 | 95.69 ± 14.71 | 101.63 ± 12.28 | .006 |
| Mild/significant mental delay, %a | ||||
| 12 mo | 15.7 | 17.0 | 15.4 | .468 |
| 24 mo | 49.3 | 60.0 | 46.7 | .076 |
| 36 mo | 32.9 | 45.5 | 29.9 | .048 |
| Mild/significant psychomotor delay, %b | ||||
| 12 mo | 14.0 | 21.7 | 12.1 | .078 |
| 24 mo | 13.2 | 13.0 | 13.3 | .595 |
| 36 mo | 10.5 | 24.4 | 7.1 | .002 |
Age-specific sample sizes ranged from 225 to 228 because of missed and/or unscorable assessments (<50).
MDI score of ≤85.
PDI score of ≤85.
Five behavioral domains were assessed with the CBCL at 36 months of age. Table 3 shows the proportions of children scoring in the problem range on each of these scales for the 2 exposure groups. A greater proportion of children with high chlorpyrifos exposure were in the clinical problem range on all 5 of the scales; the difference reached statistical significance in bivariate tests for both attention problems (P = .03) and attention-deficit/hyperactivity disorder (ADHD) problems (P = .03).
TABLE 3.
Proportions Scoring in the Clinical Problem Range on the CBCL at 36 Months According to Prenatal Chlorpyrifos Exposure Level (N = 228)
| Domain | Proportion, % | P | ||
|---|---|---|---|---|
| Total Sample |
High Exposure |
Lower Exposure |
||
| Attention problems | 3.4 | 10.6 | 1.1 | .010 |
| DSM-IV-oriented scales | ||||
| PDD problems | 4.7 | 8.5 | 3.8 | .162 |
| ADHD problems | 3.9 | 10.6 | 2.2 | .018 |
| Externalizing behavior | 9.1 | 10.6 | 8.6 | .426 |
| Internalizing behavior | 13.4 | 14.9 | 13.0 | .444 |
The sample included all children who had reached 3 years of age with data from the prenatal maternal interview, biomarkers of exposure, and the CBCL (parent report).
Table 4 shows the results of multivariate regression analyses estimating the age-specific effects of prenatal chlorpyrifos exposure on MDI scores, after adjustment for gender, ethnicity, gestational age at birth, home environment, prenatal ETS exposure, maternal education, and maternal intelligence. The adverse effect of prenatal chlorpyrifos exposure on MDI scores (−3.3 points) was marginally significant at 36 months of age (P = .06). Longer gestation was protective, despite the restricted range of gestational ages. Mother’s lack of a high school degree was associated with a statistically significant 3-point deficit in MDI scores. Black children outscored Dominican children consistently, and these effects seemed to increase with child age through 36 months (ranging from 0.2 points at 12 months to 6.3 points at 36 months). Female subjects scored, on average, significantly higher than male subjects (3.0 –3.8 points). In addition, children who lived in homes with higher HOME scores (both stimulation and interaction) scored higher scores at 36 months of age. Children exposed to ETS during the prenatal period showed a 3-point decrease in MDI scores at 24 months (P = .06), but this effect was no longer apparent at 36 months. Interaction terms for the interaction of chlorpyrifos exposure with the other exposure and sociodemographic variables were tested in the full model, and none was significant. Together, exposures and sociodemographic covariates accounted for 25% of the variance in MDI scores at 36 months.
TABLE 4.
Multivariate Linear Regression Models Testing Main and Interactive Effects of Prenatal Chlorpyrifos Exposure on 12-, 24-, and 36-Month MDI Scores, Adjusted for Race, Gender, Maternal Education, Maternal IQ, Gestational Age, and Prenatal ETS Exposure
| Variable | Model 1: 12 mo (n = 229) | Model 2: 24 mo (n = 225) | Model 3: 36 mo (n = 228) | |||
|---|---|---|---|---|---|---|
| B, Mean ± SE | P | B, Mean ± SE | P | B, Mean ± SE | P | |
| Constant | 103.893 ± 27.49 | <.001 | 10.709 ± 34.37 | NS | 52.724 ± 28.29 | .064 |
| Prenatal chemical exposures | ||||||
| ETSa | 0.456 ± 1.39 | .744 | −3.032 ± 1.66 | .069 | −0.058 ± 1.44 | .968 |
| Chlorpyrifosb | −0.344 ± 1.66 | .836 | −1.480 ± 2.03 | .466 | −3.327 ± 1.76 | .060 |
| Covariates | ||||||
| Race/ethnicityc | 0.229 ± 1.44 | .874 | 6.176 ± 1.73 | <.001 | 6.286 ± 1.47 | <.001 |
| Genderd | −2.974 ± 1.30 | .023 | −3.760 ± 1.58 | .018 | −3.680 ± 1.34 | .006 |
| Gestational age | 0.355 ± 0.44 | .421 | 1.466 ± 0.59 | .013 | 1.287 ± 0.47 | .007 |
| Maternal IQe | −5.982 ± 4.96 | .229 | 1.715 ± 5.66 | .762 | −6.751 ± 4.89 | .169 |
| Low maternal educationf | −0.278 ± 1.41 | .843 | −2.298 ± 1.70 | .179 | −2.888 ± 1.45 | .048 |
| HOME score | 0.173 ± 0.12 | .150 | 0.200 ± 0.14 | .165 | 0.589 ± 0.13 | <.001 |
| R2 | 0.040 | 0.146 | 0.251 | |||
The sample included all children who had reached 3 years of age with data from the prenatal maternal interview, biomarkers of exposure, and complete data on all covariates. Sample sizes for age-specific models ranged from 225 to 229 because of missed and/or unscorable assessments (<50). B indicates unstandardized regression coefficient; NS, not significant.
Prenatally exposed = 1; not exposed = 0.
High exposure (>6.17 pg/g) = 1; low exposure (≤6.17 pg/g) = 0.
Black = 2; Dominican = 1.
Male = 2; female = 1.
Measured with the Test of Nonverbal Intelligence, Second Edition,42 natural logarithmically transformed.
No high school degree = 1; high school degree = 0.
Table 5 shows the results of multivariate regression analyses estimating the age-specific effects of prenatal chlorpyrifos exposure on PDI scores, after adjustment for gender, ethnicity, gestational age at birth, home environment, prenatal exposure to ETS, maternal education, and maternal intelligence. A significant negative chlorpyrifos effect was seen at 36 months, in that children exposed to high prenatal levels scored, on average, 6.5 points lower on the PDI, compared with those with low levels (P = .003). Of the covariates, only race (P = .03) and gestational age (P = .03) had statistically significant effects on motor development at 36 months, consistent with the findings that maternal education and other socioeconomic status proxies have little impact on motor development in the early years.47 All interaction terms for the interaction of chlorpyrifos exposure with the other exposure and sociodemographic variables were tested in the full model, and none was significant. In particular, the lack of a significant interaction between chlorpyrifos exposure levels and HOME scores for MDI or PDI scores suggests that, although the quality of the home environment may have a significant independent effect on MDI scores (P < .001) and a marginal effect on PDI scores (P = .06), it neither protects against nor exacerbates the adverse impact of prenatal chlorpyrifos exposure on mental or motor development at 36 months of age.
