Abstract
Focal bilateral hippocampal damage typically causes severe and selective amnesia for new declarative information (facts and events), a cognitive deficit that greatly impacts the ability to live a normal, fully-independent life. We describe the case of 1846, a 48-year-old woman with profound hippocampal amnesia following status epilepticus and an associated anoxic episode at age 30. 1846 has undergone extensive neuropsychological testing on many occasions over the 18 years since her injury, and we present data indicating that her memory impairment has remained severe and stable during that time. New, high-resolution structural MRI studies of 1846's brain reveal substantial bilateral hippocampal atrophy resembling that of other well-known amnesic patients. In spite of severe amnesia, 1846 lives a full and mostly independent adult life, facilitated by an extensive social support network of family and friends. Her case provides an example of a rare and unlikely positive outcome in the face of severe memory problems.
Introduction
Studies of patients with damage to the medial temporal lobe (MTL) have provided extraordinary insight into normal memory function with the classic finding of severe impairment of long-term memory despite otherwise normal cognitive abilities (Scoville & Milner, 1957). Through comparative investigations of the memory profiles of patients with different patterns of damage within the MTL, it has been shown that the hippocampus is uniquely responsible for the normal formation of relational (associative) memories (e.g., Cohen & Eichenbaum, 1993; Davachi & Dobbins, 2008; Eichenbaum & Cohen, 2001; Ranganath, 2010), and patients with focal hippocampal damage remain vital for definitive tests of various theoretical claims regarding the neural basis of memory (Aggleton & Brown, 1999; Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001; Graham, Barense, & Lee, 2010; Moscovitch et al., 2005; Nadel & Moscovitch, 1997; Squire, Stark, & Clark, 2004). Although focal hippocampal damage is rare, a variety of etiologies can bilaterally affect the hippocampus in isolation, and among them are anoxic (hypoxic) episodes (Wilson, 1996; Zola-Morgan, Squire, & Amaral, 1986b) and status epilepticus (Dietl et al., 2004; Guerreiro, Jones-Gotman, Andermann, Bastos, & Cendes, 2001; Oxbury, Oxbury, Renowden, Squier, & Carpenter, 1997). Here we report patient 1846, who presents an extremely rare case of dense anterograde amnesia in the context of otherwise relatively preserved cognitive functioning as a result of focal bilateral hippocampal damage following an episode of combined status epilepticus and anoxia. Extensive neuropsychological and neuroanatomical studies of the patient document her condition in considerable detail. Of particular interest, we investigated factors in the patient's life circumstances and social situation that might have played (and are still playing) a major role in her unusually positive outcome relative to other memory-impaired patients.
The seminal case of MTL amnesia was HM, a man who presented with remarkably well-preserved cognitive functioning in the context of a near-complete inability to remember new facts and events (Scoville & Milner, 1957). Considering the well-known case of HM alongside our case 1846 is instructional both in service of reinforcing pertinent early findings in the neuropsychology of memory and owing to certain premorbid similarities between the two cases. Crucially, HM (Scoville & Milner, 1957) and 1846 (along with other notable MTL amnesic patients [cf. Damasio, Eslinger, Damasio, Van Hoesen, & Cornell, 1985; Feinstein et al., 2010; McCarthy, Kopelman, & Warrington, 2005; Stefanacci, Buffalo, Schmolck, & Squire, 2000; Wilson & Wearing, 1995]) have a profound deficit in remembering declarative information including facts and events, but are (or once were) able to learn and express other kinds of knowledge, collectively referred to as “procedural” or “non-declarative” memories (Cohen & Squire, 1980; Squire et al., 2004). Three prominent examples of non-declarative memory processes that have been shown to be normal in MTL amnesic patients are: priming (e.g., material seen previously is recognized more quickly [E. K. Warrington & Weiskrantz, 1968], and words studied earlier can influence responses made later [Graf, Squire, & Mandler, 1984]); motor learning (repetition of manual tasks leads to faster performance [Brooks & Baddeley, 1976; Milner, 1962]); and autonomic conditioning (e.g., conditioned stimuli evoke skin conductance responses [Bechara et al., 1995]). Even when exhibiting normal learning in the specific contexts of each task, MTL amnesic patients cannot learn facts or remember episodes related to the tasks. Patient 1846 has shown this dissociation between “knowing how” and “knowing that” (Cohen & Squire, 1980) by learning a variety of manual tasks at a normal rate without any sign of recollection across multiple exposures (Cavaco, Anderson, Allen, Castro-Caldas, & Damasio, 2004).
While damage to the hippocampus in addition to other MTL structures impairs declarative memory generally (as in HM), amnesic patients with damage limited to the hippocampus show a more specific deficit, namely an inability to knit isolated memories together into a flexible, coherent mnemonic representation as characterized by relational memory theory (Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001). That is, without the hippocampus, new information sometimes appears to have been stored normally, but that information is not readily expressed unless evoked by close reinstantiation of the learning context (Cohen & Squire, 1980; Hannula, Tranel, & Cohen, 2006; Hannula, Ryan, Tranel, & Cohen, 2007; Ryan, Althoff, Whitlow, & Cohen, 2000). Patient 1846, among other hippocampal amnesic patients, has shown normal recognition of recently-studied single items so long as contextual knowledge is not important (Konkel, Warren, Duff, Tranel, & Cohen, 2008), but exhibits a profound inability to form rich relational declarative memories (see Case Overview and Neuropsychological Summary). The pattern of mnemonic sparing and impairment demonstrated by 1846 and other hippocampal amnesic patients strongly implies that the hippocampus helps to organize knowledge through associations, thereby facilitating navigation through the vicissitudes of everyday life in ways that most people take for granted.
Etiologically, HM's case was unique for many reasons, but it is particularly interesting to note that he was and is likely to remain one of fewer than a dozen human beings to have undergone an intentional bilateral excision of MTL, which in his case notably included non-hippocampal structures (Corkin, Amaral, González, Johnson, & Hyman, 1997; Salat et al., 2006; Scoville, 1954; Scoville & Milner, 1957). Every other bilateral MTL amnesic patient has suffered unintentional, often organically-arising damage to the hippocampus and/or its surrounding structures. In the case of 1846, the hippocampus was damaged by an anoxic episode during status epilepticus, both of which are etiologies associated with hippocampal damage (e.g., Guerreiro et al., 2001; Wilson, 1996). Turning first to anoxia, episodes are almost universally systemic, exposing the entire brain to oxygen starvation and potentially causing cell death. Although hippocampal damage is common after anoxia, isolated hippocampal damage in the context of an otherwise unaffected brain is relatively rare (Auer & Sutherland, 2002; Grubb et al., 2000; Horstmann et al., 2010). Volumetric investigations of the brains of anoxic patients have noted several other regions that are also susceptible to damage under these conditions including the thalamus, pallidum, and striatum. However, pathological investigations (Zola-Morgan, Squire, & Amaral, 1986b) and neuroimaging studies suggest that severe amnesia after an anoxic episode is always associated with hippocampal atrophy, even if other brain regions are also reduced in volume (Di Paola et al., 2008; Horstmann et al., 2010).
Like anoxia, epilepsy can be accompanied by anatomical changes in the MTL, and both 1846 and HM suffered from adolescent-onset epilepsy that required treatment with medications. The premorbid status of hippocampus is not known for either patient, but chronic epilepsy is often associated with hippocampal sclerosis, particularly in cases where the seizure focus lies within the temporal lobes (Corsellis & Bruton, 1983; Meldrum, 1997; Walker, Chan, & Thom, 2007). While sclerosis manifests gradually over time, isolated but especially severe epileptic seizures like the status epilepticus 1846 experienced can damage the hippocampus very quickly. Traditionally, the term status epilepticus has been used to refer to a single clinical seizure lasting for 30 minutes or more, although in modern practice the term is used more broadly and can be applied to one continuous seizure, a series of rapidly occurring overt seizures, or even subclinical, electrographical evidence of prolonged seizure activity (Knake, Hamer, & Rosenow, 2009). Notably, the condition is frequently accompanied by anoxia owing to interruption of normal respiration (Corsellis & Bruton, 1983; Walker et al., 2007) as was the case for 1846 during her hour-long seizure (although her anoxia was medically relieved during the seizure, see Case Overview). The excessive neuronal excitation arising during such prolonged status epilepticus can cause cell death in several different portions of the hippocampus, as well as other parts of the brain (DeGiorgio, Tomiyasu, Gott, & Treiman, 1992; Meldrum, 1997; Meyer, Beck, & Shepherd, 1955). When status epilepticus, alone or in combination with other events, results in bilateral hippocampal damage, severe amnesia follows (Dietl et al., 2004; Guerreiro et al., 2001; Krumholz et al., 1995; Oxbury et al., 1997).
Patient 1846's combined etiology resulted in a profound and chronic memory deficit. She presents a compelling case of hippocampal amnesia with a distinctive history and anatomical profile, and illustrates an unusual positive outcome made possible in great part by extraordinary support by her extended family.
Case Overview
1846 is a fully right-handed (+100 on the Oldfield-Geschwind handedness questionnaire, which measures 10 handed activities [e.g., writing, throwing, eating, etc.] on scales ranging from always right [i.e., 10] to always left [i.e., −10]) female patient with 14 years of education. At the time the current report was prepared, she was 48 years old, and had been participating in research at the University of Iowa for 18 years, including a number of studies reported in previous publications from our group (Allen, Tranel, Bruss, & Damasio, 2006; Buchanan, Tranel, & Adolphs, 2005; Buchanan, Tranel, & Kirschbaum, 2009; Cavaco et al., 2004; Duff, Hengst, Tranel, & Cohen, 2007; Duff et al., 2008; Feinstein, Duff, & Tranel, 2010; Gupta et al., 2009; Hannula et al., 2006; Hannula et al., 2007; Konkel et al., 2008; Tranel & Jones, 2006; Warren, Duff, Tranel, & Cohen, 2010; Warren, Duff, Tranel, & Cohen, 2011). Her contributions have been particularly informative for the literatures of memory, language, perception, and hormonal regulation.
1846 was born in 1963, and after a medically unremarkable childhood, she began suffering severe asthma attacks and complex partial seizures at the age of 16, experiencing one overt seizure approximately every 6–8 weeks. Subjectively, 1846's seizures were accompanied by an odd sensation in her abdomen shortly before and after, while her husband reported that during seizures she often rubbed her hands together unconsciously; all of these are typical manifestations accompanying medial temporal lobe epilepsy (Guerreiro et al., 2001; Walker et al., 2007) and these signs may be specific to hippocampal epilepsy (Gil-Nagel & Risinger, 1997). Seizures persisted into her adult life, and medical professionals prescribed various medications including carbamazepine (which controlled her seizures somewhat), along with theophylline to control the asthma. This regimen allowed 1846 to complete high school and to later earn an associate's degree as a medical assistant. While pursuing that degree, 1846 met the man whom she would marry a few months after graduating, and the couple has remained together for more than two decades. 1846 was the first employee at a new medical practice (including at that time only 1846, a nurse, and a doctor), and as office manager her job involved many duties including interacting with and remembering patients, scheduling appointments, maintaining and updating medical records, and dealing with insurance claims. All of these tasks required substantial planning and memory. According to her parents and husband, 1846 enjoyed her work a great deal and intended to keep her career while starting a family.
At the age of 30, 1846 was in the hospital on several separate occasions: first, giving birth to her second child; and three months later, undergoing laparoscopic cholecystectomy. After both visits she was discharged with normal vital signs, but two days after the cholecystectomy, her family reports that she returned from a shopping trip with her mother, complained of fatigue, and retired to her room. Shortly afterward, her mother found 1846 unresponsive and, according to medical records, exhibiting twitching movements of the right face and arm alternating with generalized tonic-clonic seizure activity. Physicians' notes indicate that 1846 was rushed to the emergency room where she was immediately intubated and placed on ventilatory support to correct hypercarbia and anoxia. Seizures continued for approximately one hour and required treatment with lorazepam, diazepam, phenytoin, and phenobarbital before eventually subsiding. At that time, her lab reports indicated a toxic serum theophylline level and suspected sepsis, and she was placed on intravenous antibiotics. After spending six days in the hospital under close observation and with no evidence of further seizure activity (confirmed by EEG), she was discharged.
