Abstract
A bicyclization approach to englerin A has culminated in a formal asymmetric total synthesis. Key transformations in the 10-step sequence are a regiospecific epoxide opening and a relay ene-yne-ene metathesis that converts linear substrates specifically to Δ4,6-guaiadiene-9,10 diol derivatives. Regiospecific functionalization of the diene moiety installs the oxygen bridge required for the englerin tricyclic core.
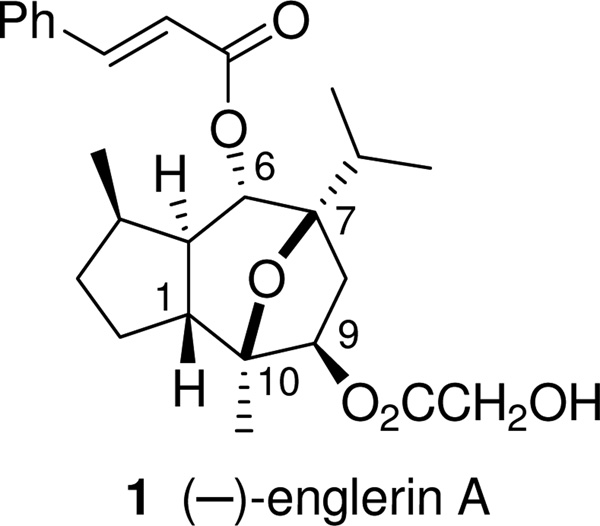
(−)-Englerin A is a natural product from Phyllathus engleri, a plant common in East Africa.1 It displays selective and potent inhibition of the growth of renal cancer cell lines in the NCI-60 screen.
The compact and intriguing structure of this new lead has inspired several total syntheses.2 The strategies conceived for the total synthesis of (−)-englerin A have provided analogs and these have contributed to the development of a preliminary structure-activity relationship (SAR).3 In light of the unique activity profile of (−)-englerin A, one can anticipate optimization of its pharmacological properties by the exploitation of mechanism of action studies and also by more extensive medicinal chemistry. In this context, each total synthesis is valuable in that it establishes proof-of-principle for a synthetic route to the tricyclic framework and also provides opportunities for variations on the scaffold itself.
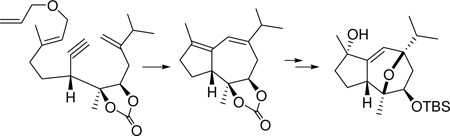
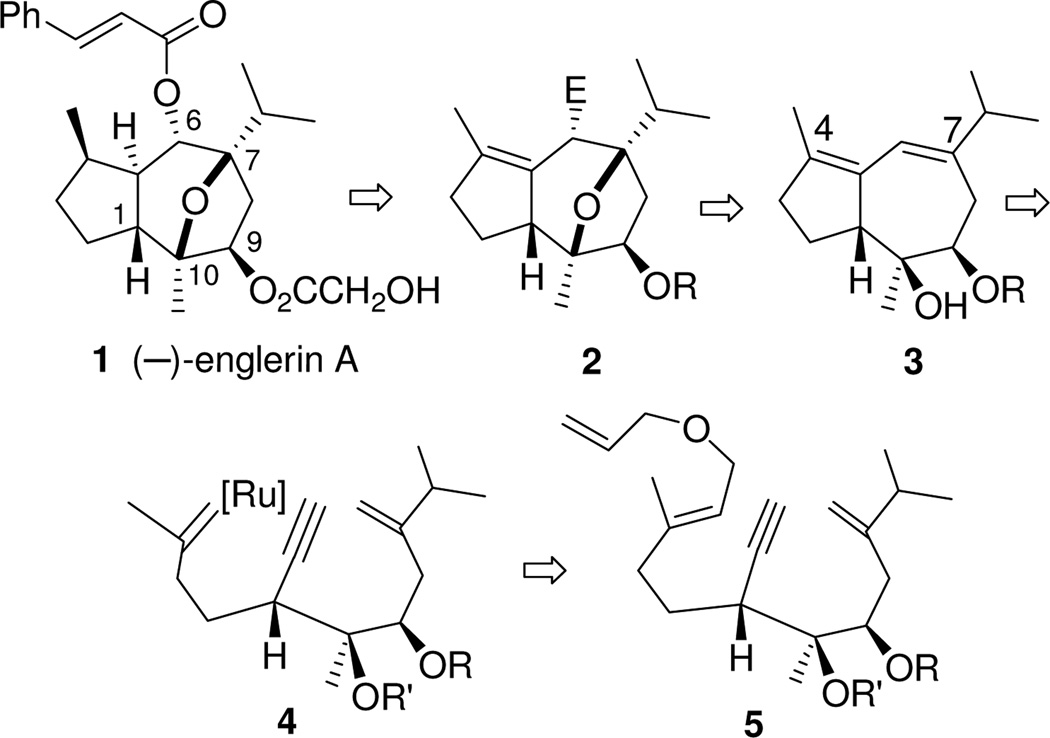
Our analysis of the structure of the englerin core (Scheme 1) led us to consider the hydroazulene 3 as the product of an ene-yne-ene metathesis-based bicyclization.4 In this approach, bicyclic 3 would arise from tandem ring closure initiated by the ruthenium carbene 4. Site-specific generation of this reactive intermediate required that our strategy be developed to include a relay metathesis step.5 Thus, we designed substrate 5. A metathesis cascade, specifically and conveniently initiated at the monosubstituted olefin of allyl ether 5, was envisioned to proceed by way of ruthenium carbene 4 to give the desired 3.