TABLE 5.
Multivariate Linear Regression Models Testing Main and Interactive Effects of Prenatal Chlorpyrifos Exposure on 12-, 24-, and 36-Month PDI Scores, Adjusted for Race, Gender, Maternal Education, Maternal IQ, Gestational Age, and Prenatal ETS Exposure
| Variable | Model 1: 12 mo (n = 228) | Model 2: 24 mo (n = 227) | Model 3: 36 mo (n = 228) | |||
|---|---|---|---|---|---|---|
| B, Mean ± SE | P | B, Mean ± SE | P | B, Mean ± SE | P | |
| Constant | 112.28 ± 34.64 | .00 | 81.05 ± 32.45 | .01 | 64.56 ± 36.64 | .079 |
| Prenatal chemical exposures | ||||||
| ETSa | 0.31 ± 1.76 | .86 | 2.83 ± 1.63 | .08 | −0.14 ± 1.79 | .940 |
| Chlorpyrifosb | −3.30 ± 2.11 | .12 | 1.17 ± 1.98 | .56 | −6.46 ± 2.18 | .003 |
| Covariates | ||||||
| Race/ethnicityc | −2.00 ± 1.81 | .27 | 2.15 ± 1.70 | .21 | 3.88 ± 1.82 | .034 |
| Genderd | 0.11 ± 1.64 | .95 | 0.08 ± 1.54 | .96 | −2.95 ± 1.66 | .077 |
| Gestational age | −0.16 ± 0.56 | .77 | 0.20 ± 0.53 | .70 | 1.38 ± 0.64 | .033 |
| Maternal IQe | −0.71 ± 6.26 | .91 | 0.09 ± 5.56 | .99 | −5.78 ± 6.08 | .343 |
| Low maternal educationf | −0.81 ± 1.77 | .65 | −1.26 ± 1.66 | .45 | 1.69 ± 1.81 | .350 |
| HOME score | −0.08 ± 0.15 | .61 | 0.09 ± 0.14 | .53 | 0.30 ± 0.16 | .057 |
| R2 | 0.024 | 0.035 | 0.107 | |||
The sample included all children who had reached 3 years of age with data from the prenatal maternal interview, biomarkers of exposure, and complete data on all covariates. Sample sizes for age-specific models varied from 227 to 228 because of missed and/or unscorable assessments (<50). B indicates unstandardized regression coefficient.
Prenatally exposed = 1; not exposed = 0.
High exposure (>6.17 pg/g) = 1; low exposure (≤6.17 pg/g) = 0.
Black = 2; Dominican = 1.
Male = 2; female = 1.
Measured with the Test of Nonverbal Intelligence, Second Edition,42 natural logarithmically transformed.
No high school degree = 1; high school degree = 0.
Because there was a significant effect of race/ethnicity on BSID-II scores in most models, and because of the risk of possible uncontrolled confounding by other unmeasured race-associated exposures, we conducted race-specific analyses at 36 months. The adverse chlorpyrifos effect on PDI scores was significant at the .05 level in both race/ethnicity groups. Specifically, among black children with high versus low exposure, the deficit was ~7 points (97.07 points vs 104.22 points). Among Dominican children with high versus low exposure, the deficit was ~6 points (93.95 points vs 99.13 points). The chlorpyrifos effect on MDI scores was significant (P < .05) for black children, with a deficit of ~6 points (88.47 points vs 94.81 points), but was not significant for Dominican children, for whom the deficit was slightly less than 2 points (85.17 points vs 86.87 points). These findings were consistent with the overall chlorpyrifos results, which were stronger for the PDI than the MDI at 36 months.
Tables 6 and 7 show the results of logistic regression analyses estimating the adjusted risks of delays (MDI and PDI) as a function of prenatal chlorpyrifos exposure, after adjustment for gestational age, gender, ethnicity, maternal education, maternal intelligence, and home environment. Before 36 months of age, highly exposed children were no more likely to exhibit mental or motor delays than were those with lower exposure. At 36 months of age, the odds of highly exposed children having mental delays were 2.4 times as great (95% CI: 1.12–5.08; P = .02) and the odds of motor delays were 4.9 times as great (95% CI: 1.78–13.72; P = .002), compared with children with lower prenatal exposures.
TABLE 6.
Logistic Regression Models Testing Effects of Chlorpyrifos on the Odds of Mental Delay at 12, 24, and 36 Months, Adjusted for Race, Gender, Gestational Age, Maternal Education, Maternal IQ, ETS Exposure, and Home Environment
| Variable | Odds Ratio (95% CI) | ||
|---|---|---|---|
| Model 1: 12 mo | Model 2: 24 mo | Model 3: 36 mo | |
| Prenatal exposures | |||
| ETSa | 0.58 (0.25–1.33) | 1.26 (0.70–2.56) | 1.29 (0.67–2.48) |
| Chlorpyrifosb | 1.22 (0.48–3.06) | 1.75 (0.86–3.60) | 2.37 (1.08–5.19) |
| Covariates | |||
| Race/ethnicityc | 1.06 (0.48–2.38) | 0.47 (0.26–0.87) | 0.35 (0.17–0.72) |
| Genderd | 1.66 (0.80–3.44) | 1.68 (0.96–2.92) | 1.90 (1.04–3.48) |
| Gestational age | 1.00 (0.79–1.27) | 0.89 (0.72–1.10) | 0.81 (0.65–1.01) |
| Maternal IQe | 1.02 (0.98–1.05) | 0.99 (0.96–1.01) | 1.01 (0.99–1.04) |
| Low maternal educationf | 1.49 (0.69–3.22) | 0.95 (0.52–1.73) | 1.40 (0.74–2.67) |
| HOME score | 0.96 (0.89–1.02) | 0.96 (0.92–1.01) | 0.90 (0.85–0.96) |
Sample sizes for age-specific models ranged from 225 to 228 because of missed and/or unscorable assessments (<50).
Prenatally exposed = 1; not exposed = 0.
High exposure (>6.17 pg/g) = 1; low exposure (≤6.17 pg/g) = 0.
Black = 2; Dominican = 1.
Male = 2; female = 1.
Measured with the Test of Nonverbal Intelligence, Second Edition,42 natural logarithmically transformed.
No high school degree = 1; high school degree = 0.
TABLE 7.