Physically, 1846 seemed largely unharmed by the episode. Family members report that her epilepsy was actually less severe after the episode, and could be controlled with medication (as long as she remembered to take it). Specifically, 1846 has variously and sequentially been prescribed phenytoin, valproic acid, and felbamate; at the time of the current report, she is taking only lamotrigine. By her husband's account, the occasional severe asthma attacks that led to repeated hospitalizations have been absent since the episode with no obvious connection beyond coincidence. Her husband also volunteered that her libido was greatly reduced or perhaps entirely eliminated. Although her physical recuperation proceeded swiftly, the lasting consequences of 1846's brain injury only became apparent gradually over the following weeks and months.
Neuropsychological Profile
The cognitive consequences of 1846's episode of anoxia and status epilepticus were referred to by her physicians immediately afterward as “impaired short term memory.” Essentially, 1846 had lost the ability to readily form lasting memories of factual information and events, to all appearances a textbook example of medial temporal lobe amnesia (Scoville & Milner, 1957). Initially, it was hoped that the severe memory loss was an acute consequence of the episode, as reflected in her neurologist's notes, for example, "I would hope that her memory would spontaneously improve and eventually she would get back to normal." Unfortunately, the full extent of her memory impairment became apparent over approximately six months of intensive testing and remediation training at the University of Iowa Hospitals and Clinics (UIHC), with little obvious improvement in typical memory functions. In fact, as noted earlier, 1846 has been participating in research at our institution for more than 18 years, and her neuropsychological profile has remained nearly unchanged over that period. Scores from an extensive neuropsychological battery illustrate the striking dissociation between her severe declarative memory deficit and other cognitive processes.
Intellectual Achievement
1846's general intellectual functioning is in the low-average range, and has remained stable since the episode. She was tested with the Wechsler Adult Intelligence Scale-Revised (WAIS-R; Wechsler, 1981) in 1993 soon after visits to UIHC began, when her full-scale intelligence quotient (FSIQ) was measured as 82; six years later the Wechsler Adult Intelligence Scale, 3rd ed. (WAIS-III; Wechsler, 1997a) measured her full-scale IQ as 84. The verbal and performance components of the WAIS-R were very similar (83 and 84); the corresponding components of the WAIS-III (89 and 79) for verbal and performance, respectively, were slightly more discrepant. The Wide Range Achievement Test-Revised (WRAT-R; Jastak & Wilkinson, 1984) showed average performance in both reading (95) and spelling (103). 1846's estimated overall low-average intelligence based on these measures is broadly commensurate with her premorbid functioning, as reflected in her high school academic transcript and standardized achievement test scores. We asked a neuropsychologist blind to all aspects of the case and this report to estimate 1846's premorbid IQ, based on academic and standardized test data. The estimate was that 1846 was in the upper low average/lower average range in terms of premorbid intellectual caliber. Table 1 provides subtest scores for all neuropsychological measures.
Table 1.
Intellectual functioning and academic achievement
| WAIS Exams (Year Administered) | WAIS-R (1993) | WAIS-III (1999) |
|---|---|---|
| Scale | Score (%tile) | |
| FSIQ | 82 (12) | 84 (14) |
| VIQ | 83 (23) | 89 (23) |
| VCI | NA | 88 (21) |
| Information | 8 | 8 |
| Digit span | 10 | 10 |
| Vocabulary | 7 | 8 |
| Arithmetic | 8 | 7 |
| Comprehension | 7 | 9 |
| Similarities | 8 | 7 |
| Letter-number sequencing | NA | 8 |
| PIQ | 84 (14) | 79 (8) |
| PRI | NA | 86 (18) |
| PSI | NA | 84 (14) |
| Picture completion | 8 | 8 |
| Picture arrangement | 7 | 5 |
| Block design | 9 | 10 |
| Object assembly | 8 | 6 |
| Digit-symbol coding | 8 | 6 |
| Matrix reasoning | NA | 5 |
| Symbol search | NA | 8 |
| WMI | NA | 90 |
| Test | Score (%ile) | Admin. |
|---|---|---|
| WRAT-R | 1993 | |
|
| ||
| Reading | 84 (12) | |
| Spelling | 95 (37) | |
| Arithmetic | 103 (58) | |
|
| ||
| NART-R (# errors) | 31 (55) | 1994 |
Abbreviations and attributions: WAIS-III, Wechsler Adult Intelligence Scale, 3rd ed. (Wechsler, 1997a); WAIS-R, Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981); WRAT-R, Wide Range Achievement Test-Revised (Jastak & Wilkinson, 1984); NART-R, (Nelson & Willison, 1991); FSIQ, full-scale intelligence quotient; VIQ, verbal IQ; VCI, verbal comprehension index; PIQ, performance IQ; PRI, perceptual reasoning index; PSI, processing speed index; WMI, working memory index.
Notes: all WAIS scores are age-corrected and scaled; WRAT-R scores are standardized; and the WAIS-III contains more subtests and subcomponents than the WAIS-R, and a lack of a WAIS-R subtest is indicated with NA.
Retrograde memory
Measurement of 1846's retrograde memory is limited by the fact that she was relatively young when many of the standard retrograde memory tests were designed (see also Oxbury et al., 1997; Tate, 2004). For example, when her retrograde memory was evaluated in 1993, 1846 was 30 years old, and most of the tests contain items extending back to the 1950s (Albert, Butters, & Brandt, 1981; Squire, Haist, & Shimamura, 1989), well before 1846 was born. Nonetheless, even when this limitation is taken into account, 1846 demonstrates a severe retrograde amnesia on all such tests across most eras, with particularly poor recall performance (see Table 2). Low-average scores were observed only in recognition performance for two decades (1950s and 1970s) addressed by the Boston Remote Memory Test (Albert et al., 1981); otherwise, all scores are borderline or defective. 1846's autobiographical memory was measured with the Iowa Autobiographical Memory Questionnaire (IMAQ; Jones, Grabowski, & Tranel, 1998), and while she was able to recall some information about her past, those facts were relatively sparse compared to norms (her answers were corroborated by a collateral, as is standard protocol with the IAMQ). Her memory performance was defective in each era for which she qualified, and there was modest evidence for a temporal gradient (Ribot, 1881; Squire & Alvarez, 1995): her recollection of early childhood and adolescence was 73% correct; but her young adulthood and recent life scores were 59% and 55%, respectively, indicating that more recent memories may have been disrupted to a greater extent. However, her scores were sufficiently poor in each era that this numerical gradient could not be confirmed in the context of normative performance.
Table 2.
Retrograde memory
| Test | Score | (see subheadings) | Administered | Interpretation |
|---|---|---|---|---|
| Boston Remote Memory Test | %ile | 1993 | ||
|
| ||||
| Recall 1950s | 12.5% | 5 | borderline | |
| Recall 1960s | 12.5% | <1 | defective | |
| Recall 1970s | 37.5% | 2 | borderline | |
| Recognition 1950s | 37.5% | 19 | low average | |
| Recognition 1960s | 37.5% | 1 | defective | |
| Recognition 1970s | 63% | 14 | low average | |
|
| ||||
| Iowa Autobiographical Memory Questionnaire | %ile | 1993 | ||
|
| ||||
| Early childhood and adolescence | 73% | <1 | defective | |
| Young adulthood | 59% | <1 | defective | |
| Recent life | 55% | <1 | defective | |
|
| ||||
| Boston Famous Faces Test | Mean for NLs | 1993 | ||
|
| ||||
| 1950s | 25% | 78 | defective | |
| 1960s | 25% | 79 | defective | |
| 1970s | 50% | 80 | defective | |
| 1980s | 12.5% | NA | defective | |
|
| ||||
| Squire's Remote Memory Test for Public Events | Mean for NLs | 1993 | ||
|
| ||||
| Recall 1950s | 0% | 54 | defective | |
| Recall 1960s | 3% | 50 | defective | |
| Recall 1970s | 4% | 48 | defective | |
| Recall 1980s | 0% | 60 | defective | |
| Recognition 1950s | 31% | 84 | defective | |
| Recognition 1960s | 30% | 86 | defective | |
| Recognition 1970s | 43% | 82 | defective | |
| Recognition 1980s | 50% | 80 | defective | |
Abbreviations and attributions: NL, normal comparison subjects; NA, not available ; Boston Remote Memory Test (Albert et al., 1981); Iowa Autobiographical Memory Questionnaire (Jones et al., 1998); Boston Famous Faces Test (Albert, Butters, & Levin, 1979); Squire's Remote Memory Test for Public Events (Squire, Haist et al., 1989).
Relative to other amnesic patients with focal hippocampal damage, 1846's retrograde memory deficit is unusually severe, with IAMQ performance at or near the bottom of the range for all reported MTL patients (Tranel & Jones, 2006). When prompted during that exam to engage in free recall of episodes that occurred during various phases of her life 1846 was frequently unable to produce any specific events (e.g., invited to recall a brief story about “an incident from grade school”, no response was given), although she was able to produce autobiographical information (e.g., her home address while attending grade school). Notably, her scores from the information (8) and vocabulary (9) subtests of the WAIS-III were average, suggesting that some aspects of her remote semantic memory are relatively intact. Other amnesic patients with damage limited to the MTL or hippocampus have generally intact retrograde memory for all but relatively recent epochs (Bright et al., 2006; Rempel-Clower, Zola, Squire, & Amaral, 1996), but the literature also reports MTL-damaged cases with extensive retrograde amnesia (Barr, Goldberg, Wasserstein, & Novelly, 1990; Sanders & Warrington, 1971). It is possible that 1846's preexisting epilepsy may have exerted an influence here, leaving her existing episodic memories more vulnerable than other MTL patients. Alternatively, 1846's exaggerated episodic memory impairment could be interpreted as support for the multiple trace theory of MTL function (Moscovitch & Nadel, 1998; Nadel & Moscovitch, 1997), which suggests that the hippocampus remains essential for episodic memory irrespective of the age of that memory, while semantic memory is acquired gradually and stored elsewhere in the brain.
Anterograde memory
1846 is severely amnesic for new declarative/relational information regardless of modality, and this is the most salient and defining feature of her entire neuropsychological presentation (see Table 3). As noted earlier, her IQ is in the low average range (WAIS-III FSIQ of 84), whereas her Wechsler Memory Scale, 3rd edition (WMS-III; Wechsler, 1997b) General Memory Index is severely defective at 57. Among the WMS-III tests, only her Immediate Memory score reached the low average range (85), and this is consistent with her working-memory scores (see “Working memory, attention, and executive function”). Memory for both verbal and visual information are both compromised, with the former evident in repeated administrations of the Rey Auditory-Verbal Learning Test (Rey, 1941) wherein her delayed recall was typically a single word (across eight unique tests her scores were 0,0,1,2,1,1,3, and 1), and the latter clearly demonstrated by a median Complex Figure Test (Osterrieth, 1944; Rey, 1941) recall score of 4 after a 30-minute delay (across seven unique tests her scores were 0,1.5,5,6,6,4, and 3).
Table 3.