Scheme 1.
Retrosynthetic Analysis
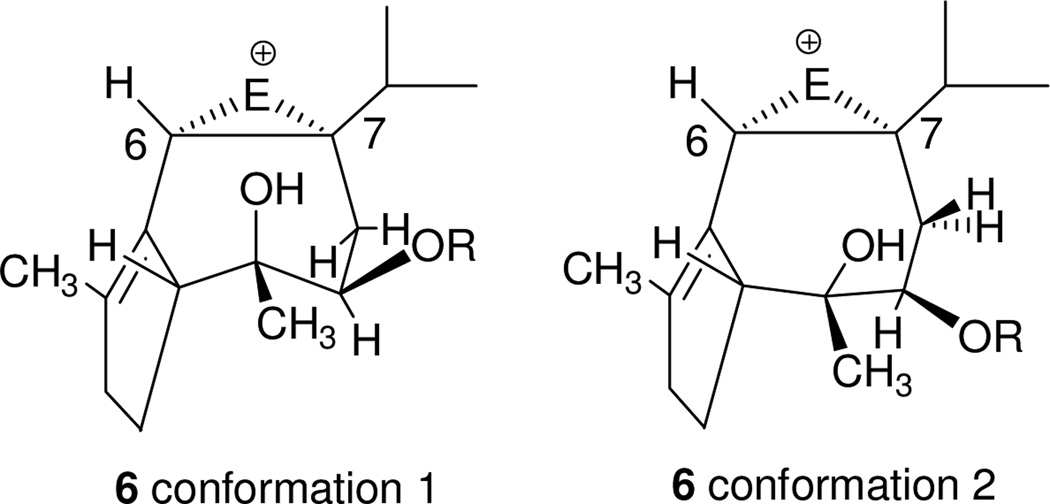
In order to complete a synthesis based on hydroazulene 3 with efficiency, we would have to differentiate the two double bonds so as to further the synthetic scheme. We postulated that reversible addition of a soft electrophile to diene 3 would be accompanied by transannular etherification across the 7-membered ring, leaving the cyclopentene olefin untouched (3 -> 2). Indeed, examination of the two unstrained conformations available to an intermediate bridged cation 6 (Figure 2) led us to believe that the hydroxyl nucleophile would effect ring opening by attack at C-7 (conformation 2) as it cannot adopt a position that would allow backside attack at C-6.
Figure 2.
Two conformations available to bridged cation 6
Our strategy was attractive because it appeared that the relay ring closing metathesis (RRCM) substrate 5 would be readily available from geraniol (7a). Opening the ring of a 2,3-disubstituted epoxy alcohol at the 3-position (see 9 -> 10) seemed likely to provide access to the desired functionality pattern for substrate 5. Implementation of a scheme based on these original concepts has now progressed to a formal total synthesis of (−)-englerin A.