Logistic Regression Models Testing Effects of Chlorpyrifos on the Odds of Psychomotor Delay at 12, 24, and 36 Months, Adjusted for Race, Gender, Gestational Age, Maternal Education, Maternal IQ, ETS Exposure, and Home Environment
| Variable | Odds Ratio (95% CI) | ||
|---|---|---|---|
| Model 1: 12 mo | Model 2: 24 mo | Model 3: 36 mo | |
| Prenatal chemical exposures | |||
| ETSa | 0.95 (0.42–2.15) | 0.77 (0.33–1.80) | 1.82 (0.69–4.79) |
| Chlorpyrifosb | 1.88 (0.78–4.53) | 1.01 (0.37–2.76) | 4.52 (1.61–12.70) |
| Covariates | |||
| Race/ethnicityc | 1.59 (0.69–3.67) | 0.86 (0.36–2.09) | 0.55 (0.19–1.54) |
| Genderd | 0.91 (0.42–1.96) | 0.74 (0.33–1.63) | 1.89 (0.75–4.75) |
| Gestational age | 1.00 (0.78–1.28) | 0.88 (0.68–1.14) | 0.97 (0.68–1.38) |
| Maternal IQe | 1.01 (0.98–1.04) | 0.98 (0.95–1.02) | 1.03 (0.99–1.06) |
| Low maternal educationf | 1.10 (0.49–2.50) | 1.24 (0.54–2.81) | 0.36 (0.12–1.10) |
| HOME score | 0.99 (0.92–1.06) | 1.01 (0.94–1.08) | 0.94 (0.86–1.02) |
Sample sizes for age-specific models varied from 227 to 228 because of missed and/or unscorable assessments (<50).
Prenatally exposed = 1; not exposed = 0.
High exposure (>6.17 pg/g) = 1; low exposure (≤6.17 pg/g) = 0.
Black = 2; Dominican = 1.
Male = 2; female = 1.
Measured with the Test of Nonverbal Intelligence, Second Edition,42 natural logarithmically transformed.
No high school degree = 1; high school degree = 0.
After examination of the age-specific results for the MDI and the PDI, GLM was conducted to explore developmental trajectories. In addition to the test of main effects, GLM tested the interaction of each independent variable with age, to obtain estimates of effects over time. Covariates were included if they were significant predictors in any age-specific model; for the MDI, these were ethnicity, maternal education, gender, gestational age, HOME scores, and ETS exposure. Covariates for the PDI were ethnicity, gender, gestational age, HOME scores, and ETS exposure. Results of the GLM analysis (n = 189) were consistent with the age-specific findings with respect to the effects of chlorpyrifos, race, and HOME scores. In addition, GLM analyses provided estimates of within-subject effects (change over time) and within-subject contrasts, indicating at exactly which age the effects occurred. Preliminary analyses indicated no significant interactions of chlorpyrifos exposure with any of the covariates, including ETS exposure and race (P > .05). Therefore, all interactions terms were excluded from the final models.
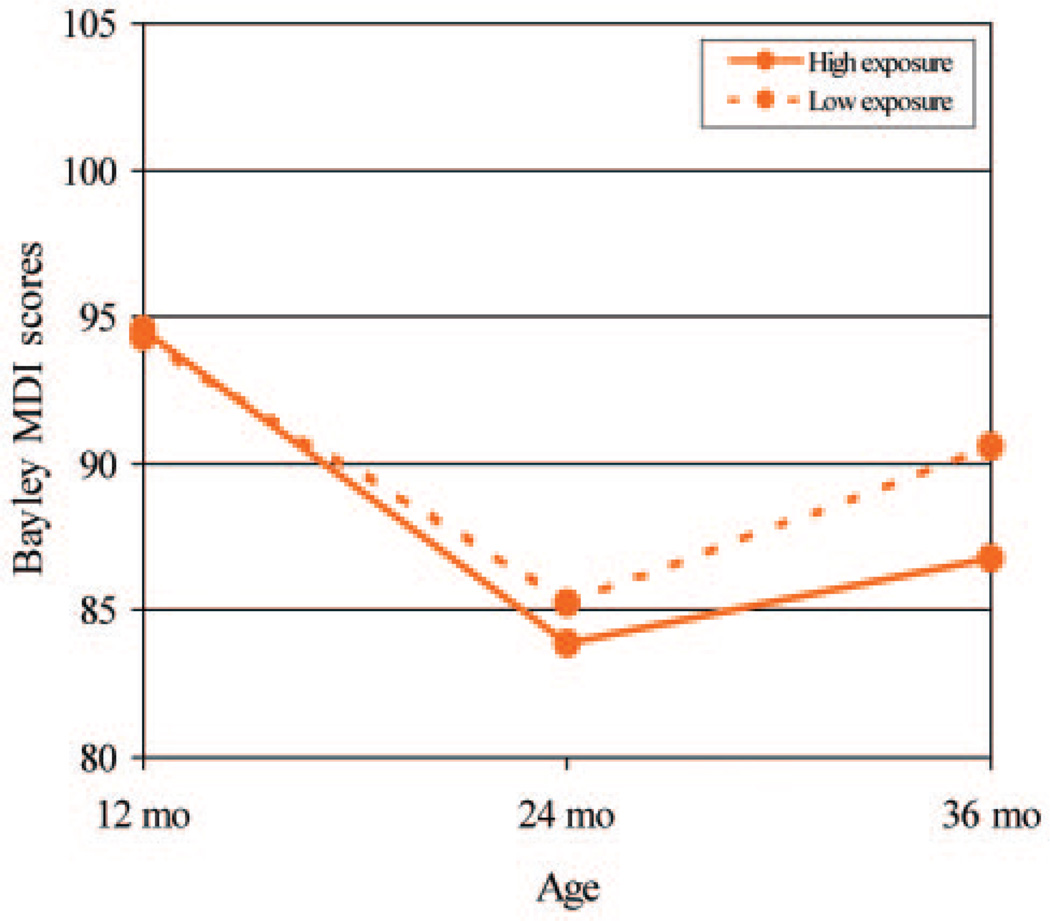
Figure 1 shows the estimated means for MDI scores over time according to prenatal chlorpyrifos exposure level, adjusted for the effects of covariates. Both high-exposure and lower-exposure groups demonstrated decreases in scores, as reported frequently for low-income populations during the toddler years, followed by increases in the later preschool period. The recovery by 3 years of age was slightly greater for children with low exposure, compared with children with high exposure, but the chlorpyrifos within-subject effect (change over time) was not significant (P = .23). The GLM analysis did indicate significant within-subject effects of both race (P = .001) and HOME scores (P = .03) on MDI scores, consistent with results at the individual time points. A second GLM analysis, including only 1 independent variable and using tests of within-subject contrasts, showed that the ethnicity effect on MDI scores emerged between 12 and 24 months (P = .001).
FIGURE 1.
Estimated effects of prenatal chlorpyrifos exposure on MDI scores for children 12 to 36 months of age according to race/ethnicity by using GLM repeated-measures analysis of variance. The models were adjusted for race/ethnicity, gender, gestational age, maternal educational level, HOME score, and ETS exposure. The high chlorpyrifos exposure group includes those with cord blood chlorpyrifos levels >6.17 pg/g, and the low group includes all those with lower levels.