Anterograde memory
| Test | Score | %ile | Admin. | Interpretation |
|---|---|---|---|---|
| WMS-R | 1993 | |||
|
| ||||
| Delayed recall index | 50 | <1 | defective | |
| General memory index | 60 | <1 | defective | |
| Verbal memory index | 66 | 1 | defective | |
| Visual memory index | 72 | 3 | borderline | |
|
| ||||
| Benton Visual Retention Test (Form C) | 1993 | |||
|
| ||||
| # Correct | 4 | NA | defective | |
| # Errors | 9 | NA | defective | |
|
| ||||
| Warrington Recognition Memory Test | 1993 | |||
|
| ||||
| Faces (#/50) | 34 | 2 | borderline | |
| Words (#/50) | 30 | 2 | borderline | |
|
| ||||
| WMS-III | See below | |||
|
| ||||
| Auditory immediate | 75 | 5 | 2000 | borderline |
| Auditory delayed | 62 | 1 | 2000 | defective |
| Auditory recognition delayed | 61 | <1 | 2000 | defective |
| General memory | 57 | <1 | 1998 | defective |
| Immediate memory | 85 | 16 | 1998 | low average |
| Visual immediate | 61 | <1 | 1998 | defective |
| Visual delayed | 72 | 3 | 1998 | borderline |
| Logical memory, prose immediate recall (#/75) | 23 | 5 | 2000 | borderline |
| Logical memory, prose delayed recall (#/50) | 0 | <1 | 2000 | defective |
| Verbal paired associates, immediate recall (#/32) | 0 | <1 | 2000 | defective |
| Verbal paired associates, delayed recall (#/8) | 0 | <1 | 2000 | defective |
|
| ||||
| AVLT | Multiple | |||
|
| ||||
| Trial 5 (#/15) | 6,2,7,7,4,6,5,4 | <1 | defective | |
| Recall, 30' (#/15) | 0,0,1,2,1,1,3,1 | <1 | defective | |
|
| ||||
| CFT | Multiple | |||
|
| ||||
| Recall, 30'(#/36) | 0,1.5,5,6,6,4,3 | <5 | defective | |
Abbreviations and attributions: NA, not available; Benton Visual Retention Test (Sivan, 1992); Warrington Recognition Memory Test (E. K. Warrington, 1984); WMS-III, Wechsler Memory Scale, 3rd ed. (Wechsler, 1997b); WMS-R, Wechsler Memory Scale-Revised (Wechsler, 1987); AVLT, Auditory-Verbal Learning Test (Rey, 1941); CFT, Rey-Osterrieth Complex Figure Test (Osterrieth, 1944; Rey, 1941).
1846 has also participated in a number of experimental investigations of the underlying nature of hippocampal-dependent memory. For example, she was tested for her memory of previously-studied items and the relations among those items, including familiar objects placed in scenes (Hannula et al., 2006), sets of faces presented simultaneously with scenic backgrounds (Hannula et al., 2007), and small sets of novels objects (Konkel et al., 2008). In each experiment, her overt behavior, and in one case her eye movements (Hannula et al., 2007), indicated that although her knowledge of individual items was relatively preserved, her memory for the relations between items was selectively impaired, an outcome that aligns with the predictions of relational memory theory (cf. Eichenbaum & Cohen, 2001). In another investigation studying memory using eye movements (Warren et al., 2011), she and other amnesic patients performed a complex visual search task less well than healthy comparison subjects, and when considered in the context of their abnormal eye movement patterns this suggested that the hippocampus may support representation of complex visual materials being held on-line (see also Voss et al., 2011; Warren et al., 2011). These investigations have contributed substantially to an emerging understanding that the hippocampus relates otherwise distinct representations together, and that it appears to do so even within very brief intervals.
Procedural memory
1846 shows preservation of procedural memory typical of patients with hippocampal damage and severe declarative amnesia (see Table 4). Her learning on a repeated mirror-tracing task (Milner, 1962) was normal despite profound amnesia for the circumstances of testing. She also exhibited normal rates of learning when asked to perform a series of manual tasks testing different aspects of non-declarative memory, including: weaving on a loom; filling cylinders with liquid; tracing moving geometric figures; navigating between targets on a screen; and tapping out a spatial sequence of key presses (Cavaco et al., 2004). As before, her normal acquisition of each of these tasks stood in stark contrast to her lack of knowledge about the learning circumstances.
Table 4.
Procedural memory
| Test | Performance | Administered | Interpretation |
|---|---|---|---|
| Mirror Tracing (Triangle-time to complete) | Time | 1994 | |
|
| |||
| Trial 1 | 24 s | normal | |
| Trial 2 | 20 s | normal | |
| Trial 3 | 16 s | normal | |
| Trial 4 | 17 s | normal | |
| Trial 5 | 16 s | normal | |
| 30' delay 1 | 18 s | normal | |
| 30'delay 2 | 20 s | normal | |
|
| |||
| Weaving * | Time (Z) | 2001 | Comments |
|
| |||
| Trial 1 | 196 s (2.29) | poor performance | |
| Trial 4 | 137 s (2.55) | normal rate of learning | |
| Trial 5 (24-hour delay) | NA | ||
|
| |||
| Geometric Figure Tracing * | Score (Z) | 2001 | Comments |
|
| |||
| Trial 1 | 133174 px (0.54) | normal performance | |
| Trial 5 | 106664 px (0.98) | normal rate of learning | |
| Trial 6 (24-hour delay) | 129720 px (1.26) | normal retention | |
| Trial 10 | 116486 px (0.43) | normal rate of learning | |
|
| |||
| Control Stick Manipulation * | Time (Z) | 2001 | Comments |
|
| |||
| Trial 1 | 67.11 s (1.61) | borderline performance | |
| Trial 5 | 43.87 s (2.63) | slow rate of learning | |
| Trial 6 (24-hour delay) | 74.43 s (3.14) | impaired retention | |
| Trial 10 | 42.07 s (2.31) | slow rate of learning | |
|
| |||
| Pouring Liquid * | Volume (Z) | 2001 | Comments |
|
| |||
| Trial 1 | 66 mL (−2.01) | poor performance | |
| Trial 5 | 84.5 mL (−1.92) | normal rate of learning | |
| Trial 6 (24-hour delay) | 78.5 mL (−1.63) | normal retention | |
| Trial 10 | 49 mL (−3.29) | unusual regression | |
|
| |||
| Spatial Sequence * | Time (Z) | 2001 | Comments |
|
| |||
| Trial 1 | 18.46 s (−0.33) | normal performance | |
| Trial 5 | 18.40 s (0.92) | no evident learning | |
| Trial 6 (24-hour delay) | NA | ||
Abbreviations and attributions: Mirror tracing (c.f. Milner, 1962); NA, not available.
These tasks are reported in more detail in another publication by our group (Cavaco et al., 2004). Z scores derived from the performance distribution of healthy comparison subjects provide normative context. In all tests except pouring liquid, improved performance is reflected in lower scores/durations, while larger poured volumes indicate improvement in the pouring task. 1846 showed a normal rate of learning and retention in most tasks apart from an unusual regression in the pouring task and stable performance in the spatial sequence task.
Working memory, attention, and executive function
Consistent with her general level of intellectual functioning, 1846 exhibits average to low-average abilities in tests of working memory, attention, and executive function (see Table 5). Classic measures of short-term memory capacity including digit span (10, WAIS-III), spatial span (10, WMS-III), and letter-number sequencing (8, WAIS-III), all indicate average ability. Measures of executive function also indicated generally average performance, for example, a Trail-Making Test Form B (Reitan & Wolfson, 1985) time of 59 seconds, and 6 completed categories in the Wisconsin Card Sorting Task (Grant & Berg, 1993) without clinically significant patterns of errors. Her score of 40 on the color-word Stroop task (Golden, 1978) is in the low-average range. In some of the more complex tasks 1846's scores fall into the defective range, although in certain cases this was due to a failure to comprehend instructions rather than difficultly with task performance (e.g., the Tower of Hanoi Task; Lucas, 1881). Her difficulty with the Controlled Oral Word Association (COWA; Benton, Hamsher, & & Sivan, 1994) task is unusual but not unprecedented among amnesic patients (e.g., patient Michelle in Tate, 2004) and may arise from difficulty with remembering previous responses.
Table 5.
Working memory, attention, and executive function
| Test | Score | %ile | Administered | Interpretation |
|---|---|---|---|---|
| Booklet Category Test (errors) | 30 | 33 | 1994 | average |
|
| ||||
| COWA (Form A) | 21 | 3 | 1993 | defective |
|
| ||||
| Tower of Hanoi | * | NA | 1993 | defective |
|
| ||||
| Stroop (T-scores) | 1994 | |||
|
| ||||
| Color Score | 46 | 34 | average | |
| Color Word Score | 40 | 16 | low average | |
| Interference Score | 48 | 45 | average | |
| Word Score | 34 | 6 | borderline | |
|
| ||||
| Trailmaking Test | 1993 | |||
|
| ||||
| A | 21s | 63 | average | |
| B | 59s | 29 | average | |
|
| ||||
| WCST | 1993 | |||
|
| ||||
| Correct | 68 | NA | average | |
| Errors | 14 | 58 | average | |
| Perseverative responses | 7 | 66 | average | |
| Nonperseverative errors | 8 | 50 | average | |
| Perseverative errors | 6 | 70 | average | |
| Categories completed | 6 | <16 | average | |
Abbreviations and attributions: *, did not finish; NA, not available; Booklet Category Test (DeFilippis & McCampbell, 1997); COWA, Controlled Oral Word Association Test (Benton et al., 1994); Tower of Hanoi (Lucas, 1881); Stroop, Color-Word Stroop Test (Golden, 1978); Trailmaking Test (Reitan & Wolfson, 1985); WCST, Wisconsin Card Sorting Test (Grant & Berg, 1993).
Speech, language, and naming
1846 demonstrates behavioral passivity in her communication, rarely initiating conversation, and although she responds enthusiastically to questions, she typically volunteers specific answers without elaboration. Her speech is perfectly comprehensible, but slightly dysarthric and characterized by hypernasality. Despite this, 1846 performs normally on most tests of speech (e.g., Multilingual Aphasia Examination Token Test [Benton et al., 1994] score of 41), but her language contains some abnormalities (see Table 6). Her vocabulary, reading, and writing are squarely in the normal range. Her naming of items from various categories of unique and non-unique entities is generally normal, although her performance on the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983) was below expectations (43). 1846 and her husband completed the La Trobe Communication Questionnaire (Douglas, Bracy, & Snow, 2007) in order to compare evaluations of communication by both the patient and a familiar partner. Both 1846's self rating (61) and her husband's rating (68) were within one standard deviation of the normative means characterizing patients with TBI (mean = 54.94, s.d. = 14.08) and their familiar partners (mean = 59.35, s.d. = 14.94), and the greater estimate of communication difficulty by 1846's husband is congruent with those data as well. Notably, the normative data for long-term TBI patients (i.e., >5 years since injury) indicate that patients and partners usually have similar ratings after more time has passed, but 1846's amnesia may interfere with her ability to evaluate her communication accurately.
Table 6.
Speech, language, and naming
| Test | Score | %ile | Administered | Interpretation |
|---|---|---|---|---|
| Benton Writing Assessment | 2 | NA | 1994 | borderline |
|
| ||||
| Boston Naming Test (#/60) | 43 | <1 | 1993 | defective |
|
| ||||
| Token Test | 41 | 33 | 1993 | average |
|
| ||||
| BDAE | 1993 | |||
|
| ||||
| Reading (#/10) | 9 | 25 | average | |
|
| ||||
| Naming | 1994 | |||
|
| ||||
| Animals | 98% | 75 | average | |
| Fruits and vegetables | 96% | 70 | average | |
| Iowa Famous Faces Test | 87% | 55 | average | |
| Iowa Famous Landmarks Test | 63% | <1 | defective | |
| Musical instruments | 93% | 19 | low average | |
| Tools/utensils | 89% | 2 | borderline | |
| Vehicles | 100% | 55 | average | |
Abbreviations and attributions: NA, not available; Benton Writing Assessment (Benton, Sivan et al., 1994); Boston Naming Test (Kaplan et al., 1983); Token Test (Benton et al., 1994); NART-R, National Adult Reading Test-Revised (Nelson & Willison, 1991); BDAE, Boston Diagnostic Aphasia Examination (Goodglass & Kaplan, 1972); Naming (Tranel, Enekwechi, & Manzel, 2005; Tranel, 2006).
Perceptual, constructional, and motor functions
1846's visuoperceptual and visuoconstructional abilities are generally in the average to low-average range (see Table 7). This is reflected in normal to low-normal judgment of line orientation, facial discrimination, block design, and grooved pegboard performance (Benton, Sivan, Hamsher, Varney, & Spreen, 1994; Matthews & Klove, 1964). Two notable exceptions are her Complex Figure Test (Osterrieth, 1944; Rey, 1941) copy scores (median=27) and her clock-drawing performance (defective). The low copy score was reliable over multiple instances of testing across nine years, and was mostly attributable to poor placement of features rather than outright omissions. Meanwhile, her clock drawing was defective due to comprehension (or attention) errors on both tested occasions (i.e., in response to the instruction “Draw a clock with hands showing the time twenty to four”, on two occasions she produced a normal clock face with hands indicating twenty after four). She performs within expectations on tests of basic psychomotor function.