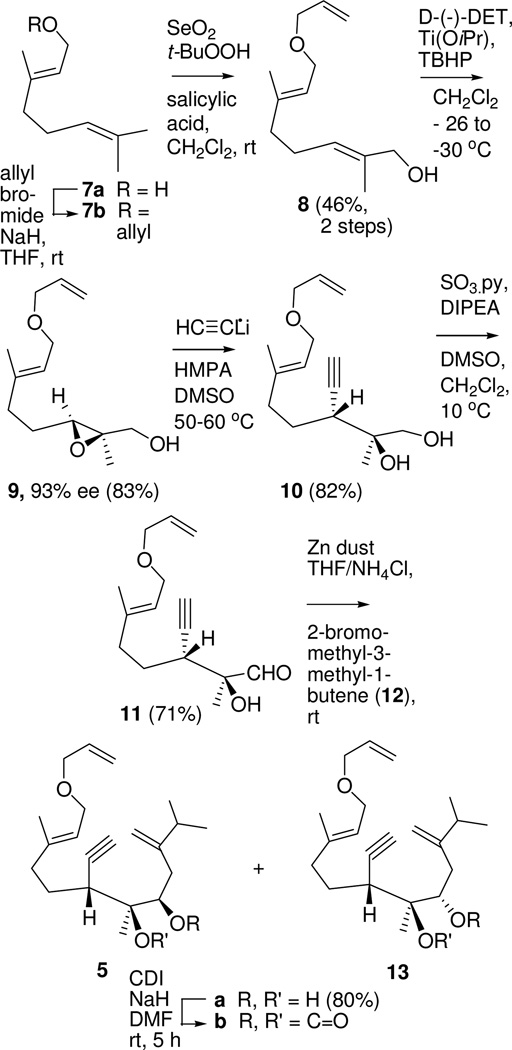
Geraniol (7a) was converted to a mixture of diol derivatives 5b and 13b in seven steps (Scheme 2). O-Allylation followed by catalytic SeO2 oxidation6 gave a useful yield of the (E)-allylic alcohol 8. Then Sharpless epoxidation7 introduced chirality8 and provided an opportunity to incorporate the required alkyne substituent with the desired stereochemistry at the latent C-1 (see 9).
Scheme 2.
Synthesis of RRCM Substrate 5
We were surprised that we were unable to find an unbiased example of an acetylide opening of a 2-alkyl 2,3-epoxy alcohol or a derivative of such an alcohol.9,10 Consequently we tested conditions reported to effect ring opening of 3-substituted 2,3-epoxy alcohols and their derivatives. Of these, the most successful was treatment of the epoxide opening with Li-acetylide ethylene diamine complex in HMPA/DMSO.11 This protocol provided diol 10 in 82% yield.
Parikh-Doering oxidation12 gave aldehyde 11 which was subjected to a Barbier addition13 with the reagent from 2-bromomethyl-3-methyl-1-butene (12).14 This produced a mixture of the desired diol 5a and its C-9 epimer, diol 13a. With the goal of obtaining derivatives that would allow us to assign unambiguously the relative stereochemistries of the two diastereomers and to test metathesis conditions, we prepared the cyclic carbonates 5b and 13b by treating the diol mixture with carbonyl diimidazole (CDI).
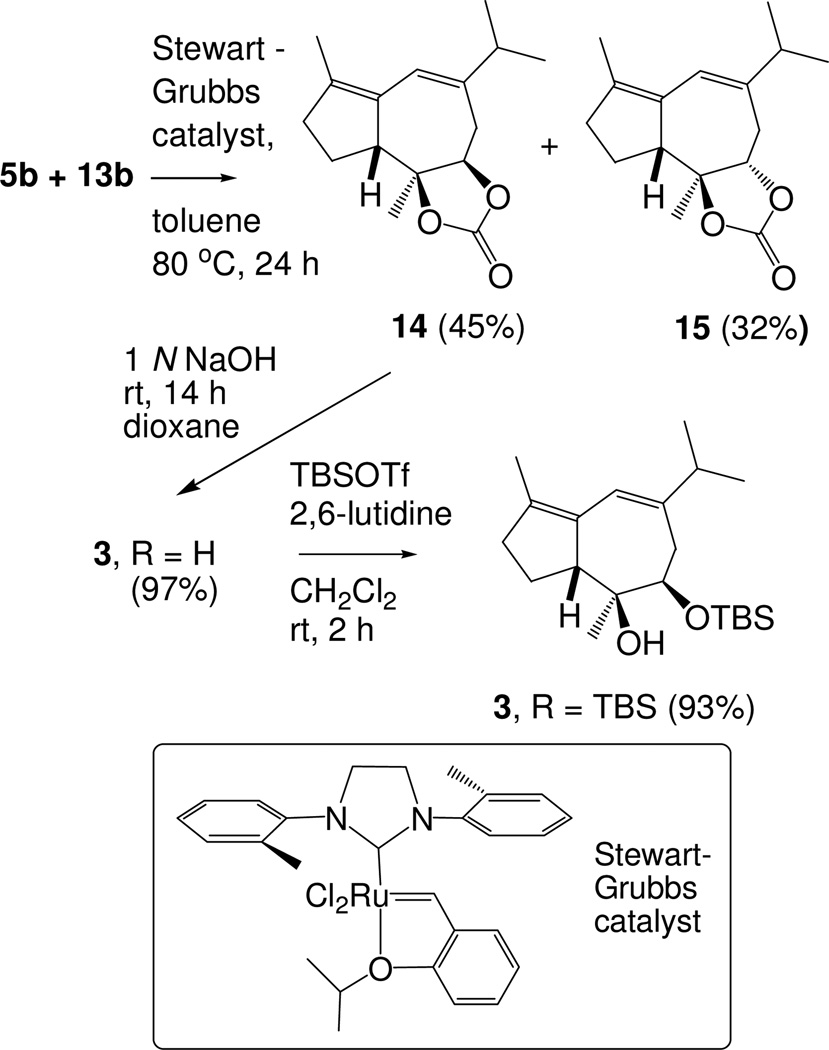
Relay ring closing metathesis (RRCM) of the carbonate mixture (5b+13b) with the Stewart-Grubbs catalyst15 gave a mixture of stereoisomers 14 and 15 (Scheme 3). This reaction is impressive in several respects: (1) only the guaiadiene ring system is generated; no decalin derivative was detected,16 (2) the formation of the cyclopentene ring illustrates the capability of relay-initiated enyne metathesis to produce tetrasubstituted olefins, and (3) both carbonate stereoisomers 5b and 13b undergo bicyclization, giving 14 and the more conformationally rigid 15, indicating the power of the Stewart-Grubbs catalyst.