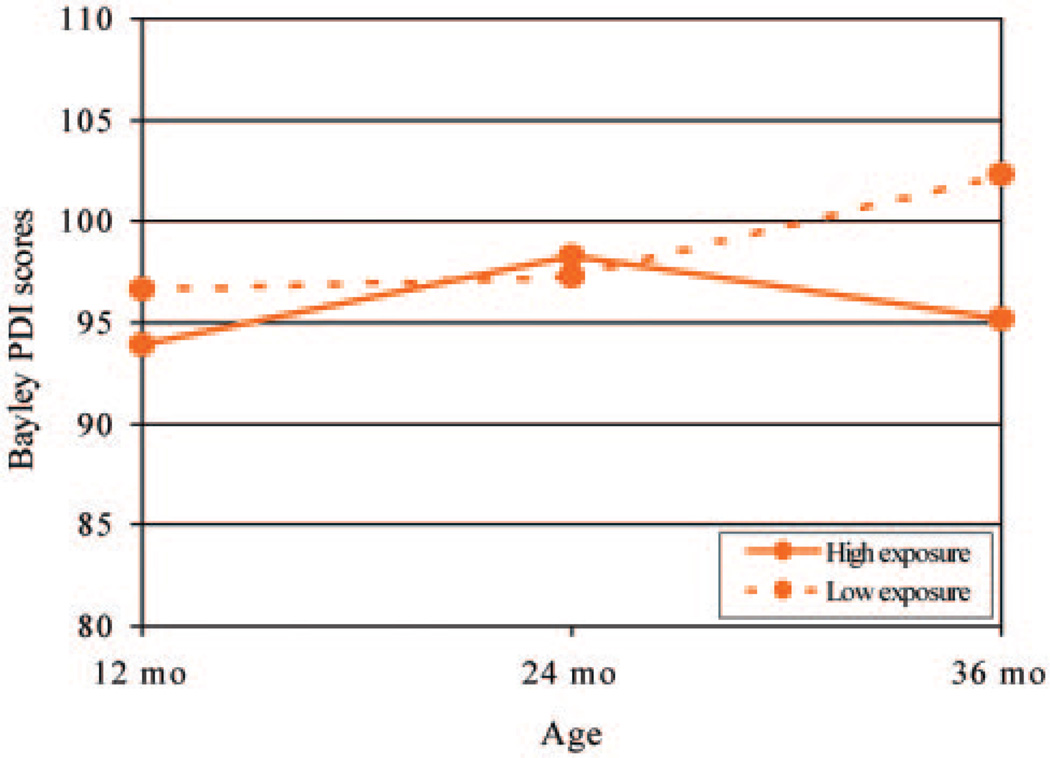
The results of the GLM analysis for PDI scores revealed a significant within-subject effect (change over time) of prenatal chlorpyrifos exposure (P = .01), with an estimated deficit of >7 points at 36 months of age among children exposed to high levels of chlorpyrifos (Fig 2). There was also a significant change over time in the effect of ethnicity, in that the difference between the mean PDI scores of the 2 groups increased over time, with Dominican children having lower mean scores regardless of exposure level (P = .001). A second GLM analysis, including only these 2 independent variables and using tests of within-subject contrasts, showed that the ethnicity effect on PDI scores emerged between 12 and 24 months (P = .001) and the chlorpyrifos effect emerged between 24 and 36 months (P = .003).
FIGURE 2.
Estimated effects of prenatal chlorpyrifos exposure on PDI scores for children 12 to 36 months of age according to race/ethnicity by using GLM repeated-measures analysis of variance. The models were adjusted for race/ethnicity, gender, gestational age, maternal educational level, maternal IQ, HOME score, and ETS exposure. The high chlorpyrifos exposure group includes those with cord blood chlorpyrifos levels >6.17 pg/g, and the low group includes all those with lower levels.
Table 8 shows the results of logistic regression analyses estimating the adjusted risk of behavior problems as a function of chlorpyrifos exposure. Significant chlorpyrifos effects were found for attention problems, ADHD problems, and pervasive developmental disorder (PDD) problems. In addition, children with prenatal ETS exposure were significantly more likely to score in the clinical range for ADHD problems. Despite the significant associations between chlorpyrifos exposure and these subscales, only 3.9% to 4.9% of the sample scored in the clinical range, which resulted in large CIs. There was no evidence of interaction between ETS exposure and chlorpyrifos exposure for these behavior problems, but small numbers limited our ability to test these effects.
TABLE 8.
Logistic Regression Models Testing Effects of Chlorpyrifos on the Odds of Behavioral Disorder at 36 Months, Adjusted for Race, Gender, Gestational Age, Maternal Education, Maternal IQ, ETS Exposure, and Home Environment (N = 228)
| Variable | Odds Ratio (95% CI) | ||
|---|---|---|---|
| Attention Problems | ADHD Problems | PDD Problems | |
| Prenatal exposures | |||
| ETSa | 2.81 (0.44–17.77) | 8.10 (1.20–54.65) | 0.75 (0.17–3.41) |
| Chlorpyrifosb | 11.26 (1.79–70.99) | 6.50 (1.09–38.69) | 5.39 (1.21–24.11) |
| Covariates | |||
| Race/ethnicityc | 0.19 (0.02–1.61) | 0.26 (0.04–1.82) | 0.06 (0.01–0.65) |
| Genderd | 2.84 (0.50–16.09) | 1.05 (0.23–4.74) | 2.40 (0.65–8.93) |
| Gestational age | 0.62 (0.39–0.99) | 0.62 (0.40–0.97) | 0.81 (0.50–1.30) |
| Maternal IQe | 0.99 (0.94–1.06) | 1.03 (0.97–1.09) | 1.00 (0.95–1.05) |
| Maternal educationf | 0.22 (0.02–2.08) | 0.35 (0.06–2.10) | 1.75 (0.46–6.61) |
| HOME score | 0.93 (0.80–1.08) | 0.88 (0.77–1.01) | 0.93 (0.82–1.05) |
Prenatally exposed = 1; not exposed = 0.
High exposure (>6.17 pg/g) = 1; low exposure (≤6.17 pg/g) = 0.
Black = 2; Dominican = 1.
Male = 2; female = 1.
Measured with the Test of Nonverbal Intelligence, Second Edition,42 natural logarithmically transformed.
No high school degree = 1; high school degree = 0.
Because the EPA ban occurred during the study period, we examined its overall effect on chlorpyrifos levels and BSID-II scores over time. The ban was actually a phase-out, beginning with announcements of the impending regulation and some early removal of products from retail stores. After the ban, it was possible that some families continued to use pesticide products purchased before the ban. It was therefore difficult to determine with precision the date when exposure stopped; however, it was possible to classify the births in the present sample into the preban period (before January 2000), the midban or phase-out period (January 2000 to December 2000), and the postban period (January 2001 and later). One-way analysis of variance of ban effects on mean logarithmically transformed chlorpyrifos levels showed significant decreases in mean chlorpyrifos blood levels at delivery in this sample across the preban (0.92 pg/g), midban (0.81 pg/g), and postban periods (0.90 pg/g) (F = 49.81; P < .001). There were significant increases in 36-month BSID-II MDI scores from the preban period (average score: 87.13 points) to the midban period (average score: 91.69 points) (F = 3.88; P = .02) and in PDI scores from the preban period (average score: 97.31 points) to the midban period (average score: 101.79 points) (F = 2.91; P = .056), but there were slight nonsignificant decreases in scores after full implementation of the ban (MDI average score: 89.48 points; PDI average score: 99.40 points). The initial decrease in chlorpyrifos levels from the preban period to the midban period corresponded to the largest increase in BSID-II scores, but larger epidemiologic studies will be needed to assess the full impact of the ban, including the longer-term effects of possible substitute pesticides.