Table 7.
Visuoperceptual, visuoconstructional, and motor functions
| Test | Score(s) | %ile | Admin. | Interpretation |
|---|---|---|---|---|
| Benton Facial Discrimination Test | 45 | 49 | 1993 | average |
|
| ||||
| Hooper Visual Organization Test (T-score) | 64 | 92 | 1993 | superior |
|
| ||||
| Judgment of Line Orientation | 22 | 22 | 1993 | low average |
|
| ||||
| CFT (copy) | 27,27,28,27,26,26,11.5 | <=8 | Many | borderline |
|
| ||||
| Drawing to dictation | 1993 | |||
|
| ||||
| Clock | 3 | NA | defective | |
| House | 1 | NA | normal | |
|
| ||||
| Grooved pegboard | 1993 | |||
|
| ||||
| Right | 65 s | 14 | low average | |
| Left | 70 s | 21 | low average | |
Abbreviations and attributions: NA, not available; Benton Facial Recognition Test (Benton, Sivan et al., 1994); Hooper Visual Organization Test (Hooper, 1958); Judgment of Line Orientation (Benton, Sivan et al., 1994); CFT, Rey-Osterrieth Complex Figure Test (Osterrieth, 1944; Rey, 1941); Grooved pegboard (Matthews & Klove, 1964).
Mood, personality, and adaptive functioning
1846 typically presents as euthymic, being both polite and friendly, although as previously mentioned she only infrequently initiates conversations and she tends to respond to questions with the requested information but little else. Her husband completed the Iowa Rating Scales of Personality Change (IRSPC; Barrash, Anderson, Jones, & Tranel, 1997) and only two items were unusual: first, he indicated that she now demonstrates a fairly severe lack of planning that was not previously present (Δ =+3), which is consistent with her amnesia; and second, he indicated that her lability and moodiness have increased dramatically (Δ =+5). In spite of the latter change, formal testing supports her having a baseline state of moderately positive mood (see Table 8). Her Beck Depression Inventory (Beck, 1996) score of 9 indicates minimal depression, while a recent administration of the Beck Anxiety Inventory (Beck, Epstein, Brown, & Steer, 1988) yielded a score of 13, suggesting no more than mild anxiety. Likewise, none of her scores on the clinical and validity scales of the Minnesota Multiphasic Personality Inventory (McKinley & Hathaway, 1944) were significantly elevated (the cutoff for significant elevation is a T-score of 65, which is 1.5 s.d. above the mean). 1846 completed the Satisfaction with Life Scale, a simple instrument designed to evaluate overall attitude toward an individual's own situation (Diener, Emmons, Larsen, & Griffin, 1985). Her responses indicated at least slight agreement with each of five positive statements, and her total score (30/35) is well above the mean of 20.8 reported for patients in the chronic phase after brain injury (Corrigan, Bogner, Mysiw, Clinchot, & Fugate, 2001). An interesting footnote to the outcomes of these mood-related instruments is the noted lack of cortisol release in response to stressful circumstances by patients with hippocampal damage, including 1846 (Buchanan et al., 2009). Despite subjective and objective indications of stress when asked to perform public speaking, neither she nor any other hippocampal amnesic patient in that study exhibited the typical increase in cortisol associated with stress. Whether this change in acute hormonal response is accompanied by chronic change in mood is unknown.
Table 8.
Mood, personality, and adaptive functioning
| Test | Score | Admin. | Interpretation |
|---|---|---|---|
| Satisfaction with Life Scale (#/35) | 30 | 1993 | more satisfied than most brain-injury patients |
|
| |||
| Beck Anxiety Inventory | 13 | 2010 | mild anxiety |
|
| |||
| Beck Depression Inventory | 9 | 1993 | minimal depression |
|
| |||
| MM PI (T-scores) | L-53, F-46, K-64, Hs-53, D-54, Hy-47, Pd-43, Mf-48, Pa-56, Pt-62, Sc-60, Ma-65, Si-53 | 1994 | no significant elevations on any validity or clinical scales |
| Mayo-Portland Adaptability lndex-4 | Rater (2003) | Score | Interpretation |
|---|---|---|---|
| Adaptability | 1846 | 49 | normal |
| Adjustment | 1846 | 45 | normal |
| Participation | 1846 | 48 | normal |
| Total | 1846 | 48 | normal |
| Adaptability | Collateral | 45 | normal |
| Adjustment | Collateral | 44 | normal |
| Participation | Collateral | 54 | normal |
| Total | Collateral | 47 | normal |
Abbreviations and attributions: Satisfaction with Life Scale (Diener et al., 1985); Beck Anxiety Inventory (Beck et al., 1988); Beck Depression Inventory (Beck, 1996); MMPI-2, Minnesota Multiphasic Personality Index (McKinley & Hathaway, 1944); Mayo-Portland Adaptability Index-4 (Malec & Lezak, 2008).
1846 and her husband both completed the Mayo-Portland Adaptability Inventory-4, an instrument designed to briefly evaluate the abilities, mood, and social participation of individuals with acquired brain injury (MPAI-4; Malec & Lezak, 2008). Overall, she and her husband agreed that across domains she experiences no more problems with the listed activities than the MPAI-4's normative mean for brain-damaged participants, with all T scores in the 44–54 range. However, both parties responded to the “Memory” item by indicating at least moderate impairment, and likewise rated her “fund of information” (i.e., knowledge of personal history) as similarly impaired. The largest difference in inter-rater T scores was on the participation subscale (Δ = 6), suggesting that 1846 reports her social participation as slightly more normal than her husband, but neither score is abnormally high (see Table 8).
Life Outcome
As the foregoing data demonstrate, 1846 has undergone extensive and repeated formal neuropsychological assessment. However, such exams do not provide a complete picture of a patient's life. In the interest of filling in some of the non-psychometric details and evaluating her overall life outcome, we informally and separately interviewed 1846, her husband, and her parents. The information that follows provides important insights into how 1846 and her family continue to cope successfully with her severe memory impairment. We begin by summarizing the rehabilitation therapies that 1846 was exposed to and their lasting impact.
Rehabilitation
As her neuropsychological profile indicates, 1846's memory performance has not improved appreciably since soon after the episode, but her exposure to rehabilitation and remediation therapy at the University of Iowa during that first year has made some lasting contributions to her outcome. Specifically, outpatient neuropsychological rehabilitation was provided, with sessions attended by 1846 and her husband. Her treatment focused on learning compensatory strategies to minimize the effects of her amnesia on real-world activities. Specific therapies included training in the systematic use of a planning book (with calendar and daily schedule), as well instruction in increased structure and consistency in her daily schedule. In addition, because her cognitive difficulties appeared to worsen significantly under stress, she was provided with training in methods of stress management based on principles of procedural memory (Suhr, Anderson, & Tranel, 1999). 1846's adherence to these rehabilitation strategies is reportedly good. According to her husband and parents, 1846 continues to use a day planner, and it suffices to organize her limited daily activities when she remembers to write down notes. The structure of her environment and daily life, imposed to some extent by her family and described below, appears to be of greater utility than her own external memory aids.
Work
While the presence of a memory deficit was evident following 1846's episode, its severity and extent became especially obvious when she tried to return to her previous job at the medical practice within two months of the episode. As reported by her husband, 1846 had managed the entire practice without difficulty, taking phone calls, filling out complex insurance forms, and completing other typical office tasks without difficulty. Suddenly, these routine tasks were overwhelming, exemplified by her attempt to reorganize the office filing system on one day only to return the next day with no knowledge of the new filing scheme. Relegated to stuffing envelopes, she eventually resigned and applied for disability benefits. Family members pointed out that she had always intended to work outside the home, and when asked, 1846 expresses some regret that she was not able to continue developing in her career. Any such disappointment must be relatively limited, however, given her generally positive mood and overall satisfaction with her life.
Parenting and familial relationships
1846 is currently a homemaker with two children, including one born only months before the episode. Despite her severe memory impairment, family members report that she was able to care for her infant without supervision, perhaps drawing on the parenting skills developed when caring for her older child. Although 1846 remains involved with her children, for example, by talking with them about their lives and attending their extracurricular events, her role in parenting has been affected by her amnesia. When she attempted to return to work, the children were put in daycare, and their enrollment at that facility continued after she resigned, reducing her parenting demands somewhat. Her husband has absorbed many responsibilities that might otherwise have been shared, and likewise her parents, who live nearby, have taken a significant part in their grandchildren's lives. For example, both of 1846's children participate in recreational sports, and their grandparents take on much of the responsibility for ferrying them to and from practices and games, facilitating extracurricular activities that would have otherwise been unfeasible. 1846's father endorsed the idea that he and his wife have served as something approaching surrogate parents to their grandchildren, and reinforced this by noting that he and his grandchildren exchange text messages several times a week over and above any inperson visits.
The family's adaptations accommodate 1846's memory problems, as when her husband attends parent-teacher conferences alone, but returns to report the important information and gist to 1846, who absorbs and comments on this information at her own pace. Her husband also reports that while 1846 did most of the cooking before the episode, he now does most of the cooking at home or otherwise arranges meals for the family. Likewise, family trips are planned mostly by 1846's husband and children. 1846's daughter has contributed individually as well, taking over some responsibility for delivering her younger brother wherever he needs to go once she had obtained her driver's license. While 1846 is a more passive parent than she might have been otherwise, she does significantly influence the lives of her children by talking with them about their problems and offering advice and opinions. The children, along with their father and grandparents, have meanwhile shouldered significant additional duties in order to compensate for 1846's memory problem. Despite this, the family remains close-knit and extremely supportive.
Mood
Severe neuropsychological deficits are frequently associated with changes in mood and attitude, and there is some evidence of this after 1846's episode, including her husband's comments and IRSPC scores related to her emotional lability. Session notes from early evaluation and rehabilitation visits to the University of Iowa offer some evidence of disturbed mood: “Acute distress was evident upon feedback regarding the seriousness of the memory defect.”; while performing the Tower of Hanoi Task, “… she admitted that it was frustrating. She did not cry at any point during or after the test…”; “Husband reports… some possible improvement in her emotional stability.”; etc., although contemporaneous Beck Depression Inventory (Beck, 1996) scores never entered the clinically significant range. Even so, her parents corroborate her husband's reports of changes after the episode, indicating that her mood was subdued for almost five years. However, there is also a consensus that she has adapted to her new circumstances well, especially within the last five to ten years, exhibiting generally neutral to positive affect. Some of these changes may be due to behavioral adjustment, while others are likely the result of a very supportive environment.
Socialization
In many respects, 1846's life circumstances are enviable by the standards of densely amnesic patients, most of whom are confined to institutions and require more or less constant supervision. 1846 is surrounded by friends and family, living within a block of many friends and acquaintances of long standing, and only a few minutes' drive from her parents. With the help of her husband and parents, she is able to manage her household shopping needs, look after her children, and maintain a relatively normal homemaker's life. Notably, while interactions with friends and family are common, her husband and parents report that 1846 has not established any close extrafamilial friendships since the episode, a pattern that is quite different from her extraverted socializing before the episode, and not uncommon after brain injury (Engberg & Teasdale, 2004; Teasdale & Engberg, 2005). Her parents note that she was always an aggressive social climber, and speculate that she would have been involved in local society if not for her memory problem. This change may be a consequence of 1846's now relatively passive behavior, a characteristic common to many patients with anterograde amnesia, particularly after anoxia (Wilson, 1996), and is reflected to some extent in items from the IRSPC indicating that she is extremely cautious in social situations (Social Appropriateness score of 1, Δ=−2, and Social Withdrawal score of 2, Δ=−1). It remains unclear whether the change is a direct result of brain injury or related to 1846's knowledge of her amnesia and concern about the perceptions of others. In any event, 1846's passivity may in turn explain why it is possible for her to spend most of her days unsupervised, as she does not drive and almost never spontaneously ventures out of the house, instead waiting for someone to accompany her. Her husband and parents state that left to her own devices during the day, 1846 completes some household chores, watches television, plays computerized solitaire, and solves word jumbles. She does not initiate novel, unfamiliar tasks, and does not put herself in harm's way.