Scheme 3.
RRCM and Preparation of Transannular Etherification Substrate
The major compound, isolated by chromatography in 45% yield, was assigned structure 14 on the basis of nuclear Overhauser effects (see the Supporting Information). Hydrolytic removal of the carbonate group gave diol 3 (R = H) and selective silylation of the secondary alcohol gave cyclization substrate 3 (R = TBS) in 90% yield for the two steps.
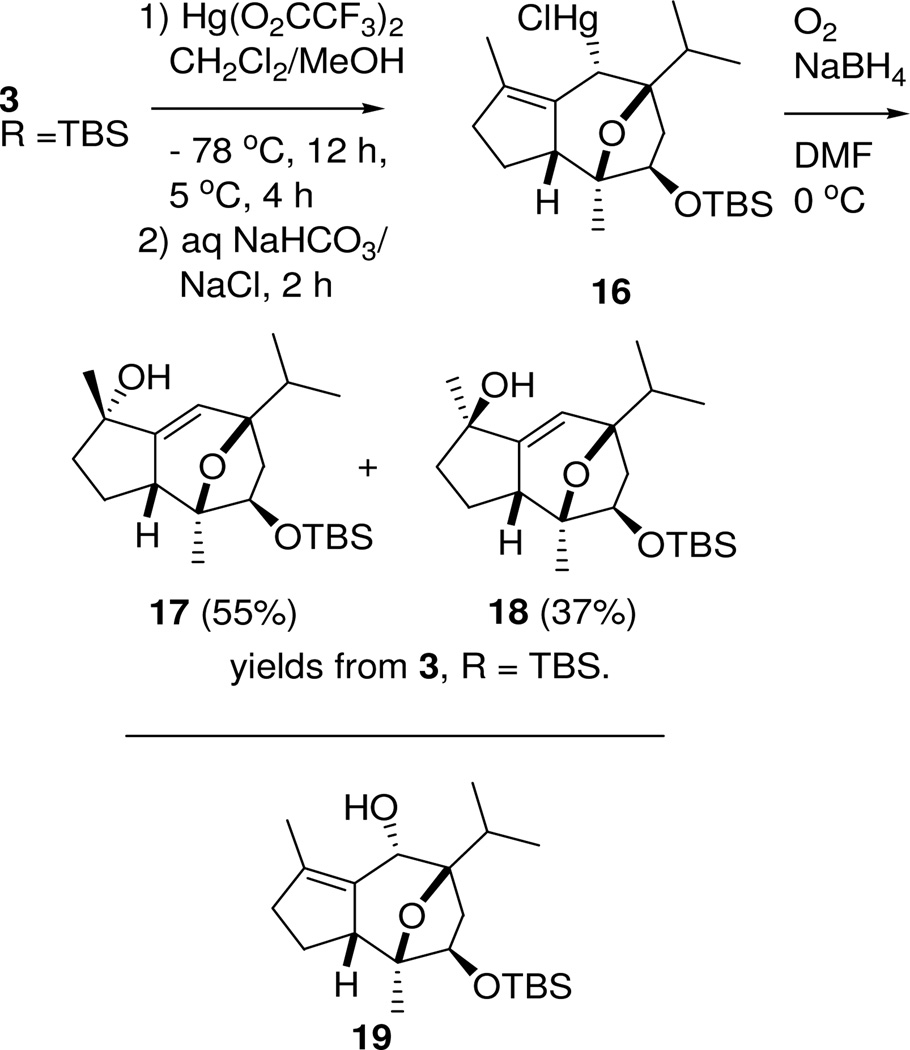
After some experimentation, we found conditions for introduction of the oxygen bridge. Our hope of effecting transannular opening of a Δ6,7 epoxide was disappointed when treatment of diene 3 (R = TBS) with mCPBA provided a product in which the olefinic proton was retained. Attempts to connect the oxygen bridge by haloetherification were uniformly unsuccessful, leading in most cases to the recovery of starting material. Oxymercuration with Hg(O2CCF3)2 followed by addition of NaCl/NaHCO3 solution17 gave the alkyl mercurial 16 from regio- and stereospecific addition to the Δ6,7 olefin18 (Scheme 4). Therefore, product formation was consistent with the proposed concerted addition portrayed in Figure 2.
Scheme 4.
Oxymercuration / oxidative demercuration of hydroazulene 3
Oxidative demercuration19 with NaBH4 and O2 in DMF provided a mixture of stereoisomeric tertiary alcohols 17 and 18 in which the functionality pattern of the allylic system has been reversed. Separation of the two diastereomers resulted in isolation of a 55% yield of the α-alcohol 17 and 37% yield of the β-isomer 18. Although the ionic transannular oxymercuration reaction was specific in the desired sense, oxidative demercuration through the radical intermediate resulted in oxygenation at C-4 rather than at C-6.