DISCUSSION
Three substantive findings emerged from the study. First, by 3 years of age, significantly greater proportions of highly exposed children scored in the range of mental and motor delays, compared with those with lower exposures. After adjustment for the effects of covariates, highly exposed children scored, on average, 6.5 points lower on the test of motor development (P = .003) and 3.3 points lower on the test of mental development (P = .06) at 3 years of age than did those with lower levels of exposure. Second, the developmental trajectories for PDI and MDI scores confirmed that adverse cognitive and psychomotor effects increased over time and these effects were present in both Dominican and black subjects, which supported the main conclusion of the study. Third, at 3 years of age, children who were exposed prenatally to high levels of pesticides were significantly more likely to score in the clinical range for attention problems, ADHD problems, and PDD problems than were children with lower levels of chlorpyrifos exposure. The significant negative effects of chlorpyrifos on developmental and behavioral indicators observed in multivariate testing were also seen in the simple bivariate tests of both continuous and dichotomous outcomes. The multivariate tests showed that the effects of chlorpyrifos were not attributable to the effects of potential known confounders, and they provided the best estimates of the negative effects of chlorpyrifos on development.
In the present study, we used chlorpyrifos levels measured in umbilical cord blood at delivery as the dosimetric measure of prenatal exposure. However, it is not clear to what extent these measures reflect critical exposures throughout pregnancy. Specifically, the half-life of the chemically specific metabolite of chlorpyrifos in blood is 27 hours, with 70% of an orally administered dose being eliminated in the urine within 4 days after exposure.48 Experimental data indicate that the half-life of chlorpyrifos in adipose tissue is somewhat longer (62 hours).49 In chronic-exposure situations, a biomarker measured at a single time point can provide a representative dosimetric measure of exposure even if the toxicant has a short half-life, such as cotinine.50–52 Recent data from the larger cohort study from which this sample was drawn showed that exposures to chlorpyrifos during pregnancy remained fairly constant, that there was little within-home variability in indoor air levels (P > .2), and that between-home variability accounted for 92% of the variance in chlorpyrifos levels (P < .001).53 In addition, it is likely that the concentrations of chlorpyrifos in maternal blood and adipose tissue are in steady state with each other and with the concentrations in fetal blood unless the blood samples are obtained very soon after exposure to chlorpyrifos.54 For these reasons, we think that cord blood provides a reasonably accurate biomarker of exposure in the present study. The dichotomization of chlorpyrifos cord blood levels (ie, using the group with highest exposure as the risk indicator), although yielding significant effects in these analyses, does sacrifice exposure information and does not allow full exploration of the possibly subtle relationship between exposure and developmental outcomes. We are also limited by the sensitivity and predictive validity of standardized developmental tests in the first 3 years of life. We fully expect that future studies, including the continued assessment of this cohort by incorporating larger sample sizes and more-targeted developmental tests at older ages, will have the capacity to explore fully the possible dose-response relationships. In addition, the present study lacks information about early childhood chlorpyrifos and lead exposure, which could affect estimates of a dose-response relationship in the present study. Such information will be available for the current cohort at older ages, when a more-informative analysis of possible dose-response relationships can be conducted.
The deficit in motor development that emerged by 3 years of age among highly exposed children might be educationally meaningful, because compromised motor performance in the preschool years is a significant precursor of subsequent educational performance deficits, such as 8-year academic achievement in reading, mathematics, and spelling.47 Continued follow-up monitoring of the present cohort should determine whether observed 3-year motor deficits are predictive of subsequent motor problems and/or educational deficits.
In the present cohort, the mean cognitive scores were well below average and showed a general decrease over the first few years. Such a decline was observed previously for infants raised in low-income families and communities, independent of chemical exposures,37,55,56 in part because toddlers from low-income homes have less access to developmentally stimulating environments and may be delayed in acquiring language and problem-solving skills.57–63 Although children with low HOME scores did have significantly lower BSID-II scores in this study, there was no indication that the chlorpyrifos effects were either exacerbated or remediated by the quality of the home environment. The finding that a large proportion of children scored in the delayed range on the MDI, although consistent with other reports for similar populations,64,65 is worrisome, especially because the MDI is often given more importance than the PDI.66,67
Children who are exposed prenatally to well-studied toxicants (such as lead, ETS, or cocaine) exhibit the same kind of decline in cognitive scores during the early preschool years as seen among low-income children.68–71 This report is the first to show that prenatal organophosphate exposure generates a similar pattern. It is not yet known whether children from low-income families have a more difficult time than less-deprived children in recovering from the effects of prenatal chlorpyrifos exposure or whether additional stimulation can compensate for some of the adverse effects of early toxic exposures on cognitive development in low-income populations. The present findings suggest that the developmental deficit is present regardless of the quality of the home environment. It is not clear whether the children with high chlorpyrifos exposure are exhibiting developmental delay (development that follows an expected pattern but is delayed) or developmental disability (an atypical rather than expected course of development over time). Subsequent testing at later ages will provide longer-term data on average developmental trajectories exhibited by this group.
The magnitude of the pesticide effect on MDI scores reported in this study was modest but comparable in magnitude to reports from some other studies of prenatal neurotoxicants such as cocaine.72,73 The PDI effect reported here was also similar to prenatal maternal smoking effects on 2-year PDI scores, risk of motor delay, balance, and fine-motor coordination at 5 years of age, as measured with the Peabody Developmental Motor Scales.74 Motor effects may persist even when adjusted for birth weight, despite the fact that lower birth weight itself has been associated with increased frequency of motor problems.75 Other studies found no adverse effects of prenatal cocaine exposure on motor development.76–78
Although the adjusted mean 36-month PDI and MDI scores for the highly and lower exposed pesticide groups differed by only 7.1 and 3.0 points, respectively, the proportion of children in the highly exposed group with developmental scores of <85 was 5 times greater than that among lower exposed children for the PDI and 2.4 times greater for the MDI. Highly exposed children were significantly more likely than lower exposed children to need (and to be eligible for) early intervention services, which are designed for children who are at potential risk for early school failure. US law mandates that early intervention services be provided to children at risk of developmental delay.79 For example, in New York City, developmentally delayed children (MDI scores of <80) are referred to an early intervention program administered by the New York State Department of Health and Mental Health. Children in the present sample who scored in the delayed range were referred to the state program. Longer-term follow-up monitoring is needed to determine whether this intervention improves developmental performance and whether this has predictive value.39
Given the diversity of possible mechanisms and target tissues, the developing brain could be vulnerable to organophosphate pesticide exposure from early embryonic life into childhood.16,80–85 According to Slotkin,22 the period of vulnerability to chlorpyrifos extends through the period of synaptic modeling, and this continues well into childhood and even adolescence. In the present report, both chlorpyrifos and lead exposures were assessed during the prenatal period only, which is a limitation. In light of the proposed mechanisms for chlorpyrifos, it is possible that at least some of the observed effect was attributable to subsequent early childhood exposures. A recent study by Grandjean et al86 of a small sample of children in Ecuador who were exposed to multiple pesticides as a result of maternal floriculture work reported that effects of prenatal exposure might be lasting and might differ from the effects of postnatal exposure. Specifically, those authors found an increase in neurologic abnormalities and lower scores on the Stanford-Binet copying test among those with prenatal pesticide exposure (assessed by maternal interview) and also among stunted children. They also found that postnatal pesticide exposure, measured as decreased total excretion of dimethyl and diethyl metabolites of organophosphates, was associated with increased simple reaction time, independent of the other risk factors. In the present cohort, chlorpyrifos levels in 2- to 3-year blood samples will be analyzed in future reports, and the impact of early childhood exposure to chlorpyrifos on neurobehavioral development will be evaluated. In addition, early childhood blood lead levels will be included in future analyses.