Everyday memory
One exception to 1846's otherwise passive (and adaptive) behavior is an occasional fixation on certain ideas or information. 1846's mother observed that her daughter will, in anticipation of upcoming events, (perhaps prompted by handwritten notes) occasionally pepper family members with repeated questions about when the event will be taking place, what preparation might be necessary, etc. Although she cannot remember the answers, or even having asked the questions, these fixations may serve 1846 as a means of very slowly acquiring new information, or simply remembering that certain events will take place at all. These bouts of intensely repetitive questioning are limited to family members, as 1846 is typically more withdrawn around others.
Although she does not often volunteer information, when asked questions, 1846 will give answers that are plausible in nature, albeit sometimes incorrect. Some examples are particularly stark, including inconsistent access to factual information about her children, as when her daughter was approaching legal driving age and would variously be reported by 1846 to have her driving permit, her license, or neither. None of the answers were implausible alone, but their mutually contradictory nature was obvious. This could be construed as confabulation if it were clinically problematic, but is more likely a coping mechanism developed gradually after the episode, one that allows 1846 to participate in a conversation without immediately revealing her memory problem to others (see also Duff et al., 2008).
It is interesting to note that although amnesia interfered with 1846's work, it did not prove problematic when the family moved to a new home some months after the episode, as by all accounts she adapted to the new surroundings with relative ease despite not being able to remember her new address or other novel factual information. The difference nicely illustrates the distinction between the rapid acquisition of declarative knowledge required, for example, in many common employment scenarios, and the more gradual, procedural behavioral modifications that amnesic patients can acquire without difficultly (cf. Brooks & Baddeley, 1976; Cohen & Squire, 1980; Duff, Hengst, Tranel, & Cohen, 2006; Glisky, Schacter, & Tulving, 1986; Wilson & Wearing, 1995).
Comparison to other cases
Having already contrasted 1846's case with that of HM, we selected another patient for close comparison, and the extensive neuropsychological, neuroanatomical, and pathological data available for anoxic amnesic patient RB (Zola-Morgan, Squire, & Amaral, 1986a) make him an ideal foil. Anatomically, the right hippocampi of the two patients are strikingly similar, showing relatively circumscribed lateral reductions in volume, but 1846's left hippocampus is markedly more atrophic than RB's (see Neuroanatomy section and Figure 2). This suggests that 1846's right hippocampus may exhibit focal cell loss in cornu ammonis-1, although post mortem histology (as for RB) would be required for confirmation. Neuropsychologically, the two patients both exhibited amnesia. RB's WMS score of 90 would suggest a less severe amnesia than 1846's, but on other tests of memory the two patients are very similar (e.g., Complex Figure Test (Osterrieth, 1944; Rey, 1941) delayed recall scores of <5, word list recall performance <10% of normative expectations, etc.). 1846's history of epilepsy and acute episode of status epilepticus likely explains both her more extensive hippocampal atrophy and her modestly greater memory impairment.
Figure 2.
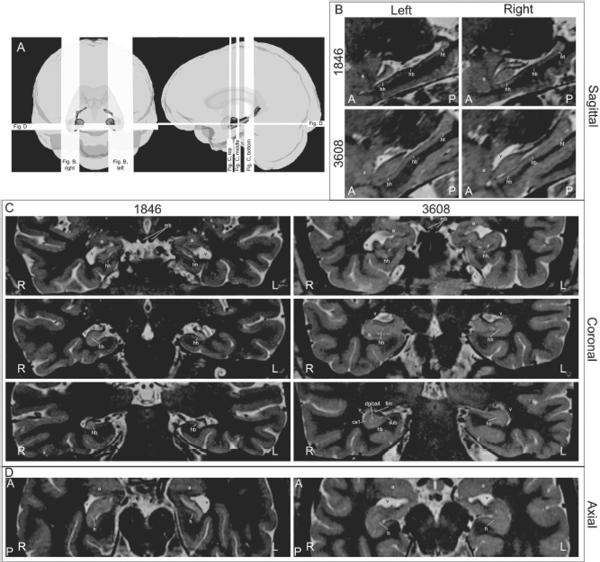
Anatomy of 1846 and healthy comparison 3608 based on high-resolution T2-weighted structural MRI scans. Panel A uses three-dimensional models of traced structures from 1846 (see Fig. 1) and planar sections to illustrate the location of the slices shown in panels BD. In panel B, sagittal sections reveal the extensive atrophy of 1846's left hippocampus along its entire length (left column). The right hippocampus is less compromised (right column). In panel C, successive coronal sections illustrate hippocampal anatomy of the anterior hippocampal head (top row), the posterior hippocampal head at the uncal apex (middle row), and the hippocampal body caudal to the uncal apex (bottom row). At bottom right, 3608's right hippocampus contains enough detail for identification of several hippocampal subfields. In panel D, axial sections reveal the severe atrophy of 1846's left hippocampus and the corresponding enlargement of the inferior horns of the lateral ventricles. Common abbreviations for panels BD: A, anterior; P, posterior; R, right; L, left; h, hippocampus; hh, hippocampal head; hb, hippocampal body; ht, hippocampal tail; a, amygdala; v, inferior horn of the lateral ventricle; mb, mammillary body/bodies; sub, subiculum; ca1, cornu ammonis-1; dg/ca4, dentate gyrus and cornu ammonis-4; fim, alveus/fimbria.
Interestingly (and as in the case of patient RB described above), reports describing severely amnesic patients have historically focused almost exclusively on men, including HM (Scoville & Milner, 1957). Other patients of particular renown include Boswell (Damasio, Tranel, & Damasio, 1989), EP (Stefanacci et al., 2000), NA (Squire, Amaral, Zola-Morgan, Kritchevsky, & Press, 1989), Clive Wearing (Wilson & Wearing, 1995), Jay (Wilson, 1999), and case 1951 (Feinstein et al., 2010), all of whom have contributed greatly to the scientific study of memory systems and to public awareness of amnesia. Despite the prominence of these amnesic men in the case report literature, a casual survey of published research suggests that men and women are equally likely to suffer from severe amnesia, or at least to participate in research studies of memory impairment. Indeed, although much of what we know about amnesia has been learned from the study of both men and women, thorough descriptions of women with amnesia are relatively rare (see also Duff, Wszalek, Tranel, & Cohen, 2008). Addressing this disparity is important, as many challenges faced by women with brain injuries are different from those faced by men with the same underlying deficit (Clark & Pompa, 2009). The case presented here illustrates some of those unique challenges, in particular child-rearing in the face of severe amnesia.
A related observation is that 1846 and at least three other reported women with severe amnesia have demonstrated relatively positive life outcomes. Her case resembles that of the patient Michelle reported by Tate (2004) to some degree in that both women have concentrated on child-rearing, possibly owing to an inability to work outside the home. The relative success of both can be attributed to excellent social support on the part of parents, and in the case of 1846, her husband. Likewise, patient Angie (Duff et al., 2008) has had success in parenting her stepchildren despite severe amnesia. However, neither 1846 nor Michelle has demonstrated Angie's ability to creatively overcome the obstacles presented by amnesia in a professional environment, and while 1846 has some knowledge of her impairment, she lacks Angie's remarkable insight. Duff et al. (2008) attribute this marked difference between Angie and other patients to the unusual neuroanatomy of that case, and 1846's extensive hippocampal damage certainly does not contradict their suggestion. Finally, patient YR's outcome has not been extensively reported (Holdstock et al., 2000; Mayes et al., 2001; Mayes, Holdstock, Isaac, Hunkin, & Roberts, 2002), but like 1846 and Michelle, YR's amnesia interfered with work outside the home while her ability to cope with home life was much less affected. Other authors have reported that women who suffer traumatic brain injury are less likely to accept rehabilitation services or vocational training, and have speculated that gender stereotyping may make a transition to the role of homemaker easier for women than for men (Bounds, Schopp, Johnstone, Unger, & Goldman, 2003). The small sample of amnesic patients discussed here generally aligns with that observation.
1846's outcome can also be considered in the context of outcomes for the other amnesic patients who have participated extensively in our research program at the University of Iowa. Of the most intensively studied and severely amnesic cohort of such patients (029, 1606, 1846, 1951, 2144, 2308, 2363, and 2563), 1846 and 2563 are the only patients who are not currently living in assisted-care facilities (although 2144 was relatively independent before her death, as was 1606 before suffering a head injury that worsened his condition). 1951, 2308, and 2363 all reside in centers for brain-injured patients or retirement homes despite being of only middle age; 029 also lived in an assisted care facility after his neurological injury. The density of 1846's amnesia might merit a similar living situation if not for the mitigating factor of her extensive social support network, but that cushion has given her the freedom to live in more engaging surroundings and to slowly adapt to her memory disorder.
Neuroanatomy
The neuroanatomy of this case is intriguing because of the combination of two etiologies, each by itself sufficient to yield hippocampal damage and severe memory problems. While the patient's cognitive outcome is quite clear, the acute interaction of these etiologies in the context of adolescent-onset epilepsy created a complex anatomical picture that we explored with in vivo structural neuroimaging techniques.
1846's neuroanatomy has been described briefly in the context of a study of a group of anoxic patients reported by our laboratory (Allen et al., 2006), based on whole-brain MRI results obtained at 1.5T, which allowed good resolution of the hippocampus as a unit and of the surrounding cortex (1.5mm slice thickness; .7mm interpixel distance). Using regression-based predictions derived from a normative sample of healthy brains (Allen, Bruss, Brown, & Damasio, 2005), Allen et al. (2006) estimated the volume and Studentized residual values of the major lobes, hippocampi, and amygdalae of each anoxic participant. 1846's bilateral hippocampal volume had a Studentized residual value of 4.23 less than that predicted by her age and sex, which was well below the normal range, and the smallest observed volume in any of the anoxic patients considered in that report, including several other severely amnesic patients.
Notably, both the original T1 scans and more recently collected T2 scans (see below) suggest that 1846 may exhibit some degree of whole-brain or cortical atrophy, perhaps related to her anoxic episode (cf. Grubb et al., 2000) or putative medial temporal lobe epilepsy (Lin et al., 2007). Studentized volumes calculated by Allen et al. (2006) indicate that after accounting for age and sex, 1846's lobar gray- and white-matter volumes were within the normal range although in many instances numerically smaller than predicted by her age and sex. Specifically, 1846's lobar gray-matter residuals were: frontal, −1.42; parietal, −1.79; temporal, −0.67, while her lobar white-matter residuals were: frontal, −1.07; parietal, −1.27; temporal, −0.10 (occipital lobe volume was not evaluated for either tissue class). These values do not indicate gross atrophy, but gray matter volumes are somewhat more reduced than white matter volumes. Volume of both gray and white matter in the temporal lobe appears to be squarely within the normal range, which implies that the cortical thinning reported in MTL epilepsy patients may not be present in 1846 (Lin et al., 2007).
The Allen et al. (2006) study documented gross hippocampal atrophy in 1846, but the consequences of her anoxia and status epilepticus for individual hippocampal subfields were not explored. To address this, we obtained new scans of 1846 and a healthy age- and sex-matched comparison participant (3608) using a 3T MRI apparatus and a novel scanning protocol that afforded very fine-grained resolution. The high-resolution T2-weighted scan was a 2D turbo spin-echo sequence with the following parameters: TE=17ms; TR=10730ms; flip=126; FOV=170×170mm; slice thickness/gap=0.8/0.0mm; matrix=384×378; bandwidth=246Hz/pixel; turbo factor=17; averages=2. This sequence was 13m16s long and was repeated 3 times. After post-processing, the product of the protocol was a partial-brain volume targeting the medial temporal lobes including the amygdala, the hippocampus, and the surrounding parahippocampal cortex with an in-plane (i.e., coronal) resolution of 0.5×0.5 mm and a slice thickness of 0.5mm (see Figure 1 & 2). Scans of both the native and upsampled resolution are more than sufficient to permit rule-based parcellation of the hippocampal subfields in healthy adults (Mueller et al., 2007; Stark, Yassa, & Stark, 2010; Wieshmann et al., 1999; Yassa et al., 2010), and we proceeded with a qualitative evaluation of 1846's medial temporal lobes, as contrasted with 3608 (healthy comparison subject, see Figure 2; quantitative analysis of the same tissue will require additional normative information, beyond the scope of the current report). We also collected and provide analysis of a lower-resolution whole-brain FLAIR volume (TE=406; TR=5000; TI=1800; FOV=256×230; Slices=176; pixel size=1×1×1mm; bandwidth=592Hz/pixel; turbo factor=2; time=5m52s).