Although we would have preferred to obtain the known Δ4,5 C-6 alcohol 1920 directly, alcohol 17 has been converted to (−)-englerin A in seven steps (by way of alcohol 19).20 Therefore, access to alcohol 17 completes a formal synthesis of (−)-englerin A (1).
The synthesis of alcohol 17 illustrates the efficient opening of the epoxide ring of a β-substituted α-epoxy alcohol under the lithium acetylide conditions and the relay ene-yne-ene metathesis method for the preparation of a bicyclic diene that is disubstituted on both ends and that contains a tetrasubstituted olefin. Furthermore, the conversion of diene 3 to the mercurial 16 provides proof-of-concept for the soft electrophile-initiated regio- and stereoselective transannular etherification of Δ4,5, Δ6,7 guaiadienes. Approaches to compounds in the englerin series remain under investigation in our laboratories.
Supplementary Material
Figure 1.
Structure of englerin A
Acknowledgment
This work was supported by the National Institutes of Health (GM 74776), by ChemMaster International, and by the National Science Foundation (CHE-0131146, NMR instrumentation). We are grateful to Robert H. Grubbs (Caltech) and Ian Stewart (currently at ExxonMobil Chemical Company) for an early gift of the Stewart-Grubbs catalyst.
Footnotes
Supporting Information Available. Experimental procedures with analytical data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ratnayake R, Covell D, Ransom TT, Gustafson KR, Beutler JA. Org. Lett. 2009;11:57. doi: 10.1021/ol802339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For completed syntheses and approaches, see the following reviews Chain WJ. Synlett. 2011:2605. Pouwer RH, Richard J-A, Tseng C-C, Chen DYK. Chem. Asian J. 2012;7:22. doi: 10.1002/asia.201100780. and Lu Y, Yao H-Q, Sun B-F, Chin J. Org. Chem. 2012;32:1. Also see Wang CL, Sun B-F, Chen S-G, Ding R, Lin G-Q, Xu J-Y, Shang Y-J. Synlett. 2012;23:263.