The finding that highly exposed children were more likely to manifest attention and ADHD problems is consistent with the hypothesized neurotoxic mechanism but should be interpreted with caution, for several reasons. First, the CBCL criteria for ADHD problems are derived from DSM-IV, but DSM-IV itself has low sensitivity for assessing the inattentiveness of preschool-aged children.87 Second, a very small number of children included in the final analyses had scores exceeding the clinical cutoff point (n = 9), which resulted in large CIs. Nevertheless, the finding is consistent with other neurotoxicant studies that reported significant risk for behavioral disorders associated with prenatal lead and ETS exposures88 and alcohol and cocaine.89 Such environmental toxicants have been generally associated with cognitive and behavioral abnormalities, including attention and memory disorders, lower IQ, poorer academic achievement, impulse control problems, frustration intolerance, and aggression. The low-dose behavioral toxicologic effects seem to be especially dangerous during the critical period of fetal development. The finding that highly exposed children were more likely to be classified as having PDD problems might be explained by the number of ADHD-related symptoms included in the criteria for PDD problems.
Despite the small sample size in the present report, findings regarding increased risk for behavioral problems are worrisome. ADHD is one of the most common childhood behavior disorders, affecting >2 million children and adolescents in the United States alone.90 According to DSM-IV, ~3% to 5% of school-aged children suffer from ADHD, but the causes of the disorder are largely unknown. ADHD is associated with altered brain functioning and is characterized by an inability to focus on tasks, as well as by impulsive hyperactive behavior, lethargic inattention, or both. Furthermore, attention problems reflect long-term patterns of functioning and tend to be correlated strongly with later functioning by 9 years of age. It is not clear, on the basis of the present report, exactly how prenatal chlorpyrifos exposure might affect the onset of clinically diagnosed ADHD or whether there is a dose-response relationship with the severity of ADHD symptoms, but this finding of significant associations between chlorpyrifos exposure and behavioral problems deserves additional study.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Environmental Health Sciences (grants 5 P01 ES009600, 5 R01 ES008977, 5 R01ES11158, 5 R01 ES012468, and 5 R01 ES10165), US EPA (grants R827027, 82860901, and RD-832141), Irving General Clinical Research Center (grant RR00645), Bauman Family Foundation, Beldon Fund, Educational Foundation of America, Horace W. Goldsmith Foundation, Irving A. Hansen Memorial Foundation, Gladys and Roland Harriman Foundation, W. Alton Jones Foundation, New York City Council Speaker’s Fund, New York Community Trust, New York Times Company Foundation, and V. Kann Rasmussen Foundation.
We acknowledge gratefully the contributions of Andria Reyes, Beatriz Plaza, Diurka Diaz, Darrell Holmes, Mejico Borjas, Susan Illman, and Eric Evans from Columbia University; Larry Needham and Richard Jackson from the Centers for Disease Control and Prevention; and the obstetrics/gynecology staff members at Harlem Hospital, Allen Pavillion, and New York-Presbyterian Hospital. We also acknowledge the biospecimen laboratory work of Xinhe Jin, Lirong Qu, and Jing Lai, under the direction of Dr Deliang Tang.
Abbreviations
- ADHD
attention-deficit/hyperactivity disorder
- BSID-II
Bayley Scales of Infant Development II
- CBCL
Child Behavior Checklist
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- EPA
Environmental Protection Agency
- ETS
environmental tobacco smoke
- HOME
Home Observation for Measurement of the Environment
- MDI
Mental Development Index
- PDD
pervasive developmental disorder
- PDI
Psychomotor Development Index
- GLM
general linear modeling
- CI
confidence interval
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.US Environmental Protection Agency. Administrator’s announcement. [Accessed August 16, 2000];2000 Available at: www.epa.gov/pesticides/announcement6800.htm.
- 2.US Environmental Protection Agency. Chlorpyrifos end-use products cancellation order. [Accessed January 25, 2002];2002 Available at: www.epa.gpv/fedrgstr/EPA-PEST/2002/January/DAY-25/p1764.htm.
- 3.Whyatt RM, Camann D, Perera FP, et al. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol Appl Pharmacol. 2005;206:246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Whyatt RM, Rauh V, Barr DB, et al. Prenatal insecticide exposure and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkowitz GS, Obel J, Deych E, et al. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect. 2003;111:79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surgan MH, Congdon T, Primi C, Lamster S, Louis-Jacques J. Pest Control in Public Housing, Schools and Parks: Urban Children at Risk: LAW 180–20134 PESP 202–7643. Albany, NY: State Department of Law, Environmental Protection Bureau; 2002. [Google Scholar]
- 7.Whyatt RM, Camann DE, Kinney PL, et al. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ Health Perspect. 2002;110:507–514. doi: 10.1289/ehp.02110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whyatt RM, Barr DB, Camann DE, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111:749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkowitz GS, Wetmur JG, Birman-Deych E, et al. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112:388–391. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107:409–419. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dam K, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: delayed targeting of DNA synthesis after repeated administration. Brain Res. 1998;108:39–45. doi: 10.1016/s0165-3806(98)00028-5. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DE, Seidler FJ, Slotkin TA. Early biochemical detection of delayed neurotoxicity resulting from developmental exposure to chloropyrifos. Brain Res Bull. 1998;45:143–147. doi: 10.1016/s0361-9230(97)00329-8. [DOI] [PubMed] [Google Scholar]
- 13.Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- 15.Richardson RJ. Assessment of the neurotoxic potential of chlorpyrifos relative to other organophosphorus compounds: a critical review of the literature. J Toxicol Environ Health. 1995;44:135–165. doi: 10.1080/15287399509531952. [DOI] [PubMed] [Google Scholar]
- 16.Slotkin SM. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–79. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huff RA, Corcoran JJ, Anderson JK, Abou-Donia MB. Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J Pharmacol Exp Ther. 1994;269:329–335. [PubMed] [Google Scholar]
- 18.Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson P, Talts U. Neonatal exposure to neurotoxic pesticides increases adult susceptibility: a review of current findings. Neurotoxicology. 2000;21:37–47. [PubMed] [Google Scholar]
- 20.Ahlbom J, Fredriksson A, Eriksson P. Exposure to an organophosphate (DFP) during a defined period in neonatal life induces permanent changes in brain muscarinic receptors and behaviour in adult mice. Brain Res. 1995;677:13–19. doi: 10.1016/0006-8993(95)00024-k. [DOI] [PubMed] [Google Scholar]
