Abstract
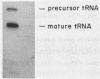
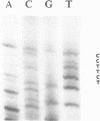
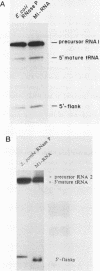
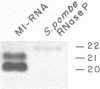
The nucleotide sequence of the gene encoding the Escherichia coli selenocysteine tRNA (tRNA(SeCys] predicts an unusually long acceptor stem of 8 base pairs (one more than other tRNAs). Here we show by in vivo experiments (Northern blots, primer extension analysis) and by in vitro RNA processing studies that E. coli tRNA(SeCys) does contain this additional basepair, and that its formation results from abnormal cleavage by RNase P.
Full text
PDF
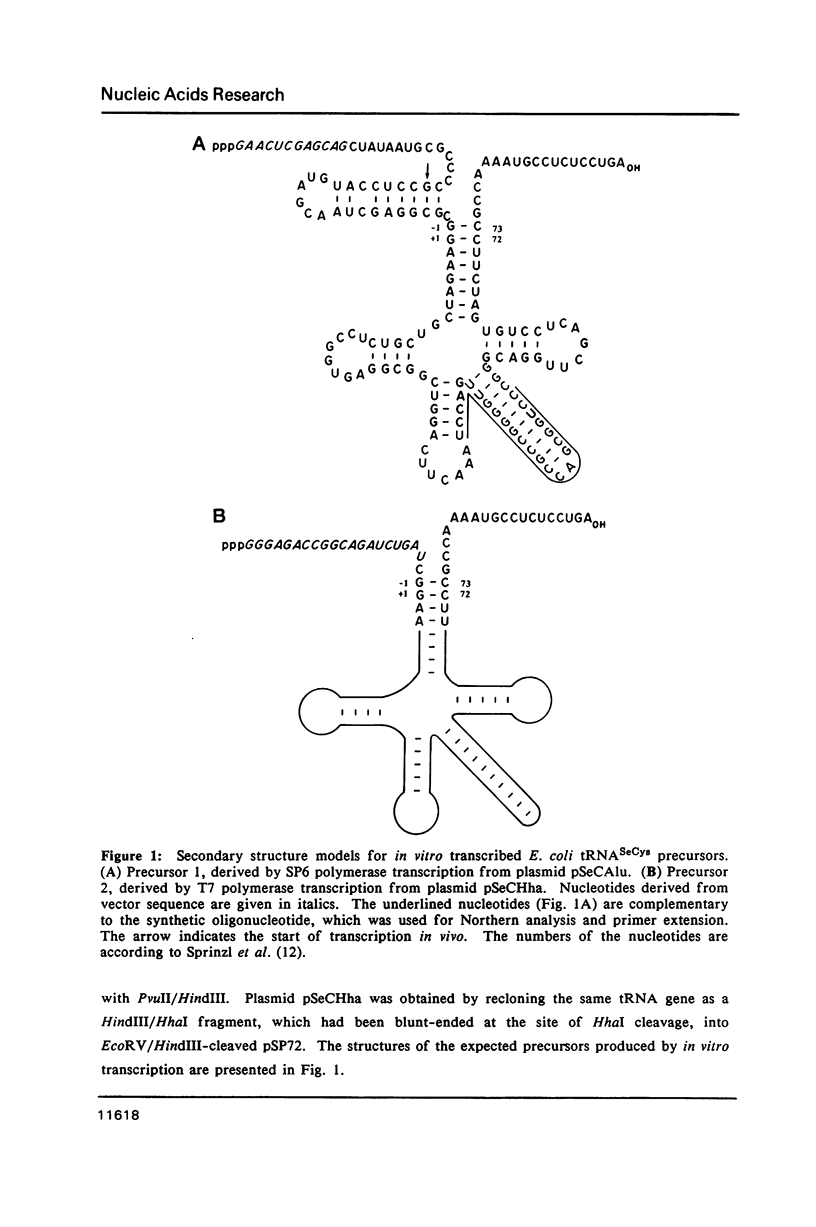


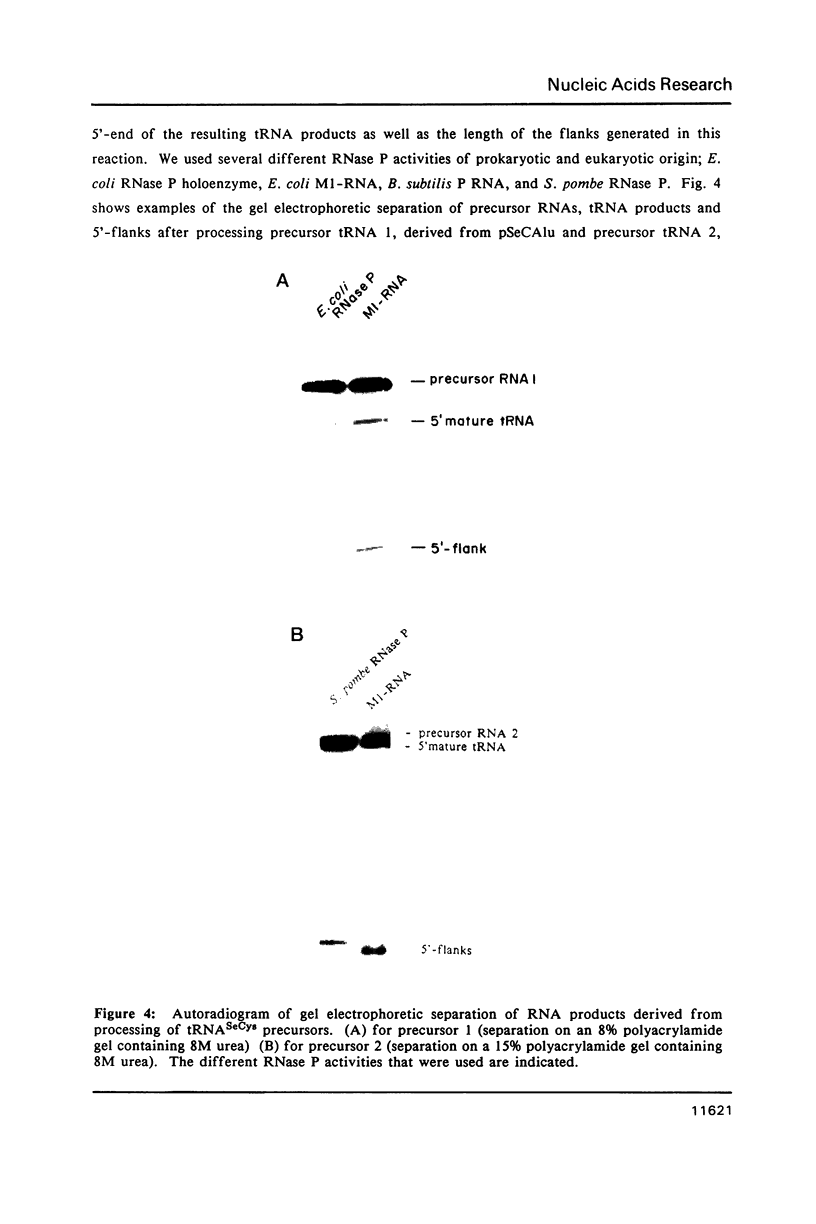



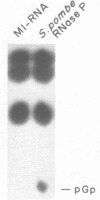
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burkard U., Willis I., Söll D. Processing of histidine transfer RNA precursors. Abnormal cleavage site for RNase P. J Biol Chem. 1988 Feb 15;263(5):2447–2451. [PubMed] [Google Scholar]
- Caillet J., Plumbridge J. A., Springer M., Vacher J., Delamarche C., Buckingham R. H., Grunberg-Manago M. Identification of clones carrying an E. coli tRNAPhe gene by suppression of phenylalanyl-tRNA synthetase thermosensitive mutants. Nucleic Acids Res. 1983 Feb 11;11(3):727–736. doi: 10.1093/nar/11.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. J., Vold B. S. Structural requirements for processing of synthetic tRNAHis precursors by the catalytic RNA component of RNase P. J Biol Chem. 1988 Jan 