- 3.a Akee RK, Ransom T, Ratnayake R, McMahon JB, Beutler JA. J. Nat. Prod. 2012;75:459. doi: 10.1021/np200905u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ushakov DB, Navickas V, Strobele M, Maichle-Mossmer C, Sasse F, Maier ME. Org. Lett. 2011;13:2090. doi: 10.1021/ol200499t. [DOI] [PubMed] [Google Scholar]; c Chan KP, Chen DY-K. ChemMedChem. 2011;6:420. doi: 10.1002/cmdc.201000544. [DOI] [PubMed] [Google Scholar]; d Xu J, Caro-Diaz EJE, Batova A, Sullivan SDE, Theodorakis EA. Chem.- Asian J. 2012;7:1052. doi: 10.1002/asia.201101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Lee D. Eur. J. Org. Chem. 2011:4269. [Google Scholar]
- 5.Hoye TR, Jeffrey CS, Tennakoon MA, Wang J, Zhao H. J. Am. Chem. Soc. 2004;126:10210. doi: 10.1021/ja046385t. [DOI] [PubMed] [Google Scholar]
- 6.a Umbreit MA, Sharpless KB. J. Am. Chem. Soc. 1977;99:5525. [Google Scholar]; b Gaich T, Mulzer J. Org. Lett. 2010;12:272. doi: 10.1021/ol902594b. [DOI] [PubMed] [Google Scholar]
- 7.a Gao Y, Hanson RM, Klunder JM, Ko SY, Masamune H, Sharpless KB. J. Am. Chem. Soc. 1987;109:5765. [Google Scholar]; b Ichige T, Okano Y, Kanoh N, Nakata M. J. Org. Chem. 2009;74:230. doi: 10.1021/jo802249k. [DOI] [PubMed] [Google Scholar]; c Bravo F, McDonald FE, Neiwert WA, Hardcastle KI. Org. Lett. 2004;6:4487. doi: 10.1021/ol048212e. [DOI] [PubMed] [Google Scholar]
- 8.The enantiomeric excess of the Sharpless product was calculated from the 1H nmr spectra of its Mosher esters; see the Supporting Information.
- 9.See however the regiospecific ring opening of vinyl substituted examples: Narjes F, Schaumann E. Liebigs Ann. Chem. 1993:841.
- 10.In addition one might note the successful addition of acetylide to the 3-position of 2-substituted 2,3-epoxy esters: Bartlett PA, Tanzella DJ, Barstow JF. J. Org. Chem. 1982;47:3941.
- 11.Parker KA, Chang W. Org. Lett. 2005;7:1785. doi: 10.1021/ol050356l. [DOI] [PubMed] [Google Scholar]; Inanaga J, Kawanami Y, Yamaguchi M. Bull. Chem. Soc. Jpn. 1986;59:1521. [Google Scholar]
- 12.Parikh JR, von Doering WE. J. Am. Chem. Soc. 1967;89:5505. [Google Scholar]
- 13.Petrier C, Einhorn J, Luche JL. Tetraheron Lett. 1985;26:1449. [Google Scholar]
- 14.The known bromide 12 was prepared from the corresponding alcohol (see the Supporting Information) by the method of Barton DH, Shioiri T, Widdowson DA. J. Chem. Soc. C. 1971:1968.
- 15.Stewart IC, Ung T, Pletnev AA, Berlin JM, Grubbs RH, Schrodi Y. Org. Lett. 2007;9:1589. doi: 10.1021/ol0705144. [DOI] [PubMed] [Google Scholar]
- 16.This result may be compared with the non-specific non-relay ene-yne-ene metathesis of a similar, highly substituted system in which reaction carried out under ethylene is presumably initiated at the acetylene; see Knueppel S, Rogachev VO, Metz P. Eur. J. Org. Chem. 2010:6145.
- 17. Broka CA, Lim Y-T. J. Org. Chem. 1988;53:5876. See also Kang SH, Kim M, Kang SY. Angew. Chem. Int. Ed. 2004;43:6177. doi: 10.1002/anie.200461289.
- 18.One should note that this result is not inconsistent with regiochemistry observed in the oxymercuration of simple conjugated dienes; see Barluenga J, Perez-Prieto J, Asensio GJ. Chem. Soc., Perkin Trans. 1: Org.Bio-Org. Chem. 1984:629.
- 19.Hill CL, Whitesides GM. J. Am. Chem. Soc. 1974;96:870. [Google Scholar]
- 20.This alcohol is a known compound, prepared from alcohol 17 in two steps and 60% yield as described in Molawi K, Delpont N, Echavarren AM. Angew. Chem. Int. Ed. 2010;49:3517. doi: 10.1002/anie.201000890.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.