- 21.Whitney KD, Sielder FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- 22.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ Health Perspect. 2003;111:536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young JG, Eskenazi B, Gladstone EA, et al. Association between in utero organophosphate exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26:199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Guillette EA, Meza MM, Aquilar MG, Soto AD, Garcia IE. An anthropological approach to the evaluation of preschool children exposed to pesticides in Mexico. Environ Health Perspect. 1998;106:347–353. doi: 10.1289/ehp.98106347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruckart PZ, Kakolewski K, Bove FJ, Kaye WE. Long-term neurobehavioral health effects of methyl parathion exposure in children in Mississippi and Ohio. Environ Health Perspect. 2004;112:46–51. doi: 10.1289/ehp.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss B, Amier S, Amier RW. Pesticides. Pediatrics. 2004;113:1030–1038. [PubMed] [Google Scholar]
- 28.Landrigan PJ. Pesticides and polychlorinated biphenyls (PCBs): an analysis of evidence that they impair children’s neurobehavioral development. Mol Genet Metab. 2001;73:11–17. doi: 10.1006/mgme.2001.3177. [DOI] [PubMed] [Google Scholar]
- 29.Perera F, Rauh VA, Tsai WY, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multi-ethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thier A, Enck J, Klossner C. Plagued by Pesticides: An Analysis of New York State and New York City’s 1997 Pesticide Use and Sales Data. New York, NY: New York Public Interest Research Group; 1998. [Google Scholar]
- 31.Perera FP, Whyatt RM, Jedrychowski W, et al. Recent developments in molecular epidemiology: a study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am J Epidemiol. 1998;147:309–314. doi: 10.1093/oxfordjournals.aje.a009451. [DOI] [PubMed] [Google Scholar]
- 32.Perera FP, Illman SM, Kinney PL, et al. The challenge of preventing environmentally related disease in young children: community-based research in New York City. Environ Health Perspect. 2002;110:197–204. doi: 10.1289/ehp.02110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barr DB, Barr JR, Maggio VL, et al. A multi-analyte method for the quantification of contemporary pesticides in human serum and plasma using high resolution mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2002;778:99–111. doi: 10.1016/s0378-4347(01)00444-3. [DOI] [PubMed] [Google Scholar]
- 34.Bayley N. Bayley Scales of Infant Development. 2nd ed. San Antonio, TX: Psychological Corp; 1993. [Google Scholar]
- 35.Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analyses of pre- and postnatal lead exposure and early cognitive development. N Engl J Med. 1987;316:1037–1044. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- 36.Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Waternaux C. Low-level lead exposure and children’s cognitive function in the preschool years. Pediatrics. 1991;87:219–227. [PubMed] [Google Scholar]
- 37.Burchinal MR, Campbell FA, Bryant DM, Wasik BH, Ramey CT. Early intervention and mediating processes in cognitive performance of children of low-income African American families. Child Dev. 1997;68:935–954. doi: 10.1111/j.1467-8624.1997.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 38.Burchinal MR, Roberts JE, Kooper S, Zeisel SA. Cumulative risk and early cognitive development: a comparison of statistical risk models. Dev Psychol. 2000;36:793–807. doi: 10.1037//0012-1649.36.6.793. [DOI] [PubMed] [Google Scholar]
- 39.Sternberg RJ, Grigorenko EL, Bandy DA. The predictive value of IQ. Merrill-Palmer Q. 2001;47:1–41. [Google Scholar]
- 40.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms and Profiles: Child Behavior Checklist & Profile for Ages 1.5–5: English. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- 42.Brown L, Sherbenou RJ, Johnson SK. Test of Non-verbal Intelligence: A Language-Free Measure of Cognitive Ability. 2nd ed. Austin, TX: PRO-ED; 1990. [Google Scholar]
- 43.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas Press; 1979. [Google Scholar]
- 44.Bradley R, Caldwell B, Rock S, et al. Home environment and cognitive development in the first 3 years of life: a collaborative study involving six sites and three ethnic groups in North America. Dev Psychol. 1989;25:217–235. [Google Scholar]
- 45.Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Low-level lead exposure, social class, and infant development. Neurotoxicol Teratol. 1988;10:497–503. doi: 10.1016/0892-0362(88)90084-0. [DOI] [PubMed] [Google Scholar]
- 46.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 47.Sullivan MC, McGrath MM. Perinatal morbidity, mild motor delay, and later school outcomes. Dev Med Child Neurol. 2003;45:104–112. [PubMed] [Google Scholar]
- 48.Nolan RJ, Rick DL, Freshour NL, Saunders JH. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol Appl Pharmacol. 1984;73:8–15. doi: 10.1016/0041-008x(84)90046-2. [DOI] [PubMed] [Google Scholar]
- 49.Smith GN, Watson BS, Fischer FS. Investigations on dursban insecticide: metabolism of [36Cl]O,O-diethly-O-35,6-trichloror-2-pyridyl phosphorothioate in rats. J Agric Food Chem. 1967;15:132–138. [Google Scholar]
- 50.Kemmeren JM, Van Poppel G, Verhoef P, Jarvis MJ. Plasma cotinine: stability in smokers and validation of self-reported smoke exposure in nonsmokers. Environ Res. 1994;66:235–243. doi: 10.1006/enrs.1994.1059. [DOI] [PubMed] [Google Scholar]
- 51.Pojer R, Whitefield JB, Poulos V, Eckhard IF, Richmond R, Hensley WJ. Carboxyhemoglobin, cotinine, and thiocyanate assay compared for distinguishing smokers from non-smokers. Clin Chem. 1984;30:1377–1380. [PubMed] [Google Scholar]
- 52.Woodward M, Tunstall-Pedoe H, Smith WC, Tavendale R. Smoking characteristics and inhalation biochemistry in the Scottish populations. J Clin Epidemiol. 1991;44:1405–1410. doi: 10.1016/0895-4356(91)90101-e. [DOI] [PubMed] [Google Scholar]
- 53.Whyatt RM, Garfinkel RS, Hoepner LA, Borjas M, Camann DE. Within and between home variability in indoor-air insecticide levels during pregnancy [abstract W2A–3]. Presented at the 15th Annual Conference of the International Society of Exposure Analysis; October 30–2013November 3, 2005; Tucson, AZ. [Google Scholar]
- 54.Needham LL. Assessing exposure to organophosphorus pesticides by biomonitoring in epidemiologic studies of birth outcomes. Environ Health Perspect. 2005;113:494–553. doi: 10.1289/ehp.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luster T, McAdoo H. Family and child influences on educational attainment: a secondary analysis of the High/Scope Perry preschool data. Dev Psychol. 1996;32:23–39. [Google Scholar]
- 56.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 57.Morisset CE, Barnard KE, Greenberg MT, Booth CL, Spieker SJ. Environmental influences on early language development: the context of social risk. Dev Psychopathol. 1990;2:127–149. [Google Scholar]
- 58.Arriaga RI, Fenson L, Cronan T, Pethick SJ. Scores on the MacArthur Communicative Development Inventory of children from low- and middle-income families. Appl Psycholinguist. 1998;19:209–223. [Google Scholar]