15;263(2):652–657. [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Krupp G., Cherayil B., Frendewey D., Nishikawa S., Söll D. Two RNA species co-purify with RNase P from the fission yeast Schizosaccharomyces pombe. EMBO J. 1986 Jul;5(7):1697–1703. doi: 10.1002/j.1460-2075.1986.tb04413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988 Feb 25;331(6158):723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- Orellana O., Cooley L., Söll D. The additional guanylate at the 5' terminus of Escherichia coli tRNAHis is the result of unusual processing by RNase P. Mol Cell Biol. 1986 Feb;6(2):525–529. doi: 10.1128/mcb.6.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich C., Gardiner K. J., Olsen G. J., Pace B., Marsh T. L., Pace N. R. The RNA component of the Bacillus subtilis RNase P. Sequence, activity, and partial secondary structure. J Biol Chem. 1986 Jun 15;261(17):7888–7893. [PubMed] [Google Scholar]
- Stadtman T. C. Specific occurrence of selenium in enzymes and amino acid tRNAs. FASEB J. 1987 Nov;1(5):375–379. doi: 10.1096/fasebj.1.5.2445614. [DOI] [PubMed] [Google Scholar]
- Söll D. Genetic code: enter a new amino acid. Nature. 1988 Feb 25;331(6158):662–663. doi: 10.1038/331662a0. [DOI] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Leinfelder W., Böck A. Cotranslational insertion of selenocysteine into formate dehydrogenase from Escherichia coli directed by a UGA codon. Proc Natl Acad Sci U S A. 1987 May;84(10):3156–3160. doi: 10.1073/pnas.84.10.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]