Figure 1.
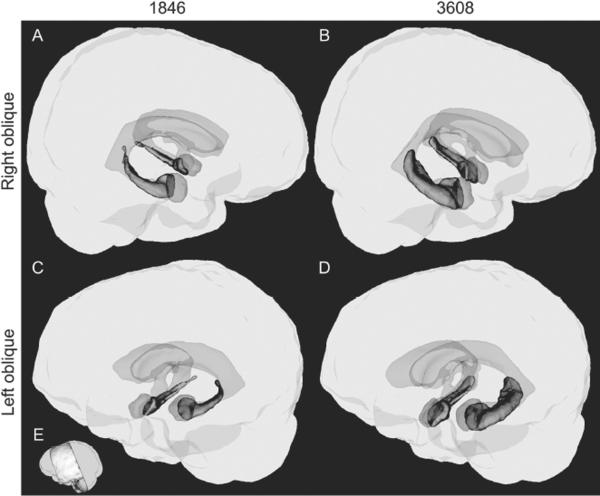
Three-dimensional tracing-based models of hippocampus, amygdala, and the ventricular system in 1846 and a healthy age- and sex-matched comparison participant, 3608. In panels A–D, the whole-brain volume is presented in gray, portions of the ventricular system within our scan volume in translucent blue, amygdala in translucent red, and hippocampus in solid green. E illustrates the extent of the high-resolution scan. Panels B and D illustrate healthy structures in 3608. The size and extent of the hippocampus is normal bilaterally. Panels A and C model the same structures in patient 1846. While the hippocampus is diminished bilaterally, right hippocampus is somewhat less atrophied than left.
Whole brain
Temporal lobe
Bilaterally, 1846's superior, middle, and inferior temporal gyri are normal. Heschl's gyri are also normal. See the further discussion of the medial temporal lobe below.
Cerebral cortex outside of temporal lobe
There is some evidence of cortical thinning in the paracentral lobule and precuneus in the T2 modality, and these regions are moderately hyperintense on FLAIR. Both are in watershed zones of the cortical arterial supply, and such regions are thought to be more susceptible to damage from anoxic events (Caine & Watson, 2000). Although neither of these specific regions is thought to be necessary for normal memory, it is possible that dysfunction of these regions may influence certain aspects of 1846's cognitive functioning.
Cortical and subcortical white matter
In the high-resolution T2 scan, perivascular (Virchow-Robin) spaces appear as hyperintense streaks; such spaces are also observed in healthy human brains. No evidence of white matter T2 hyperintensities was recognized.
Subcortical structures
Most subcortical structures are intact, but the left mammillary body is noticeably atrophied, possibly as a consequence of transsynaptic degeneration from extensive hippocampal atrophy (see Fig 2C). Although outright lesion of the mammillary bodies and other diencephalic structures is known to cause severe amnesia (e.g., Squire et al., 1989), both mammillary bodies are still present and identifiable in 1846 despite the reduced volume on the left.
Medial temporal lobe
Amygdala
1846's amygdala is normal bilaterally in terms of shape, size, and signal.
Hippocampus
The pes hippocampi are atrophied bilaterally, but particularly on the left. There, the anterior two-thirds of the hippocampus have atrophied, and the inferior horn of the lateral ventricle is correspondingly larger. The extent of the left-lateralized atrophy is sufficient that no subfields of the hippocampus appear to have been spared. On the right, the extent of the atrophy is restricted to the anterior third of the hippocampus, and is nowhere as great as that seen on the left. Based on a past report of the histology of anoxic amnesic patients (Zola-Morgan, Squire, & Amaral, 1986b), we anticipated that the cornu ammonis-1 (CA1) field would exhibit the greatest atrophy. On the left, all subfields appeared equally diminished, but on the right, the reduced lateral extent of the hippocampus does suggest focal CA1 damage. T2 and FLAIR hyperintensities are present bilaterally, typical of patients who have suffered status epilepticus (Guerreiro et al., 2001).
The volume of 1846's hippocampus was quantitatively evaluated by manual tracing using a method adapted from Pruessner et al. (2000) in consultation with an investigator who participated in the previous published report of 1846's hippocampal volume (JB from Allen et al., 2006), and the observed volumes were: left hippocampus 1080 mm3; right hippocampus 1646 mm3. The total volume is approximately 80% of that reported by Allen et al. (2006), which could reflect the greater precision of the new T2 scan, differences in apparent volume owing to imaging modality, interval increases in atrophy of the tissue, or some combination of these factors. In the matched comparison participant, the same T2-weighted scan protocol and tracing technique yielded a left hippocampal volume of 3487 mm3 and a right hippocampal volume of 3337 mm3, both of which are within the expected normal range.
Fimbria
On the right the fimbria is robust, but on the left the fimbria is somewhat attenuated. This is consistent with the extensive hippocampal atrophy and reduced volume of the mammillary body on the left, strongly suggesting loss of hippocampal efferent axons in the former and trans-synaptic degeneration in the latter.
Parahippocampal gyrus
Bilaterally, caudal to the amygdala, the anterior portion of the parahippocampal gyrus is noticeably smaller than average, and there may be some thinning of the underlying white matter.
Fusiform gyrus
Caudal to the uncus, the middle portion of the fusiform gyrus may exhibit some atrophy bilaterally. On the left, 1846's collateral sulcus is unusually shallow and disappears approximately 1 cm caudal to the uncus, and at this point the fusiform gyrus falls into the occipito-temporal sulcus. Caudal to this the collateral sulcus reemerges to define a newly expressed fusiform gyrus. On the right, the fusiform gyrus also exhibits substantial narrowing, although it never disappears completely into the occipito-temporal sulcus.
Summary
Neuropathologically, 1846 presents as a hybrid of anoxic and epileptic etiologies, with modest evidence of neocortical involvement implying anoxia combined with gross hippocampal atrophy that could have been driven by either kind of injury but is more severe than has been observed in other patients who suffered isolated anoxic episodes. While some neocortical portions of temporal lobe (fusiform and parahippocampal gyri) are smaller than average and at the low end of the normal range, the obvious atrophy of the hippocampus stands apart. Adolescent-onset epilepsy may have contributed to some of the unusual characteristics observed in 1846's medial temporal lobe, but the stark change in her neuropsychological profile after the episode of status epilepticus and anoxia strongly suggests that her neuroanatomy was greatly altered at that time.
Discussion
1846 represents a rare case of hippocampal amnesia, and her combined anoxic/epileptic etiology and generally positive outcome make her unusual even within that small population. We close this report by considering the mechanisms that directly contributed to her hippocampal damage and the circumstances that have allowed her to live a relatively normal life despite a profound memory impairment.
Hippocampal damage after an anoxic episode varies in extent, roughly in accord with the severity of oxygen deprivation, but generally conforms to an expected pattern (Auer & Sutherland, 2002). Studies using animal models have shown that the vulnerability of hippocampus is greatest after five minutes of oxygen deprivation (Brierley, Meldrum, & Brown, 1973), and moreover, the extent of damage is generally related to the duration of the anoxic episode (Radovsky, Katz, Ebmeyer, & Safar, 1997). Pathological investigations of post-anoxic hippocampal tissue from animal models have shown that subfield cornu ammonis-1 (CA1) is most vulnerable to necrotic cell death in anoxic conditions, and that this damage manifests slowly over the course of several days following acute anoxia even when normal respiration has been reestablished (Kirino, 1982). Necrotic death in CA1 is also evident in the post mortem pathology of human anoxic patients, accompanied at a gross level by CA1 atrophy (Zola-Morgan, Squire, & Amaral, 1986a), and cell death again appears to be delayed after an acute anoxic event (Petito, Feldmann, Pulsinelli, & Plum, 1987; Volpe & Petito, 1985). The precise mechanisms underlying neuronal necrosis in CA1 after acute anoxia have not been determined, although it has been shown that extracellular concentrations of glutamate are greatly increased in those conditions (Benveniste, Drejer, Schousboe, & Diemer, 1984), and given the prevalence of glutamate receptors in CA1, excitotoxic cell death has been widely accepted as an explanation (e.g., Auer & Sutherland, 2002). However, other mechanisms for CA1 damage have also been identified, and continuing research may eventually permit clinical intervention during or after anoxia to prevent CA1 damage (cf. Sun et al., 2009).
Status epilepticus (hereafter, "status") can result in a pattern of hippocampal damage very similar to that found after anoxia (Corsellis & Bruton, 1983; Meldrum, 1997; Meyer et al., 1955; Ng, Graham, Adams, & Ford, 1989), although other CA fields may be uniquely vulnerable during prolonged status (DeGiorgio et al., 1992; Fujikawa, 1996; Nevander, Ingvar, Auer, & Siesjo, 1985). This histological resemblance between status and anoxia has prompted debate over whether anoxic conditions provide a shared explanation (Brierley et al., 1973; Meldrum, 1997) as status is often accompanied by anoxia owing to interrupted respiration. Studies in animal models have shown that even well-ventilated subjects experiencing status for tens of minutes exhibit necrotic cells in CA fields (Nevander et al., 1985) thus demonstrating that seizure activity alone can produce excitotoxic cell death (for review, see Fujikawa, 2005), although it is unclear whether this purely seizure-driven pattern of damage would arise outside a laboratory. On a gross anatomical scale, status results in acute inflammation of the hippocampal tissue followed in the chronic phase by atrophy combined with scarring in CA1 and CA3 (Corsellis & Bruton, 1983; Guerreiro et al., 2001; Meldrum, 1997), both of which are apparent in 1846's MTL. Notably, while extrahippocampal brain regions can also be injured by both anoxia and status (Fujikawa, 1996; Horstmann et al., 2010; Meyer et al., 1955), 1846's brain seems relatively free of other damage.
Hippocampal damage such as that suffered by 1846 is irreversible, and no known prosthetic or therapy can completely remediate the condition. This leaves most severely memory-impaired patients with few real prospects in life, and the outcomes reviewed above indicate that confinement to the relatively impoverished environments of full-time care facilities is the modal endpoint for such patients. Why is 1846 different? Some of the key factors in her comparatively positive outcome have been the measures taken by her extended family to compensate for her cognitive deficits with their own time and resources. Her husband's success at work has enabled her to specialize in her role as a homemaker. Her family has also taken steps to make her life easier: her husband moved the immediate family to a comfortable neighborhood close to many friends, and her parents relocated to live close to 1846's new home; her mother helps 1846 with daily shopping; her husband takes care of meals; and even the children have pitched in, with 1846's daughter often chauffeuring her brother. Overall, 1846's episode seems to have drawn the entire family closer together, both physically and emotionally. Her father commented that 1846 “couldn't have a better husband,” and this respect seems to be mutual. 1846's case is a clear demonstration that social, and particularly family, support can make all the difference in a positive outcome after brain injury, and while that message is not new, it remains an important predictor of a patient's life trajectory and a sanguine note that should give other patients and their families some hope.
Summary and Conclusions
Bilateral hippocampal damage yields a profound inability to learn new information, especially when that information must be remembered in a broader context. Both anoxia and status epilepticus can cause damage to the hippocampus, and patient 1846 illustrates an informative case of simultaneous exposure to both etiologies. In fact, relative sparing of her non-mnemonic cognition makes her a textbook case of medial temporal lobe and hippocampal amnesia as initially recognized in patient HM. While many patients who are comparably (or even less severely) amnesic are confined to institutional, around-the-clock care, 1846 is able to lead a relatively full and independent life owing to extensive social support by friends and family. She has contributed to a great deal of research which has helped to our expand knowledge and understanding of the mechanisms of human memory.