- 59.Black MM, Hess CR, Berenson-Howard J. Toddlers from low-income families have below normal mental, motor, and behavior scores on the revised Bayley Scales. J Appl Dev Psychol. 2000;21:655–666. [Google Scholar]
- 60.Guo G, Harris KM. The mechanisms mediating the effects of poverty on children’s intellectual development. Demography. 2000;37:431–447. doi: 10.1353/dem.2000.0005. [DOI] [PubMed] [Google Scholar]
- 61.Bradley RH, Caldwell B. The HOME inventory and family demographics. Dev Psychol. 1984;20:315–320. [Google Scholar]
- 62.Bradley RH, Corwyn RF, McAdoo HP, Garcia-Coll C. The home environments of children in the United States, part I: variations by age, ethnicity and poverty status. Child Dev. 2001;72:1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- 63.Dubow E, Ippolito M. Effects of poverty and quality of the home environment on changes in the academic and behavioral adjustment of elementary school-age children. J Clin Child Psychol. 1994;3:401–412. [Google Scholar]
- 64.Tineo W, Huberman HS, Agin M, et al. Prevalence and predictors of delay and early intervention referral in a sample of poor, urban children. Presented at the Head Start 7th National Research Conference; June 28 to July 1, 2004; Washington, DC. [Google Scholar]
- 65.Huberman H, Tineo W, Rosenberg T, Sharif I, Mendelsohn A. Developmental delay in poor urban children: high rates of early intervention (EI) referrals in a public health-primary care intervention study sample [abstract] J Urban Health. 2003;80(suppl 2):ii119. [Google Scholar]
- 66.Cole KN, Harris SR. Instability of the intelligence quotient-motor quotient relationship. Dev Med Child Neurol. 1992;34:633–641. doi: 10.1111/j.1469-8749.1992.tb11494.x. [DOI] [PubMed] [Google Scholar]
- 67.Aylward GP, Verhulst SJ, Bell S, Gyurke JS. Cognitive and motor score differences in biologically at-risk infants. Infant Behav Dev. 1995;18:43–52. [Google Scholar]
- 68.Delaney-Black V, Covington C, Templin T, et al. Expressive language development of children exposed to cocaine prenatally: literature review and report of a prospective cohort study. J Commun Disord. 2000;33:463–480. doi: 10.1016/s0021-9924(00)00033-2. [DOI] [PubMed] [Google Scholar]
- 69.Hurt H, Brodsky NL, Betancourt L, et al. Cocaine-exposed children: follow-up through 30 months. J Dev Behav Pediatr. 1995;16:29–35. [PubMed] [Google Scholar]
- 70.Singer L, Arendt R, Farkas K, Minnes S, Huang J, Yamashita T. Relationship of prenatal cocaine exposure and maternal post-partum psychological distress to child developmental outcome. Dev Psychopathol. 1997;9:473–489. doi: 10.1017/s0954579497001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol. 2004;26:373–385. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arendt R, Angelopoulos J, Salvator A, Singer L. Motor development of cocaine-exposed children at age two years. Pediatrics. 1999;103:86–92. doi: 10.1542/peds.103.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singer LT, Arendt R, Minnes S, et al. Cognitive and motor outcomes of cocaine-exposed infants. JAMA. 2002;287:1952–1960. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trasti N, Vik T, Jacobsen G, Bakketeig LS. Smoking in pregnancy and children’s mental and motor development at age 1 and 5 years. Early Hum Dev. 1999;55:137–147. doi: 10.1016/s0378-3782(99)00017-1. [DOI] [PubMed] [Google Scholar]
- 75.Scottish Low Birthweight Study Group. The Scottish Low Birthweight Study, part I: survival, growth, neuromotor and sensory impairment. Arch Dis Child. 1992;67:675–681. doi: 10.1136/adc.67.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frank DA, Jacobs RR, Beeghly M. Level of prenatal cocaine exposure and scores on the Bayley Scales of Infant Development: modifying effects of caregiver, early intervention, and birth weight. Pediatrics. 2002;110:1143–1152. doi: 10.1542/peds.110.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kilbride H, Castor C, Hoffman E, Fuger KL. Thirty-six month outcome of prenatal cocaine exposure for term or near-term infants: impact of early case management. J Dev Behav Pediatr. 2000;21:19–26. doi: 10.1097/00004703-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 78.Messinger DS, Bauer CR, Das A, et al. The Maternal Lifestyle Study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113:1677–1685. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- 79.Kirby RS, Swanson ME, Kelleher KJ, Bradley RH, Casey PH. Identifying at-risk children for early intervention services: lessons from the Infant Health and Development Program. J Pediatr. 1993;122:680–686. doi: 10.1016/s0022-3476(06)80004-1. [DOI] [PubMed] [Google Scholar]
- 80.Dam K, Seidler FJ, Slotkin TA. Transcriptional biomarkers distinguish between vulnerable periods for developmental neurotoxicity of chlorpyrifos: implications for toxicogenomics. Brain Res Bull. 2003;59:261–265. doi: 10.1016/s0361-9230(02)00874-2. [DOI] [PubMed] [Google Scholar]
- 81.Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: effects on neurospecific proteins indicate changing vulnerabilities. Environ Health Perspect. 2003;111:297–304. doi: 10.1289/ehp.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moser VC, Padilla S. Age- and gender-related differences in the time-course of behavioral and biochemical effects produced by oral chlorpyrifos in rats. Toxicol Appl Pharmacol. 1998;149:107–119. doi: 10.1006/taap.1997.8354. [DOI] [PubMed] [Google Scholar]
- 83.Qiao D, Seidler FJ, Padilla S, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: what is the vulnerable period? Environ Health Perspect. 2002;110:1097–1103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell CG, Seidler FJ, Slotkin TA. Chlorpyrifos interferes with cell development in rat brain regions. Brain Res Bull. 1997;43:179–189. doi: 10.1016/s0361-9230(96)00436-4. [DOI] [PubMed] [Google Scholar]
- 85.Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Brain Res. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 86.Grandjean P, Harari R, Bar DB, Debes F. Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics. 2006;117(3) doi: 10.1542/peds.2005-1781. Available at: www.pediatrics.org/cgi/content/full/117/3/e546. [DOI] [PubMed] [Google Scholar]
- 87.Ogino T, Hattori J, Abiru K, Nakano K, Oka E, Ohtsuka Y. Symptoms related to ADHD observed in patients with pervasive developmental disorder. Brain Dev. 2005;27:345–348. doi: 10.1016/j.braindev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Wasserman GA, Liu X, Pine DS, Graziano JH. Contribution of maternal smoking during pregnancy and lead exposure to early child behavior problems. Neurotoxicol Teratol. 2001;23:13–21. doi: 10.1016/s0892-0362(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 89.Nordstrom Bailey B, Sood BG, Sokol RJ, et al. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicol Teratol. 2005;27:181–189. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 90.National Center for Environmental Health. Attention-Deficit/Hyperactivity Disorder: A Public Health Perspective. Atlanta, GA: Centers for Disease Control and Prevention; 1999. NCEH Publication 99–0362. [Google Scholar]