Acknowledgements
This work was supported by the following funding sources: NINDS P50 NS19632 (DT). The authors would like to express their gratitude to 1846, her husband, and her parents for consenting to participate in our research and for the interviews that informed this case report. The authors would also like to thank Dr. Steven W. Anderson, Kenneth Manzel, and Joel Bruss for their contributions to this report.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal–anterior thalamic axis. Behavioral and Brain Sciences. 1999;22(3):425–489. doi:10.1017/S0140525X99002034. [PubMed] [Google Scholar]
- Albert MS, Butters N, Brandt J. Patterns of remote memory in amnesic and demented patients. Archives of Neurology. 1981;38(8):495–500. doi: 10.1001/archneur.1981.00510080057008. [DOI] [PubMed] [Google Scholar]
- Albert MS, Butters N, Levin J. Temporal gradients in the retrograde amnesia of patients with alcoholic korsakoff's disease. Archives of Neurology. 1979;36(4):211–216. doi: 10.1001/archneur.1979.00500400065010. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26(9):1245–60. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 1279-82. doi:10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. Journal of Clinical and Experimental Neuropsychology. 2006;28(4):457–476. doi: 10.1080/13803390590949287. doi:10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- Auer RN, Sutherland GR. Hypoxia and related conditions. In: Graham DI, Lantos PL, editors. Greenfield's neuropathology. Seventh ed. Arnold; London: 2002. pp. 233–280. [Google Scholar]
- Barr WB, Goldberg E, Wasserstein J, Novelly RA. Retrograde amnesia following unilateral temporal lobectomy. Neuropsychologia. 1990;28(3):243–255. doi: 10.1016/0028-3932(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Barrash J, Anderson S, Jones R, Tranel D. The iowa rating scales of personality change: Reliability and validity. [Abstract] Journal of the International Neuropsychological Society : JINS. 1997;3(1):27–28. [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science (New York, N.Y.) 1995;269(5227):1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck depression inventory. 2nd ed. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K, Sivan A. Multilingual aphasia examination. 3rd ed. Psychological Assessment Resources; Lutz, FL: 1994. [Google Scholar]
- Benton A, Sivan A, Hamsher K, Varney SN, Spreen O. Benton laboratory of tests. Psychological Assessment Resources; Lutz, FL: 1994. [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. Journal of Neurochemistry. 1984;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Bounds TA, Schopp L, Johnstone B, Unger C, Goldman H. Gender differences in a sample of vocational rehabilitation clients with TBI. NeuroRehabilitation. 2003;18(3):189–196. [PubMed] [Google Scholar]
- Brierley JB, Meldrum BS, Brown AW. The threshold and neuropathology of cerebral “anoxic-ischemic” cell change. Archives of Neurology. 1973;29(6):367–374. doi: 10.1001/archneur.1973.00490300029003. [DOI] [PubMed] [Google Scholar]
- Bright P, Buckman J, Fradera A, Yoshimasu H, Colchester AC, Kopelman MD. Retrograde amnesia in patients with hippocampal, medial temporal, temporal lobe, or frontal pathology. Learning & Memory (Cold Spring Harbor, N.Y.) 2006;13(5):545–557. doi: 10.1101/lm.265906. doi:10.1101/lm.265906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DN, Baddeley AD. What can amnesic patients learn? Neuropsychologia. 1976;14(1):111–122. doi: 10.1016/0028-3932(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Emotional autobiographical memories in amnesic patients with medial temporal lobe damage. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2005;25(12):3151–3160. doi: 10.1523/JNEUROSCI.4735-04.2005. doi:10.1523/JNEUROSCI.4735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Kirschbaum C. Hippocampal damage abolishes the cortisol response to psychosocial stress in humans. Hormones and Behavior. 2009;56(1):44–50. doi: 10.1016/j.yhbeh.2009.02.011. doi:10.1016/j.yhbeh.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine D, Watson JD. Neuropsychological and neuropathological sequelae of cerebral anoxia: A critical review. Journal of the International Neuropsychological Society : JINS. 2000;6(1):86–99. doi: 10.1017/s1355617700611116. [DOI] [PubMed] [Google Scholar]
- Cavaco S, Anderson SW, Allen JS, Castro-Caldas A, Damasio H. The scope of preserved procedural memory in amnesia. Brain : A Journal of Neurology. 2004;127(Pt 8):1853–1867. doi: 10.1093/brain/awh208. doi:10.1093/brain/awh208. [DOI] [PubMed] [Google Scholar]
- Clark E, Pompa JL. Women and traumatic brain injury. In: Fletcher-Janzen E, editor. The neuropsychology of women. Springer; New York: 2009. pp. 69–86. doi:10.1007/978-0-387-76908-0. [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia and the hippocampal system. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia - dissociation of knowing how and knowing that. Science. 1980;210(4466):207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Corkin S, Amaral DG, González RG, Johnson KA, Hyman BT. H. M.'s medial temporal lobe lesion: Findings from magnetic resonance imaging. Journal of Neuroscience. 1997;17(10):3964–3979. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JD, Bogner JA, Mysiw WJ, Clinchot D, Fugate L. Life satisfaction after traumatic brain injury. The Journal of Head Trauma Rehabilitation. 2001;16(6):543–555. doi: 10.1097/00001199-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Corsellis JAN, Bruton CJ. Neuropathology of status epilepticus in humans. In: Delgado-Escutea AV, Wasterlain CG, Treiman DM, Porter RJ, editors. Status epilepticus. Raven Press; New York, New York: 1983. pp. 129–139. [Google Scholar]
- Damasio AR, Eslinger PJ, Damasio H, Van Hoesen GW, Cornell S. Multimodal amnesic syndrome following bilateral temporal and basal forebrain damage. Archives of Neurology. 1985;42(3):252–259. doi: 10.1001/archneur.1985.04060030070012. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Amnesia caused by herpes simplex encephalitis, infarctions in basal forebrain, Alzheimer's disease and anoxia/ischemia. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Elsevier; Amsterdam: 1989. [Google Scholar]
- Davachi L, Dobbins IG. Declarative memory. Current Directions in Psychological Science : A Journal of the American Psychological Society. 2008;17(2):112–118. doi: 10.1111/j.1467-8721.2008.00559.x. doi:10.1111/j.1467-8721.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis NA, McCampbell E. Booklet category test. Psychological Assessment Resources; Odessa, FL: 1997. [Google Scholar]
- DeGiorgio CM, Tomiyasu U, Gott PS, Treiman DM. Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia. 1992;33(1):23–27. doi: 10.1111/j.1528-1157.1992.tb02278.x. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Caltagirone C, Fadda L, Sabatini U, Serra L, Carlesimo GA. Hippocampal atrophy is the critical brain change in patients with hypoxic amnesia. Hippocampus. 2008;18(7):719–728. doi: 10.1002/hipo.20432. doi:10.1002/hipo.20432. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. Journal of Personality Assessment. 1985;49(1):71–75. doi: 10.1207/s15327752jpa4901_13. doi:10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Dietl T, Urbach H, Helmstaedter C, Staedtgen M, Szentkuti A, Grunwald T, Kurthen M. Persistent severe amnesia due to seizure recurrence after unilateral temporal lobectomy. Epilepsy & Behavior : E &B. 2004;5(3):394–400. doi: 10.1016/j.yebeh.2004.01.004. doi:10.1016/j.yebeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Douglas JM, Bracy CA, Snow PC. Measuring perceived communicative ability after traumatic brain injury: Reliability and validity of the la trobe communication questionnaire. The Journal of Head Trauma Rehabilitation. 2007;22(1):31–38. doi: 10.1097/00001199-200701000-00004. [DOI] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Development of shared information in communication despite hippocampal amnesia. Nature Neuroscience. 2006;9(1):140–146. doi: 10.1038/nn1601. [DOI] [PubMed] [Google Scholar]
- Duff MC, Hengst JA, Tengshe C, Krema A, Tranel D, Cohen NJ. Hippocampal amnesia disrupts the flexible use of procedural discourse in social interaction. Aphasiology. 2008;22(7–8):866–880. doi: 10.1080/02687030701844196. doi:10.1080/02687030701844196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst JA, Tranel D, Cohen NJ. Talking across time: Using reported speech as a communicative resource in amnesia. Aphasiology. 2007;21(6–8):702716. doi: 10.1080/02687030701192265. doi:10.1080/02687030701192265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Wszalek T, Tranel D, Cohen NJ. Successful life outcome and management of real-world memory demands despite profound anterograde amnesia. Journal of Clinical and Experimental Neuropsychology. 2008:1–15. doi: 10.1080/13803390801894681. doi:10.1080/13803390801894681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. Oxford University Press; New York, New York: 2001. [Google Scholar]
- Engberg AW, Teasdale TW. Psychosocial outcome following traumatic brain injury in adults: A long-term population-based follow-up. Brain Injury : [BI] 2004;18(6):533–545. doi: 10.1080/02699050310001645829. doi:10.1080/02699050310001645829. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Duff MC, Tranel D. Sustained experience of emotion after loss of memory in patients with amnesia. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(17):7674–7679. doi: 10.1073/pnas.0914054107. doi:10.1073/pnas.0914054107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Rudrauf D, Khalsa SS, Cassell MD, Bruss J, Grabowski TJ, Tranel D. Bilateral limbic system destruction in man. Journal of Clinical and Experimental Neuropsychology. 2010;32(1):88–106. doi: 10.1080/13803390903066873. doi:10.1080/13803390903066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa DG. The temporal evolution of neuronal damage from pilocarpine-induced status epilepticus. Brain Research. 1996;725(1):11–22. doi: 10.1016/0006-8993(96)00203-x. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG. Prolonged seizures and cellular injury: Understanding the connection. Epilepsy & Behavior : E&B. 2005;7(Suppl 3):S3–11. doi: 10.1016/j.yebeh.2005.08.003. doi:10.1016/j.yebeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Gil-Nagel A, Risinger MW. Ictal semiology in hippocampal versus extrahippocampal temporal lobe epilepsy. Brain : A Journal of Neurology. 1997;120(Pt 1):183–192. doi: 10.1093/brain/120.1.183. Pt 1. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Schacter DL, Tulving E. Learning and retention of computer-related vocabulary in memory-impaired patients: Method of vanishing cues. Journal of Clinical and Experimental Neuropsychology. 1986;8(3):292–312. doi: 10.1080/01688638608401320. [DOI] [PubMed] [Google Scholar]
- Golden C. Stroop color and word test. Stoelting; Chicago, IL: 1978. [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Lea & Febiger; Philadelphia, PA: 1972. [Google Scholar]
- Graf P, Squire LR, Mandler G. The information that amnesic patients do not forget. Journal of Experimental Psychology.Learning, Memory, and Cognition. 1984;10(1):164–178. doi: 10.1037//0278-7393.10.1.164. [DOI] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48(4):831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. doi:10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Grant D, Berg E. Wisconsin card sorting test. Psychological Assessment Resources; Lutz, FL: 1993. [Google Scholar]
- Grubb NR, Fox KA, Smith K, Best J, Blane A, Ebmeier KP, O'Carroll RE. Memory impairment in out-of-hospital cardiac arrest survivors is associated with global reduction in brain volume, not focal hippocampal injury. Stroke; a Journal of Cerebral Circulation. 2000;31(7):1509–1514. doi: 10.1161/01.str.31.7.1509. [DOI] [PubMed] [Google Scholar]
- Guerreiro CA, Jones-Gotman M, Andermann F, Bastos A, Cendes F. Severe amnesia in epilepsy: Causes, anatomopsychological considerations, and treatment. Epilepsy & Behavior : E&B. 2001;2(3):224–246. doi: 10.1006/ebeh.2001.0167. doi:10.1006/ebeh.2001.0167. [DOI] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47(7):1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. doi:10.1016/j.neuropsychologia.2009.02.007 ER. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ryan JD, Tranel D, Cohen NJ. Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. Journal of Cognitive Neuroscience. 2007;19:1690–1705. doi: 10.1162/jocn.2007.19.10.1690. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. Journal of Neuroscience. 2006;26(32):8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Cezayirli E, Isaac CL, Aggleton JP, Roberts N. A comparison of egocentric and allocentric spatial memory in a patient with selective hippocampal damage. Neuropsychologia. 2000;38(4):410–425. doi: 10.1016/s0028-3932(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hooper HE. The hooper visual organization test: Manual. Western Psychological Service; Los Angeles: 1958. [Google Scholar]
- Horstmann A, Frisch S, Jentzsch RT, Muller K, Villringer A, Schroeter ML. Resuscitating the heart but losing the brain: Brain atrophy in the aftermath of cardiac arrest. Neurology. 2010;74(4):306–312. doi: 10.1212/WNL.0b013e3181cbcd6f. doi:10.1212/WNL.0b013e3181cbcd6f. [DOI] [PubMed] [Google Scholar]
- Jastak S, Wilkinson G. The wide range achievement test: Manual of instructions. Jastak Associates; Wilmington, DE: 1984. [Google Scholar]
- Jones R, Grabowski T, Tranel D. The neural basis of retrograde memory: Evidence from positron emission tomography for the role of non-mesial temporal lobe structures. Neurocase. 1998;4 [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The boston naming test. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Research. 1982;239(1):57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Knake S, Hamer HM, Rosenow F. Status epilepticus: A critical review. Epilepsy & Behavior : E&B. 2009;15(1):10–14. doi: 10.1016/j.yebeh.2009.02.027. doi:10.1016/j.yebeh.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Frontiers in Human Neuroscience. 2008;2:15. doi: 10.3389/neuro.09.015.2008. doi:10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz A, Sung GY, Fisher RS, Barry E, Bergey GK, Grattan LM. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology. 1995;45(8):1499–1504. doi: 10.1212/wnl.45.8.1499. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Thompson PM. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cerebral Cortex (New York, N.Y.: 1991) 2007;17(9):2007–2018. doi: 10.1093/cercor/bhl109. doi:10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- Lucas E. R é cr é ations math é matiques. Gauthier-Villars; Paris: 1881. [Google Scholar]
- Malec JF, Lezak MD. Manual for the mayo-portland adaptability inventory (MPAI-4) 2008 [Google Scholar]
- Matthews CG, Klove H. Instruction manual for the adult neuropsychology battery. University of Wisconsin Medical School; Madison, WI: 1964. [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12(3):325–340. doi: 10.1002/hipo.1111. doi:10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Isaac CL, Holdstock JS, Hunkin NM, Montaldi D, Downes JJ, Roberts JN. Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cognitive Neuropsychology. 2001;18(2):97–123. doi: 10.1080/02643290125897. doi:10.1080/02643290125897. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Kopelman MD, Warrington EK. Remembering and forgetting of semantic knowledge in amnesia: A 16-year follow-up investigation of RFR. Neuropsychologia. 2005;43(3):356–372. doi: 10.1016/j.neuropsychologia.2004.06.024. doi:10.1016/j.neuropsychologia.2004.06.024. [DOI] [PubMed] [Google Scholar]
- McKinley JC, Hathaway SR. The minnesota multiphasic personality inventory. V. hysteria, hypomania and psychopathic deviate. Journal of Applied Psychology. 1944;23(2):153–174. doi:10.1037/h0059245. [Google Scholar]
- Meldrum BS. First alfred meyer memorial lecture. epileptic brain damage: A consequence and a cause of seizures. Neuropathology and Applied Neurobiology. 1997;23(3):185–201. discussion 201-2. [PubMed] [Google Scholar]
- Meyer A, Beck E, Shepherd M. Unusually severe lesions in the brain following status epilepticus. Journal of Neurology, Neurosurgery, and Psychiatry. 1955;18(1):24–33. doi: 10.1136/jnnp.18.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Psychologie de l'hippocampe. Centre National de la Recherche Scientifique; Paris, France: 1962. Les trouble de la memoire accompagnant des lesions hippocampiques balaterals; p. 272. [Google Scholar]
- Moscovitch M, Nadel L. Consolidation and the hippocampal complex revisited: In defense of the multiple-trace model. Current Opinion in Neurobiology. 1998;8(2):297–300. doi: 10.1016/s0959-4388(98)80155-4. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account based on multiple trace theory. Journal of Anatomy. 2005;207(1):35–66. doi: 10.1111/j.1469-7580.2005.00421.x. doi:10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Stables L, Du AT, Schuff N, Truran D, Cashdollar N, Weiner MW. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4T. Neurobiology of Aging. 2007;28(5):719–726. doi: 10.1016/j.neurobiolaging.2006.03.007. doi:10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997;7(2):217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Willison J. National adult reading test (NART) test manual. 2nd ed. NFER-Nelson; London: 1991. [Google Scholar]
- Nevander G, Ingvar M, Auer R, Siesjo BK. Status epilepticus in well-oxygenated rats causes neuronal necrosis. Annals of Neurology. 1985;18(3):281–290. doi: 10.1002/ana.410180303. doi:10.1002/ana.410180303. [DOI] [PubMed] [Google Scholar]
- Ng T, Graham DI, Adams JH, Ford I. Changes in the hippocampus and the cerebellum resulting from hypoxic insults: Frequency and distribution. Acta Neuropathologica. 1989;78(4):438–443. doi: 10.1007/BF00688181. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe. Arch. Psychol. 1944;30:206. [Google Scholar]
- Oxbury S, Oxbury J, Renowden S, Squier W, Carpenter K. Severe amnesia: An usual late complication after temporal lobectomy. Neuropsychologia. 1997;35(7):975–988. doi: 10.1016/s0028-3932(97)00025-0. [DOI] [PubMed] [Google Scholar]
- Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37(8):1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: Minimizing the discrepancies between laboratories. Cerebral Cortex (New York, N.Y.: 1991) 2000;10(4):433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Radovsky A, Katz L, Ebmeyer U, Safar P. Ischemic neurons in rat brains after 6, 8, or 10 minutes of transient hypoxic ischemia. Toxicologic Pathology. 1997;25(5):500–505. doi: 10.1177/019262339702500512. [DOI] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. doi:10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead – Reitan neuropsychological test battery. Neuropsychology Press; Tucson, AZ: 1985. [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1996;16(16):5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Arch. Psychol. 1941;28:286. [Google Scholar]
- Ribot T. Les maladies de la memoire. Germer Bailliere; Paris: 1881. [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychological Science. 2000;11(6):454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Salat DH, van der Kouwe AJ, Tuch DS, Quinn BT, Fischl B, Dale AM, Corkin S. Neuroimaging H.M.: A 10-year follow-up examination. Hippocampus. 2006;16(11):936–945. doi: 10.1002/hipo.20222. doi:10.1002/hipo.20222. [DOI] [PubMed] [Google Scholar]
- Sanders HI, Warrington EK. Memory for remote events in amnesic patients. Brain : A Journal of Neurology. 1971;94(4):661–668. doi: 10.1093/brain/94.4.661. [DOI] [PubMed] [Google Scholar]
- Scoville WB. The limbic lobe in man. Journal of Neurosurgery. 1954;11(1):64–66. doi: 10.3171/jns.1954.11.1.0064. doi:10.3171/jns.1954.11.1.0064. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery & Psychiatry. 1957;(20):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan AD. Benton visual retention test. 5th ed. The Psychological Corporation; New York, New York: 1992. [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Current Opinion in Neurobiology. 1995;5(2):169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Amaral DG, Zola-Morgan S, Kritchevsky M, Press G. Description of brain injury in the amnesic patient N.A. based on magnetic resonance imaging. Experimental Neurology. 1989;105(1):23–35. doi: 10.1016/0014-4886(89)90168-4. [DOI] [PubMed] [Google Scholar]
- Squire LR, Haist F, Shimamura AP. The neurology of memory: Quantitative assessment of retrograde amnesia in two groups of amnesic patients. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1989;9(3):828–839. doi: 10.1523/JNEUROSCI.09-03-00828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learning & Memory (Cold Spring Harbor, N.Y.) 2010;17(6):284–288. doi: 10.1101/lm.1768110. doi:10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Buffalo EA, Schmolck H, Squire LR. Profound amnesia after damage to the medial temporal lobe: A neuroanatomical and neuropsychological profile of patient E. P. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2000;20(18):7024–7036. doi: 10.1523/JNEUROSCI.20-18-07024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhr J, Anderson S, Tranel D. Progressive muscle relaxation in the management of behavioural disturbance in alzheimer's disease. Neuropsychological Rehabilitation. 1999;9(1):31–44. doi:10.1080/713755590. [Google Scholar]
- Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Tymianski M. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nature Neuroscience. 2009;12(10):1300–1307. doi: 10.1038/nn.2395. doi:10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- Tate R. Emotional and social consequences of memory disorders. In: Baddeley AD, Kopelman MD, Wilson BA, editors. The essential handbook of memory disorders for clinicians. Wiley & Sons; Chichester, UK: 2004. pp. 329–352. [Google Scholar]
- Teasdale TW, Engberg AW. Psychosocial consequences of stroke: A long-term population-based follow-up. Brain Injury : [BI] 2005;19(12):1049–1058. doi: 10.1080/02699050500110421. doi:10.1080/02699050500110421. [DOI] [PubMed] [Google Scholar]
- Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20(1):1–10. doi: 10.1037/0894-4105.20.1.1. doi:10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- Tranel D, Enekwechi N, Manzel K. A test for measuring recognition and naming of landmarks. Journal of Clinical and Experimental Neuropsychology. 2005;27(1):102–126. doi: 10.1080/138033990513663. doi:10.1080/138033990513663. [DOI] [PubMed] [Google Scholar]
- Tranel D, Jones RD. Knowing “what” and knowing “when”. Journal of Clinical and Experimental Neuropsychology. 2006;28(1):43–66. doi: 10.1080/13803390490919344. doi:10.1080/13803390490919344. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Petito CK. Dementia with bilateral medial temporal lobe ischemia. Neurology. 1985;35(12):1793–1797. doi: 10.1212/wnl.35.12.1793. [DOI] [PubMed] [Google Scholar]
- Voss JL, Warren DE, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Spontaneous revisitation during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):E402–9. doi: 10.1073/pnas.1100225108. doi:10.1073/pnas.1100225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M, Chan D, Thom M. Hippocampus and human disease. In: Anderson P, Morris R, Amaral DG, O'Keefe J, editors. The hippocampus book. Oxford University Press; New York, New York: 2007. pp. 769–812. [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ. Medial temporal lobe damage impairs representation of simple stimuli. Frontiers in Human Neuroscience. 2010;4:35. doi: 10.3389/fnhum.2010.00035. doi:10.3389/fnhum.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ. Observing degradation of visual representations over short intervals when medial-temporal lobe is damaged. Journal of Cognitive Neuroscience. 2011 doi: 10.1162/jocn_a_00089. doi:10.1162/jocn_a_00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK. Recognition memory test. NFER-Nelson; Berkshire, UK: 1984. [Google Scholar]
- Warrington EK, Weiskrantz L. New method of testing long-term retention with special reference to amnesic patients. Nature. 1968;217(5132):972–974. doi: 10.1038/217972a0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-revised manual. The Psychological Corporation; New York, New York: 1981. [Google Scholar]
- Wechsler D. Wechsler memory scale-revised manual. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Wechsler D. Wechsler adult intelligence Scale. Third edition The Psychological Corporation; New York, New York: 1997a. [Google Scholar]
- Wechsler D. Wechsler memory Scale. Third edition The Psychological Corporation; New York, New York: 1997b. [Google Scholar]
- Wieshmann UC, Symms MR, Mottershead JP, MacManus DG, Barker GJ, Tofts PS, Shorvon SD. Hippocampal layers on high resolution magnetic resonance images: Real or imaginary? Journal of Anatomy. 1999;195(Pt 1):131–135. doi: 10.1046/j.1469-7580.1999.19510131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BA. Cognitive functioning of adult survivors of cerebral hypoxia. Brain Injury : [BI] 1996;10(12):863–874. doi: 10.1080/026990596123846. [DOI] [PubMed] [Google Scholar]
- Wilson BA. Jay: Compensating for amnesia. In: Wilson BA, editor. Case studies in neuropsychological rehabilitation. Oxford University Press; New York: 1999. pp. 43–54. [Google Scholar]
- Wilson BA, Wearing D. Prisoner of consciousness: A state of just awakening following herpes simplex encephalitis. In: Campbell R, Conway MA, editors. Broken memories: Case studies in memory impairment. Blackwell; Cambridge, MA: 1995. pp. 14–30. [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. NeuroImage. 2010;51(3):1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. doi:10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1986a;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1986b;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]